Abstract
Alzheimer's disease (AD) is a common neurodegenerative disorder that can destroy the memory of sufferers and lead to distress for the individual and society. Brain-derived neurotrophic factor (BDNF) and butyrylcholinesterase (BCHE) are two genes associated with β-amyloid plaques and neurofibrillary tangles that are two key factors in the pathophysiology of AD. The aim of the current meta-analysis was to evaluate the association between BDNF Val66Met (rs6265), BDNF C270T (rs2030324) and BCHE-K (rs1803274) polymorphisms and AD. A comprehensive meta-analysis was performed using the online database PubMed without a time limitation. A total of 56 articles evaluating 12,563 cases and 12,622 controls were selected for the current meta-analysis. The results showed a moderate association of the BDNF C270T polymorphism with the risk of AD in Asians under a dominant model (P=0.03; odds ratio, 1.88; 95% confidence interval, 1.08–3.27). No other significant association was found during the meta-analysis for the other two polymorphisms (P>0.05). The current meta-analysis suggests that BDNF C270T is a risk factor for AD in Asians. This meta-analysis has been, to the best of our knowledge, the most comprehensive meta-analysis of BDNF Val66Met, BDNF C270T and BCHE-K to date.
Keywords: Alzheimer's disease, meta-analysis, BDNF Val66Met, BDNF C270T, BCHE-K, Asians
Introduction
Alzheimer's disease (AD) is a common neurodegenerative disorder that is the main cause of dementia. The clinical presentation of AD is characterized by progressive memory disorder and cognitive dysfunction (1). The worldwide prevalence of AD was 26.6 million in 2006, and this number is predicted to quadruple by 2050. The rapidly increased AD incidence is likely lead to a significant burden on family and society (2).
AD is a complex disease involving the interaction of genetic and environmental factors. It has been shown that AD development is contributed to by several elements, such as senile plaques, neurofibrillary tangles (NFTs), abnormally aggregated ‘reactive’ proteins like β-amyloid (Aβ) and tau, exposure to aluminum and brain inflammation (3). A genome-wide association study revealed that multiple mutations in candidate genes greatly increase the chance of developing AD (4). Genes such as brain-derived neurotrophic factor (BDNF) and butyrylcholinesterase (BCHE) are believed to play a significant role in AD progression (5–7).
BDNF is a member of the neurotrophic factor family and is encoded by a gene located on chromosome 11p13 (8). A previous study demonstrated that the levels of BDNF and its receptor, tyrosine receptor kinase B, were decreased in the frontal cortex and hippocampus of patients with AD (9). BDNF is known to protect against the neurotoxicity of the Aβ peptide and neural cell death by the aggregation of Aβ and tau proteins (10,11). Several single nucleotide polymorphisms (SNPs), such as Val66Met and C270T (rs2030324), in BDNF have been reported to be associated with AD (12–19).
BCHE is located on chromosome 3q26 (20), spanning over 73 kb with four exons and three large introns (21). The protein, BCHE, is an acetylcholine-hydrolyzing enzyme. BCHE is considered to be relevant to the progressive memory disorder and dementia in AD (22,23) and has been associated with NFTs and Aβ in the pathology of AD (24). In addition, BCHE has been found to play an important role in AD plaque maturation (25).
Inconsistent results have been reported in the previous studies on the association of BDNF and BCHE polymorphisms with AD. For BDNF Val66Met, there have been five positive results in Europeans (12–15) and Asians (16), and 21 negative results (among 18 studies) in Europeans (26–37), Asians (19,38–42) and Africans (29). For BDNF C270T, there have been four positive results in Europeans (13,17) and Asians (18,19) and 14 negative results in Europeans (28–31,34–37,43–45), Asians (40,41) and Africans (29). For BCHE-K variants, there have been six positive results in Europeans (46–51) and 22 negative results in Europeans (5,52–67) and Asians (68–72). Discrepancies among the previous association studies may have been the result of limited power, different ethnic backgrounds or the different processes and status in patients with AD. Meta-analysis can strengthen the power by combining data from different studies and can draw a more comprehensive conclusion by analyzing studies in different ethnicities (73–75). The aim of the current meta-analysis was to assess the association between the three polymorphisms and AD.
Materials and methods
Article retrieval
Articles were retrieved in January 2014 by searching PubMed without time or language restrictions. The following keywords were used: ‘Alzheimer disease BCHE association or Alzheimer disease BCHE polymorphism’ and ‘Alzheimer disease BDNF association or Alzheimer disease BDNF polymorphism’. The current meta-analysis included studies that met the following criteria: i) An original case-control study assessing the association of BDNF and BDHE with AD in humans; ii) a study containing sufficient information for the odds ratios (ORs) and 95% confidence intervals (CIs) to be obtained; iii) a study in which the genotype distribution of each polymorphism in controls met the Hardy-Weinberg equilibrium (HWE); iv) a study in the cumulative number of stages for one genetic locus was at least three. From each study, the following data were extracted or calculated: First author, publication year, country, ethnicity, number of cases and controls, HWE for controls, reported association results and the power of each study.
Data analysis
Arlequin software (76) was used to test whether the genotyping distribution in the controls was in HWE. The power of each study was calculated by a Power and Sample Size Calculation program (77). Cochran's Q statistic and I2 test (78) were used to evaluate the statistical heterogeneity. A fixed-effect model was used for the studies with minimal to moderate heterogeneity (I2<50%), and the random-effect model was used for the studies with significant heterogeneity (I2≥50%), with the exception of the allelic analysis. Subgroup analyses were performed in different inheritable models that contained dominant, recessive and additive models. Review Manager 5 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) was used to estimate the combined ORs and CIs (79). Funnel plots were drawn to observe the potential publication bias. P<0.05 was considered to indicate a statistically significant difference.
Results
Article retrieval
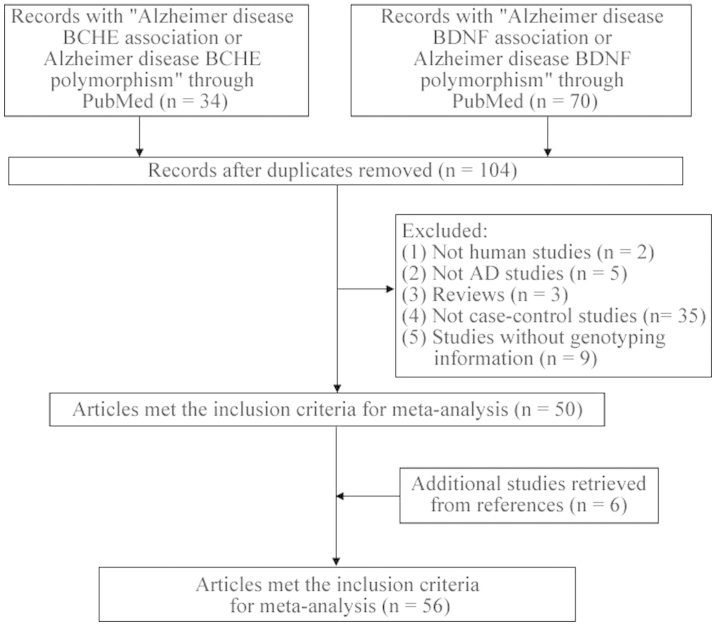
As shown in Fig. 1, 104 articles were obtained from the two searches. Two non-human studies were excluded, as were five non-AD studies, three reviews, 35 studies that only focused on patients and nine case-control studies without genotyping information. In addition, six studies were included from the references. The genotypes in the controls of the case-control studies met the HWE. Finally, 56 articles (5,12–19,26–72) with 12,563 cases and 12,622 controls among 72 stages were involved in the present meta-analysis. The characteristics of the included studies are shown in Table I.
