Abstract
Type 2 diabetes (T2D) is characterized by progressive and inexorable β-cell dysfunction, leading to insulin deficiency. Novel strategies to preserve the remaining β-cells and restore β-cell function for the treatment of diabetes are urgently required. Mesenchymal stem cells (MSCs) have been exploited in a variety of clinical trials aimed at reducing the burden of immune-mediated disease. The aim of the present clinical trial was to assess the safety and efficacy of umbilical cord-derived MSC (UCMSC) transplantation for patients with T2D. The safety and efficacy of UCMSC application were evaluated in six patients with T2D during a minimum of a 24-month follow-up period. Following transplantation, the levels of fasting C-peptide, the peak value and the area under the C-peptide release curve increased significantly within one month and remained high during the follow-up period (P<0.05). Three of the six patients became insulin free for varying lengths of time between 25 and 43 months, while the additional three patients continued to require insulin injections, although with a reduced insulin requirement. Fasting plasma glucose and 2-h postprandial blood glucose levels were relatively stable in all the patients following transplantation. There was no immediate or delayed toxicity associated with the cell administration within the follow-up period. Therefore, the results indicated that transplantation of allogeneic UCMSCs may be an approach to improve islet function in patients with T2D. There were no safety issues observed during infusion and the long-term monitoring period.
Keywords: diabetes, mesenchymal stem cells, cytotherapy
Introduction
Diabetes mellitus is caused by absolute insulin deficiency due to autoimmune destruction of insulin secreting pancreatic β-cells (type 1 diabetes, T1D) or by relative insulin deficiency due to decreased insulin sensitivity (type 2 diabetes, T2D). Treatment for T1D and T2D often involves regular insulin injections and oral medication with sulfonylurea. However, this treatment method neither precisely controls the blood sugar levels, nor prevents complications associated with diabetes. Reduction of β-cell mass in the pancreas is the hallmark of the development of diabetes. Regeneration and maintenance of pancreatic endocrine tissue following the onset of islet destruction can have a considerable therapeutic impact on diabetes (1). Transplantation therapies for T1D include whole organ transplantation, islet transplantation and regeneration therapy (2–5). The success of the Edmonton protocol for pancreatic islet transplantation has sparked new interests in the treatment of T1D (3). However, the requirement for immunosuppression and the scarcity of organs available for processing and transplantation has hindered the widespread use of this therapy. Therefore, the search for alternative approaches is of paramount clinical interest.
Mesenchymal stem cells (MSCs) have already made their mark in the field of regenerative medicine, since they have the capacity of proliferation and differentiation into the mesenchymal lineage, secreting a variety of cytokines and growth factors, as well as a profound immunosuppressive capability in vitro (6–8). MSCs can be easily derived from various tissues, and their therapeutic worth has already been validated for a number of clinical conditions (9). Unlike embryonic stem cells, neither their procurement nor their use is deemed controversial (10). Thus, there has been great interest in the potential clinical applications of MSCs (11,12). There is increasing evidence from animal studies and clinical trials indicating the therapeutic effects of MSC transplantation in a number of diseases, such as myocardial infarction (13,14), stroke (15), spinal cord injury (16), brain injury (17), refractory systemic lupus erythematosus (18–20), liver disease (21), diabetes mellitus (10,22,23) and acute and chronic graft-versus-host disease (GVHD) (24,25). Human umbilical cord-derived mesenchymal stem cells (UCMSCs) possess greater pluripotency compared with adult stem cells, expressing the pluripotency markers, Oct-4, Sox-2 and c-Myc (26), and share similar in vitro immunosuppressive properties as MSCs obtained from the bone marrow. Unlike embryonic stem cells, however, UCMSCs do not form tumors when transplanted (27,28). Furthermore, UCMSCs are immune-privileged, immune-suppressive and readily available as a cell source with few ethical disputes (29,30). Thus, UCMSCs may provide a novel source of cell therapy for diabetes. However, to date, few clinical investigations have focused on the treatment of T2D with UCMSCs. Therefore, the present study reports the preliminary experience of the application of an intravenous infusion of UCMSCs in six patients with T2D.
Materials and methods
Patient enrollment
In total, six male patients with different histories of T2D were enrolled in the study between April 2010 and December 2011 at Weifang People's Hospital (Weifang, China). Their clinical characteristics are outlined in Table I. The mean age at infusion was 40.5±3.76 years (range, 27–51 years), and the mean duration of time from the symptoms of hyperglycemia to transplantation (first infusion) was 64.7±23.8 weeks (range, 4–157 weeks). All the recipients had previously been treated with insulin injections. Despite this treatment, their blood glucose and glycated hemoglobin (HbA1c) levels were poorly controlled, and insulin secretion was relatively insufficient under the condition of insulin resistance prior to infusion. The study protocol was evaluated and approved by the Medical Ethics Board of Weifang People's Hospital affiliated to Weifang Medical College, under the auspices of the Development Plan Project of Science and Technology of Weifang. Prior to transplantation, all the patients were informed of the process concerning the UCMSC infusion and volunteered to receive this treatment. Written informed consent was obtained from all the involved diabetic patients. Exclusion criteria were as follows: i) Severe comorbidity, such as cardiopulmonary, renal or liver dysfunction, or systemic infection; ii) leukocyte, platelet or hemoglobin levels of <3.0×109/l, 75×109/l and 100 g/l, respectively; iii) serum positive serum for HIV, the surface antigen of hepatitis B (HBV; HBsAg), hepatitis C (HCV) or tumor markers; iv) unwillingness to participate in the clinical trial; v) previous enrollment in other clinical trials in the last three months; vi) lactating or pregnant females; vii) prior history of severe allergic reactions; and viii) other clinical conditions that the investigators considered not appropriate for enrollment in this study.
