Abstract
Cocaine abuse has been shown to have broad-ranging effects on human immunity. With regards to HIV infection, in vitro studies have shown that cocaine enhances infection of stimulated lymphocytes. Moreover, cohort studies in the pre- and post-HAART era have linked stimulant abuse with increased HIV pathogenesis. The latter data, however, have been undermined by a series of confounding factors underscoring the importance of controlled in vivo models to fully assess the impact of cocaine use and abuse on HIV infection and pathogenesis. Here, we have infected humanized mice with HIV-1 following acute cocaine exposure to assess the impact on infection. Stimulant exposure resulted in increased inflammatory cytokine expression, accelerated HIV infection, while blunting effector function of cytotoxic T lymphocytes. These data demonstrate cocaine’s multifactorial impact on HIV infection that extends beyond high-risk behavior.
Stimulants such as cocaine and methamphetamine are known co-factors in human disease pathogenesis and significant modulators of immune responses1,2,3,4,5,6. Cocaine has been shown to alter the phenotype and function of antigen presenting cells, macrophages, T cells, and the cytokines that they produce1,2,3,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27. Many of these effects appear to directly impact on HIV infection and replication8,26,28. A compelling link between cocaine and HIV replication was first described in vitro studies in the early 1990’s8,10,11. Lymphocytes from healthy donors were stimulated in the presence or absence of cocaine, and evaluated for their ability to support HIV-1 infection and replication. HIV replication was enhanced up to 3-fold at cocaine concentrations between 0.3 pg/ml to 0.3 ng/ml (10−9 to 10−6 M). Subsequently, the same investigators found that addition of TGF-β to control cells induced similar changes in HIV replication, and the stimulatory effects of cocaine were completely blocked using neutralizing antibodies against TGF-β8,10. However, other cytokines and pathways have been implicated in the interaction between cocaine, T cells and HIV. For example, studies have demonstrated that cocaine can act via the intracellular sigma-1 receptor (σ1R) to up-regulate the expression of IL-10 and down-regulate the expression of IFN-γ, two cytokines centrally involved in HIV pathogenesis24,26,29. While the above studies point to a dampened inflammatory state, cohort studies reveal higher levels of inflammation demonstrated by increased levels of neopterin3, IFN-γ as well as TNF-α in the presence of cocaine30,31. Finally, our group recently demonstrated that cocaine exposure of quiescent T cells renders them permissive to HIV infection32. These effects were mediated in part by the σ1R and mainly through the dopamine D4 receptor (D4R)32. Therefore, cocaine exposure has both direct and indirect effects on HIV replication in CD4 T cells.
While in vitro studies have yielded valuable information on cocaine’s impact in HIV infection, they are limited in scope. Clinical and epidemiological studies have tackled the issue looking at various cohorts. While the data from a number of studies suggest that poor HAART adherence is the main factor enhancing HIV disease progression in cocaine users33,34,35,36,37, others have suggested that it may not a sole factor. In studies controlling for HAART adherence, cocaine users were more likely to have higher viral loads, display quicker and more robust viral rebounds upon cessation of HAART and exhibit higher morbidity3,4,38,39,40,41. While the data obtained from the cohort studies are quite valuable, they are confounded by factors including but not limited to HAART adherence, multidrug use and co-infections such as HCV and/or TB. Meanwhile, early chimeric mouse models were used to develop more controlled in vivo systems to study the effects of cocaine on HIV infection. In these studies, systemic daily administration of cocaine significantly increased the percentage of HIV-infected human lymphocytes and dramatically increased total viral loads22,26. However, the lack of peripheral reconstitution in these animals prevented more extensive studies on HIV pathogenesis and drug abuse. Advances in the development of humanized mice have allowed for such in-depth analysis of HIV pathogenesis and latency42,43 as well as immune function44,45. To this end, we have employed the BLT humanized mouse model to comprehensively assess the impact of cocaine on HIV infection. Following a 5-day cocaine pre-treatment, subsets of mice were infected with HIV-189.6 followed by continuous cocaine administration. Mice were sacrificed two weeks after infection, at which we collected various tissues to measure levels of infection and examine other immunological parameters. Based on our data, pretreatment with cocaine resulted in an increased expression of inflammatory cytokines leading to accelerated HIV infection. Moreover, HIV infected, cocaine treated animals continuously expressed virus in peripheral blood and their effector activity of cytotoxic T cells was impaired. Our data demonstrate that cocaine exposure enhances early acute HIV infection both directly, as shown in our previous in vitro studies, and indirectly through changes in the immune phenotype of T cells.
Results
Acute exposure of humanized mice to cocaine results in increased immune activation
The latest advances in humanized mice have led to the development of models that closely mimic human immunity and allow for the study of chronic infections. Using the BLT humanized mouse model, we examined in vivo the impact of cocaine exposure on HIV infection. Chimeric mice were constructed as previously described44,45 and shown in Fig. 1. Following reconstitution with human cells, cohorts were separated into 4 different groups based on treatment: (i) NON (saline, no HIV), (ii) COCAINE (cocaine 5 mg/kg/day, no HIV), (iii) HIV (saline, HIV), and (iv) COCAINE-HIV (cocaine 5 mg/kg/day, HIV-189.6 300–400 IU). We treated mice with 5 mg/kg of cocaine or saline every day for 5 days by intraperitoneal injection. At the end of 5 days, the HIV and COCAINE-HIV groups were infected intraperitoneally with HIV-189.6. Cocaine or saline administration continued for 2 weeks after infection at which point mice were sacrificed to assess levels of infection and lymphocyte phenotype in various tissues (Fig. 1).
Figure 1. A schematic diagram of the experimental set up.