Figure 1.
Flowchart of the selection process in the meta-analysis. AD, Alzheimer's disease; BCHE, butyrylcholinesterase; BDNF, brain-derived neurotrophic factor.
Table I.
Characteristics of the case-control studies in the current meta-analysis.
| First author (ref) | Year | Country | Ethnicity | Cases/controls (n/n) | HWE | Result | Power | |
|---|---|---|---|---|---|---|---|---|
| BDNF Val66Met | ||||||||
| Ventriglia (12) | 2002 | Italy | Europeans | 130/111 | Yes | S | 0.052 | |
| Saarela (13) | 2006 | Finland | Europeans | 97/101 | Yes | S | 0.086 | |
| Nacmias (26) | 2004 | Italy | Europeans | 83/97 | Yes | NS | 0.117 | |
| Combarros (27) | 2004 | Spain | Europeans | 237/218 | Yes | NS | 0.197 | |
| Tsai (38) | 2004 | China | Asians | 163/89 | Yes | NS | 0.164 | |
| Bian (39) | 2005 | China | Asians | 203/239 | Yes | NS | 0.266 | |
| Lee (28) | 2005 | USA | Europeans | 95/70 | Yes | NS | 0.119 | |
| Nishimura (19) | 2005 | Japan | Asians | 172/275 | Yes | NS | 0.262 | |
| Desai 1 (29) | 2005 | USAa | Europeans | 995/671 | Yes | NS | 0.516 | |
| Desai 2 (29) | 2005 | USAb | Africans | 64/45 | Yes | NS | 0.054 | |
| Matsushita (16) | 2005 | Japan | Asians | 487/471 | Yes | S | 0.512 | |
| Vepsäläinen (30) | 2005 | Finland | Europeans | 375/460 | NA | NS | 0.254 | |
| Bodner (31) | 2005 | USA | Europeans | 256/194 | Yes | NS | 0.192 | |
| Li 1 (32) | 2005 | UK | Europeans | 359/396 | Yes | NS | 0.285 | |
| Li 2 (32) | 2005 | USAc | Europeans | 188/361 | Yes | NS | 0.222 | |
| Li 3 (32) | 2005 | USAd | Europeans | 388/349 | Yes | NS | 0.271 | |
| Forero (33) | 2006 | Colombia | Europeans | 101/168 | Yes | NS | 0.111 | |
| Akatsu (40) | 2006 | Japan | Asians | 95/108 | Yes | NS | 0.146 | |
| Zhang (34) | 2006 | USA | Europeans | 295/250 | Yes | NS | 0.224 | |
| Tsai (41) | 2006 | China | Asians | 175/189 | Yes | NS | 0.229 | |
| Huang (35) | 2007 | USA | Europeans | 220/128 | Yes | NS | 0.124 | |
| He (42) | 2007 | China | Asians | 513/575 | Yes | NS | 0.564 | |
| Cozza (37) | 2008 | Italy | Europeans | 251/97 | Yes | NS | 0.139 | |
| Feher (14) | 2009 | Hungary | Europeans | 160/164 | Yes | S | 0.211 | |
| Pivac (15) | 2011 | Croatia | Europeans | 211/402 | Yes | S | 0.235 | |
| Boiocchi (36) | 2013 | Italy | Europeans | 191/408 | Yes | NS | 0.262 | |
| BDNF C270T | ||||||||
| Kunugi (18) | 2001 | Japan | Asians | 170/498 | Yes | S | 0.084 | |
| Riemenschneider (43) | 2002 | Germany | Europeans | 210/188 | Yes | NS | 0.076 | |
| Nishimura (44) | 2004 | Brazil | Europeans | 188/188 | Yes | NS | 0.088 | |
| Olin (17) | 2005 | US | Europeans | 212/202 | Yes | S | 0.076 | |
| Lee (28) | 2005 | USA | Europeans | 106/73 | Yes | NS | 0.063 | |
| Nishimura (19) | 2005 | Japan | Asians | 172/275 | Yes | S | 0.073 | |
| Desai 1 (29) | 2005 | USAa | Europeans | 719/523 | Yes | NS | 0.207 | |
| Desai 2 (29) | 2005 | USAb | Africans | 58/42 | Yes | NS | 0.056 | |
| Vepsäläinen (30) | 2005 | Finland | Europeans | 375/460 | Yes | NS | 0.457 | |
| Bodner (31) | 2005 | USA | Europeans | 256/194 | Yes | NS | 0.088 | |
| Akatsu (40) | 2006 | Japan | Asians | 95/108 | Yes | NS | 0.065 | |
| Zhang (34) | 2006 | USA | Europeans | 295/250 | Yes | NS | 0.113 | |
| Saarela (13) | 2006 | Finland | Europeans | 97/101 | Yes | S | 0.089 | |
| Tsai (41) | 2006 | China | Asians | 175/189 | Yes | NS | 0.096 | |
| Huang (35) | 2007 | USA | Europeans | 220/128 | Yes | NS | 0.081 | |
| Cozza (37) | 2008 | Italy | Europeans | 251/97 | Yes | NS | 0.091 | |
| Cousin (45) | 2011 | France | Europeans | 425/470 | Yes | NS | 0.152 | |
| Boiocchi (36) | 2013 | Italy | Europeans | 192/384 | Yes | NS | 0.308 | |
| BCHE-K | ||||||||
| Lehmann (51) | 1997 | UK | Europeans | 74/104 | NA | S | 0.083 | |
| Singleton (63) | 1998 | UK | Europeans | 119/83 | Yes | NS | 0.111 | |
| Crawford (62) | 1998 | USA | Europeans | 391/201 | Yes | NS | 0.182 | |
| Brindle (64) | 1998 | USA | Europeans | 188/165 | NA | NS | 0.161 | |
| Piccardi (55) | 2007 | Italy | Europeans | 158/118 | Yes | NS | 0.109 | |
| Kehoe (60) | 1998 | UK | Europeans | 181/71 | Yes | NS | 0.093 | |
| Ki (71) | 1999 | Korea | Asians | 78/74 | NA | NS | 0.231 | |
| Wiebusch (50) | 1999 | Canada | Europeans | 135/70 | Yes | S | 0.094 | |
| Grubber (59) | 1999 | USA | Europeans | 245/241 | NA | NS | 0.137 | |
| Tilley (58) | 1999 | UK | Europeans | 177/118 | Yes | NS | 0.145 | |
| McIlroy (49) | 2000 | Ireland | Europeans | 175/187 | Yes | S | 0.175 | |
| Yamamoto (70) | 1999 | Japan | Asians | 203/288 | NA | NS | 0.087 | |
| Lee (69) | 2000 | China | Asians | 89/101 | NA | NS | 0.086 | |
| Mattila (57) | 2000 | Finland | Europeans | 80/67 | Yes | NS | 0.077 | |
| Bi | 2001 | China | Asians | 38/40 | Yes | NS | 0.171 | |
| Prince (56) | 2001 | Sweden | Europeans | 201/166 | Yes | NS | 0.103 | |
| Raygani (47) | 2004 | Iran | Europeans | 105/129 | Yes | S | 0.146 | |
| Combarros (46) | 2005 | Spain | Europeans | 187/172 | Yes | S | 0.134 | |
| Deniz-Naranjo (54) | 2007 | Spain | Europeans | 282/312 | Yes | NS | 0.248 | |
| Mateo (53) | 2008 | Spain | Europeans | 231/221 | Yes | NS | 0.144 | |
| Scacchi (5) | 2009 | Italy | Europeans | 471/254 | Yes | NS | 0.279 | |
| Simão-Silva | 2013 | Brazil | Europeans | 78/80 | Yes | NS | 0.108 | |
| Russ (65) | 1998 | UK | Europeans | 203/122 | NA | NS | 0.100 | |
| Hiltunen (61) | 1998 | Finland | Europeans | 59/59 | Yes | NS | 0.090 | |
| Yamada (72) | 1998 | Japan | Asians | 48/107 | Yes | NS | 0.096 | |
| Alvarez-Arcaya (48) | 2000 | Spain | Europeans | 202/249 | NA | S | 0.141 | |
| Helbecque (66) | 1998 | Variouse | Europeans | 336/344 | Yes | NS | 0.272 | |
| Laws (67) | 1999 | Australia | Europeans | 237/348 | NA | NS | 0.239 |
Caucasian descent
African-American.