Table I.
Administered UCMSC dose and pretreatment characteristics of the type 2 diabetes patients.
| Pretreatment characteristic | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (years) | Duration (months) | First dose (×106cells/kg) | Second dose (×106cells/kg) | Interval (days) | Follow-up (months) | Insulin-free period (months) | BMI (kg/m2) | HbA1c (%) | Fasting C-peptide (ng/ml) | Cmax (ng/ml) | Insulin dose (IU/kg/day) | Diabetic complications | |
| 1 | 47 | 4 | 0.88 | 0.85 | 16 | 44 | 43 | 24.25 | 10.1 | 1.08 | 5.07 | 0.225 | None | |
| 2 | 42 | 36 | 0.95 | 0.94 | 14 | 36 | RI | 24.31 | 8.9 | 1.26 | 4.03 | 0.296 | None | |
| 3 | 51 | 84 | 0.86 | 0.87 | 17 | 35 | RI | 23.60 | 7.4 | 0.56 | 1.32 | 0.779 | None | |
| 4 | 27 | 12 | 0.90 | 0.87 | 16 | 32 | 29 | 23.24 | 6.3 | 0.91 | 3.61 | 0.405 | None | |
| 5 | 32 | 48 | 0.81 | 0.79 | 14 | 28 | 25 | 22.50 | 9.8 | 1.42 | 5.69 | 0.267 | None | |
| 6 | 44 | 72 | 0.88 | 0.90 | 15 | 24 | RI | 24.10 | 8.8 | 0.96 | 2.16 | 0.619 | None | |
| Averagea | 40.5±3.76 | 42.7±13.02 | 0.88±0.02 | 0.87±0.02 | 15.3±0.49 | 33.2±2.82 | 23.7±0.29 | 8.55±0.59 | 1.03±0.12 | 3.65±0.68 | 0.43±0.09 | None | ||
Results are expressed as the mean ± standard error. RI, reduced insulin requirement; UCMSC, umbilical cord mesenchymal stem cell; BMI, body mass index; HbA1c, glycated hemoglobin; Cmax, peak concentration (C-peptide).
Preparation of UCMSCs
Umbilical cord samples were collected from full-term cesarean section patients with their consent, and approval was granted by Weifang People's Hospital. All procedures were conducted in accordance with the guidelines of the Medical Ethics Committee of the Health Bureau (31). UCMSCs were isolated from the gelatinous tissue surrounding the vein and the artery. In addition, a 10-ml sample of cord blood was analyzed for communicable diseases, including HBV, HCV, HIV, cytomegalovirus (CMV) and syphilis. Virus-free umbilical cords were transferred for cell preparation in a good manufacturing practice laboratory. Briefly, the primary culture was initiated by seeding chopped tissue (2–3-mm3 sections) onto 100-mm dishes in growth medium, containing α-MEM (Gibco Life Technologies, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS), 10 ng/ml basic fibroblast growth factor (Invitrogen Life Technologies, Carlsbad, CA, USA), 50 I.U./ml penicillin and 50 µg/ml streptomycin. UCMSCs were incubated at 37°C in a humidified, 5% CO2 atmosphere. When the cells reached 70–80% confluence (∼10 days), they were harvested with 0.25% trypsin (Gibco Life Technologies) and subcultured with the same culture medium. In addition, a 30-ml sample of venous blood was collected from the participants for serum extraction (5810R; Eppendorf, Hamburg, Germany). The blood sample was allowed to clot by leaving it undisturbed at room temperature for 15–30 min, and then, the serum was extracted by spinning at 2,000 × g for 10 min in a refrigerated centrifuge.
After two passages of culture, the FBS was removed from the culture medium and replaced with 3% autologous serum. UCMSCs at passages 3–5 were used in the study. To ensure the quality of the UCMSCs, cell growth was regularly monitored during the cultivation. The cell viability of harvested UCMSCs was determined by trypan blue testing. Briefly, the cell suspension was diluted as 1:1 using a 0.4% trypan blue solution (Bio-Rad Laboratories, Shanghai, China) and incubated at room temperature for 1–2 min. The percentage of non-viable cells (blue) was assessed by counting cells under the microscope.
