Abstract
Traditional medicinal plants have been used in the treatment of various diseases for centuries. A number of plant-derived compounds have been proposed as anticancer agents and are currently undergoing medical development. Petasites japonicus (PJ), also known as Butterbur, is a herb cultivated in East Asia that is used as a traditional herbal medicine. The aim of the present study was to investigate whether a methanol extract of PJ demonstrated anticancer activity against Hep3B hepatocellular carcinoma (HCC) cells. The anticancer property and underlying mechanism of the extract were evaluated by assessing the effect on cell viability, nuclear morphology and the expression of phosphorylated (p)-mTOR, p-Akt, β-catenin and p-glycogen synthase kinase-3β, which are markers for cancer cell proliferation and metastasis. These results were obtained by the MTT assay, fluorescence microscopy and Western blot analysis. The methanol extract of PJ was shown to decrease the cell viability in a concentration-dependent manner. In addition, the methanol extract of PJ was found to inhibit the growth of Hep3B HCC cells through inhibiting the Akt/mTOR and Wnt signaling pathways. These results suggest that the methanol extract of PJ exerts an anticancer effect on Hep3B HCC cells.
Keywords: Petasites japonicas, hepatocellular carcinoma, Wnt, Akt, mTOR
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common solid tumor and a leading cause of cancer mortality worldwide. The majority of HCC cases (80%) arise in Eastern Asia and Africa. The risk factors of HCC include a high metastasis potential and tumor recurrence (1,2). Previously, several studies focused on the factors involved in the molecular pathogenesis of HCC, including p53, Ras/extracellular signal-regulated kinase, phosphatidylinositide 3-kinase (PI3K)/Akt and Wnt signaling (3–5). The Wnt proteins and downstream molecules regulate multiple processes that have a critical role in cancer cell development, tumor growth, proliferation, differentiation and metastasis (6). Previous studies have shown that aberrant activation of Wnt signaling is associated with cancer development, as demonstrated in cervical, colon and breast cancer cells (7,8). Upon activation of the Wnt canonical signaling pathway, β-catenin becomes phosphorylated at the Ser33/37 residue by glycogen synthase kinase (GSK)-3β in the Axin complex, triggering subsequent proteasomal degradation. β-catenin translocates into the nucleus, where the protein activates target genes coding for proteins that are associated with cell proliferation (9). Several studies have demonstrated that the phosphorylation of GSK3β (inactive form) by Akt promotes angiogenesis, metastasis and cell survival. In addition, the Akt/mTOR signaling pathway has been shown to be associated with cell survival, and the pathway has been found to be frequently activated in various cancer cell types (10,11). Therefore, targeting the Akt/mTOR and Wnt signaling pathways may be a promising approach in the molecular therapy of cancer.
Petasites japonicus (PJ), also known as Butterbur, is cultivated in Eastern Asia and is used as both a traditional medicine and vegetable. Petasiphenol can be isolated from the scapes of PJ, and has been shown to possess antimutagenic and microbicidal activities (12). In addition, pyrrolizidine alkaloids from the stalks of PJ have been shown to inhibit tumor formation (13). PJ is also known for its anti-inflammatory and antiallergenic effects (14,15). The roots of PJ are used in Korea as traditional medicines to treat anodynia and as an antidote for food poisoning. However, to the best of our knowledge, there has been no systematic study investigating the bioactivity of PJ. In particular, there are no studies in the literature investigating the in vitro and in vivo anticancer properties of PJ roots. Thus, it was hypothesized that the methanol extract of PJ roots (PJE) may modulate molecular signaling pathways and inhibit the proliferation of HCC cells.
In the present study, the effect of PJE on the rate of apoptosis and growth of Hep3B HCC cells was evaluated in vitro and in vivo. In addition, the underlying mechanisms were investigated by analyzing the expression levels of key molecules involved in the Akt/mTOR and Wnt signaling pathways. The aim of the current study was to further the understanding of the anticancer mechanisms of PJE and demonstrate that PJE contains natural herb materials that may be used to develop anticancer drugs. The active compounds responsible for the cytotoxic effect of PJE require further investigation.
