Abstract
Recent studies have indicated that the C-reactive protein/ albumin (CRP/Alb) ratio is associated with clinical outcomes in patients with hepatocellular carcinoma (HCC). We examined the prognostic value of this ratio in patients with small-cell lung cancer (SCLC). In this retrospective study, a total of 367 eligible SCLC patients were analyzed and the correlation between the pretreatment CRP/Alb ratio and overall survival (OS) was investigated. The optimal cutoff level of CRP/Alb ratio was at 0.441. A low and high CRP/Alb ratio was assigned to 65.1% and 34.9% of patients, respectively. The median OS of patients with a high CRP/Alb ratio was worse than those in the low group (13.70 vs 18.90 months HR, 1.34; p = 0.005). Disease stage (p < 0.001), performance status (PS) (p < 0.001) and pretreatment LDH (p < 0.001) were also significant predictors of OS. Multivariate analyses showed that the CRP/Alb ratio is an independent prognostic factor (p = 0.025). This study demonstrated that the CRP/Alb ratio could independently predict OS in patients with SCLC, and had comparable prognostic value to other known prognostic markers. Therefore, the CRP/Alb ratio could have prognostic value and be a measurable biomarker in patients with SCLC.
Lung cancer is a commonly diagnosed cancer and is the leading cause of cancer-related deaths. In 2014, approximately 224,210 new lung cancer cases are predicted and 159,260 will die from this malignancy in the United States1. Of the lung cancers diagnosed, 15% are classified as small-cell lung cancer (SCLC)2,3. Staging of patients with SCLC is determined by the Veteran Affairs Lung Study Group (VALSG) staging system4. First-line treatment using etoposide-based chemotherapy produces a high response rate in patients with SCLC. However, the 5-year overall survival rate for patients with limited and extensive staging is 25% and 7.8%, respectively5,6. Currently, although thoracic radiotherapy and prophylactic cranial irradiation have improved the clinical outcome of patients with SCLC, a proportion of patients will develop relapse and/or distant metastases7. These patients have a poorer prognosis. Therefore, identifying factors with prognostic value in patients currently treated for SCLC may help improve their clinical outcomes.
Various laboratory and clinical markers include baseline serum carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), neuron-specific enolase (NSE), performance status (PS), disease extent, age, and gender. These prognostic markers are associated with overall survival (OS) in SCLC8,9,10. Of the prognostic factors, PS and disease stage are important prognostic indicators for SCLC11,12. However, conflicting results of these prognostic factors have been reported and there remains no optimal prognostic index for SCLC.
Although environmental and genetic factors contribute to the development of cancer, there is increasing evidence which demonstrates the role of inflammation in the initiation and progression of cancer13. The prognostic value of many inflammation–based scores, such as the modified Glasgow Prognostic Score (mGPS), prognostic Nutritional Index (PNI), and neutrophil Lymphocyte Ratio (NLR), have been validated in many types of cancer, including SCLC14,15,16. Additionally, several studies have indicated that the levels of C-reactive protein (CRP) correlates with the disease progression in a number of cancers. It has been reported that serum albumin could be a marker of tumor recurrence17,18,19. The prognostic value of CRP and serum albumin is unknown and may have independent prognostic value. A previous study showed that CRP/Alb ratio is an independent risk factor of mortality in patients with sepsis20. Moreover, it has been shown that the C-reactive protein/ Albumin (CRP/Alb) ratio is significantly associated with the outcome of patients with cancer21.
In this retrospective study, we examine the prognostic value of CRP/Alb ratio in patients with SCLC. Additionally, we further investigate the relationship between CRP/Alb ratio and clinical outcomes.
Materials and Methods
Participant Identification
We enrolled 665 patients diagnosed with SCLC in Sun Yat-sen University Cancer Center (SYSUCC) between January 2006 and December 2011. The inclusion criteria of this study were as follow: 1) cytologically or histologically diagnosed as SCLC, 2) an age of at least 18 years, 3) having at least one measurable lesion, 4) having pre-treatment blood sampling for CRP and albumin measurement, and 5) staged on the basis of VALSG staging system. Patients were excluded if there is detectable inflammatory disease. Moreover, the propensity score is used to adjust for potential selection bias of the participants. Then a total of 367 eligible patients were enrolled into the study. All clinical and follow-up records were reviewed retrospectively. This study was approved by the Institutional Review Board of SYSUCC and written informed consent was obtained for each patient.
