Abstract
A syndrome of hepatosplenomegaly, thrombocytopenia, and anemia and the presence of sea-blue histiocytes in bone marrow has been associated with parenteral soybean oil administration in patients receiving long-term total parenteral nutrition (TPN). A case is described here where this syndrome was observed in a pediatric patient who received long-term parenteral fish oil nutrition.
INDEX TERMS: fatty acids, fish oils, omega-3, parenteral nutrition, sea-blue histiocyte syndrome, soybean oil
INTRODUCTION
Sea-blue histiocyte syndrome is a rare disorder of abnormal lipid metabolism and the finding of macrophages in the liver and bone marrow that contain unsaturated, intracytoplasmic phospholipid that stains a deep blue color upon application of Giemsa solution.1 Clinical characteristics of the condition include thrombocytopenia, anemia, splenomegaly, and hepatomegaly. Approximately 15% of cases progress to liver cirrhosis and portal hypertension.2 The disorder has been reported as a secondary phenomenon in diseases that either increase the production of lipid, such as chronic myeloid leukemia, idiopathic thrombocytopenic purpura, and myelodysplastic syndromes, or decrease the catabolism of lipid, as in the Niemann-Pick and Gaucher lysosomal storage diseases.3 Sea-blue histiocyte syndrome has also been reported in adults receiving long-term parenteral nutrition with soybean oil emulsion.4,5 We describe a case here of a pediatric patient who developed sea-blue histiocyte syndrome associated with the chronic parenteral administration of fish oil emulsion.
CASE REPORT
A 1360-g female was born at 30 weeks gestation by Cesarean section to a 21-year-old primigravida mother, because of fetal distress. The newborn was operated on emergently after birth because of obvious necrotic bowel with gastroschisis. Intraoperatively, the patient was found to have midgut volvulus and intestinal atresia. Subsequent surgery left her with less than 10 cm of bowel, a gastrostomy feeding tube, and dependence on total parenteral nutrition (TPN). Congenital cytomegalovirus infection was also diagnosed. Intravenous soybean oil was used as the fat component of her nutrition because it was the only US Food and Drug Administration-approved parenteral fat available in the United States.
By 10 weeks of age, she developed parenteral nutrition associated hepatic cholestasis and jaundice. The lipid source of her TPN was converted from 3 g/kg/day parenteral soybean oil to 1 g/kg/day parenteral fish oil in August 2008 under an investigational protocol, with resolution of hyperbilirubinemia. Over the course of the next 5.5 years, the patient maintained a normal leukocyte concentration, an average platelet count of 127,000 cells/μL and a low hemoglobin concentration (average: 10.8 g/dL) that was attributed to anemia of chronic disease and, at times, non-adherence to enteral iron supplementation. Her peripheral blood triglyceride concentration remained below 50 mg/dL, on average, throughout her life. Triglyceride concentrations higher than 100 mg/dL were recorded on 2 brief occasions.
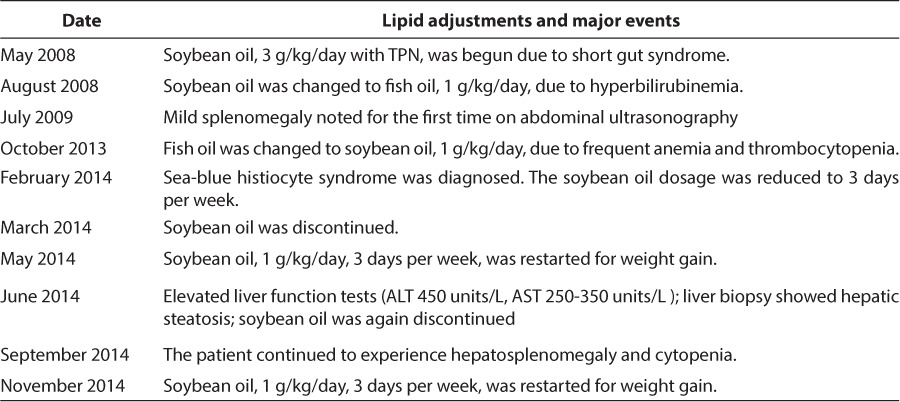
The patient experienced 11 episodes of central line-associated sepsis in her first 5.5 years of life, caused by infection with various mainly gram-negative bacteria. With each episode, she developed fever and elevated C-reactive protein concentration with increasingly prominent anemia and thrombocytopenia and temporarily increased triglycerides and bilirubin (Table 1). Each of these values reversed spontaneously after the administration of antibiotics in all cases. Leukocytes increased significantly during 7 of the septic episodes and declined during 4 of them. Intravenous fish oil nutrition dosage was changed to 1 g/kg/day soybean oil in October 2013 because the hepatic risks were thought to be similar, but the fish oil was causing repeated episodes of sepsis-associated anemia and thrombocytopenia. A summary of TPN lipid changes and associated clinical sequelae can be found in Table 2.