UCMSCs used for treatment were subject to pass quality control tests, including immunophenotype identification and microbiological analysis. The cluster of differentiation (CD) marker expression was identified for the immunophenotype identification of UCMSCS, including CD34, CD44, CD45 and CD90. The fluorescein isothiocyanate (FITC) conjugated CD antibodies and their corresponding isotypes were purchased from eBiosciences (San Diego, CA, USA) and used according to the manufacturer's instructions. The cells were detected by flow cytometry with FACScan EPICS XL-ADC (Beckman Coulter, USA) as previously described (32). Routine microbiological tests were also performed before cell transplantation, including tests for endotoxin, aerobic and anaerobic bacteria and fungus. Any contaminated cell preparation was eliminated upon identification.
UCMSCs transplantation
USMSCs used for transplantation were collected between passages 3 and 5, depending on the cell growth status of the individual sample. Approximately 106 UCMSCs/kg body weight were suspended in saline (40 ml) and filtered through a cell strainer (40 µm; BD Falcon, BD Biosciences, Bedford, MA, USA). The infusion was administered intravenously through the cubital vein over 15 min, and the patients were discharged after 6 h of observation. Each patient received treatment with UCMSCs two times at a two-week interval. The patients were gender-matched with the infant whom the umbilical cord was obtained.
Assessment of efficacy and follow-up
Fasting plasma glucose (FPG) levels were checked daily for 3 months, followed by weekly check-ups for an additional 3 months. Exogenous insulin requirements were timely adjusted according to the levels of FPG. The daily insulin dose and duration were monitored and recorded for all the patients throughout the entire study period. The oral glucose tolerance test was performed using a standard 75-g glucose load, and blood samples were collected at 0, 0.5, 1 and 2 h after the test load. The area under the curve (AUC), relating to the C-peptide level, during the 2-h oral glucose tolerance test was calculated using the following formula: AUC = 0.25 × (fasting value) + 0.5 × (0.5-h value) + 0.75 × (1-h value) + 0.5 × (2-h value) (33). All associated parameters were routinely monitored, including the fasting and 2-h C-peptide level, the HbA1c level, the peak value of C-peptide during the 75-g oral glucose tolerance test (Cmax), the AUC and the levels of glutamic acid decarboxylase antibody (GAD-Ab), islet cell antibody (ICA) and insulin autoantibody (IAA). The temporal changes of exogenous insulin requirement were recorded at the baseline (prior to therapy) and at 1, 3, 6, 12, 18 and 24 months post-transplantation. In addition, transplant complications were recorded following the procedure. Clinical characteristics, including the body mass index, duration of disease and diabetic complications were also recorded (Table I). The follow-up period was different for each patient due to different times of patient enrollment, however, a minimum of a 24-month follow-up period was required for the present study.
Blood glucose was measured using a glucometer (OneTouch Ultra2, LifeScan, Milpitas, CA, USA), and the serum C-peptide levels were detected using a chemoluminescent immunoassay with a commercially available kit (DiaSorin S.P.A., Saluggia, Italy). HbA1c levels were determined using high-pressure liquid chromatography (Shanghai Huachen Medical Technology, Co., Ltd., Shanghai, China). Detection of the serum levels of GAD-Ab, ICA and IAA were conducted using a combinatorial islet autoantibody workshop kit (Shenzhen Sciarray BioTech Co., Ltd., Shenzhen, China) at the clinical endocrinology laboratories of Weifang People's Hospital.
Safety evaluation of UCMSC therapy
Clinical, laboratory and radiographic measurements were used to assess the safety of the UCMSC transplantation therapy. Immediate reactions included fever, respiratory failure, headache and systemic complications (systemic infections), while delayed reactions included tumor formation. Examinations were conducted prior to and following transplantation at 1, 3, 6, 12, 18 and 24 months. The assessments included routine blood and urine tests, liver and kidney function assessment and blood lipid content analysis. In addition, the presence of tumor markers in the blood serum was assessed, including α-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 125 and carbohydrate antigen 199. The presence of HBV, HCV, HIV, CMV and syphilis infections was also analyzed. Finally, an electrocardiogram, chest X-ray examination and abdominal color Doppler ultrasound examination were performed.
Statistical analysis
Clinical and biochemical data are expressed as the mean ± standard error, and comparisons of the changes from the baseline conditions were analyzed using the Student's t-test (two-tailed, paired). Differences among groups and follow-up times after transplantation (1, 3, 6, 12, 18 and 24 months) were determined with one-way analysis of variance. The baseline data were reported individually in a tabular form. All data were analyzed using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA), and P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
In total, six patients ranging in age between 27 and 51 years (average, 40.5±3.76 years) were enrolled in the study. All the patients were diagnosed with T2D based on the World Health Organization criteria (34), with a mean diesease duration of 42.7±13.02 months. The mean UCMSC dose (including the first and second infusion) was 0.88±0.05×106 cells/kg (range, 0.79–0.95×106/kg). The mean interval between the first and second infusion was 15.3±0.49 days and the follow-up time varied between 24 and 44 months (mean, 33.2±2.82 months). Patients 5 and 6, who tested serum positive for GAD-Ab prior to treatment, were found to test negative at 1 month after the transplantation, which was maintained for 24 months. Serum ICA and IAA levels were negative in all the patients prior to and following transplantation. Patients 1, 4 and 5, who presented with diabetic ketoacidosis at diagnosis, overcame this condition at 1 month after the UCMSC transplantation. Table I outlines the diabetes-associated parameters of all the involved patients prior to USMSC administration.