Materials and methods
Cell culture and reagents
Hep3B cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were grown in Dulbecco's modified Eagle's medium (WelGENE, Seoul, Korea), containing 10% fetal bovine serum (FBS) and 1% antibiotics (Gibco®, Invitrogen Life Technologies, Grand Island, NY, USA), at 37°C in a 5% CO2 incubator. The 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrzolium bromide (MTT) dye, and propidium iodide (PI) staining solution were obtained from Sigma-Aldrich (St. Louis, MO, USA). BIO and XAV 939 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Rapamycin and LY294002 were purchased from Calbiochem (San Diego, CA, USA)
Preparation of the PJ extract and fractions
The dried roots of PJ were purchased from Kapdang Co. (Seoul, Korea). In total, 50 g of the powdered root of PJ was extracted with 500 ml methanol (95%) for 48 h. The extract solution was evaporated to dryness under reduced pressure to yield the crude methanol extract. Subsequently, the PJE was suspended in H2O and partitioned successively with n-Hexane, ethyl acetate and ethanol. Each extract was evaporated to dryness under reduced pressure to yield the respective extracts.
Measurement of cell viability
Cells were seeded in 12-well plates at 1×105 cells/ml and incubated with 0, 50, 100, 200 µg/ml PJ for 24 or 48 h. The respective medium was removed, and the samples were incubated with 20 µl MTT solution (5 mg/ml) in phosphate-buffered saline (PBS) for 1 h. Converted purple formazan dye from MTT was solubilized in dimethyl sulfoxide, and the optical densities were measured using a microplate reader (Bio-Rad, Hercules, CA, USA) at 595 nm.
Nuclear morphology
Cells were seeded in 12-well plates and treated with PJE for 24 h at 50 or 100 µg/ml concentrations. The cells were stained with Hoechst 33342 (Sigma, St. Louis, MO, USA) for 30 min. Slides were washed with PBS, and mounting fluid was poured over the slide. The slides were covered with a cover slip and sealed with lacquer. Cells were observed under a fluorescence microscope (Olympus Optical Co., Tokyo, Japan) to assess any alterations in the nuclear morphology.
Apoptosis analysis
Cells were seeded in 60-mm plates at 1×106 cells/ml and incubated for 24 h. PJE was applied at the indicated concentrations and the samples were incubated for 24 h. The cells were subsequently harvested and resuspended with PBS. Apoptotic cells were identified using a FITC-Annexin V Apoptosis Detection kit (BD Biosciences, San Jose, CA, USA). The stained cells were analyzed by fluorescence-activated cell sorting (BD Biosciences, San Jose, CA, USA) to determine the percentages of Annexin V-positive cells.
Western blot analysis
Cells were seeded in six-well plates (2×106 cells/ml) and were pretreated with the following inhibitors for 30 min: 50 nM rapamycin, 10 nM LY294002, 3 µM XAV 939 and 0.5 µM BIO. The cells were subsequently treated with PJE for 24 h. Cells were washed with PBS and lysed with radioimmunoprecipitation assay lysis buffer [50 mM Tris-HCl (pH 8.0), 1% NP 40, 0.5% sodium deoxycholate, 150 mM NaCl and 1 mM phenylmethylsulfonyl fluoride). Protein concentrations were determined using the Bradford assay. All samples were separated by sodium dodecylsulfate polyacrylamide gel electrophoresis, and the proteins were transferred onto a nitrocellulose membrane. The membrane was incubated overnight with primary antibodies (dilution, 1:1,000) at 4°C. The membrane was then incubated with secondary IgG antibodies (dilution, 1:10,000) conjugated to horseradish peroxidase at room temperature for 90 min, and the proteins were visualized by enhanced chemiluminescence (Intron Biotechnology, Inc., Seongnam, Korea). The following antibodies were used: Polyclonal rabbot anti-human phosphorylated (p)-mTOR (2971S), monoclonal mouse anti-human p-Akt (4051S), polyclonal rabbit anti-human p-GSK3β (9336S) and monoclonal rabbit anti-human total form of β-catenin (9582S) were purchased from Cell Signaling Technology (Danvers, MA, USA).