Table 1.
Bacteremia Type and Cytopenia
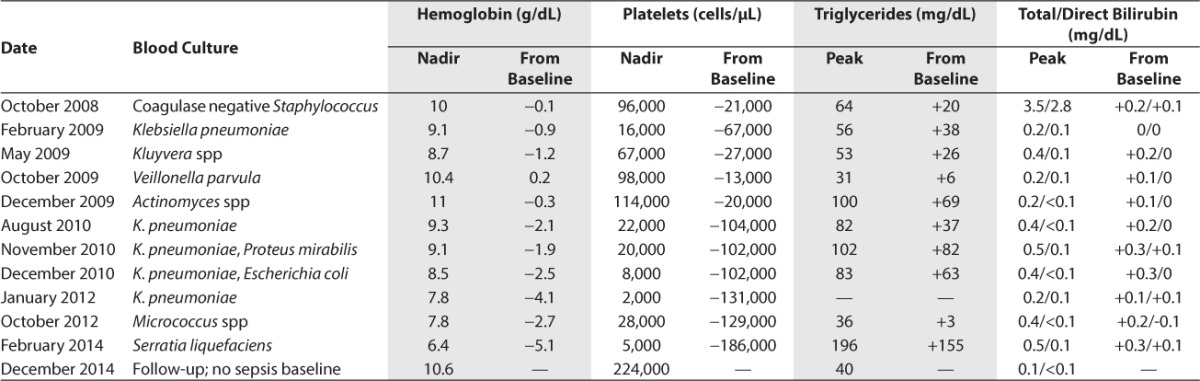
Table 2.
Lipid Adjustments and Major Events Timeline
A more in-depth work up of the patient's cytopenia was conducted during a February 2014 episode of sepsis. A summary of the work up is provided here. The patient was significantly and acutely anemic but did not exhibit any signs of bleeding. Hepatosplenomegaly was palpated on physical examination. A follow-up abdominal ultrasonography confirmed significant hepatomegaly and splenomegaly with some gallstones. Vascular interrogation did not show portal vein thrombosis or other hepatic vascular obstruction. The patient's kidneys were at the upper limits of the normal range in size. A liver biopsy assay showed significant capsule, fibrous tissue, and smooth muscle but no evidence of cirrhosis. Further characterization could not be done due to a small sample size. A gallbladder biopsy showed the usual undulating surface mucosa. Minimal inflammation was identified. The muscular wall was intact.
The patient's platelet concentration was significantly and acutely lower than her normal baseline value. Mean platelet volume was 15.5 fL, suggesting active bone marrow release into the systemic circulation. Liver function tests, bilirubin, coagulation studies, and basic metabolic panel values were within normal limits. A bone marrow biopsy analysis showed an increased number of sea-blue histiocytes, 90% to 95% marrow cellularity with trilineage hematopoiesis and slightly increased megakaryocytic numbers. The myeloid-to-erythroid ratio was 1.6:1. Peripheral blood morphology showed slight to moderate normochromic normocytic anemia with rare dacrocytes, leukopenia without absolute neutropenia and relative monocytosis with occasional activated monocytes. Results from a comprehensive lysosomal enzyme panel and a bone marrow chromosome analysis were normal.
Having ruled out the typical causes of sea-blue histiocyte syndrome, it was postulated that parenteral fat from intravenous nutrition was contributory to this patient's sepsis-related anemia and thrombocytopenia. The dosage of intravenous soybean oil nutrition was reduced to 3 days per week on hospital discharge. Intravenous fat was discontinued altogether in May 2014 as continuous enteral feeding was advanced.
The patient was readmitted to the hospital for a hepatitis work-up in response to moderately elevated liver function test results found on routine surveillance in June 2014. Hepatosplenomegaly was palpated. A liver biopsy examination showed grade 6/8, stage 3/4, nonalcoholic hepatic steatosis according to nonalcoholic steatohepatitis clinical research network criteria.6 Other causes of hepatitis were ruled out.