Samples collected from the University of California, San Diego, CA, USA
samples collected from Washington University Alzheimer's Disease Research Center patient registry (Seattle, WA, USA).
France, UK, Spain, Italy and the Netherlands. HWE, Hardy-Weinberg equilibrium; NS, not significant; S, significant; NA, not applicable; BDNF, brain-derived neurotrophic factor; BCHE, butyrylcholinesterase.
BDNF Val66Met
A total of 23 articles with 26 stages involving 6,504 patients with AD and 6,636 controls were included for the meta-analysis of BDNF Val66Met. Significant statistical heterogeneity was found at the allelic level (I2=58%), under the dominant model (I2=56%) and the additive model (I2=53%). The major allele frequency of BDNF Val66Met was 0.805 in Europeans [International Haplotype Mapping Project panel derived from Utah residents with Northern and Western European ancestry (HapMap-CEU)], higher than the frequency in Asians [HapMap-Han Chinese in Beijing, China (HCB), 0.733; HapMap-Japanese in Tokyo Japan (JPT), 0.682] and Africans [HapMap-Yoruba in Ibadan, Nigeria (YRI), 0.004]. The ethnic differences for BDNF Val66Met were low between different populations [Fixation index (Fst)=0.1006]; therefore, the meta-analysis was also performed by ethnicity. No significant association was found in the meta-analysis on allelic analysis (P=0.99; OR, 1.00; 95% CI, 0.91–1.10; Table II) or under the other models for combined and stratified populations (P>0.05, Table II).
Table II.
Meta-analysis of the association of the BDNF Val66Met, BDNF C270T and BCHE-K polymorphisms with Alzheimer's disease.
| A, BDNF Val66Met polymorphism | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genetic model | Cases/controls (n/n) | Ethnicity | No. of studies | OR (95% CI) | P-value | I2 (%) | Power | |||||||
| Overall (M vs. V) | 6504/6636 | Overall | 26 | 1.00 (0.91–1.10) | 0.99 | 58 | 1.000 | |||||||
| 4632/4645 | Europeans | 18 | 1.01 (0.88–1.15) | 0.89 | 65 | 0.999 | ||||||||
| 1808/1946 | Asians | 7 | 0.95 (0.87–1.04) | 0.26 | 38 | 0.976 | ||||||||
| 64/45 | Africans | 1 | 1.18 (0.27–5.06) | 0.82 | NA | 0.054 | ||||||||
| Dominant (MM/MV vs. VV) | 6129/5982 | Overall | 25 | 1.03 (0.90–1.18) | 0.65 | 56 | 1.000 | |||||||
| 4257/4185 | Europeans | 17 | 1.03 (0.87–1.22) | 0.72 | 64 | 1.000 | ||||||||
| 1808/1946 | Asians | 7 | 0.98 (0.85–1.13) | 0.82 | 16 | 0.938 | ||||||||
| 64/45 | Africans | 1 | 1.19 (0.27–5.24) | 0.82 | NA | 0.060 | ||||||||
| Recessive (MM vs. MV/VV) | 6129/6176 | Overall | 25 | 0.90 (0.80–1.02) | 0.43 | 42 | 0.997 | |||||||
| 4257/4185 | Europeans | 17 | 0.95 (0.78–1.15) | 0.58 | 44 | 0.880 | ||||||||
| 1808/1946 | Asians | 7 | 0.87 (0.74–1.02) | 0.09 | 44 | 0.918 | ||||||||
| 64/45 | Africans | 1 | NA | NA | NA | NA | ||||||||
| Additive (MM vs. VV) | 3650/3753 | Overall | 25 | 0.92 (0.71–1.18) | 0.50 | 53 | 0.988 | |||||||
| 2160/2419 | Europeans | 17 | 0.82 (0.57–1.18) | 0.28 | 59 | 0.934 | ||||||||
| 1393/1271 | Asians | 7 | 1.11 (0.85–1.45) | 0.43 | 9 | 0.631 | ||||||||
| 97/63 | Africans | 1 | 0.85 (0.33–2.15) | 0.73 | NA | 0.085 | ||||||||
| B, BDNF C270T polymorphism | ||||||||||||||
| Genetic model | Cases/controls (n/n) | Ethnicity | No. of studies | OR (95% CI) | P-value | I2 (%) | Power | |||||||
| Overall (T vs. C) | 4216/4370 | Overall | 18 | 1.12 (0.91–1.37) | 0.30 | 63 | 0.986 | |||||||
| 3546/3258 | Europeans | 13 | 1.01 (0.83–1.24) | 0.92 | 58 | 0.982 | ||||||||
| 612/1070 | Asians | 4 | 1.80 (0.99–3.27) | 0.06 | 62 | 0.154 | ||||||||
| 58/42 | Africans | 1 | 0.71 (0.17–2.94) | 0.64 | NA | 0.056 | ||||||||
| Dominant (TT/TC vs. CC) | 4216/4370 | Overall | 18 | 1.10 (0.87–1.39) | 0.40 | 62 | 0.999 | |||||||
| 3546/3258 | Europeans | 13 | 0.99 (0.78–1.25) | 0.91 | 58 | 0.998 | ||||||||
| 612/1070 | Asians | 4 | 1.88 (1.08–3.27) | 0.03a,b | 53 | 0.241 | ||||||||
| 58/42 | Africans | 1 | 0.70 (0.17–2.99) | 0.63 | NA | 0.064 | ||||||||
| Recessive (TT vs. TC/CC) | 4216/4370 | Overall | 18 | 1.12 (0.88–1.42) | 0.37 | 0 | 0.725 | |||||||
| 3546/3258 | Europeans | 13 | 1.12 (0.88–1.43) | 0.37 | 0 | 0.737 | ||||||||
| 612/1070 | Asians | 4 | 1.33 (0.03–51.71) | 0.88 | 64 | 0.062 | ||||||||
| 58/42 | Africans | 1 | NA | NA | NA | NA | ||||||||
| Additive (TT vs. CC) | 3528/3609 | Overall | 18 | 1.17 (0.88–1.54) | 0.29 | 0 | 0.722 | |||||||
| 2929/2560 | Europeans | 13 | 1.17 (0.88–1.55) | 0.29 | 0 | 0.738 | ||||||||
| 545/1011 | Asians | 4 | 1.40 (0.03–57.09) | 0.86 | 64 | 0.063 | ||||||||
| 54/38 | Africans | 1 | NA | NA | NA | NA | ||||||||
| C, BCHE-K polymorphism | ||||||||||||||
| Genetic model | Cases/controls (n/n) | Ethnicity | No. of studies | OR (95% CI) | P-value | I2 (%) | Power | |||||||
| Overall (K vs. W) | 4769/4242 | Overall | 27 | 1.07 (0.94–1.21) | 0.31 | 56 | 1.000 | |||||||