Animals
Eight nude mice (4-weeks-old) were obtained from SLC (Tokyo, Japan). Of these, four mice made up the control group and the other four mice made up the experimental group. All mice housed in Hannam University Animal Research Center. Hep3B cells (2×106 cells/ml) were subcutaneously inoculated in four-week-old nu/nu mice at the left flank. After one week, the experimental mice were treated with an injection of PJE (30 g/g/day). The control group received daily injections with vehicle only (0.2 cc PBS). Tumor size was measured in two perpendicular diameters using a caliper every three days. The tumor volume was calculated using the following formula: Volume = 1/2(length × width2). The body weight of each animal was measured at a set time, once per week. All the animal experiments were approved by the Ethics Committee for Animal Experimentation of Hannam University (Daejeon, Korea).
Statistical analysis
All the experiments were repeated at least three times, and the results are expressed as the mean ± standard deviation for each group. The Student's t-test was used to evaluate statistical significance, where P<0.05 was considered to indicate a statically significant difference. All analyses were carried out using Microsoft Excel (Redmond, Washington, USA).
Results
Cytotoxic effect of PJE on Hep3B cells
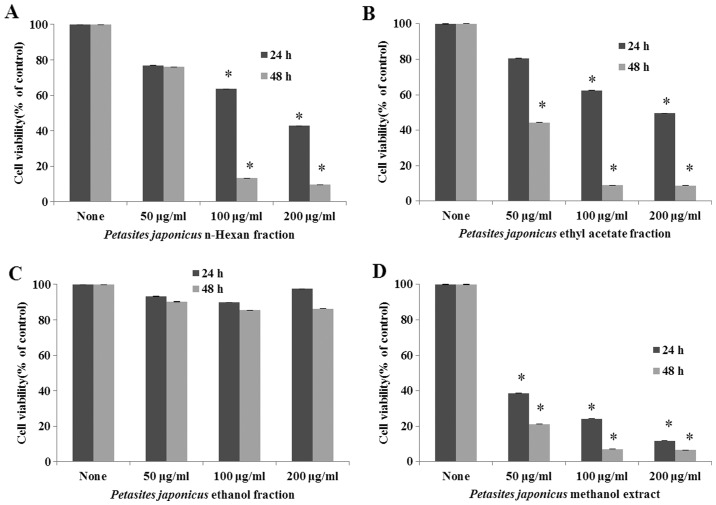
To determine the cytotoxic effects on Hep3B cells, the methanol extract and various fractions of PJ were applied at two concentrations (50 and 100 µg/ml) for 24 and 48 h (Fig. 1A-D). PJE was shown to be the most potent, inhibiting cell proliferation in a dose-dependent manner. The ethanol fraction did not exhibit any antiproliferative activity. The results indicated that PJE exerted an antiproliferative effect on Hep3B cells.
Figure 1.
Antiproliferative effect of Petasites japonicus (A) n-Hexan fraction, (B) ethyl acetate fraction, (C) ethanol fraction and (D) methanol extract on Hep3B cells. Hep3B cells were treated with different concentrations of the various fractions for different time periods, and the cell viability was detected using an MTT assay. Results are expressed as the mean ± standard deviation.
PJE induces apoptosis in HCC cells
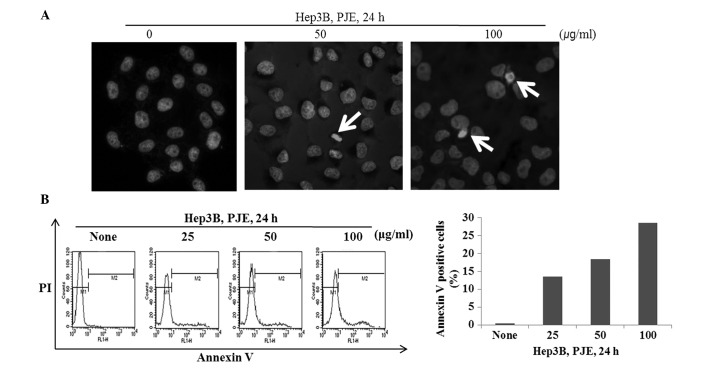
In order to determine whether the PJE-induced decrease in cell viability was caused by cell apoptosis, a staining procedure was employed that used the fluorescent DNA-binding dye, Hoechst 33342. As shown in Fig. 2A, when treated with PJE at 100 µg/ml, an increased number of Hep3B cells formed apoptotic bodies when compared with the control cells. As shown in Fig. 2B and C, the number of Annexin V-positive cells increased markedly following treatment with the high concentration of PJE.
Figure 2.