Clinical Data extraction
We collected baseline characteristic of participants, including age, gender, disease stage, PS, smoking status, and histology by using a standard data extraction system. Patients who had more than 100 cigarettes were defined as smokers. Stage was determined based on the VALSG staging system, which divides patients into limited and extensive stage. Blood samples were tested prior to initial treatment for levels of CRP, albumin, and LDH. The CRP/Alb ratio was calculated by dividing the serum CRP level by the serum albumin level20,22.
Patients Follow-up
All patients were carefully followed after pathological diagnosis. Patients received dynamic computed tomography (CT) scan every 2 cycles of chemotherapy or every 6 weeks. The response of treatment was evaluated by a systematical radiologic review committee according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.0). We compared the difference in survival by means of OS, defined as the time elapsed from the date of pathological diagnosis to the time of death for any cause or at last follow-up. The initiation of follow-up was the date of finishing the anti-tumor treatment, and the end date of follow-up was March 30, 2014 or death from any cause. Patients who did not die at the time of last follow-up were censored.
Statistical Analysis
Continuous variables were presented as median value and range, and were transformed into dichotomous variables at median value. The Chi-Square or Fisher’s exact test was used to compare the Categorical variables, which was presented as the numbers and percentages of patients. The optimal cutoff level of CRP/Alb ratio was determined by a web-based system, R software-engineered, designed by Budczies J et al. ( http://molpath.charite.de/cutoff/)23. The relationship between OS and prognostic factors, which includes cancer stage, LDH, CRP/Alb ratio and PS, was calculated using the Kaplan–Meier method respectively. Univariate analyses were conducted using the log-rank test analysis. Multivariate analysis of these variables in survival was performed using the Cox proportional hazards model. All statistical analyses were performed using SPSS 21.0 software (IBM, Armonk, NY). All tests were two-sided and a P value < 0.05 was considered statistically significant.
Results