| 4313/3632 | Europeans | 22 | 1.06 (0.92–1.22) | 0.45 | 63 | 0.994 | ||||||||
| 456/610 | Asians | 5 | 1.17 (0.92–1.47) | 0.20 | 0 | 0.341 | ||||||||
| Dominant (KK/KW vs. WW) | 3659/2992 | Overall | 20 | 1.01 (0.84–1.22) | 0.89 | 65 | 0.999 | |||||||
| 3573/2845 | Europeans | 18 | 1.02 (0.83–1.24) | 0.88 | 69 | 0.998 | ||||||||
| 86/147 | Asians | 2 | 0.97 (0.55–1.73) | 0.93 | 0 | 0.151 | ||||||||
| Recessive (KK vs. KW/WW) | 3452/2628 | Overall | 19 | 1.15 (0.85–1.54) | 0.36 | 0 | 0.455 | |||||||
| 3366/2481 | Europeans | 17 | 1.19 (0.88–1.61) | 0.25 | 0 | 0.433 | ||||||||
| 86/147 | Asians | 2 | 0.35 (0.05–2.22) | 0.26 | 0 | 0.073 | ||||||||
| Additive (KK vs. WW) | 2430/1995 | Overall | 19 | 1.19 (0.90–1.58) | 0.23 | 0 | 0.439 | |||||||
| 2372/1891 | Europeans | 17 | 1.23 (0.92–1.65) | 0.15 | 0 | 0.418 | ||||||||
| 58/104 | Asians | 2 | 0.36 (0.06–2.29) | 0.28 | 0 | 0.073 | ||||||||
P≤0.05
significance of P-value lost following correction by multiple testing. BDNF, brain-derived neurotrophic factor; BCHE, butyrylcholinesterase; NA, not applicable; OR, odds ratio; CI, confidence interval.
BDNF C270T
A total of 17 articles with 18 stages involving 4,216 patients with AD and 4,370 controls were included for the meta-analysis of the BDNF C270T polymorphism. Significant heterogeneity was observed in the meta-analysis at the allelic level (I2=63%) and under the dominant model (I2=62%). The frequency of the BDNF C270T allele was 0.667 in Chinese subjects (HapMap-HCB), higher than that in the Japanese group (HapMap-JPT, 0.455), Europeans (HapMap-CEU, 0.570) and Africans (HapMap-YRI, 0.550). Further analysis showed a low ethnic difference for BDNF C270T (Fst=0.0230). No significant association between BDNF C270T and AD was observed at the allelic level (P=0.30; OR, 1.12; 95% CI, 0.91–1.37; Table II). A further subgroup meta-analysis by ethnicity showed a significant association between BDNF C270T and AD in Asians under a dominant model (P=0.03; OR, 1.88; 95% CI, 1.08–3.27; Table II and Fig. 2).
Figure 2.
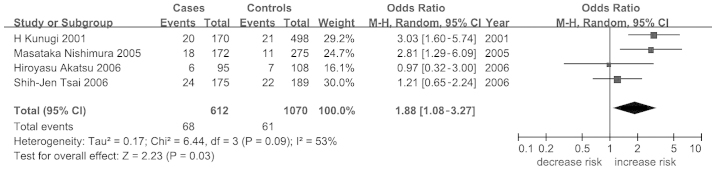
Forest plot of the association of the brain-derived neurotrophic factor C270T polymorphism with Alzheimer's disease in Asians. M-H, Mantel-Haenszel; CI, confidence interval.
BCHE-K
A total of 28 articles with 4,894 patients with AD and 4,367 controls were included for the meta-analysis of the BCHE-K variant. Although minimal ethnic differences were found in Europeans and Asians (Fst=0.0009), significant heterogeneity was found in the meta-analysis on the allelic level (I2=56%) and under the dominant model (I2=65%); however, no significant association was found at the allelic level (P=0.31; OR, 1.07; 95% CI, 0.94–1.21; Table II). Subgroup meta-analysis also did not yield any significant results (P>0.05, Table II).
Power analyses
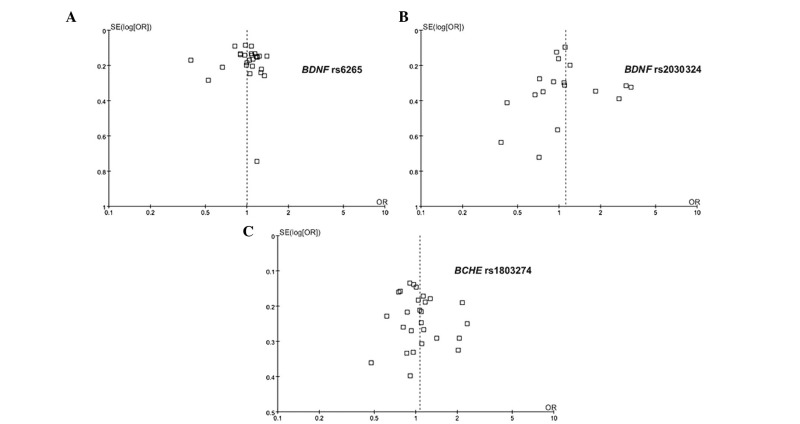
The power analyses in this meta-analysis were calculated under a moderate risk of AD (OR, 1.2; Tables I and II). The power was 1.000 for BDNF Val66Met, 0.986 for BDNF C270T and 1.000 for the BCHE-K variant (Table II), which was considerably higher than that in each of the individual studies (Table I). No publication bias was found in the meta-analyses of the three SNPs (Fig. 3).
Figure 3.
Funnel plots of the association of the (A) BDNF Val66Met, (B) BDNF C270T and (C) BCHE-K polymorphisms with Alzheimer's disease. BDNF, brain-derived neurotrophic factor; BCHE, butyrylcholinesterase; SE, standard error; OR, odds ratio.
Discussion
In the present study, 56 studies (72 stages) among 12,563 cases and 12,622 controls were analyzed to assess the association of the BDNF Val66Met, BDNF C270T and BCHE-K variants with AD. The results showed a moderate association between BDNF C270T and AD in Asians (P=0.03; OR, 1.88; 95% CI, 1.08–3.27; Table II and Fig. 2) but no significant associations were observed in the other meta-analyses.
The BDNF Val66Met polymorphism has been shown to impair the secretion of BDNF (80), and to be able to change brain morphology and cognitive function (81). Previous studies have reported five positive results (12–16) and 21 negative results (19,26–42) between the BDNF Val66Met polymorphism and AD. In the present meta-analysis, no significant association was found between BDNF Val66Met and AD (P>0.05, Table II). This was consistent with a former meta-analysis (82). The current meta-analysis of BDNF Val66Met included 23 articles, seven more than the previous study. In addition, meta-analyses were performed under various genetic models, including dominant, recessive and additive models. Subgroup meta-analysis by ethnicity was also conducted, although no statistically significant results were obtained.