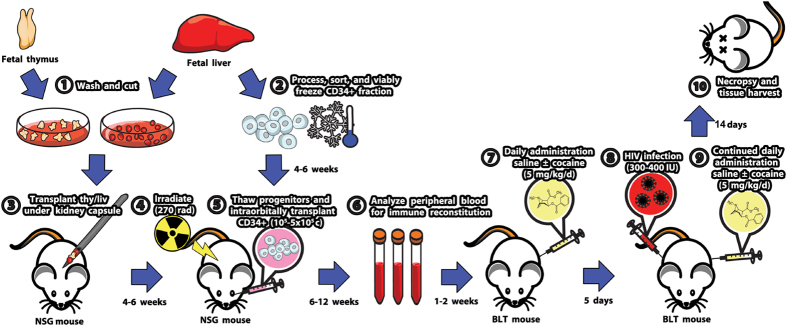
BLT mice were generated and treated as described in the Materials and Methods section. Cocaine treated and untreated mice were sacrificed at two weeks after infection at which we harvested blood, spleen and thymus to assess levels of HIV infection and characterize immune cells.
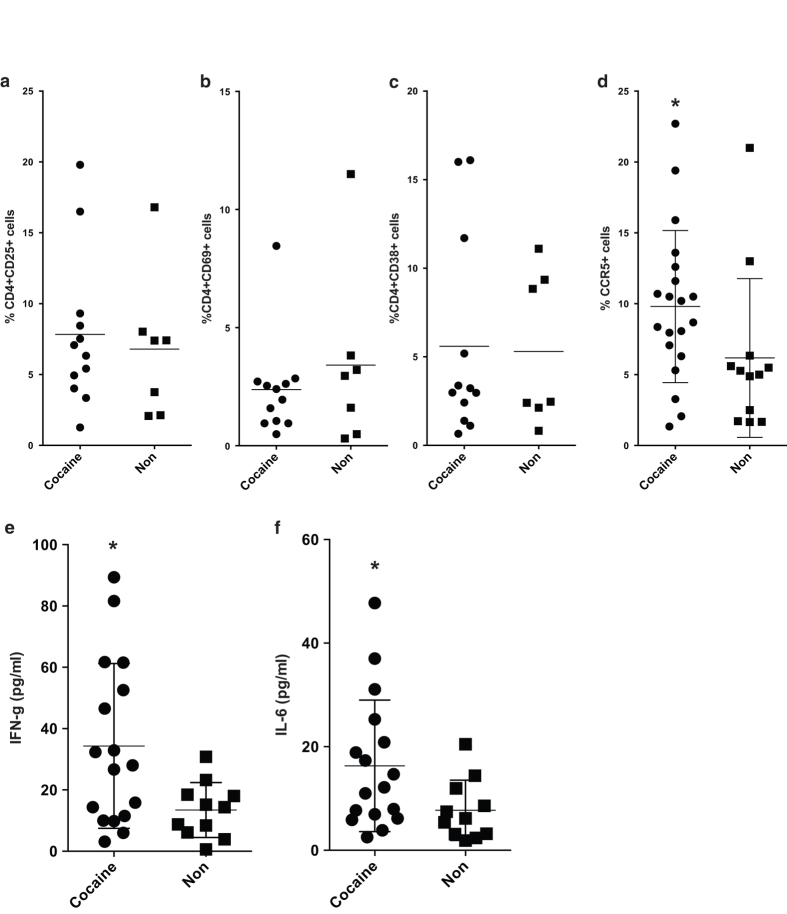
To assess the effect of acute cocaine exposure prior to infection, blood samples were collected from mice and analyzed by flow cytometry. Based on our results, expression of CD25, CD69 and HLADR on CD4 T cells (Fig. 2a–c) was not altered in cocaine treated animals, a phenotype seen in our previous in vitro studies32. However, cocaine exposure resulted in upregulation of CCR5 expression on CD4 T cells, a phenotype previously seen in our in vitro studies (Fig. 2d)32. There was no effect on CXCR4 expression (data not shown). Furthermore, we collected blood samples to assess plasma cytokine levels. Cocaine treated animals demonstrated elevated levels of IFN-γ and IL-6 (Fig. 2e,f). These data suggest that exposure of humanized mice to cocaine promotes an inflammatory state capable of facilitating HIV infection.
Figure 2. Five day acute cocaine exposure results in subtle but significant changes in CD4 T cell phenotype.
(a-c) Blood samples collected after 5 days of cocaine exposure were used for flow cytometric analysis of T cell activation markers on CD4 T cells. There were no changes on the expression of CD25, CD69 and CD38. (d) Blood samples were also tested for CCR5 expression. The data are from three mouse cohorts. Cocaine treated animals demonstrated increased levels of the receptor (Cocaine – n = 20, Non – n = 12). (e-f) Plasma from the same mice was collected and used to examine levels of Th1/Th2/Th17 cytokines (Cocaine – n = 20, Non – n = 12). For all figures, *p < 0.05, **p < 0.01, using Mann-Whitney or Kruskal-Wallis test (for 3 or more groups).
