Abstract
Bone fracture is accompanied with poor oxygen supply and nutrient deficiency in the local fracture site, and oxygen supply is an important factor that can affect fracture healing. Hypoxia-inducible factor-1 (HIF-1) plays a key role in the regulation of oxygen homeostasis. HIF-1α is rapidly upregulated in response to hypoxia and antagonizes hypoxia-induced apoptosis. In the present study, the viability of an osteoblast cell line, MC3T3-E1, and the expression of HIF-1α protein in the MC3T3-E1 cells was examined under hypoxic conditions. The HIF-1α level was then manipulated and the reduction in the viability of the MC3T3-E1 cells in response to the hypoxia was re-evaluated. In addition, the regulation of HIF-1α in the adaptation of MC3T3-E1 cells to hypoxia was explored. The results showed that the viability of MC3T3-E1 cells decreased and the expression of HIF-1α protein increased under hypoxic conditions. Furthermore, the reduction in the viability of MC3T3-E1 cells post-hypoxia was attenuated by HIF-1α overexpression, while HIF-1α-knockdown by small interfering RNA enhanced the hypoxia-induced decrease in cell viability. It was additionally found that the forced expression of HIF-1α inhibited the hypoxia-induced cell apoptosis. These findings indicate that the forced expression of HIF-1α inhibits hypoxia-induced apoptosis and thus attenuates the hypoxia-induced decrease in cell viability.
Keywords: hypoxia-inducible factor-1α, hypoxia, apoptosis, osteoblast cell
Introduction
Fracture not only directly destroys bone integrity, but also causes damage to local soft tissues and interrupts blood flow, which is followed by the onset of ischemic-hypoxia in the local bone tissue. The poor oxygen supply and nutrient deficiency at the fracture site affects the fracture healing, particularly without timely treatment, as the ischemic-hypoxia deteriorates the physiological status of local osteoblast cells and inhibits bone repair (1). Oxygen deprivation under ischemic conditions causes functional impairment of the cells and often structural tissue damage (2). Furthermore, ischemia at fracture sites is the key cause of delayed union or non-union fracture healing, and it is rarely a solitary factor affecting fracture repair (3). Studies have shown that the early stages of fracture in humans are characterized by inflammation and hypoxia, and the initial inflammatory phase of fracture represents a critical step for the outcome of the healing process (4–6). Hypoxia-inducible factor-1α (HIF-1α) has a regulatory function during inflammation resolution in vivo (7,8).
HIF-1 is a transcription factor that acts as a master regulator in oxygen homeostasis, existing as a heterodimer composed of α and β subunits. HIF-1β, an aryl hydrocarbon receptor nuclear translocator, is expressed in normoxic cells constitutively; HIF-1α is continuously synthesized and only present in hypoxic cells, due to rapid degradation by the ubiquitin-proteasome system under normoxic conditions (9). HIF-1α plays a key role in the cellular response to hypoxia and is involved in glucose metabolism, vascular remodeling and erythropoiesis via gene activation (10), in addition to being required for solid tumor formation and embryonic vascularization (11). When cells are exposed to hypoxia, HIF-1α initiates the protective and adaptive mechanism; if this is not sufficient to rescue cells from the severe hypoxia, the cells die via apoptosis and even necrosis (12).
Apoptosis, which is also called programmed cell death, is induced by hypoxic conditions, which cause decreases in the mitochondrial membrane potential and the release of cytochrome c (13). The released cytochrome c then stimulates the protein caspase 9, which activates the apoptosis executioner caspase 3, thus leading to cell death (14). It has been reported that the activation of HIF-1α delays inflammation resolution by reducing neutrophil apoptosis (7). It has also been demonstrated that HIF-1α may act as a protective factor in the apoptotic process of cardiac fibroblasts and represent a potential therapeutic target for heart remodeling following injury due to hypoxia (15). HIF-1α plays a role in hypoxia-induced apoptosis and does not only stimulate, but may also prevent apoptosis (16).
In the present study, the viability of the osteoblast cell line MC3T3-E1 was investigated following exposure to hypoxia, and HIF-1α protein expression was determined. The HIF-1α level was then manipulated and the reduction in the viability of the MC3T3-E1 cells in response to the hypoxia was re-evaluated.