Properties of the UCMSCs
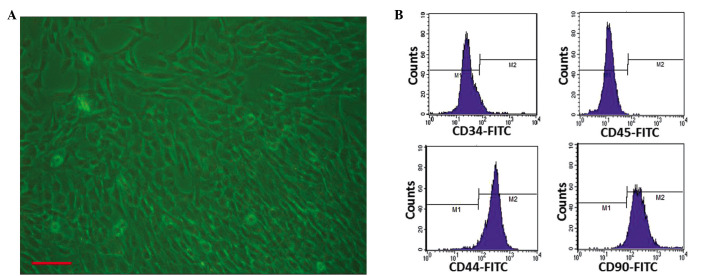
Using an umbilical cord tissue block culture attachment method, primary UCMSCs were successfully isolated from the Wharton's jelly of human umbilical cords. The UCMSCs exhibited typical fibroblast-like morphology (Fig. 1A), and the cell viability was ≥85%, as determined with trypan blue testing. Each preparation was negative for pathogenic microorganisms, including aerobic and anaerobic bacteria and fungus. Endotoxin was ≤0.25 EU/ml in the supernatants of each cell preparation. HBsAg, HCV-Ab, HIV-Ab, human CMV-IgM and syphilis antibody tests were all negative. At passage 3, the cultured cells showed positive expression of CD44 and CD90 (>90%) and negative expression of CD34 and CD45 (<3.0%; Fig. 1B), exhibiting similar characteristics to those of previously reported bone marrow-derived MSCs (17). These results were consistent with the immunophenotypic characteristics of UCMSCs, which had been reported in previous studies (35,36).
Figure 1.
Isolation and identification of umbilical cord mesenchymal stem cells (UCMSCs). (A) Appearance of UCMSCs after passaging (scale bar, 100 µm). (B) Identification of third-passage UCMSCs revealed positive expression of CD44 (97.1%) and CD90 (95.8%), and negative expression of CD34 (3.0%) and CD45 (0.04%), as analyzed by flow cytometry. FITC, fluorescein isothiocyanate.
Therapeutic effects of UCMSCs
Table II outlines the insulin dosages administered for all the studied patients. The average daily insulin requirements were significantly decreased following the UCMSC transplantation (P<0.05, vs. pretreatment; Fig. 2). Three of six patients (1, 4 and 5; 50.0%) became insulin free (defined as the insulin-free group) for the whole follow-up period (>24 months). The insulin requirement of the additional three patients (2, 3 and 6) reached the lowest levels at 3 months (0.252 IU/kg/day) after the transplantation, but then gradually increased between 12 and 24 months (defined as the insulin-dependent group). Notably, the insulin-free patients had presented with diabetic ketoacidosis at diagnosis, and the insulin-dependent patients continued to require insulin injections; however, their insulin requirement was significantly reduced for a short period (6 months; Fig. 2).
Table II.
Insulin dosage of the individual patients (IU/kg/day).
| Patients | Month 0 | Month 1 | Month 3 | Month 6 | Month 12 | Month 18 | Month 24 |
|---|---|---|---|---|---|---|---|
| 1 | 0.225 | 0.17 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0.296 | 0.26 | 0.2 | 0.24 | 0.25 | 0.279 | 0.298 |
| 3 | 0.779 | 0.602 | 0.56 | 0.71 | 0.78 | 0.8 | 0.82 |
| 4 | 0.405 | 0.28 | 0.16 | 0 | 0 | 0 | 0 |
| 5 | 0.267 | 0.14 | 0.03 | 0 | 0 | 0 | 0 |
| 6 | 0.619 | 0.52 | 0.56 | 0.58 | 0.633 | 0.74 | 0.915 |
| Averagea | 0.432±0.090 | 0.329±0.077 | 0.252±0.102 | 0.255±0.130 | 0.277±0.143 | 0.303±0.154 | 0.339±0.174 |
Results are expressed as the mean ± standard error of the mean.
Figure 2.
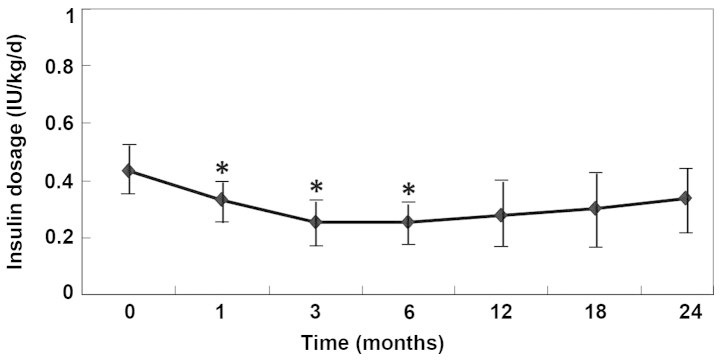
Insulin dose (IU/kg/day) administered in type 2 diabetes patients following umbilical cord mesenchymal stem cell therapy. *P<0.05, vs. month 0 (pretreatment).