Cocaine treated animals demonstrated increased kinetics of HIV infection
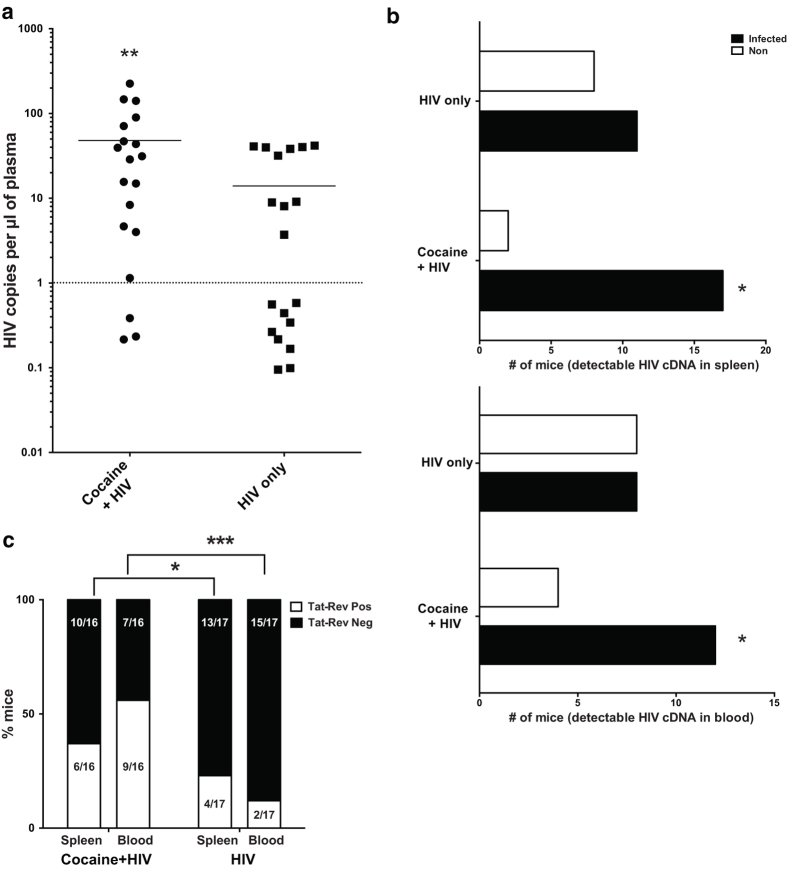
At the end of the 5-day drug pre-treatment, HIV and COCAINE-HIV mice were infected with HIV-189.6 (Fig. 1). Cocaine administration continued for the treated animals as indicated above. Two weeks after infection, the mice were sacrificed to assess the rate and level of infection. At this early timepoint using this route of inoculation, levels of infection in untreated humanized mice are typically low or undetectable46. Plasma samples were collected from all mice to measure viral loads. Based on our data, the COCAINE + HIV group had higher levels of viral loads than the HIV group (Fig. 3a). Most importantly, a higher fraction (9/19) of the untreated mice tested below the limit of detection for viral loads, while in the cocaine treated group only 3/19 had undetectable viral loads. Thus, cocaine exposure accelerated HIV replication in treated mice. In addition to plasma viral loads, we examined the presence and levels of viral cDNA in the blood and spleen of these animals. As shown in Fig. 3b, a higher fraction of cocaine treated animals had detectable levels of viral cDNA in these tissues. Thus, our model, in support of cohort studies, demonstrates that cocaine exposure, in the absence of other confounding factors such as co-infection or other drug use, accelerates viral replication.
Figure 3. Acute cocaine exposure results in accelerated HIV infection of humanized mice.
(a) After 5-day treatment, mice were infected with HIV89.6 followed by continued treatment with cocaine or saline for two more weeks. At the end of two weeks, mice were sacrificed; plasma samples were tested for levels of viral RNA. Cocaine treated mice had higher viral loads and a higher fraction of them had detectable levels of virus. (b) Samples from spleen and blood were tested for levels of viral cDNA the proportion of mice with detectable levels of viral cDNA were higher in the cocaine treated animals. (c) A higher fraction of cocaine treated animals had detectable levels of tat-rev mRNA in both spleen and blood. For all figures, *p < 0.05, **p < 0.01, using Mann-Whitney or Kruskal-Wallis test (for 3 or more groups). For proportion data, we used a Chi-square test.
Cocaine treated animals express higher levels of spliced viral mRNA in peripheral blood
To further characterize viral replication in our mouse cohorts, in addition to viral cDNA, we examined the levels of multiply spliced RNA in blood and spleen samples. We focused our analysis on the animals that had detectable viremia based on the viral load assays. Expression of multiply spliced viral RNA is associated with ongoing viral replication47. As the majority of lymphocytes in blood are resting, we expected, based on previous studies, to see very minimal levels of tat-rev mRNA47. While this was true in the untreated mice, a significantly higher fraction of COCAINE + HIV mice had detectable tat-rev mRNA in the blood (Fig. 3c). The patterns were similar but not as striking in the spleen. This could be attributed to the presence of other activation signals present in a densely packed tissue such as the spleen that would allow for ongoing replication48. Thus, at this early stage of the infection, in the presence of cocaine there are higher levels of spliced viral RNA which are potentially contributing increased viral spread.
Cocaine presence in HIV infected animals dulls CTL function
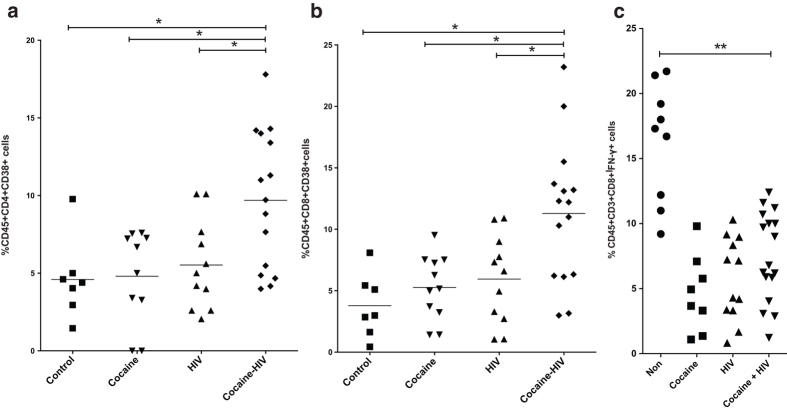
To further characterize the impact of cocaine on immunity during early acute HIV infection, we analyzed tissue samples for various immunological parameters such as T cell activation and plasma cytokine levels. Splenocytes of humanized mice with detectable viral loads were analyzed for T cell activation. Based on our data, the COCAINE-HIV group had elevated CD4+CD38+ T cells (Fig. 4a). Interestingly, the levels of activated CD8+CD38+ T cells were also higher in these animals (Fig. 4b). Since we detected increased levels of CD8+CD38+ T cells in the COCAINE-HIV group, we examined whether this increased activation would translate to robust effector cytokine release after ex vivo stimulation. This would suggest that the effector functions during early acute infection in the presence of cocaine may not be impaired. Thus, we stimulated ex vivo splenocytes from these cohorts using PMA/Ionomycin for 3 days. The COCAINE-HIV group did not exhibit significantly higher levels of IFN-γ production from the HIV only group despite the more activated phenotype (Fig. 4c).
Figure 4. Increased activation persists in the cocaine treated, HIV infected animals.