The BDNF C270T polymorphism is located in a non-coding region and may affect the BDNF expression in the neural soma, dendrites or axonal regions (83). Heterozygous carriers of the T-allele tend to have a higher risk of developing AD than non-carriers (36). There have been a total of four significant results (13,17–19) and 14 non-significant results (28–31,34–37,40,41,43–45) among the previous association studies between the BDNF C270T polymorphism and AD. In the present meta-analysis, the BDNF C270T polymorphism was found to increase the risk of AD by 88% in Asians under the dominant model. No significant association was found in the other analyses (P>0.05, Table II). A strong power was shown in the meta-analysis of BDNF C270T polymorphism (0.986). Compared with a former meta-analysis that showed no positive results (82), the current meta-analysis of BDNF C270T included 18 studies, more than the 12 in the previous study (82); the meta-analyses were performed under various genetic models, and subgroup meta-analyses by ethnicity were also conducted. With a larger sample size and more comprehensive analysis, the present study showed a more reliable conclusion than the previous study.
The K variant alone does not decrease BCHE activity, but acts in synergy with APOE4 polymorphism to increase the risk of AD (84). Additionally, the BCHE-K variant promotes fibril formation by participating in the transformation of Aβ from an initially benign form to an eventually malignant form. The BCHE-K variant acts as a general candidate risk factor of AD (84,85). Six significant results (46–51) and 22 non-significant results (5,52–72) were found in previous studies on the association between the BCHE-K variant and AD. The power of BCHE-K was 1.000, which was sufficiently strong for a precise conclusion to be drawn. The current study showed no significant association between the BCHE-K variant and AD; this was consistent with a previous meta-analysis (86). The present study involved 27 stages, six more than the former study. The studies involved in the present meta-analysis met the HWE and were performed under various genetic models with subgroup meta-analysis by ethnicity. With stricter inclusion criteria, a stronger power and more comprehensive analysis, the present meta-analysis of BCHE-K was an improvement on the former one.
There were certain limitations in the meta-analysis. Firstly, publication bias may exist, as the negative-result studies are less likely to be published and may be missed, which may influence the results. Secondly, the majority of studies investigating the association between the three polymorphisms and AD were carried out in the European and Asian populations. The number of studies in other populations, such as Africans, was limited. Future studies in other ethnic populations are warranted. Thirdly, AD is a complex disease. Different statuses in AD may affect the results of the study; however, no detailed information of the AD diagnostic criteria was available from previous studies. Future case-control studies with more comprehensive information are required. Fourthly, there are 5,724 polymorphisms in BDNF and 5,059 polymorphisms in BCHE. The current study only focused on two polymorphisms of BDNF and one polymorphism of BCHE, which may not fully show the function of those two genes. Studies investigating a wider range of polymorphisms are required to improve the representation of the two genes. Fifthly, APOE is a known pivotal gene in the AD pathogenesis but no APOE genotype was included in any of the studies. Thus, any hidden interaction of the APOE genotype with the tested three polymorphisms may have been missed in the current meta-analysis. Sixthly, although a moderate association of the BDNF C270T polymorphism with the risk of AD was observed in Asians under a dominant model (P=0.03; OR, 1.88; 95% CI, 1.08–3.27), the significance was not retained following correction by multiple testing. This result should therefore be taken with caution. Finally, there was high heterogeneity in the BDNF C270T variant under the dominant model in Asians (P=0.03, I2=53%, Table II). We speculated that the number of participants was the source of the heterogeneity, as the studies with limited samples tended to produce negative results (n<200, P>0.05) in contrast to significant results produced in the studies with sufficient samples (n>200, P<0.05).
In conclusion, the present comprehensive meta-analysis suggested a moderate association between the BDNF C270T polymorphism and AD in Asians under the dominant model. Further studies focusing on a wider range of ethnic populations are required to confirm the results of the study.
Acknowledgements
The study was supported by grants from the National Natural Science Foundation of China (nos. 31100919 and 81371469), the 973 program from the Ministry of Science and Technology of China (no. 2013CB835100), the Natural Science Foundation of Zhejiang Province (no. LR13H020003), the Disciplinary Project of Ningbo University (no. B01350104900), the KC Wong Magna Fund in Ningbo University, the Program for Professor of Special Appointment (Eastern Scholars) at Shanghai Institutions of Higher Learning and the Key Basic Research Foundation of Science and Technology Commission of Shanghai Municipality (no. 13JC1403700).
References
- 1.Mucke L. Neuroscience: Alzheimer's disease. Nature. 2009;461:895–897. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong RA. What causes Alzheimer's disease. Folia Neuropathol. 2013;51:169–188. doi: 10.5114/fn.2013.37702. [DOI] [PubMed] [Google Scholar]
- 4.Bertram L, Tanzi RE. Genome-wide association studies in Alzheimer's disease. Hum Mol Genet. 2009;18:R137–R145. doi: 10.1093/hmg/ddp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scacchi R, Gambina G, Moretto G, Corbo RM. Variability of AChE, BChE, and ChAT genes in the late-onset form of Alzheimer's disease and relationships with response to treatment with Donepezil and Rivastigmine. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:502–507. doi: 10.1002/ajmg.b.30846. [DOI] [PubMed] [Google Scholar]
- 6.Borroni B, Costanzi C, Padovani A. Genetic susceptibility to behavioural and psychological symptoms in Alzheimer disease. Curr Alzheimer Res. 2010;7:158–164. doi: 10.2174/156720510790691173. [DOI] [PubMed] [Google Scholar]
- 7.Serretti A, Olgiati P, De Ronchi D. Genetics of Alzheimer's disease. A rapidly evolving field. J Alzheimers Dis. 2007;12:73–92. doi: 10.3233/jad-2007-12108. [DOI] [PubMed] [Google Scholar]