A total of 367 enrolled patients were diagnosed with SCLC. Pre-treatment evaluation of CRP and albumin was performed between January 2006 and December 2011. The assessment of PS and disease stage was taken at time of patient admission. The majority of the patients enrolled were males (n = 316, 86.1%) and smokers (n = 302, 82.3%), and had a PS of 0-1 (n = 337, 91.8%). The median age was 59 years (range: 23-82 years). Among these, 180 (49.0%) patients were in limited stage and 187 (51.0%) patients were in extensive stage. Most of the enrolled patients received etoposide-based chemotherapy as first-line treatment (n = 315, 85.8%), while 81 (22.1%) patients had prophylactic cranial irradiation (PCI) after chemotherapy. The baseline characteristics of the 367 patients are shown in Table 1.
Table 1. Basic characteristics of all patients and univariate survival analysis.
| Variables | Cases, N | Proportion, % | Median OS, months (95% CI) | P valueb |
|---|---|---|---|---|
| Age, years | 59 (23-82)a | 0.049 | ||
| Gender | ||||
| Male | 316 | 86.1 | 16.7(14.75-18.65) | 0.077 |
| Female | 51 | 13.9 | 18.7(11.61-27.39) | |
| Smoking | ||||
| Smoker | 302 | 82.3 | 16.8(14.69-18.91) | 0.917 |
| Never-smoker | 65 | 17.7 | 16.0(12.61-19.33) | |
| Disease stage | ||||
| Limited stage | 180 | 49.0 | 22.1(17.10-27.10) | <0.001 |
| Extensive stage | 187 | 51.0 | 14.2(11.61-16.72) | |
| PS | ||||
| 0 | 195 | 53.1 | 17.0(15.01-19.06) | <0.001 |
| 1 | 142 | 38.7 | 18.7(16.14-21.20) | |
| 2 | 30 | 8.2 | 9.3(7.94-10.73) | |
| CRP/Alb | ||||
| ≥0.441 | 128 | 34.9 | 13.7(11.86-15.61) | 0.005 |
| <0.441 | 239 | 65.1 | 18.9(16.93-20.87) | |
| LDH, U/L | ||||
| Normal range | 220 | 59.9 | 19.2(17.52-20.89) | <0.001 |
| Abnormally elevated | 147 | 40.1 | 13.3(11.06-15.61) | |
| Chemotherapy regimen | ||||
| Etoposide-based | 315 | 85.8 | 17.2(15.34-19.13) | 0.685 |
| Others | 52 | 14.2 | 13.2(7.31-19.16) | |
| Prophylacticcranial irradiation | 0.361 | |||
| Yes | 81 | 22.1 | 16.2(12.85-19.55) | |
| No | 286 | 77.9 | 17.0(15.08-18.99) | |
Note: aMedian (range); bLog-rank test
Abbreviations: OS, overall survival; CI, confidence interval; PS, performance status; CRP/Alb, C-reactive protein/albumin; LDH, lactate dehydrogenase
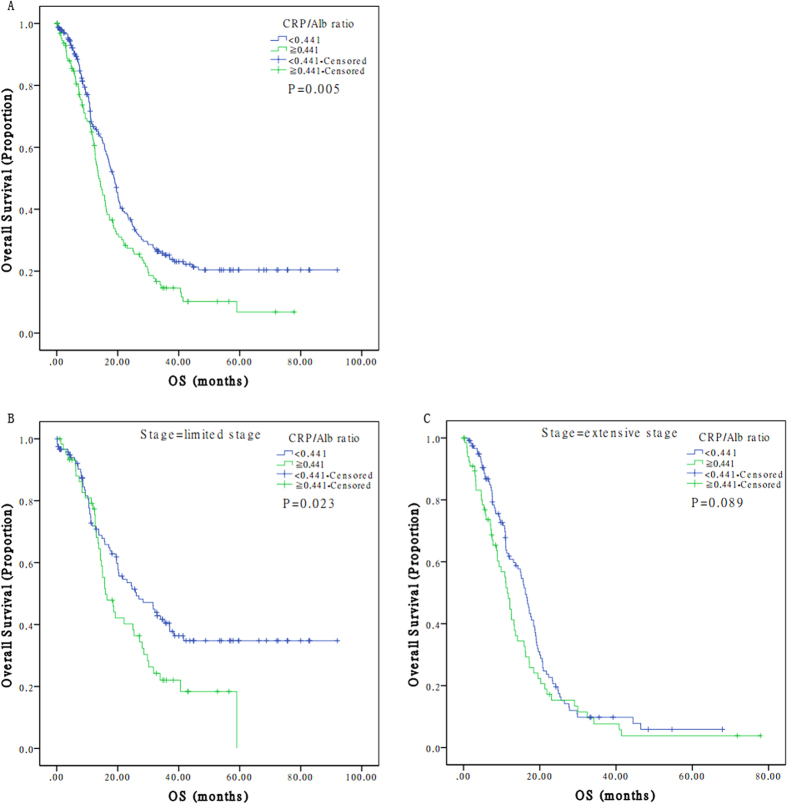
Using the biostatistical tool, Cutoff Finder, we found that the range of cutoff value for CRP/Alb ratio was wide and determined 0.441 as the optimal cutoff level for assessing OS23 (Fig. 1). Patients were divided into two groups based on the cutoff value of CRP/Alb ratio ≥ 0.441 (n = 128, 34.9%) and CRP/Alb ratio <0.441 (n = 239, 65.1%).
Figure 1.
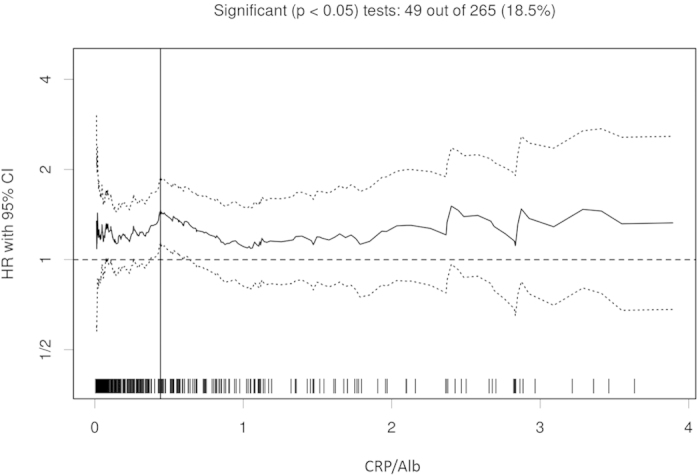
Hazard ratio (HR) for overall survival (OS) independent of cutoff point for CRP/Alb ratio in patients with small-cell lung cancer. The vertical line designates the optimal cutoff point with the most significant (log-rank test) split. The plots were generated using Cutoff Finder.