Splenocytes from the sacrificed mice were also used for flow cytometric analysis of lymphocyte phenotype. The COCAINE-HIV group of mice has higher levels of (a) CD4CD38 and (b) CD8CD38. (c) To assess functionality of CD8 T cells, we stimulated ex vivo splenocytes in the presence of GolgiPlug to measure levels of IFN-γ production. The COCAINE-HIV group had comparable levels to HIV despite the higher numbers of CD8CD38 T cells. For all figures, *p < 0.05, **p < 0.01, using Mann-Whitney or Kruskal-Wallis test (for 3 or more groups).
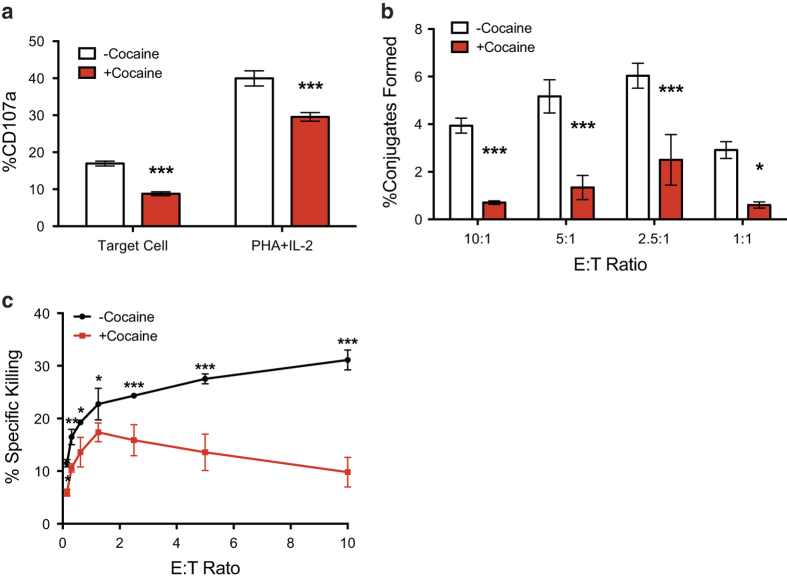
To further confirm our observations that CTL activity is dampened due to cocaine exposure, we carried out a series of in vitro experiments to determine if cocaine can directly impact CTL effector function such as killing HIV infected targets. To this end, we generated transgenic CTL to express a TCR specific for the HIV Gag epitope SL949. These CTLs were then exposed to cocaine for 3 days as previously described32 and used in a series of assays to assess their effector activity. More specifically, these effectors were co-incubated with labeled, HIV infected U1 targets to measure their cytolytic activity. As shown in Fig. 5A, CTLs treated with cocaine demonstrated decreased levels of CD107a degranulation. Moreover, we observed decreased levels of conjugate formation (Fig. 5b), an indication that CTLs are targeting antigen expressing targets. Finally, as expected, the cocaine treated CTLs failed to effectively kill the HIV-infected U1 cells (Fig. 5c). Thus, despite an activated phenotype the effector function of CTLs seems to be severely blunted.
Figure 5. HIV-specific CTL are deficient in their ability to degranulate CD107a, form conjugates and kill HIV-infected cells upon treatment with cocaine.
(a) HIV-specific CTL were co-incubated with HIV-expressing U1 cells at an 1:1 effector to target ratio or were treated with 10 μg/ml PHA and 100 U/ml IL-2 for 6 hr in the presence of protein transport inhibitors and antibodies to CD107a. Cocaine treatment of CTL resulted in a two-fold reduction in CD107a degranulation when CTL were co-cultured with HIV-infected cells and 26% less CD107a degranulation when CTL were maximally stimulated with PHA and IL-2 treatment. (b and c) HIV-specific CTL were co-incubated with HIV-expressing U1 cells at the defined effector to target ratios for 4 hr and subsequently assessed for their ability to induce (b) conjugate formation with or (c) apoptosis in target U1 cells. Cocaine treatment of CTL resulted in significantly impaired conjugate formation at all E:T ratios tested, which was further underscored by the significant loss in apoptosis induction of U1 target cells. Statistical significance was assessed by two-way ANOVA analysis, *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
Cocaine abuse has been linked to increased HIV pathogenesis, disease progression and morbidity. While there is a plethora of epidemiological studies supporting this phenomenon, this valuable information is hampered by a series of cofounding factors such as concurrent drug use and co-infections4. Moreover, these studies do not go beyond a mere measurement of indicators such as viral loads and CD4 counts. Thus, the mechanisms behind the potentiating effects of cocaine in HIV infection have not been fully examined, thus, hampering potentially more effective interventions for drug using populations.
One way of addressing the challenges as well as the limited breadth of data is the development of controlled in vivo models that would allow us to study the relationship between HIV infection and cocaine abuse. Previous studies have used chimeric mouse models demonstrating the potentiating effect of cocaine22,26. However, these models were limited as they lacked peripheral immune reconstitution. To this end, we decided to use the BLT humanized mouse model to examine the effects of cocaine on HIV infection. In this model, there is full reconstitution of all immune lineages, which allows for the study of HIV infection and immune responses. The model has been used in a variety of settings such as cancer, gene therapy and vaccine development42,43,44,45,46,49,50,51,52.
Following the generation of humanized mice, we exposed a subset of them to cocaine for 5 days. On day 5, the animals were bled to assess immune activation and a subset of them was infected with HIV. Untreated, HIV infected animals were also included alongside untreated and cocaine only treated groups. Five-day acute pre-exposure of humanized mice to cocaine resulted in an overall increase of immune activation. However, as seen in our recent in vitro studies, the level and type of activation does not conform to the typical responses seen after TcR engagement. More specifically, we did not observe upregulation of classical T cell activation markers; we observed elevated expression of IFN-γ and IL-6. These data were in agreement with previous studies that have shown in cocaine users elevated serum neopterin as well as IFN-γ and IL-6 expression3,30. In addition, we observed an upregulation of CCR5 expression in CD4 T cells, a pattern also seen in vitro. Thus, based on our previous work32 and the current studies, the presence of cocaine directly and indirectly allows for the virus to more efficiently infect its target cell populations and replicate.