Materials and methods
Cell culture and treatment
Osteoblastic MC3T3-E1 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in α-Minimum Essential Media (αMEM; Invitrogen Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Invitrogen Life Technologies) at 37°C in 5% CO2. Subsequent to reaching 85–95% confluence, the MC3T3-E1 cells were washed with 0.1% phosphate-buffered saline (PBS) and detached with 0.25% trypsin (dissolved in 0.1% PBS; Ameresco Inc., Framingham, MA, USA) with 0.025% EDTA and subcultured. To upregulate the HIF-1α, a murine HIF-1α coding sequence was amplified and cloned into a eukaryotic expression vector, pcDNA3.1 (+) (Invitrogen Life Technologies), and confirmed by sequencing. HIF-1α-pcDNA3.1 (+), or chloramphenicol acetyl transferase (CAT)-pcDNA3.1 (+) vectors were then transfected into MC3T3-E1 cells to upregulate the HIF-1α level or act as a control, respectively. The positive clone, MC3T3-E1 (HIF-1α), and MC3T3-E1 (Con) were selected in the presence of 800 µg/ml G418 and maintained in medium containing G418 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 400 µg/ml. To suppress HIF-1α expression, HIF-1α-specific small interfering (si)RNAs and siRNA control (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) were utilized at a concentration of 40 nM. Each siRNA was transfected into the MC3T3-E1 cells using Lipofectamine® 2000 (Invitrogen Life Technologies).
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total mRNA was extracted from the MC3T3-E1 or MC3T3-E1 (HIF-1α) cells with the RNeasy® Mini kit (Qiagen, Valencia, CA, USA), and an RNase inhibitor (Promega Corp., Madison, WI, USA) was then added. A SYBR® Green RT-qPCR kit (Takara, Tokyo, Japan) was used for the RT-qPCR analysis of HIF-1α mRNA, and tubulin was used as a reference gene. The ∆∆Ct method was used for relative quantification (17).
Protein sample isolation and western blot analysis
Whole MC3T3-E1 or MC3T3-E1 (HIF-1α) cells were collected and lyzed with a cell lysis reagent (Pierce, Rockford, IL, USA). Protein samples were then treated with a protease inhibitor cocktail kit (Roche Biochemicals, Basel, Switzerland) and quantified with a bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc., Rockford, IL, USA). SDS-PAGE gel (8–12%) was used to separate the protein samples, which were then transferred to a polyvinylidene difluoride membrane. HIF-1α and tubulin protein levels were detected by immunoblot analysis using rabbit polyclonal antibodies against mouse HIF-1α (#ab82832) or tubulin (#ab18251; 1:500; Abcam, Cambridge, UK). Goat anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (Pierce) and an enhanced chemiluminescence detection system (SuperSignal® West Femto; Pierce) were used for detection. The HIF-1α level was expressed as a percentage relative to tubulin expression.
Cell viability determination by MTT assay
MC3T3-E1 cells with overexpression of HIF-1α or CAT were seeded in 96-well plates. Upon reaching 85% confluence, the medium was substituted with αMEM containing 2% FBS. At different time-points post-normoxia or -hypoxia treatment, with or without siRNA transfection, the MTT assay (Invitrogen Life Technologies) was conducted according to the manufacturer's instructions. The optical density was then measured at 570 nm using a spectrophotometer.
Determination of caspase activation
MC3T3-E1 or MC3T3-E1 (HIF-1α) cells were seeded on six-well plates and treated with hypoxia for 24 or 48 h. The activity of caspase 3 was determined as previously described (18). Briefly, MC3T3-E1 or MC3T3-E1 (HIF-1α) cells were pelleted and resuspended in lysis buffer, prior to being incubated with Ac-DEVD-AMC fluorogenic peptide substrates (BD Pharmingen, San Diego, CA, USA) for caspase 3 for 30 to 60 min at 37°C. The yellow-green fluorescence of the reaction product was monitored on a spectrofluorometer by setting the excitation and emission wavelengths to 380 and 440 nm, respectively. The amount of yellow-green fluorescence was proportional to the amount of active caspase 3 present in the samples. The increase in caspase activity was expressed as a relative value to the control group.
Detection of apoptotic cells
MC3T3-E1 or MC3T3-E1 (HIF-1α) cells were seeded into Nunc™ LabTek™ II chamber slides (Nalge Nunc International Corp., Rochester, NY, USA) and subjected to hypoxia with or without siRNA transfection. The cells were then fixed, washed and stained with 1 µg/ml Hoechst 33528 (Invitrogen Life Technologies) using standard procedures (19). Apoptotic cells were screened and counted under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany) using a 4,6-diamidino-2-phenylindole filter set.