PJE induces apoptosis in Hep3B cells. (A) Cells were treated with PJE for 24 h, stained with 10 µM Hoechst 33342 and analyzed with fluorescence microscopy. (B) Cells were treated with PJE for 24 h, and the apoptotic effects were analyzed using Annexin V-PI staining. PI, propidium iodide; PJE, methanol extract of Petasites japonicus.
PJE suppresses HCC cell growth in vitro and in vivo through inhibiting the Akt/mTOR and Wnt signaling pathways
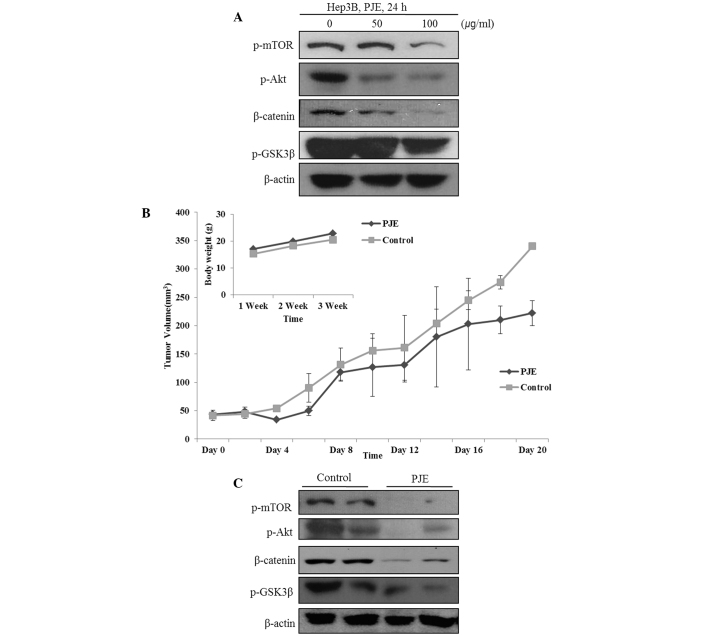
Akt/mTOR and Wnt signaling pathways play a critical role in the growth and survival of HCC cells. Therefore, whether PJE inhibited the Akt/mTOR and Wnt signaling pathways in Hep3B cells was investigated by analyzing the expression levels of Akt, mTOR, β-catenin and GSK3β. As shown in Fig. 3A, PJE suppressed the expression of β-catenin and phosphorylated Akt, mTOR and GSK3β.
Figure 3.
PJE impairs HCC cell growth through the inhibition of the Akt/mTOR and Wnt signaling pathway in vitro and in vivo. (A) With increasing concentrations of PJE, the expression levels of p-mTOR, p-Akt, β-catenin and p-GSK3β were shown to significantly decrease in the Hep3B cells. (B) PJE group exhibited reduced tumor growth compared with the control group. (C) Western blot analysis of each tumor lysate at the end of the experiment. PJE, methanol extract of Petasites japonicus; HCC, hepatocellular carcinoma; p, phosphorylated; GSK, glycogen synthase kinase.
To investigate the therapeutic effects of PJE in vivo, mice with implanted Hep3B tumors were injected subcutaneously with PJE on consecutive days. As shown in Fig. 3B, changes in the body weight and tumor size of the mice were monitored. After the animals were sacrificed, the primary tumor nodules were isolated. As shown in Fig. 3C, the expression levels of β-catenin, p-mTOR, p-Akt and p-GSK3β decreased significantly in the PJE-treated group when compared with the control group.
PJE reduces HCC cell growth through the inactivation of Akt/mTOR activity or the Wnt signaling pathway
Western blot analysis was used to clarify the mechanism by which PJE-induced suppression of specific molecules mediates the suppression of the Akt/mTOR or Wnt signaling pathways in Hep3B cells. The results revealed that PJE significantly suppressed the expression levels of p-mTOR, p-Akt, β-catenin and p-GSK3β. In addition, pretreatment with rapamycin and LY294002, which are specific inhibitors of mTOR and PI3K, respectively, followed by PJE treatment, effectively reduced the expression levels of β-catenin and phosphorylated mTOR, Akt and GSK3β (Fig. 4A). As shown in Fig. 4B, suppression of key molecules in the Wnt signaling pathway by PJE led to decreased growth of HCC cells. In addition, β-catenin and p-GSK3β were suppressed further when simultaneously treated with inhibitors such as XAV 939 or BIO, which are specific inhibitors of β-catenin and GSK3β. These results suggest that PJE may significantly suppress the expression levels of p-mTOR, p-Akt, β-catenin and p-GSK3β.