Based on the above, we anticipated HIV infection kinetics would be enhanced. As shown in our data, the presence of cocaine accelerated the progression of HIV infection as a higher fraction of animals had detectable levels of virus in serum. This was further supported by the detectable levels of viral cDNA in both spleen and blood samples. Thus, this supports the notion that cocaine alone can accelerate HIV infection4.
Furthermore, the presence of cocaine result in increased levels of detectable spliced viral mRNA. The latter correlates well with efficient viral spread47. Based on our data, we saw increased levels of tat-rev mRNA in both blood and spleen in the cocaine treated animals as opposed to the non-treated. This is quite interesting as CD4 T cells in blood are normally in a resting state and studies have shown that the majority of them are not expressing detectable levels of multiply spliced viral mRNA47. Thus, these data suggest that in the presence of cocaine, there are higher levels of viral expression allowing for more efficient viral replication.
To elucidate other means by which cocaine could influence HIV infection, we analyzed the immunological profiles of immune cells and plasma cytokine levels in the HIV infected cohorts. The cocaine exposed animals had higher levels of CD4+CD38+ T cells pointing to a more activated state that would support efficient and continuous HIV replication. Interestingly, these mice had elevated numbers of CD8+CD38+ T cells. Therefore, these cells should be able to limit spread of infection by killing infected targets in early acute infection. However, when we activated splenocytes ex vivo, we saw that the CD8 T cells did not express higher levels of IFN-γ in the COCAINE-HIV group suggesting that effector responses may be blunted by the presence of cocaine. Our in vitro assays demonstrated that cocaine can directly diminsh CTL effector function. The mechanisms of this phenotype will be further investigated.
In conclusion, using the BLT humanized mouse model, we demonstrate that acute cocaine exposure results in accelerated HIV replication, increased viral expression, enhanced inflammation, and yet potentially blunted CTL activity. More importantly, this study is a proof of principle that we can use the BLT mouse model to explore in full all aspects of cocaine abuse and its impact on HIV pathogenesis and disease progression. Finally, these studies demonstrate that cocaine exposure does not simply enhance HIV infection through high risk behavior, but also importantly through significant changes on the physiology and function of human immune cells that allow the virus to replicate and spread efficiently.
Materials and Methods
Ethics statement
Peripheral blood mononulear cells was obtained at the University of California, Los Angeles in accordance with UCLA Institutional Review Board (IRB) approved protocols under written informed consent using an IRB-approved written consent form by the UCLA/CFAR Virology Laboratory and was distributed for this study without personal identifying information. Human fetal tissue was purchased from the UCLA/CFAR Gene Therapy Core, was obtained without identifying information and did not require IRB approval for use. Animal research carried out in this manuscript was performed under the written approval of the UCLA Animal Research Committee (ARC) in accordance to all federal, state, and local guidelines. Specifically, the experiments were performed strictly according to the guidelines in The Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and the accreditation and guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care (AALAC) International under UCLA ARC Protocol Number 1997-176-53.
Cells
U1 cells (NIH AIDS Reagent Program) are a subclone of the promonocytic cell line U937 that is chronically infected with HIV and makes replication competent virus upon activation53. J.RT3-T3.5 (ATCC, Manassas, VA) are a subclone of leukemia T cell line Jurkat mutated in the T cell receptor beta chain locus such that these cells fail to express surface CD3 and TCR. U1 and J.RT3-T3.5 cells were maintained in culture media containing RPMI 1640 (Invitrogen, Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (Omega Scientific, Tarzana, CA) and 100 U/ml penicillin and 100 μg streptomycin (GIBCO, Life Technologies).
Virus stocks
For all the studies, we used HIV-189.6, a dual tropic HIV strain that infects cells expressing CXCR4 and/or CCR5 co-receptors. The strain was selected to ensure infection of all susceptible cell populations. Stocks of HIV-1 molecular clone 89.6 were obtained from 24-h harvests of supernatants from infected CEMx174. Supernatants were filtered and treated with DNase (2 μg/ml) (Worthington, Lakewood, N.J.) for 30 min at room temperature in the presence of 0.01 M MgCl2. Viral infectivity was determined by limiting dilution titration on GHOST (3) X4/R5 (NIH AIDS Reagent Program). Lentiviral vectors expressing a TCR specific for the HIV Gag epitope SL9 were produced by calcium phosphate transfection of 293FT cells with the pCCL.PPT.SFFV.1.9.IRES. dLNGFR54 in conjunction with the lentiviral packaging vectors pMDLg/pRRE and pCMV-VSV-G. Supernatants were harvest on day 2 and passed through a 0.45 micron filter and concentrated by ultracentrifugation. Infectivity was assessed by titration of lentiviral vectors on J.RT3-T3.5 cells and flow cytometric analysis for the co-expression of SL9TCR (SL9 iTAg MHC Class-I tetramer-PE, Beckman Coulter, Brea, CA) and dLNGFR (CD271-FITC, Stem Cell Technologies, Vancouver, British Columbia, Canada).
Flow cytometry
Fluorochrome-conjugated mAb, specific for human CD4, CD8, CD25, CD38, CD45, CD69, CCR5, CXCR4, HLA-DR were obtained from BD Biosciences (San Diego, CA). For each analysis, cells were labeled with mAb, fixed with 2% paraformaldehyde, and analyzed using BD LSRII Fortessa flow cytometer (BD Biosciences) using FACSDiva software. Gating on human anti-CD45-stained cells was used to exclude contaminating murine cells. Subsequent analyses were performed using FlowJo software (Tree Star, Ashland, OR).
Serum cytokine assay
Blood samples from our cohorts were recovered via puncture of the retro-orbital venous plexus using EDTA coated capillaries. Samples were spun at 3000 rpm for 5 min and serum was collected. Cytokine levels were determined using a cytometric bead array assay (BD CBA Human Th1/Th2/Th17 Cytokine Kit, BD Biosciences) specific for human IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ, and IL-17A. Samples were acquired on a BD LSR Fortessa flow cytometer (BD Biosciences) with FACSDiva software, and subsequent analyses were performed using FCAP Array software.