The clinicopathological characteristics of patients based on CRP/Alb ratio are described in Table 2. An elevated CRP/Alb ratio was significantly associated with the abnormally higher LDH level (p = 0.010). However, compared to the high CRP/Alb ratio group, gender (p = 0.637), age (p = 0.659), PS (p = 0.734), chemotherapy regimen (p = 0.432) and smoking status (p = 0.669) of patients were similar in the low CRP/Alb ratio group.
Table 2. Clinicopathological characteristics of patients stratified by CRP/Alb ratio.
| Variables | CRP/Alb ratio ≥0.441, n (%) | CRP/Alb ratio <0.441, n (%) | P value |
|---|---|---|---|
| Age( years) | 0.659 | ||
| <59 | 108 (66.7) | 54 (33.3) | |
| ≥59 | 131 (63.9) | 74 (36.1) | |
| Gender | 0.637 | ||
| Male | 204 (64.6) | 112 (35.4) | |
| Female | 35 (68.6) | 16 (31.4) | |
| Smoking | 0.669 | ||
| smoker | 195 (64.6) | 107 (35.4) | |
| Never smoker | 44 (67.7) | 21 (32.3) | |
| PS at diagnosis | 0.734 | ||
| 0 | 130 (66.7) | 65 (33.3) | |
| 1 | 91 (64.1) | 51 (35.9) | |
| 2 | 18 (60.0) | 12 (40.0) | |
| Stage | 0.584 | ||
| Limited disease | 120 (66.7) | 60 (33.3) | |
| Extensive disease | 119 (63.6) | 68 (36.4) | |
| LDH at diagnosis, U/L | 0.010 | ||
| Normal range | 155 (70.5) | 65 (29.5) | |
| Abnormally elevated | 84 (57.1) | 63 (42.9) | |
| Chemotherapy regimen | 0.432 | ||
| Etoposide-based | 208 (66.0) | 107 (34.0) | |
| others | 31 (59.6) | 21 (40.4) | |
| Prophylactic cranial irradiation | 0.793 | ||
| Yes | 185 (64.7) | 101 (35.3) | |
| No | 54 (66.7) | 27 (33.3) |
Abbreviations: PS, performance status; LDH, lactate dehydrogenase; CRP/Alb, C-reactive protein / albumin.
A total of 128 (34.9%) patients were categorized into the high level group of CRP/Alb ratio, while the remaining 239 (65.1%) patients were stratified into the low level group. In contrast to the patients with high CRP/Alb ratio, patients with low CRP/Alb ratio had longer overall survival (18.9 vs 13.7 months, p = 0.005) (Table 1). Similarly, longer overall survival was also observed when patients were stratified into limited stage (p = 0.023), but not extensive stage (p = 0.089) (Fig. 2A–C).
Figure 2.
The prognostic value of CRP/Alb ratio on overall survival curves in patients with limited or extensive stage disease. A. Comparison of OS on patients with high CRP/Alb ratio vs low CRP/Alb ratio. B. Comparison of OS on patients in limited stage with high CRP/Alb vs low CRP/Alb ratio. C. Comparison of OS on patients in extensive stage with high CRP/Alb vs low CRP/Alb ratio
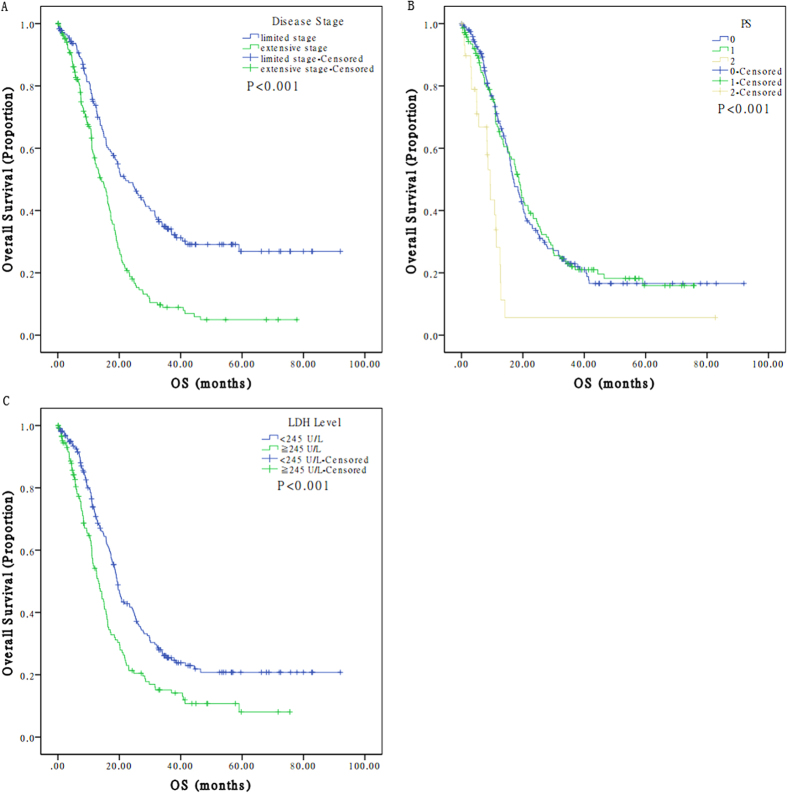