Figure 4.
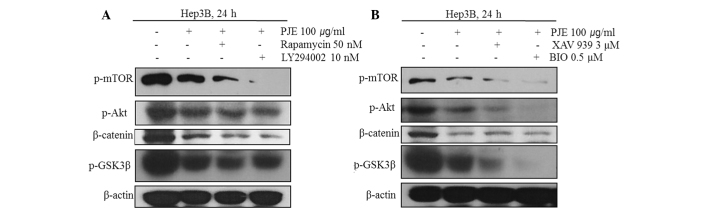
PJE suppresses HCC cell growth via the modulation of Akt/mTOR and β-catenin/p-GSK3β activity. Cells were pretreated with inhibitors for 30 min, and subsequently with PJE for 24 h. Effect of PJE following (A) pretreatment with LY294002 or rapamycin and (B) pretreatment with XAV 939 or BIO on the expression levels of p-mTOR, p-Akt, β-catenin and p-GSK3β in Hep3B cells. PJE, methanol extract of Petasites japonicus; HCC, hepatocellular carcinoma; p, phosphorylated; GSK, glycogen synthase kinase.
Discussion
Phytotherapy refers to the use of herbal medicine in the treatment of diseases. For hundreds of years, individuals have used plants to treat infirmities worldwide. Currently, traditional herbal medicine is a promising alternative to chemotherapy, and science has opened the doors for basic research in the field of phytomedicine. PJ has been used as a traditional herbal remedy and food source. In addition, previous studies have reported that PJ extract suppresses the viability of various cancer cell types, including colon, breast and cervical cancer (16–18). To the best of our knowledge, the present study demonstrated for the first time that a methanol extract of PJ roots exhibits anticancer activities in HCC by modulating cellular molecular signaling pathways. It was hypothesized that PJE suppressed cell proliferation by inhibiting the Akt/mTOR and Wnt signaling pathways. In cancer cells, a number of signaling pathways may be potential targets for anticancer therapy. Previous studies have reported the frequency of Akt/mTOR and Wnt signal activation in cancer cells (19–21). In the canonical Wnt signaling pathway, β-catenin translocates to the nucleus and binds to T cell factor (TCF), which subsequently causes the phosphorylation of GSK3 and the transcription of oncogenes. The β-catenin/TCF complex activates the transcription of genes that promote cell growth, and has been found to be associated with tumorigenesis (22,23). Akt functions partially through the phosphorylation of GSK3α and β, which in turn, regulates cell metabolism. The phosphorylation of Akt at Ser473 results in its activation and in elevated levels of p-mTOR (24). The Akt/mTOR pathway regulates several cellular functions that are critical for carcinogenesis, cell growth and mobility (25). Thus, the Akt/mTOR and Wnt signaling pathways are potentially important therapeutic targets for anticancer therapy. The results of the present study demonstrated that PJE suppressed cell proliferation in vitro by inhibiting the Akt/mTOR and Wnt signaling pathways. In addition, the in vitro data correlated with the inhibition of tumor growth. Thus, the results confirmed that PJE induces apoptosis and suppresses the expression levels of p-mTOR, p-Akt, β-catenin and p-GSK3β.
In conclusion, to the best of our knowledge, the present study was the first to isolate and screen material from PJ and examine its potent anticancer activity. In vitro methods, including a cell proliferation assay and cell cycle analysis, were used to demonstrate that PJE induces apoptosis and suppresses the Akt/mTOR and Wnt signaling pathways by reducing the expression levels of β-catenin, p-Akt, p-mTOR and p-GSK3β. In addition, the in vivo experiments further confirmed that PJE suppressed the Akt/mTOR and Wnt signaling pathways. Therefore, these observations highlight the potential value of PJE as an anticancer agent.