Generation of BLT mice and treatment
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were initially purchased from Jackson Laboratories and bred and maintained under laminar flow conditions in the Mouse/Human Chimera Core Facility at the University of California, Los Angeles (UCLA). Humanized mice were prepared as previously described44. Mice were then monitored for human cell engraftment 6 - 10 weeks post-injection. Upon reconstitution the animals were treated with cocaine for 5 days. Cocaine hydrochloride (5 mg/ml in saline) was obtained from the National Institute on Drug Abuse [NIDA; National Institutes of Health (NIH), Bethesda, MD] and diluted in saline prior to use. This dose of cocaine was selected on the basis of prior dose/response experiments (0.1, 5 or 10 mg/kg)7,55 in which the 5 mg/kg dose was shown to have no effects on engraftment yet significantly enhanced HIV infection22. Cocaine was delivered by intraperitoneal (i.p.) injection beginning 5 days pre-infection (4 - 8 weeks post human tissue implantation). After the 5-day pretreatment, a subset of animals was infected with HIV-189.6, followed by continuous cocaine administration until day 14 post-infection when the animals were sacrificed. Each experiment utilized humanized mice that were made from human tissue from the same donor and the donor tissue was unique experiment to experiment. Animals exhibiting any symptoms of graft versus host disease (GVHD) are removed from our analyses.
Viral load assay
Peripheral blood was collected by cardiac puncture and transferred into microcentrifuge tubes containing 330 mM EDTA. Viral RNA was extracted from plasma using the QIAamp Viral RNA Mini Kit (Qiagen). Quantitative RT-PCR was performed using the following primers/probe specific for gag sequences: NG1F (position 453–480) 28 bp (5′-GAGCTAGAACGATTCGCAGTTAATCCTG-3′), NG1R (position 570–534) 37 bp (5′-ATAATGATCTAAGTTCTTCTGATCCTGTCTGAAGGGA-3), NG1Z probe (position 482–520) 39 bp (FAM-5′ -CCTTTTAGAGACATCAGAAGGCTGTAGACAAATACTGGG-3-BHQ). Reverse transcription was performed using the Superscript II kit (Invitrogen). Real-time, quantitative PCR was performed on a BioRad CFX96 thermocycler. Results from samples were interpolated within the quantitation derived from the RNA standards.
Tat/rev real time RT-PCR
Expression of viral genomic RNA was measured by quantitative real-time RT-PCR and compared to in vitro-transcribed RNA standards specific for multiply spliced Tat-Rev RNA as previously described56. The 18S primer/probe set was obtained from Applied Biosystems specific for eukaryotic 18S rRNA as an endogenous control to allow relative gene expression quantification. The reaction conditions were carried out using the iScript One-Step RT-PCR Kit for Probes (Bio-Rad)56.
Quantitative real time PCR
We used quantitative real-time PCR to detect the presence of viral DNA as previously described56. Briefly, cells were harvested and DNA was subsequently isolated to be used in a quantitative real time PCR using primers specific for HIV-1 sequences56. A primer-probe pair specific for the human β-globin gene was utilized to determine the input of cellular DNA as an endogenous control to allow relative gene quantification56. The reactions were carried out using the TaqMan Core Reagents Kit (ABI Biosystems)56.
Intracellular cytokine assay
1 × 106 cells/well were seeded in 48-well plates in RPMI-10 media and stimulated for 6 hours with PMA/Inonomycin at 37 °C, 5% CO2. Following incubation, the cells were washed and stained with antibodies against CD3, CD4, CD8, CD45, CD56 and IFN-γ (BDBiosciences) to measure levels of cytokine release. Samples were run on an LSRII Fortessa (BD Biosciences) and analyzed using FlowJo.
Generating HIV-specific CTL
CD8+ T cells were isolated by magnetic bead isolation (EasySep Human CD8+ Selection Kit, Stem Cell Technologies) from the PBMC of healthy donors obtained by the UCLA CFAR Virology Core and activated overnight in 50 ng/ml anti-CD3 (clone OKT3, Imgenex, San Diego, CA) and 300 U/ml IL-2 (NIH AIDS Reagent Program). Activated CD8+ were then transduced with a lentiviral vector containing HIV SL9-specific TCR and cultured in AIM-V media (Invitrogen, Life Technologies) supplemented with 5% human AB serum (Omega Scientific), 20 ng/ml IL-7 and 20 ng/ml IL-15 (both Invitrogen, Life Technologies). CD8+ T cells expressing the SL9-specific TCR were purified by magnetic bead separation based on dLNGFR expression (EasySep Human CD271 Selection Kit, Stem Cell Technologies) and confirmed >85% by flow cytometric analysis.
Cytotoxicity Assay
Purified SL9TCR-expressing CD8+ T cells were incubated in culture media (described above) for 3 days with 10 μM cocaine (NIDA Drug Supply Program) or not. On the second day, they were labeled with 2 μM Vybrant CFDA-SE (Invitrogen, Life Technologies) according to manufacturer’s suggested protocol and maintained in fresh culture media with 10 μM cocaine overnight. On the same day, target U1 cells were labeled with 5 μM CellTrace| Violet (Invitrogen, Life Technologies) according to manufacturer’s suggested protocol and activated to produce HIV with 10 μM prostratin (LC Labs, Woburn MA). The following day, cocaine or mock treated CTL were co-incubated with prostratin-activated U1 cells for 4 hr at the described effector to target ratios. Target cell sensitivity to CTL was assessed by the intracellular expression of cleaved caspase 3 via flow cytometric analysis. Percent specific killing was assessed by subtracting the %cleaved caspase 3 in target cells cultured with CTL from the %cleaved caspase 3 in target cells cultured alone. Conjugates of effector CTL and target U1 cells were assessed by flow cytometric analysis of Vybrant CFDA-SE and CellTrace Violet double positive for cells.