DISCUSSION
Prolonged parenteral nutrition is associated with numerous complications. One of the more serious complications is parenteral nutrition-associated cholestasis and liver disease that ranges from hepatic steatosis to cirrhosis with liver failure. The most effective method to prevent parenteral nutrition associated liver disease is advancement to full enteral feeds and discontinuation of parenteral nutrition. However, this was not possible for the patient in this case because of the short length and poor function of her bowel. Although the cause of the disease is multifactorial and unclear, evidence suggests that higher doses of soybean oil are more associated with liver disease, whereas lower doses of soybean oil or fish oil may be more preventative.7–13
The composition of fatty acids and excipients found in soybean oil differs from that in fish oil. The major components of soybean oil are 44% to 62% omega-6 fatty acids, 4% to 11% omega-3 fatty acids, 2.25% glycerin, and 1.2% egg phospholipids. The remaining fats in the emulsion are composed of omega-9 and −11c fatty acids and saturated fat. Fish oil contains approximately 4% to 13% omega-6 fatty acids, 30% to 70% omega-3 fatty acids, 2.5% glycerin, and 1.2% egg phospholipids. The minor components of the emulsion are omega-7 and −9 fatty acids and saturated fat. It is interesting to note the presence of sea-blue histiocyte syndrome in this patient who received a lipid emulsion that was weighted more toward the presence of omega-3 fatty acids than omega-6 fatty acids.
Sea-blue histiocyte syndrome caused by chronic soybean oil administration has been reported to occur in adults from 6 to 131 months (median: 64 months) after the initiation of TPN.5 The authors postulated that high blood lipid levels could lead to lipid laden histiocytes, incomplete degradation and accumulation within the cell. In another publication, sea-blue histiocyte syndrome was reported in two adults with splenomegaly, apolipoprotein E mutation and mild-to-moderate hypertriglyceridemia. Macrophage uptake of mutant apolipoprotein E prevented severe hypertriglyceridemia until splenectomy.14 None of the patients in these two publications had lecithin acyltransferase deficiency or lipid storage disease. However, the patient in our case did not have elevated peripheral blood triglyceride concentrations, making both of these mechanisms of sea-blue histiocytes less likely, although it could be argued that the increased dosage of omega-3 fatty acids in the fish oil preparation caused increased chylomicron triglyceride clearance in our patient, masking another disorder.
Sea-blue histiocytes have been described in patients with lysosomal storage diseases (e.g., Niemann-Pick) and chronic myeloid leukemia. However, a genetics consultation found lysosomal enzymes in this patient's peripheral leucocytes were normal and a hematology-oncology consult ordered bone marrow aspirate showed no evidence of chromosomal abnormalities or malignancies. Further, the marrow cellularity and the presence of megakaryocytes showed no chronic bone marrow suppression. To the contrary, the increased peripheral blood mean platelet volume indicated that the cause of the thrombocytopenia was likely a destructive systemic process rather than myelosuppression.
Lecithin acyltransferase deficiency is a cause of splenomegaly with sea-blue histiocytes. Typical clinical manifestations are corneal opacification from corneal cholesterol distribution, decreased high-density lipoprotein, renal failure, and hypertriglyceridemia.15 However, physical examination of our patient did not reveal corneal opacification, and renal dysfunction and hypertriglyceridemia were not present outside of the acute periods when she was septic.
The presence of marked anemia and thrombocytopenia during septic episodes suggests that either elements of bacterial infection, such as endotoxin, or the systemic inflammatory response could have triggered the cellular destruction. Splenomegaly found on physical examination and ultrasonography along with the presence of peripheral dacrocytes indicate the spleen as the most likely source of destruction. It is possible that endotoxin caused the activation of abnormal, phospholipidladen splenic monocytes, initiating phagocytosis of red blood cells and platelets. If true, parenteral immune globulin, a corticosteroid, a tumor necrosis factor blocker, or interleukin-1 blocker could be effective in minimizing cytopenia in the future, although each of these treatments carries risk in the setting of sepsis.
The patient in this case received long-term parenteral fish oil for nutrition because of the development of parenteral nutrition-associated cholestasis. Anemia and thrombocytopenia were noted, with episodes of sepsis prior to the switch back to soybean oil in October 2013. Another episode of anemia and thrombocytopenia occurred with the February 2014 septic episode, 120 days after the discontinuation of fish oil. The episode showed the most significant acute, sepsis-related cytopenia to date with no resolution of the syndrome after switching from fish oil to soybean oil. The only published case of parenteral lipid-induced sea-blue histiocyte syndrome that showed resolution of cytopenia over time found that normal cell values were not present up to 190 days after discontinuation of parenteral lipid.16 The lack of improvement in cytopenia after switching from fish oil to soybean oil in our patient suggests that the mechanism of parenteral lipid-induced sea-blue histiocyte syndrome is similar whether or not the patient receives fish oil or soybean oil.