- 8.Maisonpierre PC, Le Beau MM, Espinosa R, III, Ip NY, Belluscio L, de la Monte SM, Squinto S, Furth ME, Yancopoulos GD. Human and rat brain-derived neurotrophic factor and neurotrophin-3: Gene structures, distributions, and chromosomal localizations. Genomics. 1991;10:558–568. doi: 10.1016/0888-7543(91)90436-I. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer I, Marín C, Rey MJ, Ribalta T, Goutan E, Blanco R, Tolosa E, Martí E. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J Neuropathol Exp Neurol. 1999;58:729–739. doi: 10.1097/00005072-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Aicardi G, Argilli E, Cappello S, Santi S, Riccio M, Thoenen H, Canossa M. Induction of long-term potentiation and depression is reflected by corresponding changes in secretion of endogenous brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 2004;101:15788–15792. doi: 10.1073/pnas.0406960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 12.Ventriglia M, Bocchio Chiavetto L, Benussi L, Binetti G, Zanetti O, Riva MA, Gennarelli M. Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer's disease. Mol Psychiatry. 2002;7:136–137. doi: 10.1038/sj.mp.4000952. [DOI] [PubMed] [Google Scholar]
- 13.Saarela MS, Lehtimaki T, Rinne JO, Huhtala H, Rontu R, Hervonen A, Roytta M, Ahonen JP, Mattila KM. No association between the brain-derived neurotrophic factor 196 G>;A or 270 C>T polymorphisms and Alzheimer's or Parkinson's disease. Folia Neuropathol. 2006;44:12–16. [PubMed] [Google Scholar]
- 14.Fehér A, Juhász A, Rimanóczy A, Kálmán J, Janka Z. Association between BDNF Val66Met polymorphism and Alzheimer disease, dementia with Lewy bodies, and Pick disease. Alzheimer Dis Assoc Disord. 2009;23:224–228. doi: 10.1097/WAD.0b013e318199dd7d. [DOI] [PubMed] [Google Scholar]
- 15.Pivac N, Nikolac M, Nedic G, et al. Brain derived neurotrophic factor Val66Met polymorphism and psychotic symptoms in Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:356–362. doi: 10.1016/j.pnpbp.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita S, Arai H, Matsui T, Yuzuriha T, Urakami K, Masaki T, Higuchi S. Brain-derived neurotrophic factor gene polymorphisms and Alzheimer's disease. J Neural Transm. 2005;112:703–711. doi: 10.1007/s00702-004-0210-3. [DOI] [PubMed] [Google Scholar]
- 17.Olin D, MacMurray J, Comings DE. Risk of late-onset Alzheimer's disease associated with BDNF C270T polymorphism. Neurosci Lett. 2005;381:275–278. doi: 10.1016/j.neulet.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Kunugi H, Ueki A, Otsuka M, Isse K, Hirasawa H, Kato N, Nabika T, Kobayashi S, Nanko S. A novel polymorphism of the brain-derived neurotrophic factor (BDNF) gene associated with late-onset Alzheimer's disease. Mol Psychiatry. 2001;6:83–86. doi: 10.1038/sj.mp.4000792. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura M, Kuno S, Kaji R, Kawakami H. Brain-derived neurotrophic factor gene polymorphisms in Japanese patients with sporadic Alzheimer's disease, Parkinson's disease, and multiple system atrophy. Mov Disord. 2005;20:1031–1033. doi: 10.1002/mds.20491. [DOI] [PubMed] [Google Scholar]
- 20.Allderdice PW, Gardner HA, Galutira D, Lockridge O, LaDu BN, McAlpine PJ. The cloned butyrylcholinesterase (BCHE) gene maps to a single chromosome site, 3q26. Genomics. 1991;11:452–454. doi: 10.1016/0888-7543(91)90154-7. [DOI] [PubMed] [Google Scholar]
- 21.Arpagaus M, Kott M, Vatsis KP, Bartels CF, La Du BN, Lockridge O. Structure of the gene for human butyrylcholinesterase. Evidence for a single copy. Biochemistry. 1990;29:124–131. doi: 10.1021/bi00453a015. [DOI] [PubMed] [Google Scholar]
- 22.Perry E, McKeith I, Ballard C. Butyrylcholinesterase and progression of cognitive deficits in dementia with Lewy bodies. Neurology. 2003;60:1852–1853. doi: 10.1212/01.WNL.0000068336.84399.9E. [DOI] [PubMed] [Google Scholar]
- 23.Arendt T, Brückner MK, Lange M, Bigl V. Changes in acetylcholinesterase and butyrylcholinesterase in Alzheimer's disease resemble embryonic development - a study of molecular forms. Neurochem Int. 1992;21:381–396. doi: 10.1016/0197-0186(92)90189-X. [DOI] [PubMed] [Google Scholar]
- 24.Carson KA, Geula C, Mesulam MM. Electron microscopic localization of cholinesterase activity in Alzheimer brain tissue. Brain Res. 1991;540:204–208. doi: 10.1016/0006-8993(91)90508-S. [DOI] [PubMed] [Google Scholar]
- 25.Darvesh S, Cash MK, Reid GA, Martin E, Mitnitski A, Geula C. Butyrylcholinesterase is associated with β-amyloid plaques in the transgenic APPSWE/PSEN1dE9 mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2012;71:2–14. doi: 10.1097/NEN.0b013e31823cc7a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nacmias B, Piccini C, Bagnoli S, Tedde A, Cellini E, Bracco L, Sorbi S. Brain-derived neurotrophic factor, apolipoprotein E genetic variants and cognitive performance in Alzheimer's disease. Neurosci Lett. 2004;367:379–383. doi: 10.1016/j.neulet.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Combarros O, Infante J, Llorca J, Berciano J. Polymorphism at codon 66 of the brain-derived neurotrophic factor gene is not associated with sporadic Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;18:55–58. doi: 10.1159/000077736. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Fukumoto H, Orne J, Klucken J, Raju S, Vanderburg CR, Irizarry MC, Hyman BT, Ingelsson M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp Neurol. 2005;194:91–96. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Desai P, Nebes R, DeKosky ST, Kamboh MI. Investigation of the effect of brain-derived neurotrophic factor (BDNF) polymorphisms on the risk of late-onset Alzheimer's disease (AD) and quantitative measures of AD progression. Neurosci Lett. 2005;379:229–234. doi: 10.1016/j.neulet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Vepsӓlӓinen S, Castren E, Helisalmi S, Iivonen S, Mannermaa A, Lehtovirta M, Hӓnninen T, Soininen H, Hiltunen M. Genetic analysis of BDNF and TrkB gene polymorphisms in Alzheimer's disease. J Neurol. 2005;252:423–428. doi: 10.1007/s00415-005-0667-5. [DOI] [PubMed] [Google Scholar]
- 31.Bodner SM, Berrettini W, van Deerlin V, Bennett DA, Wilson RS, Trojanowski JQ, Arnold SE. Genetic variation in the brain derived neurotrophic factor gene in Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:1–5. doi: 10.1002/ajmg.b.30154. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Rowland C, Tacey K, et al. The BDNF Val66Met polymorphism is not associated with late onset Alzheimer's disease in three case-control samples. Mol Psychiatry. 2005;10:809–810. doi: 10.1038/sj.mp.4001702. [DOI] [PubMed] [Google Scholar]
- 33.Forero DA, Benítez B, Arboleda G, Yunis JJ, Pardo R, Arboleda H. Analysis of functional polymorphisms in three synaptic plasticity-related genes (BDNF, COMT AND UCHL1) in Alzheimer's disease in Colombia. Neurosci Res. 2006;55:334–341. doi: 10.1016/j.neures.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Ozbay F, Lappalainen J, et al. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer's disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang R, Huang J, Cathcart H, Smith S, Poduslo SE. Genetic variants in brain-derived neurotrophic factor associated with Alzheimer's disease. J Med Genet. 2007;44:e66. doi: 10.1136/jmg.2006.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boiocchi C, Maggioli E, Zorzetto M, Sinforiani E, Cereda C, Ricevuti G, Cuccia M. Brain-derived neurotrophic factor gene variants and Alzheimer disease: An association study in an Alzheimer disease Italian population. Rejuvenation Res. 2013;16:57–66. doi: 10.1089/rej.2012.1381. [DOI] [PubMed] [Google Scholar]