IFN-γ Production and CD107a Degranulation Assays
Purified SL9TCR-expressing CD8+T cells were incubated in culture media (described above) for 3 days with 10 μM cocaine or not. On the second day, they were labeled with 2 μM Vybrant CFDA-SE (Invitrogen, Life Technologies) according to manufacturer’s suggested protocol and maintained in fresh culture media with 10 μM cocaine overnight. On the same day, target U1 cells were labeled with 5 μM CellTrace Violet (Invitrogen, Life Technologies) according to manufacturer’s suggested protocol and activated to produce HIV with 10 μM prostratin (LC Labs, Woburn MA). The following day, cocaine or mock treated CTL were co-incubated with prostratin-activated U1 cells for 6 hr at an 1:1 effector to target ratio in the presence of GolgiStop and GolgiPlug (BD Biosciences, San Jose, CA) and PerCP/Cy5.5-anti-CD107a (1:50, Biolegend, San Diego, CA). Some CTL were also treated with phytohemagglutinin, PHA, (10 μg/ml) and IL-2 (100 U/ml) to induce maximal expression of CD107a and IFN-γ. After 6 hr, IFN-γ expression was assessed by intracellular cytokine staining.
Statistical analysis
All statistical analyses were carried out using GraphPad Prism 6. For two groups, we used Mann-Whitney test or one-tailed Student’s t-test, while for groups of 3 or more we used a Kruskal-Wallis (with Dunn’s multiple comparisons test) or one-way ANOVA (with a Tukey post-test). For most of our analysis, unless otherwise indicated, we ran non-parametric tests. For our proportion analysis, we used a Fisher’s exact test.
Additional Information
How to cite this article: Kim, S. G. et al. Cocaine-mediated impact on HIV infection in humanized BLT mice. Sci. Rep. 5, 10010; doi: 10.1038/srep10010 (2015).
Acknowledgments
This work was supported by NIH grants R21DA031036-01A1 (DNV), 5P30 AI028697 (UCLA/CFAR, JAZ), and R01AI070010, (J.A.Z.). We would like to thank the UCLA/CFAR Virology Core Laboratory for blood products, real time PCR and p24 ELISA support; the UCLA/CFAR Mouse/Human Chimera core for the preparation of humanized mice. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: Ghost(3)X4/R5 from Drs. Vineet N. KewalRamani and Dan R. Littman; U1/HIV-1 from Dr. Thomas Folks; Human rIL-2 from Dr. Maurice Gately, Hoffmann - La Roche Inc. Cocaine hydrochloride was obtained from the NIDA Drug Supply Program.
Footnotes
Author Contributions S.G.K., E.L., D.D., C.S.Y., I.K., J.B.J., R.R., D.N.V. performed the experiments. S.G.K. and D.N.V. wrote the manuscript. J.A.Z. and D.N.V. designed the experimental plan.
References
- Di Francesco P. et al. Effect of acute or daily cocaine administration on cellular immune response and virus infection in mice. Nat. Immun. Cell Growth Regul. 9, 397–405 (1990). [PubMed] [Google Scholar]
- Delafuente J. C. & DeVane C. L. Immunologic effects of cocaine and related alkaloids. Immunopharmacol. Immunotoxicol. 13, 11–23 (1991). [DOI] [PubMed] [Google Scholar]
- Carrico A. W. et al. Stimulant use is associated with immune activation and depleted tryptophan among HIV-positive persons on anti-retroviral therapy. Brain Behav Immun. 22, 1257–1262 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico A. W. Substance use and HIV disease progression in the HAART era: Implications for the primary prevention of HIV. Life Sci. 88, 940–947 (2011). [DOI] [PubMed] [Google Scholar]
- Liang H. et al. Methamphetamine enhances HIV infection of macrophages. Am. J. Pathol. 172, 1617–1624 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino T. & Bayer B. M. In vivo effects of cocaine on immune cell function. J. Neuroimmunol. 83, 139–147 (1998). [DOI] [PubMed] [Google Scholar]
- Ou D. W., Shen M. L. & Luo Y. D. Effects of cocaine on the immune system of Balb/C mice. Clin. Immunol. Immunopathol. 52, 305–312 (1989). [DOI] [PubMed] [Google Scholar]
- Peterson P. K. et al. Cocaine potentiates HIV-1 replication in human peripheral blood mononuclear cell cocultures. Involvement of transforming growth factor-beta. J. Immunol. 146, 81–84 (1991). [PubMed] [Google Scholar]
- Di Francesco P. et al. In vivo cocaine administration influences lymphokine production and humoral immune response. Immunol. Res. 11, 74–79 (1992). [DOI] [PubMed] [Google Scholar]
- Peterson P. K. et al. Cocaine amplifies HIV-1 replication in cytomegalovirus-stimulated peripheral blood mononuclear cell cocultures. J. Immunol. 149, 676–680 (1992). [PubMed] [Google Scholar]
- Bagasra O. & Pomerantz R. J. Human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells in the presence of cocaine. J. Infect Dis. 168, 1157–1164 (1993). [DOI] [PubMed] [Google Scholar]
- Caiaffa W. T. et al. Drug smoking, Pneumocystis carinii pneumonia, and immunosuppression increase risk of bacterial pneumonia in human immunodeficiency virus-seropositive injection drug users. Am. J. Respir Crit Care Med. 150, 1493–1498 (1994). [DOI] [PubMed] [Google Scholar]
- Chiappelli F. et al. Cocaine blunts human CD4+ cell activation. Immunopharmacology 28, 233–240 (1994). [DOI] [PubMed] [Google Scholar]
- Wang Y., Huang D. S. & Watson R. R. In vivo and in vitro cocaine modulation on production of cytokines in C57BL/6 mice. Life Sci. 54, 401–411 (1994). [DOI] [PubMed] [Google Scholar]
- Mao J. T. et al. Cocaine down-regulates IL-2-induced peripheral blood lymphocyte IL-8 and IFN-gamma production. Cell Immunol. 172, 217–223 (1996). [DOI] [PubMed] [Google Scholar]
- Baldwin G. C. et al. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am. J. Respir Crit. Care Med. 156, 1606–1613 (1997). [DOI] [PubMed] [Google Scholar]
- Mao J. T. et al. Cocaine inhibits human endothelial cell IL-8 production: the role of transforming growth factor-beta. Cell Immunol. 181, 38–43 (1997). [DOI] [PubMed] [Google Scholar]
- Baldwin G. C., Roth M. D. & Tashkin D. P. Acute and chronic effects of cocaine on the immune system and the possible link to AIDS. J. Neuroimmunol. 83, 133–138 (1998). [DOI] [PubMed] [Google Scholar]
- Di Francesco P. et al. Effects of cocaine administration to influenza virus-immunized mice on cytokine profiles of individual splenic CD4+ and CD8+ T cells. Clin Exp. Immunol. 118, 428–434 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Flick T., Mitchel J., Knowles C. & Ault K. Cocaine effects on immunocompetent cells: an observation of in vitro cocaine exposure. Int. J. Immunopharmacol. 21, 463–472 (1999). [DOI] [PubMed] [Google Scholar]