Statistical analysis
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analyses. The Student's t-test was used to analyze the difference between two groups. Data are presented as the mean ± standard error of the mean, and P<0.05 was considered to indicate a statistically significant difference.
Results
Viability and HIF-1α expression of MC3T3-E1 cells under normoxic and hypoxic conditions
To explore the effect of hypoxia on the MC3T3-E1 cell line, the viability and HIF-1α protein levels of the cells were examined. In hypoxic and normoxic conditions, the viability of the cells was observed by MTT assay. The relative cell viability decreased significantly after 16 h in hypoxia, as compared with the cell viability in the normoxic condition (Fig. 1A). When the MC3T3-E1 cells were cultured in 1% O2 conditions for 8 h or longer, the relative HIF-1α mRNA level became higher than that of cells cultured in 20% O2 conditions, particularly when the cells were cultured for >16 h, as demonstrated by fluorescence qPCR (Fig. 1B). Western blot analysis was also conducted to analyze HIF-1α expression at the protein level, as shown in Fig. 1C. The HIF-1α expression in the MC3T3-E1 cells was significantly higher when the cells were cultured under hypoxic conditions for 16 and 24 h. These results suggest that the hypoxic condition reduces the viability of MC3T3-E1 cells and induces HIF-1α protein expression.
Figure 1.
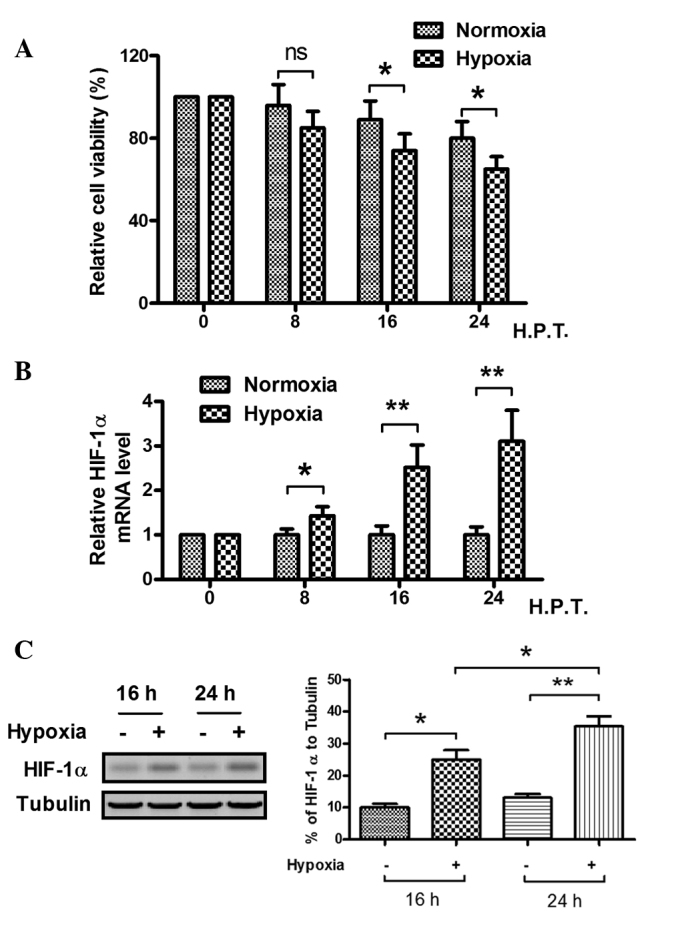
Hypoxia reduces the viability of MC3T3-E1 cells and induces HIF-1α expression. (A) MC3T3-E1 cells were cultured under normoxia (20% O2) and hypoxia (1% O2) for 24 h, and the viability of the cells was determined by MTT assay. (B) HIF-1α mRNA expression induced under hypoxia in MC3T3-E1 cells, as assessed by fluorescence quantitative polymerase chain reaction analysis. (C) Hypoxia-induced HIF-1α protein expression in MC3T3-E1 cells, as assessed by western blotting. *P<0.05 and **P<0.01. ns, no significance; HIF-1α, hypoxia-inducible factor-1α; H.P.T., hours post treatment‥
Effect of upregulated HIF-1α expression on the hypoxia-induced decrease in cell viability
As stated previously, the viability of cells was decreased and the expression of HIF-1α was increased by the hypoxic condition. In order to elucidate the effect of HIF-1α expression on the viability decrease in the MC3T3-E1 cell line caused by hypoxia, the viability of cells with forced expression of HIF-1α was investigated using an MTT assay. As shown in Fig. 2A and B, significantly high levels of HIF-1α expression were confirmed in the HIF-1α-pcDNA3.1-transfected cells, as compared with the hypoxic and control groups. In addition, as shown in Fig. 2C, the MTT assay demonstrated that the viability of the MC3T3-E1 cells was increased by the forced expression of HIF-1α. These results showed that the effect of the forced HIF-1α expression was in contrast to the effect of hypoxia on the viability of MC3T3-E1 cells.