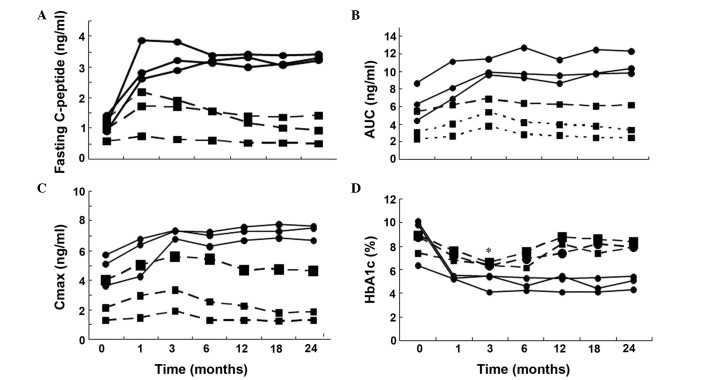
In the insulin-free group (Fig 3A-C), the C-peptide production, AUC and Cmax were significantly increased at 1, 3, 6, 12 and 24 months after the transplantation (P<0.05, vs. pretreatment), while there was no statistically significant changes observed in the insulin-dependent group (P>0.05, vs. pretreatment). Furthermore, the insulin-free patients exhibited significantly higher levels of fasting C-peptide, AUC and Cmax at different follow-up time points after the infusion of UCMSCs. The HbA1c level was shown to decrease significantly 3 months after the transplantation, and was maintained at a stable reduced level for 24 months of the follow-up period (P<0.01, vs. pretreatment). In addition, the FPG and 2 h postprandial blood glucose levels obtained 3 months after the transplantation were more stable compared with those observed 7 days prior to the treatment. In the insulin-dependent group, the HbA1c level significantly decreased only at 3 months after the transplantation (P=0.014, vs. pretreatment; Fig. 3D).
Figure 3.
Time course of (A) fasting C-peptide, (B) AUC, (C) Cmax and (D) HbA1c in the six type 2 diabetes patients. The x-axis represents the time-course relative to the transplantation of umbilical cord mesenchymal stem cells. AUC, area under the curve (C-peptide); Cmax, peak concentration (C-peptide); HbA1c, glycated hemoglobin. Black circles, patients avoiding insulin injection after the cellular therapy during the follow-up period (defined as insulin-free patient). Black squares, patients requiring reduced-dose of insulin injection after the cellular therapy (defined as insulin-dependent patient). (A-C) All black circle points are P<0.05 vs. month 0 (pretreatment) for the insulin-free patients. (D) All black square points are P<0.01 vs. month 0 (pretreatment) for the insulin-dependent patients with the exception of * (month 3) which is P<0.05 vs. month 0 (pretreatment).
Evaluation of the feasibility and safety of MSC administration
Clinical and laboratory evaluations of the UCMSC-treated T2D patients revealed no mortality or cell-related adverse side effects. In addition, there was no immediate or delayed toxicity associated with UCMSC administration within the 24-month follow-up period. Results of the physiological examinations are presented in Table III. There was no positive detection with regard to the tested viruses, including HIV, HBV, HCV, CMV and syphilis, and no infections were observed during the follow-up period. Tumor markers were not found to be positively detectable following UCMSC transplantation (Table IV), and no tumor was identified via imaging examinations in any of the patients. Throughout the entire procedure, no immunosuppressive drugs were administered for the UCMSC transplantation and GVHD was not observed.
Table III.
Results of the predominant laboratory examination.
| Items | Baseline | Month 1 | Month 3 | Month 6 | Month 12 | Month 18 | Month 24 | P-value |
|---|---|---|---|---|---|---|---|---|
| WBC (×109/l) | 6.83±0.41 | 6.47±0.36 | 6.70±0.35 | 6.63±0.38 | 6.77±0.40 | 6.70±0.39 | 6.72±0.37 | >0.05 |
| N (×109/l) | 5.21±0.22 | 5.08±0.20 | 5.30±0.32 | 5.19±0.30 | 5.32±0.27 | 5.26±0.23 | 5.31±0.33 | >0.05 |
| L (×109/l) | 2.30±0.13 | 2.11±0.10 | 2.32±0.12 | 2.25±0.11 | 2.31±0.13 | 2.33±0.14 | 2.22±0.12 | >0.05 |
| Hb (g/l) | 132.1±2.9 | 133.3±2.7 | 130.6±2.5 | 134.1±2.8 | 132.8±2.6 | 134.3±2.2 | 133.5±3.0 | >0.05 |
| PLT (×109/l) | 218.8±14.2 | 220.6±10.7 | 217.3±12.1 | 212.9±11.1 | 222.3±14.4 | 220.7±13.5 | 221.9±11.2 | >0.05 |
| ALT (U/l) | 22.5±2.0 | 22.0±1.7 | 23.4±2.3 | 21.9±1.6 | 23.0±2.1 | 22.4±1.9 | 23.1±2.2 | >0.05 |
| AST (U/l) | 21.6±1.7 | 22.5±2.1 | 23.0±2.0 | 22.5±2.2 | 21.8±1.9 | 23.0±2.3 | 22.6±2.1 | >0.05 |
| BUN (mnol/l) | 5.23±0.18 | 5.30±0.13 | 4.97±0.16 | 4.99±0.17 | 5.05±0.18 | 5.20±0.15 | 4.98±0.14 | >0.05 |
| sCr (µmol/l) | 68.5±3.3 | 67.0±2.8 | 64.8±3.0 | 67.5±3.9 | 66.0±3.1 | 65.4±3.1 | 66.1±3.4 | >0.05 |
Data are presented as the mean ± standard error of the mean (n=6). WBC, white blood cell; N, neutrophilic granulocyte; L, lymphocytes; Hb, hemoglobin; PLT, platelet; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; sCr, serum creatinine.