The median follow-up time was 29.40 months (range: 0.03-116.07 months). During the follow-up period, 258 (70.3%) patients died, and the ratio for loss to follow-up was 9.5% (n = 35). The median OS of the 367 eligible patients was 13.8 months (range: 0.03-92.03 months). Disease stage (p < 0.001), LDH level (p < 0.001), age (p = 0.049) and PS score (p < 0.001) were significantly associated with OS by univariate analyses (Fig. 3A–C). Furthermore, PS had distinct prognostic value in patients with limited (p = 0.024) or extensive staging (p < 0.001). However, when stratified by staging, a significant correlation between LDH level and OS was found in patients with extensive (P = 0.003), but not limited staging (p = 0.097). There were no significant association between OS and gender (p = 0.077), smoking status (p = 0.917), chemotherapy regimen (p = 0.685), and prophylactic cranial irradiation (p = 0.361) (Table 1).
Figure 3.
Overall survival curves comparing patients with A. limited disease vs extensive disease; B. good vs bad PS; C. high LDH vs low LDH
Using multivariate analyses, we then tested for correlation among the different variables. The analyses revealed that CRP/Alb ratio is an independent prognostic factor in patients with SCLC (p = 0.025). Compared to patients with a low CRP/Alb ratio ( < 0.441), those with high CRP/Alb ratio ( ≥ 0.441) were estimated to have 1.34 times higher risk of death (HR, 1.34; 95% CI, 1.04-1.73; p = 0.025). Moreover, cancer stage (p < 0.001), PS (p < 0.001) and LDH level (p = 0.008) also independently predicted OS (Table 3).
Table 3. Results from Cox Regression Model (Adjusted for Age, Sex, Disease stage, PS and the CRP/Alb ratio).
| Variables | Hazard Ratio |
95% CI |
P value | |
|---|---|---|---|---|
| LL | UL | |||
| Age (per 10 years’ increment) | 1.26 | 0.98 | 1.62 | 0.073 |
| ECOG-PS | ||||
| 0 | 1.00 | - | - | - |
| 1 | 0.94 | 0.73 | 1.22 | 0.654 |
| 2 | 3.13 | 1.94 | 5.05 | <0.001 |
| CRP/Alb ratio | ||||
| ≥0.442 | 1.00 | - | - | - |
| <0.442 | 1.34 | 1.04 | 1.73 | 0.025 |
| Disease stage | ||||
| Limited stage | 1.00 | - | - | - |
| Extensive stage | 2.13 | 1.65 | 2.76 | <0.001 |
| LDH (per 100U/L increment) | ||||
| Normal range | 1.00 | - | - | - |
| Abnormally elevated | 1.41 | 1.10 | 1.83 | 0.008 |
Abbreviations: CI, confidence interval; LL, lower limit; UL, upper limit; PS, performance status; CRP/Alb, C-reactive protein / albumin; LDH, lactate dehydrogenase.Ratio of C-Reactive Protein/Albumin is An Inflammatory Prognostic Score for Predicting Overall Survival of Patients with Small-cell Lung Cancer
Discussion
In this study, we retrospectively analyzed the prognostic power of CRP/Alb ratio in 367 eligible patients with SCLC at our cancer center. To our knowledge, this is the first study to analyze the correlation between CRP/Alb ratio and OS in patients with SCLC. The results of this demonstrated that CRP/Alb ratio is an independent prognostic indicator for patients with SCLC.