Figure 2.
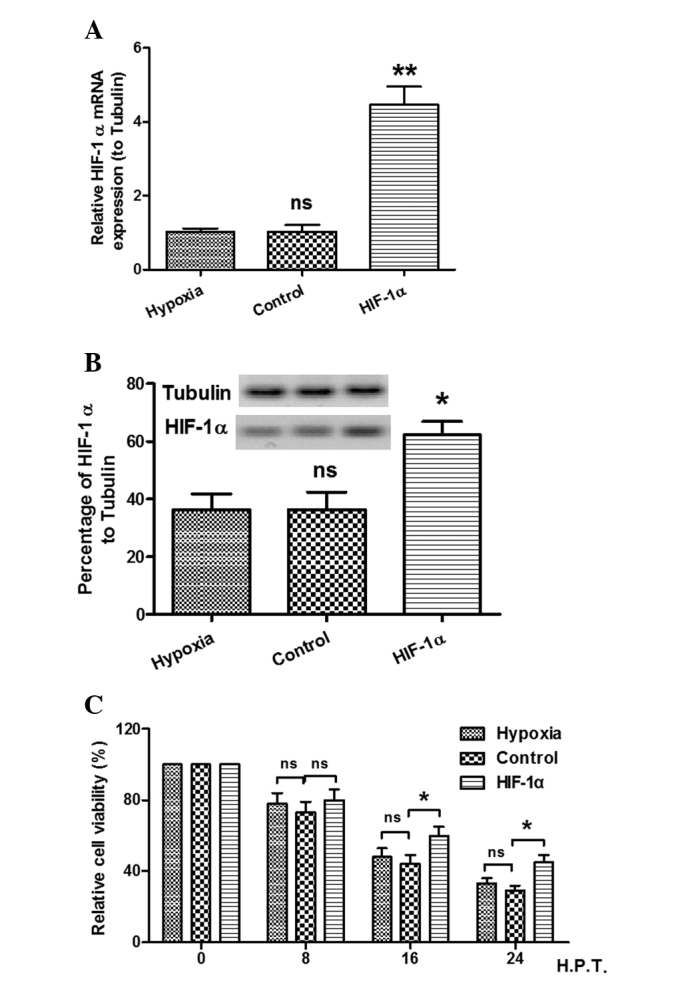
Effects of forced expression of HIF-1α on the decrease in cell viability caused by hypoxia. Three groups of cells were cultured under hypoxic (1% O2) conditions: Hypoxic group (hypoxia), chloramphenicol acetyl transferase-overexpressed group (control) and HIF-1α-overexpressed group (HIF-1α). (A and B) Expression of HIF-1α (A) mRNA and (B) protein under hypoxic conditions. *P<0.05 and **P<0.01 versus hypoxia. (C) Forced expression of HIF-1α attenuated the hypoxia-induced decrease in cell viability. **P<0.01. ns, no significance; HIF-1α, hypoxia-inducible factor-1α; H.P.T., hours post treatment.
Effect of HIF-1α-knockdown on the hypoxia-induced decrease in cell viability
To detect the role of HIF-1α in the hypoxia-induced decrease in cell viability, MC3T3-E1 cells were cultured under hypoxic conditions and transfected with siRNA. The MTT assay and western blot analysis were then conducted to confirm the relative expression levels of HIF-1α to tubulin and determine cell viability. As shown in Fig. 3A, low levels of HIF-1α mRNA expression were found post-siRNA transfection. The western blotting results also demonstrated that the HIF-1α expression in the cells transfected with HIF-1α-siRNA was significantly lower than that in the siRNA-control group (Fig. 3B). The viability of the MC3T3-E1 cells post-siRNA transfection under hypoxic conditions was determined by MTT assay. Fig. 3C shows that the viability of the cells was reduced by HIF-1α-knockdown. These results suggest that HIF-1α-knockdown enhances the hypoxia-induced decrease in cell viability.