- 37.Cozza A, Melissari E, Iacopetti P, Mariotti V, Tedde A, Nacmias B, Conte A, Sorbi S, Pellegrini S. SNPs in neurotrophin system genes and Alzheimer's disease in an Italian population. J Alzheimers Dis. 2008;15:61–70. doi: 10.3233/jad-2008-15105. [DOI] [PubMed] [Google Scholar]
- 38.Tsai SJ, Hong CJ, Liu HC, Liu TY, Hsu LE, Lin CH. Association analysis of brain-derived neurotrophic factor Val66Met polymorphisms with Alzheimer's disease and age of onset. Neuropsychobiology. 2004;49:10–12. doi: 10.1159/000075332. [DOI] [PubMed] [Google Scholar]
- 39.Bian JT, Zhang JW, Zhang ZX, Zhao HL. Association analysis of brain-derived neurotrophic factor (BDNF) gene 196 A/G polymorphism with Alzheimer's disease (AD) in mainland China. Neurosci Lett. 2005;387:11–16. doi: 10.1016/j.neulet.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Akatsu H, Yamagata HD, Kawamata J, Kamino K, Takeda M, Yamamoto T, Miki T, Tooyama I, Shimohama S, Kosaka K. Variations in the BDNF gene in autopsy-confirmed Alzheimer's disease and dementia with Lewy bodies in Japan. Dement Geriatr Cogn Disord. 2006;22:216–222. doi: 10.1159/000094933. [DOI] [PubMed] [Google Scholar]
- 41.Tsai SJ, Hong CJ, Liu HC, Liu TY, Liou YJ. The brain-derived neurotrophic factor gene as a possible susceptibility candidate for Alzheimer's disease in a Chinese population. Dement Geriatr Cogn Disord. 2006;21:139–143. doi: 10.1159/000090673. [DOI] [PubMed] [Google Scholar]
- 42.He XM, Zhang ZX, Zhang JW, Zhou YT, Tang MN, Wu CB, Hong Z. Lack of association between the BDNF gene Val66Met polymorphism and Alzheimer disease in a Chinese Han population. Neuropsychobiology. 2007;55:151–155. doi: 10.1159/000106473. [DOI] [PubMed] [Google Scholar]
- 43.Riemenschneider M, Schwarz S, Wagenpfeil S, Diehl J, Müller U, Förstl H, Kurz A. A polymorphism of the brain-derived neurotrophic factor (BDNF) is associated with Alzheimer's disease in patients lacking the Apolipoprotein E epsilon4 allele. Mol Psychiatry. 2002;7:782–785. doi: 10.1038/sj.mp.4001073. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura AL, Oliveira JR, Mitne-Neto M, Guindalini C, Nitrini R, Bahia VS, de Brito-Marques PR, Otto PA, Zatz M. Lack of association between the brain-derived neurotrophin factor (C-270T) polymorphism and late-onset Alzheimer's disease (LOAD) in Brazilian patients. J Mol Neurosci. 2004;22:257–260. doi: 10.1385/JMN:22:3:257. [DOI] [PubMed] [Google Scholar]
- 45.Cousin E, Macé S, Rocher C, et al. No replication of genetic association between candidate polymorphisms and Alzheimer's disease. Neurobiol Aging. 2011;32:1443–1451. doi: 10.1016/j.neurobiolaging.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Combarros O, Riancho JA, Infante J, Sañudo C, Llorca J, Zarrabeitia MT, Berciano J. Interaction between CYP19 aromatase and butyrylcholinesterase genes increases Alzheimer's disease risk. Dement Geriatr Cogn Disord. 2005;20:153–157. doi: 10.1159/000087065. [DOI] [PubMed] [Google Scholar]
- 47.Raygani AV, Zahrai M, Soltanzadeh A, Doosti M, Javadi E, Pourmotabbed T. Analysis of association between butyrylcholinesterase K variant and apolipoprotein E genotypes in Alzheimer's disease. Neurosci Lett. 2004;371:142–146. doi: 10.1016/j.neulet.2004.08.057. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez-Arcaya A, Combarros O, Llorca J, Sánchez-Guerra M, Berciano J, Fernández-Viadero C, Peña N. The butyrylcholinesterase K variant is a protective factor for sporadic Alzheimer's disease in women. Acta Neurol Scand. 2000;102:350–353. doi: 10.1034/j.1600-0404.2000.102006350.x. [DOI] [PubMed] [Google Scholar]
- 49.McIlroy SP, Crawford VL, Dynan KB, McGleenon BM, Vahidassr MD, Lawson JT, Passmore AP. Butyrylcholinesterase K variant is genetically associated with late onset Alzheimer's disease in Northern Ireland. J Med Genet. 2000;37:182–185. doi: 10.1136/jmg.37.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiebusch H, Poirier J, Sévigny P, Schappert K. Further evidence for a synergistic association between APOE epsilon4 and BCHE-K in confirmed Alzheimer's disease. Hum Genet. 1999;104:158–163. doi: 10.1007/s004390050929. [DOI] [PubMed] [Google Scholar]
- 51.Lehmann DJ, Johnston C, Smith AD. Synergy between the genes for butyrylcholinesterase K variant and apolipoprotein E4 in late-onset confirmed Alzheimer's disease. Hum Mol Genet. 1997;6:1933–1936. doi: 10.1093/hmg/6.11.1933. [DOI] [PubMed] [Google Scholar]
- 52.Simão-Silva DP, Bertolucci PH, de Labio RW, Payão SL, Furtado-Alle L, Souza RL. Association analysis between K and −116A variants of butyrylcholinesterase and Alzheimer's disease in a Brazilian population. Chem Biol Interact. 2013;203:358–360. doi: 10.1016/j.cbi.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Mateo I, Llorca J, Infante J, Rodríguez-Rodríguez E, Berciano J, Combarros O. Gene-gene interaction between 14-3-3 zeta and butyrylcholinesterase modulates Alzheimer's disease risk. Eur J Neurol. 2008;15:219–222. doi: 10.1111/j.1468-1331.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 54.Déniz-Naranjo MC, Muñoz-Fernández C, Alemany-Rodríguez MJ, del Carmen Pérez-Vieitez M, Aladro-Benito Y, Irurita-Latasa J, Sánchez-García F. Butyrylcholinesterase, ApoE and Alzheimer's disease in a population from the Canary Islands (Spain) Neurosci Lett. 2007;427:34–38. doi: 10.1016/j.neulet.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 55.Piccardi M, Congiu D, Squassina A, Manconi F, Putzu PF, Mereu RM, Chillotti C, Del Zompo M. Alzheimer's disease: Case-control association study of polymorphisms in ACHE, CHAT, and BCHE genes in a Sardinian sample. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:895–899. doi: 10.1002/ajmg.b.30548. [DOI] [PubMed] [Google Scholar]
- 56.Prince JA, Feuk L, Sawyer SL, Gottfries J, Ricksten A, Nӓgga K, Bogdanovic N, Blennow K, Brookes AJ. Lack of replication of association findings in complex disease: An analysis of 15 polymorphisms in prior candidate genes for sporadic Alzheimer's disease. Eur J Hum Genet. 2001;9:437–444. doi: 10.1038/sj.ejhg.5200651. [DOI] [PubMed] [Google Scholar]
- 57.Mattila KM, Rinne JO, Röyttӓ M, Laippala P, Pietilӓ T, Kalimo H, Koivula T, Frey H, Lehtimӓki T. Dipeptidyl carboxypeptidase 1 (DCP1) and butyrylcholinesterase (BCHE) gene interactions with the apolipoprotein E epsilon4 allele as risk factors in Alzheimer's disease and in Parkinson's disease with coexisting Alzheimer pathology. J Med Genet. 2000;37:766–770. doi: 10.1136/jmg.37.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tilley L, Morgan K, Grainger J, Marsters P, Morgan L, Lowe J, Xuereb J, Wischik C, Harrington C, Kalsheker N. Evaluation of polymorphisms in the presenilin-1 gene and the butyrylcholinesterase gene as risk factors in sporadic Alzheimer's disease. Eur J Hum Genet. 1999;7:659–663. doi: 10.1038/sj.ejhg.5200351. [DOI] [PubMed] [Google Scholar]