SCLC is an aggressive malignancy that is sensitive to cytotoxic agents and radiotherapy. However, most patients will die from the rapid growth of the cancer and acquired drug resistance associated with treatment3. Overall survival is an important index for evaluating clinical efficacy of different types of therapy. An inapposite predictor of OS may underestimate therapeutic benefits. Therefore, an accurate prognostic indicator to select those who are likely to benefit from anti-tumor treatment is needed.
Previous studies have reported that PS score, disease extent, LDH level, CEA and NSE significantly correlates with overall survival benefit in patients with SCLC8,24,25,26. These findings are consistent with the results of our studies, which demonstrated that better PS, limited staging, and normal serum levels of LDH were significantly associated with a longer survival than those with patients with a poor PS, extensive staging, and elevated serum LDH levels (Table 1). However, it remains uncertain whether these clinical variables have prognostic value in patients with SCLC. Furthermore, limitations in PS, heterogeneity of patients in the same disease stage, and uncertainties regarding increased LDH level may limit the prognostic value of these factors in predicting OS in SCLC.
Recent studies have showed a potential relationship between chronic inflammation and cancer. Inflammatory cells may alter the tumor microenvironment, which can promote tumorigenesis by increasing the proliferation, migration, and immune escape of tumor cells27. These observations suggest a correlation between chronic inflammation and poor OS in patients with cancer. Several studies have shown a link between chronic inflammation and tumorigenesis28,29. IL-6 is an important proinflammatory cytokine that plays a key role in the development of cancer and closely correlates with CRP levels. It has been reported that CRP is a sensitive and reliable prognostic marker for systemic inflammation that is also convenient for testing with standardized parameters established in clinical laboratories30,31. Hong et al. observed that high CRP level is associated with poor prognosis of patients with SCLC32. Moreover, serum albumin level was also a prognostic factor in SCLC patients. Low level of albumin is associated with malnutrition and weight loss, which results in a poor PS and increased cancer-related mortality33.
We hypothesized that merging CRP and albumin into a new index may have prognostic value in inflammation, and better predict overall survival of patients with cancer. Fairclough et al. proposed the concept of CRP/Alb ratio22. Otavio et al. indicated that CRP/Alb ratio showed predicted mortality in patients in ICU20. Furthermore, Akiyoshy et al. demonstrated that CRP/Alb ratio was a prognostic factor in patients with hepatocellular carcinoma (HCC). Therefore, we proposed that the CRP/Alb ratio could be a prognostic factor for patients with SCLC. In this study, a 0.441 cutoff value for CRP/Alb ratio was used for predicting overall survival in SCLC. In our study, a univariate analysis showed that the CRP/Alb ratio is associated with poor prognosis (p= 0.005) (Table 1). Compared with patients who had CRP/Alb ratio < 0.441, those with CRP/Alb ratio ≥ 0.441 had a 1.34 times higher chance of death (Table 3). By multivariate analysis, when adjusted for other variables, including cancer stage, CRP/Alb ratio independently predicted the overall survival of patients with SCLC (p = 0.025) (Table 3). Subgroup analysis suggested that OS in CRP/Alb ratio <0.441 group was significantly longer than those with CRP/Alb ratio ≥0.441 in limited stage (p = 0.023). To our knowledge, this is the first study to evaluate the prognostic value of CRP/Alb ratio in patients with lung cancer. Furthermore, it is the first study to indicate that CRP/Alb ratio can predict the overall survival in SCLC. Other than PS, the CRP/Alb ratio is more objectively determined, and would be a simple, optimal, and inexpensive prognostic indicator in patients with SCLC. Although first-line treatment with chemotherapy and radiotherapy provide much clinical benefit for patients with SCLC, they are associated with severe adverse reaction. These include myelosuppression, anorexia, and radiation pneumonitis, which may suppress the immune system and negatively affect the nutritional status of patients. Therefore, it is essential that therapeutic decisions should take into consideration the curative effects versus treatment-induced toxicities. In this study, we demonstrate that CRP/Alb ratio may serve as a screening method to choose the appropriate treatment for patients with SCLC.