Table IV.
Results of the blood tumor markers.
| Items | Baseline | Month 1 | Month 3 | Month 6 | Month 12 | Month 18 | Month 24 | P-value |
|---|---|---|---|---|---|---|---|---|
| AFP (ng/ml) | 4.52±0.50 | 4.61±0.42 | 4.31±0.33 | 4.67±0.40 | 3.94±0.26 | 4.11±0.44 | 4.45±0.39 | >0.05 |
| CEA (ng/ml) | 3.32±0.16 | 3.15±0.26 | 2.85±0.18 | 3.03±0.27 | 2.95±0.20 | 3.35±0.17 | 3.22±0.15 | >0.05 |
| CA125 (U/ml) | 18.5±1.5 | 17.4±1.3 | 18.0±1.6 | 16.9±1.8 | 17.8±1.5 | 18.1±1.7 | 16.8±1.2 | >0.05 |
| CA199 (U/ml) | 20.5±0.7 | 21.2±0.9 | 20.0±0.6 | 21.1±0.8 | 20.9±1.0 | 21.3±0.8 | 20.3±0.6 | >0.05 |
Data are presented as the mean ± standard error of the mean (n=6). AFP, α-fetoprotein; CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CA199, carbohydrate antigen 199.
Discussion
Diabetes has become a major cause of mortality in individuals aged <60 years (37). Type 2 diabetes (T2D) accounts for the majority of the diabetic population (>90%). Insulin resistance and defects in pancreatic β-cell function have been recognized as major pathophysiological abnormalities underlying the majority of T2D cases. T2D is a complex disorder that is affected by multiple genetic and environmental factors. Although complexity and heterogeneity exist in the pathogenesis of T2D, the disease is characterized by progressive and inexorable β-cell dysfunction, which subsequently leads to insulin deficiency. β-cell failure is central to the development and progression of T2D, particularly during the later stages of the disease (38). Insulin secretion decreases due to the glucotoxicity and lipotoxicity effects on pancreatic β-cells (39). Thus, intense research has focused on possible mechanisms to promote the expansion of existing pancreatic β-cells and β-cell development from either endogenous or exogenous bone marrow-residing stem cells (40,41). C-peptide levels are considered to be a critical indicator of the efficacy of novel therapies for T2D. Since genetic and environmental risk factors are involved in human T2D, transplantation of endogenous or exogenous stem cells may be beneficial for patients with diabetes (42,43).
A number of studies have suggested that bone marrow cells (BMCs) and mesenchymal stem cells (MSCs) are able to differentiate into islet β-cells and influence their regeneration in diabetic animals (23,44,45). Dong et al (23) reported that allogeneic MSC transplantation can reduce blood glucose levels in recipient rats. A relatively small number of transplanted MSCs survived and subsequently transdifferentiated into insulin-producing cells in the pancreas of the recipient rats. Furthermore, Banerjee et al (46) successfully treated streptozotocin (STZ)-induced diabetes with multiple infusions of unfractionated BMCs; however, the authors did not suggest a mechanism for the recovery. In the study by Lee et al (47), repeated transplantation with a high number of MSCs (2.5×106) induced the repair of pancreatic islets and renal glomeruli in non-obese diabetic/scid mice suffering from STZ-induced diabetes. Urbán et al (22) reported that cotransplantation of syngeneic unfractionated BMCs and syngeneic or allogeneic culture-expanded MSCs was able to reverse STZ-induced diabetes in mice; however, neither BMC nor MSC transplantation was effective alone. In 2008, Tsai et al reported that UCMSCs were capable of differentiating into pancreatic lineage cells in vitro and functioning as insulin-producing cells in vitro and in vivo. Furthermore, after the transplantation of differentiated cells into the portal vein of the diabetic rats using a Port-A catheter, blood sugar levels were shown to decrease. Insulin-producing cells containing human C-peptide and human nuclei were observed in the liver (48). In the study by Trivedi et al (49), five patients with type 1 diabetes mellitus were successfully infused with unfractionated cultured bone marrow plus human adipose-tissue-derived, insulin-making MSCs. There were not any untoward effects observed, and a 30–50% decrease in insulin requirements with a 4-26-fold increase in serum C-peptide levels was achieved, with a mean follow-up period of 2.9 months.