- 59.Grubber JM, Saunders AM, Crane-Gatherum AR, et al. Analysis of association between Alzheimer disease and the K variant of butyrylcholinesterase (BCHE-K) Neurosci Lett. 1999;269:115–119. doi: 10.1016/S0304-3940(99)00426-7. [DOI] [PubMed] [Google Scholar]
- 60.Kehoe PG, Williams H, Holmans P, Wilcock G, Cairns NJ, Neal J, Owen MJ. The butyrylcholinesterase K variant and susceptibility to Alzheimer's disease. J Med Genet. 1998;35:1034–1035. doi: 10.1136/jmg.35.12.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hiltunen M, Mannermaa A, Helisalmi S, Koivisto A, Lehtovirta M, Ryynӓnen M, Riekkinen P, Soininen H. Butyrylcholinesterase K variant and apolipoprotein E4 genes do not act in synergy in Finnish late-onset Alzheimer's disease patients. Neurosci Lett. 1998;250:69–71. doi: 10.1016/S0304-3940(98)00453-4. [DOI] [PubMed] [Google Scholar]
- 62.Crawford F, Fallin D, Suo Z, Abdullah L, Gold M, Gauntlett A, Duara R, Mullan M. The butyrylcholinesterase gene is neither independently nor synergistically associated with late-onset AD in clinic- and community-based populations. Neurosci Lett. 1998;249:115–118. doi: 10.1016/S0304-3940(98)00423-6. [DOI] [PubMed] [Google Scholar]
- 63.Singleton AB, Smith G, Gibson AM, Woodward R, Perry RH, Ince PG, Edwardson JA, Morris CM. No association between the K variant of the butyrylcholinesterase gene and pathologically confirmed Alzheimer's disease. Hum Mol Genet. 1998;7:937–939. doi: 10.1093/hmg/7.5.937. [DOI] [PubMed] [Google Scholar]
- 64.Brindle N, Song Y, Rogaeva E, et al. Analysis of the butyrylcholinesterase gene and nearby chromosome 3 markers in Alzheimer disease. Hum Mol Genet. 1998;7:933–935. doi: 10.1093/hmg/7.5.933. [DOI] [PubMed] [Google Scholar]
- 65.Russ C, Powell J, Lovestone S, Holmes C. K variant of butyrycholinesterase and late-onset Alzheimer's disease. Lancet. 1998;351:881. doi: 10.1016/S0140-6736(05)70292-0. [DOI] [PubMed] [Google Scholar]
- 66.Helbecque N. The butyrylcholinesterase K variant is not associated with Alzheimer's. Alzheimers Rep. 1998;1:309–313. [Google Scholar]
- 67.Laws SM. Evidence that the butyrylcholinesterase K variant can protect against late-onset Alzheimer's disease. Alzheimers Rep. 1999;2:219–223. [Google Scholar]
- 68.Bi S, Zhang Y, Wu J, Wang D, Zhao Q. Association between low-density lipoprotein receptor-related protein gene, butyrylcholinesterase gene and Alzheimer's disease in Chinese. Chin Med Sci J. 2001;16:71–75. [PubMed] [Google Scholar]
- 69.Lee DW, Liu HC, Liu TY, Chi CW, Hong CJ. No association between butyrylcholinesterase K-variant and Alzheimer disease in Chinese. Am J Med Genet. 2000;96:167–169. doi: 10.1002/(SICI)1096-8628(20000403)96:2<167::AID-AJMG8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto Y, Yasuda M, Mori E, Maeda K. Failure to confirm a synergistic effect between the K-variant of the butyrylcholinesterase gene and the epsilon4 allele of the apolipoprotein gene in Japanese patients with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1999;67:94–96. doi: 10.1136/jnnp.67.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ki CS, Na DL, Kim JW, Kim HJ, Kim DK, Yoon BK. No association between the genes for butyrylcholinesterase K variant and apolipoprotein E4 in late-onset Alzheimer's disease. Am J Med Genet. 1999;88:113–115. doi: 10.1002/(SICI)1096-8628(19990416)88:2<113::AID-AJMG2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 72.Yamada M, Sodeyama N, Itoh Y, Suematsu N, Otomo E, Matsushita M, Mizusawa H. Butyrylcholinesterase K variant and cerebral amyloid angiopathy. Stroke. 1998;29:2488–2490. doi: 10.1161/01.STR.29.12.2488. [DOI] [PubMed] [Google Scholar]
- 73.Xu X, Wang Y, Wang L, et al. Meta-analyses of 8 polymorphisms associated with the risk of the Alzheimer's disease. PLoS One. 2013;8:e73129. doi: 10.1371/journal.pone.0073129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dai D, Wang Y, Wang L, et al. Polymorphisms of DRD2 and DRD3 genes and Parkinson's disease: A meta-analysis. Biomed Rep. 2014;2:275–281. doi: 10.3892/br.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou J, Huang Y, Huang RS, et al. A case-control study provides evidence of association for a common SNP rs974819 in PDGFD to coronary heart disease and suggests a sex-dependent effect. Thromb Res. 2012;130:602–606. doi: 10.1016/j.thromres.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 76.Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol Bioinform Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 77.Gibson E, Fenster A, Ward AD. The impact of registration accuracy on imaging validation study design: A novel statistical power calculation. Med Image Anal. 2013;17:805–815. doi: 10.1016/j.media.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Coory MD. Comment on: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39:932–933. doi: 10.1093/ije/dyp157. [DOI] [PubMed] [Google Scholar]
- 79.Kawalec P, Mikrut A, Wiśniewska N, Pilc A. The effectiveness of tofacitinib, a novel Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2013;32:1415–1424. doi: 10.1007/s10067-013-2329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McHughen SA, Rodriguez PF, Kleim JA, Kleim ED, Marchal Crespo L, Procaccio V, Cramer SC. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20:1254–1262. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukumoto N, Fujii T, Combarros O, et al. Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer's disease: New data and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:235–242. doi: 10.1002/ajmg.b.30986. [DOI] [PubMed] [Google Scholar]
- 83.Nagata T, Shinagawa S, Nukariya K, Ochiai Y, Kawamura S, Agawa-Ohta M, Kasahara H, Nakayama K, Yamada H. Association between brain-derived neurotrophic factor (BDNF) gene polymorphisms and executive function in Japanese patients with Alzheimer's disease. Psychogeriatrics. 2011;11:141–149. doi: 10.1111/j.1479-8301.2011.00364.x. [DOI] [PubMed] [Google Scholar]
- 84.Guillozet AL, Smiley JF, Mash DC, Mesulam MM. Butyrylcholinesterase in the life cycle of amyloid plaques. Ann Neurol. 1997;42:909–918. doi: 10.1002/ana.410420613. [DOI] [PubMed] [Google Scholar]
- 85.Lehmann DJ, Nagy Z, Litchfield S, Borja MC, Smith AD. Association of butyrylcholinesterase K variant with cholinesterase-positive neuritic plaques in the temporal cortex in late-onset Alzheimer's disease. Hum Genet. 2000;106:447–452. doi: 10.1007/s004390000277. [DOI] [PubMed] [Google Scholar]
- 86.Lehmann DJ, Williams J, McBroom J, Smith AD. Using meta-analysis to explain the diversity of results in genetic studies of late-onset Alzheimer's disease and to identify high-risk subgroups. Neuroscience. 2001;108:541–554. doi: 10.1016/S0306-4522(01)00464-X. [DOI] [PubMed] [Google Scholar]