Acknowledgements
The study was supported by the 2014 Hannam University Research Fund.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Chen GG, Merchant JL, Lai P, Ho RL, Hu X, Okada M, et al. Mutation of p53 in recurrent hepatocellular carcinoma and its association with the expression of ZBP-89. Am J Pathol. 2003;162:1823–1829. doi: 10.1016/S0002-9440(10)64317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesenauer CA, Yip-Schneider MT, Wang Y, Schmidt CM. Multiple anticancer effects of blocking MEK-ERK signaling in hepatocellular carcinoma. J Am Coll Surg. 2004;198:410–421. doi: 10.1016/j.jamcollsurg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Tan D, Zhang Z, Liang JJ, Brown RE. Activation of Akt-mTOR-p70S6K pathway in angiogenesis in hepatocellular carcinoma. Oncol Rep. 2008;20:713–719. [PubMed] [Google Scholar]
- 6.Teufel A, Staib F, Kanzler S, Weinmann A, Schulze-Bergkamen H, Galle PR. Genetics of hepatocellular carcinoma. World J Gastroenterol. 2007;13:2271. doi: 10.3748/wjg.v13.i16.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 8.Fatima S, Lee NP, Luk JM. Dickkopfs and Wnt/β-catenin signalling in liver cancer. World J Clin Oncol. 2011;2:311–325. doi: 10.5306/wjco.v2.i8.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esufali S, Bapat B. Cross-talk between Rac1 GTPase and dysregulated Wnt signaling pathway leads to cellular redistribution of β-catenin and TCF/LEF-mediated transcriptional activation. Oncogene. 2004;23:8260–8271. doi: 10.1038/sj.onc.1208007. [DOI] [PubMed] [Google Scholar]
- 10.Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16:797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]
- 11.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Iriye R, Furukawa K, Nishida R, KiM CS, Fukami H. Isolation and synthesis of a new bio-antimutagen, petasiphenol, from scapes of Petasites japonicum. Biosci Biotechnol Biochem. 1992;56:1773–1775. doi: 10.1271/bbb.56.1773. [DOI] [PubMed] [Google Scholar]
- 13.Schoental R. Toxicology and carcinogenic action of pyrrolizidine alkaloids. Cancer Res. 1968;28:2237–2246. [PubMed] [Google Scholar]
- 14.Lee JS, Yang EJ, Yun CY, Kim DH, Kim IS. Suppressive effect of Petasites japonicus extract on ovalbumin-induced airway inflammation in an asthmatic mouse model. J Ethnopharmacol. 2011;133:551–557. doi: 10.1016/j.jep.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 15.Lee KP, Kang S, Park SJ, Choi YW, Lee YG, Im DS. Anti-allergic and anti-inflammatory effects of bakkenolide B isolated from Petasites japonicus leaves. J Ethnopharmacol. 2013;148:890–894. doi: 10.1016/j.jep.2013.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Heo BG, Park YS, Chon SU, Lee SY, Cho JY, Gorinstein S. Antioxidant activity and cytotoxicity of methanol extracts from aerial parts of Korean salad plants. Biofactors. 2007;30:79–89. doi: 10.1002/biof.5520300202. [DOI] [PubMed] [Google Scholar]
- 17.Kang HG, Jeong SH, Cho JH. Antimutagenic and anticarcinogenic effect of methanol extracts of Petasites japonicus Maxim leaves. J Vet Sci. 2010;11:51–58. doi: 10.4142/jvs.2010.11.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirono I, Mori H, Yamada K, Hirata Y, Haga M. Carcinogenic activity of petasitenine, a new pyrrolizidine alkaloid isolated from Petasites japonicus Maxim. J Natl Cancer Inst. 1977;58:1155–1157. doi: 10.1093/jnci/58.4.1155. [DOI] [PubMed] [Google Scholar]
- 19.Murayama K, Kimura T, Tarutani M, Tomooka M, Hayashi R, Okabe M, et al. Akt activation induces epidermal hyperplasia and proliferation of epidermal progenitors. Oncogene. 2007;26:4882–4888. doi: 10.1038/sj.onc.1210274. [DOI] [PubMed] [Google Scholar]
- 20.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 21.Chen RH, Ding WV, McCormick F. Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem. 2000;275:17894–17899. doi: 10.1074/jbc.M905336199. [DOI] [PubMed] [Google Scholar]
- 22.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 23.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 24.Kim D, Chung J. Akt: versatile mediator of cell survival and beyond. J Biochem Mol Biol. 2002;35:106–115. doi: 10.5483/BMBRep.2002.35.1.106. [DOI] [PubMed] [Google Scholar]
- 25.Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276:17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]