Despite vigorous investigation of known causes of sea-blue histiocyte syndrome in this patient, the most likely identifiable cause was administration of parenteral lipid emulsion. It did not appear that there was a difference between fish oil and soybean oil with regard to the condition. More evidence from other patients will be required to better identify a correlation between parenteral lipids and the condition and any potential treatments for the cytopenia associated with it.
ACKNOWLEDGMENTS
The authors wish to acknowledge the advice of Bruce Bostrom, MD, and Coleen Nelson, PharmD, in the preparation of this manuscript.
ABBREVIATIONS
- ALT
alanine transaminase
- AST
aspartate aminotransferase
- TPN
total parenteral nutrition
Footnotes
Disclosures The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Silverstein MN, Ellefson RD, Ahern EJ. The syndrome of the sea-blue histiocyte. N Engl J Med. 1970;282(1):1–4. doi: 10.1056/NEJM197001012820101. [DOI] [PubMed] [Google Scholar]
- 2.Hirayama Y, Kohda K, Andoh M et al. Syndrome of the sea-blue histiocyte. Intern Med. 1996;35(5):419–421. doi: 10.2169/internalmedicine.35.419. [DOI] [PubMed] [Google Scholar]
- 3.Candoni A, Grimaz S, Doretto P et al. Sea-blue histiocytosis secondary to Niemann-Pick disease type B: a case report. Ann Hematol. 2001;80(10):620–622. doi: 10.1007/s002770100354. [DOI] [PubMed] [Google Scholar]
- 4.Bigorgne C, Le Tourneau A, Vahedi K et al. Sea-blue histiocyte syndrome in bone marrow secondary to total parenteral nutrition. Leuk Lymphoma. 1998;28(5–6):523–529. doi: 10.3109/10428199809058360. [DOI] [PubMed] [Google Scholar]
- 5.Bigorgne C, Le Tourneau A, Messing B et al. Sea-blue histiocyte syndrome in bone marrow secondary to total parenteral nutrition including fat-emulsion sources: a clinicopathologic study of seven cases. Br J Haematol. 1996;95(2):258–262. doi: 10.1046/j.1365-2141.1996.d01-1907.x. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM, Janney CG, Di Bisceglie AM et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 7.Puder M, Valim C, Meisel JA et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition associated liver injury. Ann Surg. 2009;250(3):395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cober MP, Killu G, Brattain A et al. Intravenous fat emulsions reduction for patients with parenteral nutrition-associated liver disease. J Pediatr. 2012;160(3):421–427. doi: 10.1016/j.jpeds.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 9.Premkumar MH, Carter BA, Hawthorne KM et al. High rates of resolution of cholestasis in parenteral nutrition-associated liver disease with fish oil-based lipid emulsion monotherapy. J Pediatr. 2013;162(4):793–798. doi: 10.1016/j.jpeds.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez SE, Braun LP, Mercer LD et al. The effect of lipid restriction on the prevention of parenteral nutrition-associated cholestasis in surgical infants. J Pediatr Surg. 2013;48(3):573–578. doi: 10.1016/j.jpedsurg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin JI, Namgung R, Park MS, Lee C. Could lipid infusion be a risk for parenteral nutrition-associated cholestasis in low birth weight neonates? Eur J Pediatr. 2008;167(2):197–202. doi: 10.1007/s00431-007-0454-7. [DOI] [PubMed] [Google Scholar]
- 12.Colomb V, Jobert-Giraud A, Lacaille F et al. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. J Parenter Enteral Nutr. 2000;24(6):345–350. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 13.Rollins MD, Ward RM, Jackson WD et al. Effect of decreased parenteral soybean lipid emulsion on hepatic function in infants at risk for parenteral nutrition-associated liver disease: a pilot study. J Pediatr Surg. 2013;48(6):1348–1356. doi: 10.1016/j.jpedsurg.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TT, Kruckeberg KE, O'Brien JF et al. Familial splenomegaly: macrophage hypercatabolism of lipoproteins associated with apolipoprotein E mutation [apolipo-protein E (delta149 Leu)] J Clin Endocrinol Metab. 2000;85(11):4354–4358. doi: 10.1210/jcem.85.11.6981. [DOI] [PubMed] [Google Scholar]
- 15.Naghashpour M, Cualing H. Splenomegaly with sea-blue histiocytosis, dyslipidemia, and nephropathy in a patient with lecithin-cholesterol acyltransferase deficiency: a clinicopathologic correlation. Metabolism. 2009;58(10):1459–1464. doi: 10.1016/j.metabol.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Meiklejohn DJ, Baden H, Greaves M. Sea-blue histiocytosis and pancytopaenia associated with chronic total parenteral nutrition administration. Clin Lab Haem. 1997;19(3):219–221. [PubMed] [Google Scholar]