There are several limitations to our study. This includes being a retrospective and single-center study, which may limit the prognostic value of the CRP/Alb ratio. To minimize selection bias, we enrolled consecutive patients between January 2006 and December 2011. We also observed that by ultivariate analysis, the prognostic value of CRP/Alb ratio significantly correlates with limited stage in patients with SCLC. This suggests that CRP/Alb ratio is a prognostic factor, though a large-scale prospective validation study is needed. In summary, this study demonstrated that a systemic inflammation-based marker, such as CRP/Alb ratio, is an independent predictor of overall survival for patients with SCLC. The CRP/Alb ratio could be used to better predict prognosis in patients with SCLC.
Additional Information
How to cite this article: Zhou, T. et al. Ratio of C-Reactive Protein/Albumin is An Inflammatory Prognostic Score for Predicting Overall Survival of Patients with Small-cell Lung Cancer. Sci. Rep. 5, 10481; doi: 10.1038/srep10481 (2015).
Acknowledgments
This work was supported by: Wu Jieping Medical Foundation Project (Grant No: 08-JC-003), Innovative drug R&D center based on real-time high-throughput cell-based screening platform and large capacity compound library (Grant No: 2013ZX09401003-002), National Natural Science Funds of China (Grant No: 81372502) and National High Technology Research and Development Program of China (Grant No: 2012AA02A502). All the grand supporters have no roles in study design, data collection and analysis, and manuscript preparation.
Footnotes
Author Contributions Z.T. wrote manuscript and analyzed data; Z.L. performed research and analyzed data; Z.J.H. and H.S.D. designed research and analyzed data; Z.H.Y., H.Z.H., F.W.F. and Z.Y.Y. designed research; H.Y. and M.Y.X. analyzed data; Q.T., H.X.B., and Y.Y.P. performed research.
References
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians . 64, 9–29, 10.3322/caac.21208 (2014). [DOI] [PubMed] [Google Scholar]
- Herbst R. S., Heymach J. V. & Lippman S. M. Lung cancer. New. Engl. J. Med. 359, 1367–1380, 10.1056/NEJMra0802714 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally B. E., Urbanic J. J., Blackstock A. W., Miller A. A. & Perry M. C. Small cell lung cancer: have we made any progress over the last 25 years? The oncologist . 12, 1096–1104, 10.1634/theoncologist.12-9-1096 (2007). [DOI] [PubMed] [Google Scholar]
- Mountain C. F. Revisions in the International System for Staging Lung Cancer. Chest . 111, 1710–1717 (1997). [DOI] [PubMed] [Google Scholar]
- Stinchcombe T. E. & Gore E. M. Limited-stage small cell lung cancer: current chemoradiotherapy treatment paradigms. The oncologist . 15, 187–195, 10.1634/theoncologist.2009-0298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M. et al. [First-line chemotherapy and its survival analysis of 394 patients with extensive-stage small cell lung cancer in a single institute]. Zhongguo fei ai za zhi = Chinese journal of lung cancer. 17, 8–14, 10.3779/j.issn.1009-3419.2014.01.02 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- F J. B. & Costa A. F. [Small cell lung cancer-state of the art and future perspectives]. Rev. Port. Pneumol . 13, 587–604 (2007). [DOI] [PubMed] [Google Scholar]
- Stokkel M. P., van Eck-Smit B. L., Zwinderman A. H., Willems L. N. & Pauwels E. K. Pretreatment serum lactate dehydrogenase as additional staging parameter in patients with small-cell lung carcinoma. J. Cancer. Rres. Clin . 124, 215–219 (1998). [DOI] [PubMed] [Google Scholar]
- Fizazi K. et al. Normal serum neuron specific enolase (NSE) value after the first cycle of chemotherapy: an early predictor of complete response and survival in patients with small cell lung carcinoma. Cancer . 82, 1049–1055 (1998). [DOI] [PubMed] [Google Scholar]
- Paesmans M. et al. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer . 89, 523–533 (2000). [DOI] [PubMed] [Google Scholar]
- Rawson N. S. & Peto J. An overview of prognostic factors in small cell lung cancer. A report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Research. Brit J. Cancer . 61, 597–604 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. Survival and prognostic factors in small cell lung cancer. Medical oncology (Northwood, London, England) . 27, 73–81, 10.1007/s12032-009-9174-3 (2010). [DOI] [PubMed] [Google Scholar]