To the best of our knowledge, the present study was the first to use UCMSC-based therapy for T2D patients. Of the six patients included in the current study, three patients achieved insulin independence that remained controlled for a median time of 29 months, with the longest insulin dependence observed in one patient for 43 months ongoing. The insulin-free group presented an increase in C-peptide levels (fasting, C-peptide AUC and Cmax) for up to 2 years during the follow-up period. A marked increase in C-peptide production indicates an improved β-cell function. However, the six patients with T2D enrolled in the present study were heterogeneous, and a differential clinical response was identified with three patients continuing to require insulin injections, although C-peptide levels were found to increase in the first month after the UCMSC transplantation. However, these levels decreased quickly after the third month (Fig. 4A). Previous studies have demonstrated that the self-renewal, survival and differentiation abilities of stem cells are predominantly regulated by their microenvironment (12,50). In insulin-dependent patients, a long diabetic history, older age, lower serum C-peptide level at pretreatment and any other complications may indicate the presence of a less conducive microenvironment for the transplanted cells, leading to the short-term recovery of β-cell function, which impacts the treatment efficiency of UCMSC transplantation. Although a prolonged follow-up is required, the results of the present study indicate that an intravenous infusion of UCMSCs is beneficial for the restoration of β-cell function in patients with T2D.
In the insulin-free patients, the serum C-peptide levels were shown to continuously improve, which may have been due to a mild immune injury and a better microenvironment for the transplanted UCMSCs in these patients. The possible explanations for the success of this treatment are two-fold. Firstly, the transplanted UCMSCs may induce the regeneration of recipient-derived pancreatic insulin-secreting cells. Secondly, UCMSCs can inhibit T-cell-mediated immune responses against newly formed β-cells, which in turn, are able to survive in this altered immunological milieu (10,22,51). The administration of UCMSCs for the treatment of systemic lupus erythematosus has provided additional evidence for their immunoregulatory role (52), supporting their use in controlling autoimmunity and triggered inflammation. However, the mechanisms underlying the regenerative process and the appropriate conditions for UCMSC therapy in diabetes remain poorly understood. Furthermore, the efficacy of this treatment may be associated with the duration of diabetes, the microenvironment conditions, the remaining β-cells, the severity of complications, times of infusion and the UCMSC delivery method. Therefore, further well-controlled studies with an increased number of cases are required to clarify the efficacy and safety of UCMSC intravenous infusion for the treatment of T2D.
Patient safety and the potential benefit to risk ratio are always the foremost considerations in clinical practice. In the present study, a novel approach for the treatment of T2D was attempted using the transplantation of UCMSCs. Systemic follow-ups were undertaken to evaluate the safety and efficacy of UCMSC therapy in human subjects. The safety measurements indicated that UCMSC administration via intravenous infusion was well-tolerated at the indicated doses without immediate effects. During the follow-up period, there were no immunological reactions and tumor formation was not identified. Thus, the results indicated that the risk-benefit ratio of UCMSC-based therapy in T2D appears to be favorable. This observation is in accordance with previous studies performed by Shi et al (53) and Lv et al (54), where the results also suggested that transplantation with UCMSCs may be a safe approach. However, further observations of possible transplant complications are required.
In conclusion, the present clinical trial-based study transplanted allogeneic UCMSCs in patients with T2D. After two UCMSC infusions, all the studied patients exhibited a significant improvement in the diabetic status, as indicated by changes in the C-peptide and HbA1c levels. In addition, a reduction in the insulin requirement or insulin independence were achieved during the follow-up period. Cell infusion-related immediate and long-term side effects were not observed during the treatment. Therefore, the present study provided a novel cell therapeutic protocol for the treatment of T2D with allogeneic UCMSC transplantation, without the application of immunosuppressive drugs.
Acknowledgements
This study was supported by a grant from the Science and Technology Development Foundation of Weifang (no. 201102009). The authors thank Dr Chang-Shan Liu and Dr Lin Liu for their helpful suggestions and are grateful to Li Gao, Zhi-Cui Liu, Nan Jia, Feng-Fei Yu, Na Li and Gui-Lan Ma for their technical assistance.