Figure 3.
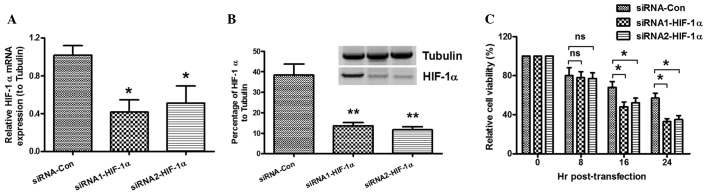
HIF-1α-knockdown enhances the hypoxia-induced decrease in cell viability. Three groups of cells were respectively transfected with control siRNA (siRNA-Con) and two HIF-1α-specific siRNAs: siRNA1-HIF-1α and siRNA2-HIF-1α. (A and B) Expresion of HIF-1α (A) mRNA and (B) protein following siRNA transfection. *P<0.05 and **P<0.01 versus siRNA-Con. (C) HIF-1α-knockdown enhanced the hypoxia-induced decrease in osteoblast viability. *P<0.05 and **P<0.01. ns, no significance; siRNA, small interfering RNA; HIF-1α, hypoxia-inducible factor-1α.
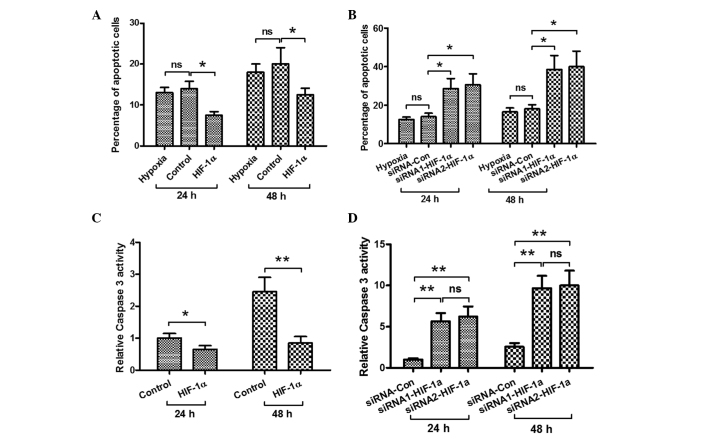
Effect of HIF-1α on the hypoxia-induced osteoblast apoptosis and caspase 3 activity
In order to explore the possible mechanism by which HIF-1α attenuates the hypoxia-induced decrease in MC3T3-E1 cell viability, the effects of HIF-1α on the hypoxia-induced osteoblast apoptosis and the activity of caspase 3 were investigated. The cells were transfected with pcDNA3.1 (+) (control) and HIF-1α-pcDNA3.1 (+) under hypoxic conditions. As shown in Fig. 4A, forced HIF-1α expression significantly suppressed the hypoxia-induced apoptosis after 24 and 48 h. By contrast, when the cells were transfected with siRNA (control) and two types of siRNA-HIF-1α during hypoxia, the levels of HIF-1α in the siRNA-HIF-1α-transfected cell groups were decreased and the percentage of cells undergoing apoptosis was increased significantly (Fig. 4B). In addition, the activity of caspase 3 was examined; as shown in Fig. 4C, the activity of caspase 3 was inhibited in the cells with forced HIF-1α expression under hypoxia after 24 and 48 h. By contrast, in the siRNA-HIF-1α-transfected osteoblasts, the hypoxia-induced caspase 3 activity was enhanced (Fig. 4D). These results show that HIF-1α inhibits hypoxia-induced osteoblast apoptosis.
Figure 4.
HIF-1α inhibits hypoxia-induced osteoblast apoptosis. The cell groups were cultured under hypoxic conditions. (A) Forced HIF-1α expression inhibited hypoxia-induced osteoblast apoptosis. (B) HIF-1α-knockdown enhanced hypoxia-induced osteoblast apoptosis. (C) Forced HIF-1α expression inhibited the hypoxia-induced caspase 3 activity in osteoblasts. (D) HIF-1α-knockdown enhanced the hypoxia-induced casepase 3 activity in osteoblasts. *P<0.05 and **P<0.01. ns, no significance; siRNA, small interfering RNA; HIF-1α, hypoxia-inducible factor-1α.