- Nair M. P. et al. Cocaine differentially modulates chemokine production by mononuclear cells from normal donors and human immunodeficiency virus type 1-infected patients. Clin. Diagn Lab. Immunol. 7, 96–100 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. D. et al. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J. Infect Dis. 185, 701–705 (2002). [DOI] [PubMed] [Google Scholar]
- Halpern J. H. et al. Diminished interleukin-6 response to proinflammatory challenge in men and women after intravenous cocaine administration. J. Clin. Endocrinol. Metab. 88, 1188–1193 (2003). [DOI] [PubMed] [Google Scholar]
- Gardner B. et al. Cocaine modulates cytokine and enhances tumor growth through sigma receptors. J. Neuroimmunol. 147, 95–98 (2004). [DOI] [PubMed] [Google Scholar]
- Nair M. P. et al. Cocaine modulates dendritic cell-specific C type intercellular adhesion molecule-3-grabbing nonintegrin expression by dendritic cells in HIV-1 patients. J. Immunol. 174, 6617–6626 (2005). [DOI] [PubMed] [Google Scholar]
- Roth M. D., Whittaker K. M., Choi R., Tashkin D. P. & Baldwin G. C. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J. Leukoc Biol. 78, 1198–1203 (2005). [DOI] [PubMed] [Google Scholar]
- Reynolds J. L. et al. Proteomic analysis of the effects of cocaine on the enhancement of HIV-1 replication in normal human astrocytes (NHA). Brain Res. 1123, 226–236 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekker G. et al. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. Int. Immunopharmacol. 6, 1029–1033 (2006). [DOI] [PubMed] [Google Scholar]
- Guitart X., Codony X. & Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology (Berl) 174, 301–319 (2004). [DOI] [PubMed] [Google Scholar]
- Fox H. C. et al. Immune system inflammation in cocaine dependent individuals: implications for medications development. Human Psychopharmacology: Clinical and Experimental 27, 156–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X. et al.. Cocaine infusion increases interferon-gamma and decreases interleukin-10 in cocaine-dependent subjects. Clin. Immunol. Immunopathol. 89, 181–190 (1998). [DOI] [PubMed] [Google Scholar]
- Kim S. G. et al. Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. J. Leukoc Biol. 94, 835–843 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison L. S. et al. Short-term discontinuation of HAART regimens more common in vulnerable patient populations. AIDS Research and Human Retroviruses 24, 1347–1355 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin C. H. et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS and Behavior 11, 185–194 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten J. H. et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. Journal of General Internal Medicine 17, 377–381 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA - Journal of the American Medical Association 300, 550–554 (2008). [DOI] [PubMed] [Google Scholar]
- Wood E. et al. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ 169, 656–661 (2003). [PMC free article] [PubMed] [Google Scholar]
- Baum M. K. et al. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. Journal of Acquired Immune Deficiency Syndromes 50, 93–99 (2009). [DOI] [PubMed] [Google Scholar]
- Carrico A. W. et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosomatic Medicine 69, 785–792 (2007). [DOI] [PubMed] [Google Scholar]
- Cook J. A. et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS 22, 1355–1363 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D., Keruly J. C. & Chaisson R. E. Differences in HIV Disease Progression by Injecting Drug Use in HIV-Infected Persons in Care. Journal of Acquired Immune Deficiency Syndromes 35, 46–51 (2004). [DOI] [PubMed] [Google Scholar]
- Denton P. W. et al. Generation of HIV latency in humanized BLT mice. J. Virol. 86, 630–634 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton P. W. et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5, e16 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatakis D. N. et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 108, E1408–1416 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkus M. W. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12, 1316–1322 (2006). [DOI] [PubMed] [Google Scholar]
- Nischang M. et al. Humanized mice recapitulate key features of HIV-1 infection: a novel concept using long-acting anti-retroviral drugs for treating HIV-1. PLoS One 7, e38853 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace M. J. et al. Directly infected resting CD4+ T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog. 8, e1002818 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein D. A. et al. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity 15, 671–682 (2001). [DOI] [PubMed] [Google Scholar]
- Kitchen S. G. et al. In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLoS Pathog 8, e1002649 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon C. C. et al. HIV-1 infection of hematopoietic progenitor cells in vivo in humanized mice. Blood 122, 2195–2204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S. et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood 115, 1534–1544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z. et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204, 705–714 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M. et al. Characterization of a promonocyte clone chronically infected with HIV and inducible by 13-phorbol-12-myristate acetate. J. Immunol. 140, 1117–1122 (1988). [PubMed] [Google Scholar]
- Kitchen S. G. et al. Engineering antigen-specific T cells from genetically modified human hematopoietic stem cells in immunodeficient mice. PLoS One 4, e8208 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy L. E. et al. The distribution of cocaine in mice differs by age and strain. Neurotoxicol. Teratol. 26, 839–848 (2004). [DOI] [PubMed] [Google Scholar]
- Vatakis D. N., Bristol G., Wilkinson T. A., Chow S. A. & Zack J. A. Immediate activation fails to rescue efficient human immunodeficiency virus replication in quiescent CD4+ T cells. J. Virol. 81, 3574–3582 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]