References
- 1.Jung KY, Kim KM, Lim S. Therapeutic approaches for preserving or restoring pancreatic β-cell function and mass. Diabetes Metab J. 2014;36:426–436. doi: 10.4093/dmj.2014.38.6.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsen JL. Pancreas transplantation: indications and consequences. Endocr Rev. 2004;25:919–946. doi: 10.1210/er.2002-0036. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Lakey JR, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 5.Yamaoka T. Regeneration therapy of pancreatic beta cells: towards a cure for diabetes? Biochem Biophys Res Commun. 2002;296:1039–1043. doi: 10.1016/S0006-291X(02)02000-4. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis; Proc Natl Acad Sci USA; 2002; pp. 4397–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 9.Sharma RR, Pollock K, Hubel A, et al. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418–1437. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domínguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C. Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med. 2012;1:59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 13.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: The IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 14.Schächinger V, Erbs S, Elsässer A, et al. REPAIR-AMI Investigators: Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 15.Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/S1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Kim DY, Sung IY, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70:1238–1247. doi: 10.1227/NEU.0b013e31824387f9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZX, Guan LX, Zhang K, et al. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy. 2008;10:134–139. doi: 10.1080/14653240701883061. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang J, Zhang H, Hua B, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423–1429. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 21.Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 22.Urbán VS, Kiss J, Kovács J, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–253. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 23.Dong QY, Chen L, Gao GQ, et al. Allogeneic diabetic mesenchymal stem cells transplantation in streptozotocin-induced diabetic rat. Clin Invest Med. 2008;31:E328–E337. doi: 10.25011/cim.v31i6.4918. [DOI] [PubMed] [Google Scholar]
- 24.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K, Frassoni F, Ball L, et al. Developmental Committee of the European Group for Blood and Marrow Transplantation: Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 26.Carlin R, Davis D, Weiss M, et al. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4:8. doi: 10.1186/1477-7827-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torrente Y, Polli E. Mesenchymal stem cell transplantation for neurodegenerative diseases. Cell Transplant. 2008;17:1103–1113. doi: 10.3727/096368908787236576. [DOI] [PubMed] [Google Scholar]
- 28.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 29.Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joyce N, Annett G, Wirthlin L, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao YM. Medical ethics: unavoidable issue in medical research. Zhonghua Yi Xue Za Zhi. 2005;85:424–426. [PubMed] [Google Scholar]
- 32.Moniri MR, Sun XY, Rayat J, et al. TRAIL-engineered pancreas-derived mesenchymal stem cells: characterization and cytotoxic effects on pancreatic cancer cells. Cancer Gene Ther. 2012;19:652–658. doi: 10.1038/cgt.2012.46. [DOI] [PubMed] [Google Scholar]
- 33.Haffner SM, Stern MP, Hazuda HP, et al. Hyperinsulinemia in a population at high risk for non-insulin-dependent diabetes mellitus. N Engl J Med. 1986;315:220–224. doi: 10.1056/NEJM198607243150403. [DOI] [PubMed] [Google Scholar]
- 34.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Majore I, Moretti P, Stahl F, et al. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 2011;7:17–31. doi: 10.1007/s12015-010-9165-y. [DOI] [PubMed] [Google Scholar]
- 36.Tong CK, Vellasamy S, Tan BC, et al. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35:221–226. doi: 10.1042/CBI20100326. [DOI] [PubMed] [Google Scholar]
- 37.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halban PA, Polonsky KS, Bowden DW, et al. β-cell failure in type 2 diabetes: Postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–1758. doi: 10.2337/dc14-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison LB, Adams-Huet B, Raskin P, Lingvay I. β-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care. 2012;35:1406–1412. doi: 10.2337/dc11-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763–770. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 41.Nir T, Dor Y. How to make pancreatic beta cells-prospects for cell therapy in diabetes. Curr Opin Biotechnol. 2005;16:524–529. doi: 10.1016/j.copbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem. 2011;57:241–254. doi: 10.1373/clinchem.2010.157016. [DOI] [PubMed] [Google Scholar]
- 43.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous hematopoietic stem cell transplantation for type 1 diabetes. Ann N Y Acad Sci. 2008;1150:220–229. doi: 10.1196/annals.1447.048. [DOI] [PubMed] [Google Scholar]
- 44.Tang DQ, Cao LZ, Burkhardt BR, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–1732. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh SH, Muzzonigro TM, Bae SH, et al. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest. 2004;84:607–617. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- 46.Banerjee M, Kumar A, Bhonde RR. Reversal of experimental diabetes by multiple bone marrow transplantation. Biochem Biophys Res Commun. 2005;328:318–325. doi: 10.1016/j.bbrc.2004.12.176. [DOI] [PubMed] [Google Scholar]
- 47.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice; Proc Natl Acad Sci USA; 2006; pp. 17438–17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai PJ, Wang HS, Shyr YM, et al. Transplantation of insulin-producing cells from umbilical cord mesenchymal stem cells for the treatment of streptozotocin-induced diabetic rats. J Biomed Sci. 2012;19:47. doi: 10.1186/1423-0127-19-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trivedi HL, Vanikar AV, Thakker U, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc. 2008;40:1135–1139. doi: 10.1016/j.transproceed.2008.03.113. [DOI] [PubMed] [Google Scholar]
- 50.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 51.Cutler AJ, Limbani V, Girdlestone J, Navarrete CV. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J Immunol. 2010;185:6617–6623. doi: 10.4049/jimmunol.1002239. [DOI] [PubMed] [Google Scholar]
- 52.Liang J, Gu F, Wang H, et al. Mesenchymal stem cell transplantation for diffuse alveolar hemorrhage in SLE. Nat Rev Rheumatol. 2010;6:486–489. doi: 10.1038/nrrheum.2010.80. [DOI] [PubMed] [Google Scholar]
- 53.Shi M, Zhang Z, Xu R, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725–731. doi: 10.5966/sctm.2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cord-derived mesenchymal stem cells in autism. J Transl Med. 2013;11:196. doi: 10.1186/1479-5876-11-196. [DOI] [PMC free article] [PubMed] [Google Scholar]