Discussion
Secondary or indirect bone healing typically involves four phases, known as the inflammatory, soft callus, hard callus and remodeling phases (20). Numerous factors can affect fracture healing, including the coordination of multiple cell types (such as osteoblasts and chondrocytes); cytokines (such as transforming growth factor-β, basic fibroblast growth factor and platelet-derived growth factor), which have a regulatory effect on the initiation and development of the fracture repair process (21–23); and the oxygen level of the tissues at the fracture site. Since oxygen plays a critical role as a participant in multiple basic cellular processes, hyperbaric oxygen therapy is one of the methods used to promote fracture healing by delivering 100% oxygen at pressures greater than one atmosphere (24). Low-intensity pulsed ultrasound (LIPUS) can also accelerate fracture healing by inducing the homing of circulating osteogenic progenitors to the fracture site (25); furthermore, LIPUS treatment combined with functional electrical stimulation treatment has shown better effects in accelerating new bone formation (26). In addition, improvements in the adaptation of osteoblasts and chondrocytes to hypoxia ameliorate the physiological status of these cells, which are subject to hypoxia (27).
The protective role of HIF-1α has been confirmed in various types of cells (28). Cells with high HIF-1α levels showed more resistance to apoptosis caused by hypoxia and glucose deprivation than did cell lines with low HIF-1α expression under normoxia (28). It can thus be concluded that HIF-1α plays a role in hypoxia-induced apoptosis, and acts as an antiapoptotic factor (12). In the present study, the viability of MC3T3-E1 cells decreased and the expression of HIF-1α protein in the MC3T3-E1 cells increased under hypoxic conditions. It was also found that the viability of HIF-1α-transfected MC3T3-E1 cells was higher than that in cells without forced expression of HIF-1α (Fig. 2C), whereas HIF-1α-knockdown by siRNA in MC3T3-E1 cells enhanced the hypoxia-induced decrease in cell viability (Fig. 3C). It was ascertained that the forced expression of HIF-1α in the MC3T3-E1 cell line attenuated the hypoxia-induced decrease in cell viability by inhibiting apoptosis. These results indicate that HIF-1α plays a key role in the hypoxia-induced decrease in osteoblast viability.
In conclusion, the viability of the MC3T3-E1 cell line decreased under hypoxia and HIF-1α expression was upregulated. The forced expression of HIF-1α in the MC3T3-E1 cell line attenuated the hypoxia-induced decrease in osteoblast viability by inhibiting apoptosis. These present findings provide novel insight into the mechanism underlying the hypoxia-induced decrease in cell viability, and indicate that HIF-1α expression affects cell viability by inhibiting apoptosis.
References
- 1.Lu C, Wang X, Sinha A, et al. The role of oxygen during fracture healing. Bone. 2013;52:220–229. doi: 10.1016/j.bone.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scaringi R, Piccoli M, Papini N, et al. NEU3 sialidase is activated under hypoxia and protects skeletal muscle cells from apoptosis through the activation of the epidermal growth factor receptor signaling pathway and the hypoxia-inducible factor (HIF)-1α. J Biol Chem. 2013;288:3153–3162. doi: 10.1074/jbc.M112.404327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C, Hu D, Miclau T, Marcucio RS. Ischemia leads to delayed-union during fracture healing: a mouse model. J Orthop Res. 2007;25:51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolar P, Gaber T, Perka C, Duda GN, Buttgereit F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin Orthop Relat Res. 2011;469:3118–3126. doi: 10.1007/s11999-011-1865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoff P, Maschmeyer P, Gaber T, et al. Human immune cells' behavior and survival under bioenergetically restricted conditions in an in vitro fracture hematoma model. Cell Mol Immunol. 2013;10:151–158. doi: 10.1038/cmi.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoff P, Gaber T, Schmidt-Bleek K, et al. Immunologically restricted patients exhibit a pronounced inflammation and inadequate response to hypoxia in fracture hematomas. Immunol Res. 2011;51:116–122. doi: 10.1007/s12026-011-8235-9. [DOI] [PubMed] [Google Scholar]