- Mantovani A. Cancer: Inflaming metastasis. Nature . 457, 36–37, 10.1038/457036b (2009). [DOI] [PubMed] [Google Scholar]
- Zhou T. et al.A systemic inflammation-based prognostic scores (mGPS) predicts overall survival of patients with small-cell lung cancer. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine, 10.1007/s13277-014-2623-4 (2014). [DOI] [PubMed] [Google Scholar]
- Kidney cancer: Prognostic nutritional index predicts survival. Nature reviews. Urology, 10.1038/nrurol.2014.292 (2014). [DOI]
- Lin G. N. et al. Elevated neutrophil-to-lymphocyte ratio predicts poor outcome in patients with advanced non-small-cell lung cancer receiving first-line gefitinib or erlotinib treatment. Asia-Pac. J. clin. onco, 10.1111/ajco.12273 (2014). [DOI] [PubMed] [Google Scholar]
- Roxburgh C. S. & McMillan D. C. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future oncol. (London, England) . 6, 149–163, 10.2217/fon.09.136 (2010). [DOI] [PubMed] [Google Scholar]
- Jin Y., Zhao L. & Peng F. Prognostic impact of serum albumin levels on the recurrence of stage I non-small cell lung cancer. Clinics . 68, 686–693, 10.6061/clinics/2013(05)17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz-Zajac M. et al. Comparative evaluation of serum C-reactive protein (CRP) levels in the different histological subtypes of esophageal cancer (squamous cell carcinoma and adenocarcinoma of esophagus). J. Clin. Lab. Anal. 26, 73–81, 10.1002/jcla.21486 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzani O. T., Zampieri F. G., Forte D. N., Azevedo L. C. & Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PloS one . 8, e59321, 10.1371/journal.pone.0059321 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A. et al.The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol., 10.1245/s10434-014-4048-0 (2014). [DOI] [PubMed] [Google Scholar]
- Fairclough E., Cairns E., Hamilton J. & Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin. Med. 9, 30–33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budczies J. et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PloS one . 7, e51862, 10.1371/journal.pone.0051862 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculier J. P. et al.Carcinoembryonic antigen: a useful prognostic marker in small-cell lung cancer. J. Clin. Oncol. 3, 1349–1354 (1985). [DOI] [PubMed] [Google Scholar]
- Petrovic M. et al. The prognostic significance of the circulating neuroendocrine markers chromogranin A, pro-gastrin-releasing peptide, and neuron-specific enolase in patients with small-cell lung cancer. Med. Oncol. (Northwood, London, England) . 31, 823, 10.1007/s12032-013-0823-1 (2014). [DOI] [PubMed] [Google Scholar]
- Bremnes R. M. et al. The value of prognostic factors in small cell lung cancer: results from a randomised multicenter study with minimum 5 year follow-up. Lung cancer . 39, 303–313 (2003). [DOI] [PubMed] [Google Scholar]
- Colotta F., Allavena P., Sica A., Garlanda C. & Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis . 30, 1073–1081, 10.1093/carcin/bgp127 (2009). [DOI] [PubMed] [Google Scholar]
- Balkwill F. & Mantovani A. Inflammation and cancer: back to Virchow? Lancet . 357, 539–545, 10.1016/S0140-6736(00)04046-0 (2001). [DOI] [PubMed] [Google Scholar]
- Coussens L. M. & Werb Z. Inflammation and cancer. Nature . 420, 860–867, 10.1038/nature01322 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povoa P. C-reactive protein: a valuable marker of sepsis. Intens. Care. Med. 28, 235–243, 10.1007/s00134-002-1209-6 (2002). [DOI] [PubMed] [Google Scholar]
- Kanoh Y., Abe T., Masuda N. & Akahoshi T. Progression of non-small cell lung cancer: diagnostic and prognostic utility of matrix metalloproteinase-2, C-reactive protein and serum amyloid A. Oncol. Rep. 29, 469–473, 10.3892/or.2012.2123 (2013). [DOI] [PubMed] [Google Scholar]
- Hong S., Kang Y. A., Cho B. C. & Kim D. J. Elevated serum C-reactive protein as a prognostic marker in small cell lung cancer. Yonsei. Med. J. 53, 111–117, 10.3349/ymj.2012.53.1.111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roxburgh C. S. & McMillan D. C. Cancer and systemic inflammation: treat the tumour and treat the host. Brit. J. cancer. 110, 1409–1412, 10.1038/bjc.2014.90 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]