- 7.Elks PM, van Eeden FJ, Dixon G, et al. Activation of hypoxia-inducible factor-1α (hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood. 2011;118:712–722. doi: 10.1182/blood-2010-12-324186. [DOI] [PubMed] [Google Scholar]
- 8.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-inducud changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 10.Corn PG, Ricci MS, Scata KA, et al. Mxi1 is induced by hypoxia in a HIF-1-dependent manner and protects cells from c-Myc-induced apoptosis. Cancer Biol Ther. 2005;4:1285–1294. doi: 10.4161/cbt.4.11.2299. [DOI] [PubMed] [Google Scholar]
- 11.Ryan HE, Lo J, Johnson RS. HIF-1alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piret JP, Mottet D, Raes M, Michiels C. Is HIF-1alpha a pro-or an anti-apoptotic protein? Biochem Pharmacol. 2002;64:889–892. doi: 10.1016/S0006-2952(02)01155-3. [DOI] [PubMed] [Google Scholar]
- 13.Sinha K, Das J, Pal PB, Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 14.Yang TM, Qi SN, Zhao N, et al. Induction of apoptosis through caspase-independent or caspase-9-dependent pathway in mouse and human osteosarcoma cells by a new nitroxyl spin-labeled derivative of podophyllotoxin. Apoptosis. 2013;18:727–738. doi: 10.1007/s10495-013-0819-5. [DOI] [PubMed] [Google Scholar]
- 15.Yang B, He K, Zheng F, et al. Over-expression of hypoxia-inducible factor-1 alpha in vitro protects the cardiac fibroblasts from hypoxia-induced apoptosis. J Cardiovasc Med (Hagerstown) 2014;15:579–586. doi: 10.2459/JCM.0b013e3283629c52. [DOI] [PubMed] [Google Scholar]
- 16.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(ΔΔC(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Seong GJ, Park C, Kim CY, et al. Mitomycin-C induces the apoptosis of human Tenon's capsule fibroblast by activation of c-Jun N-terminal kinase 1 and caspase-3 protease. Invest Ophthalmol Vis Sci. 2005;46:3545–3552. doi: 10.1167/iovs.04-1358. [DOI] [PubMed] [Google Scholar]
- 19.Sareen D, van Ginkel PR, Takach JC, et al. Mitochondria as the primary target of resveratrol-induced apoptosis in human retinoblastoma cells. Invest Ophthalmol Vis Sci. 2006;47:3708–3716. doi: 10.1167/iovs.06-0119. [DOI] [PubMed] [Google Scholar]
- 20.Kumar G, Narayan B. The biology of fracture healing in long bones. In: Banaszkiewicz P, Kader D, editors. Classic Papers in Orthopaedics. Springer; London: 2014. pp. 531–533. [Google Scholar]
- 21.Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200:165–170. doi: 10.3181/00379727-200-43410A. [DOI] [PubMed] [Google Scholar]
- 22.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Hara Y, Tagawa M, et al. Recombinant human basic fibroblast growth factor accelerates fracture healing by enhancing callus remodeling in experimental dog tibial fracture. J Bone Miner Res. 1998;13:942–949. doi: 10.1359/jbmr.1998.13.6.942. [DOI] [PubMed] [Google Scholar]
- 24.Bennett MH, Stanford RE, Turner R. Hyperbaric oxygen therapy for promoting fracture healing and treating fracture non-union. Cocbrane Database Syst Rev. 2012;11:CD004712. doi: 10.1002/14651858.CD004712.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumagai K, Takeuchi R, Ishikawa H, et al. Low-intensity pulsed ultrasound accelerates fracture healing by stimulation of recruitment of both local and circulating osteogenic progenitors. J Orthop Res. 2012;30:1516–1521. doi: 10.1002/jor.22103. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Qu J, Xu D, Zhang T, Qin L, Lu H. Combined application of low-intensity pulsed ultrasound and functional electrical stimulation accelerates bone-tendon junction healing in a rabbit model. J Orthop Res. 2014;32:204–209. doi: 10.1002/jor.22505. [DOI] [PubMed] [Google Scholar]
- 27.Steinbrech DS, Mehrara BJ, Saadeh PB, et al. Hypoxia regulates VEGF expression and cellular proliferation by osteoblasts in vitro. Plast Reconstr Surg. 1999;104:738–747. doi: 10.1097/00006534-199909010-00019. [DOI] [PubMed] [Google Scholar]
- 28.Akakura N, Kobayashi M, Horiuchi I, et al. Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Res. 2001;61:6548–6554. [PubMed] [Google Scholar]