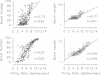
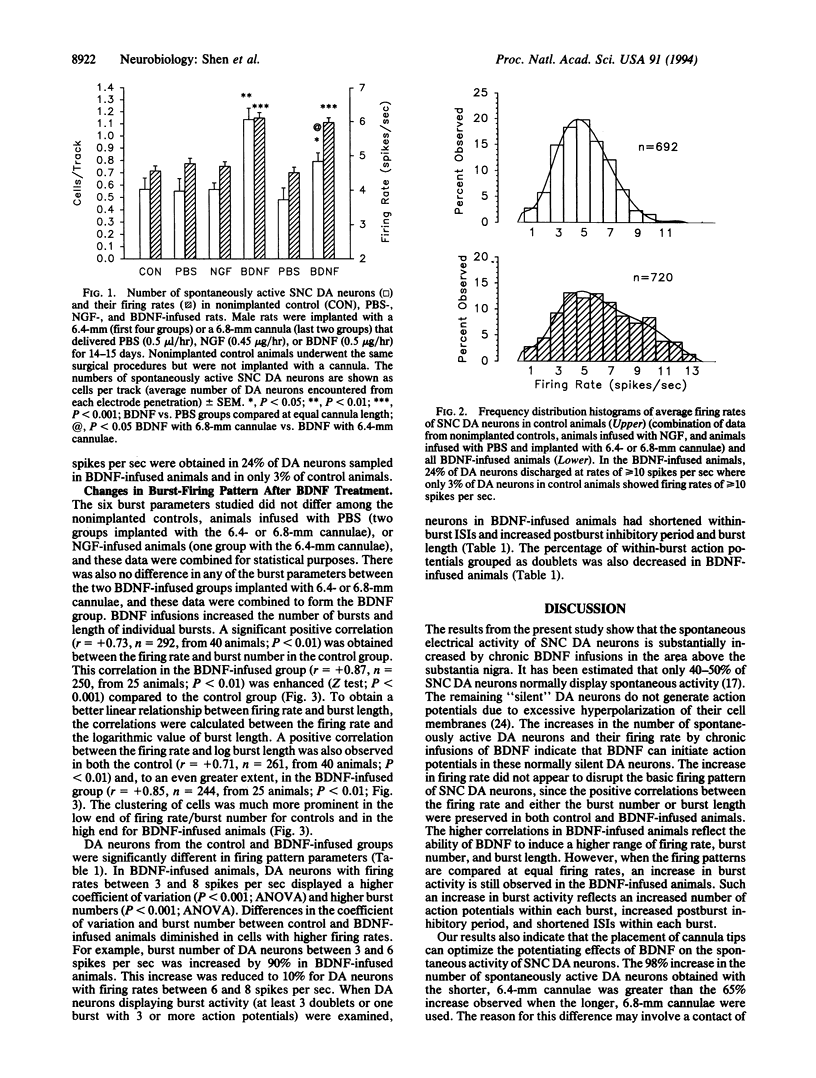
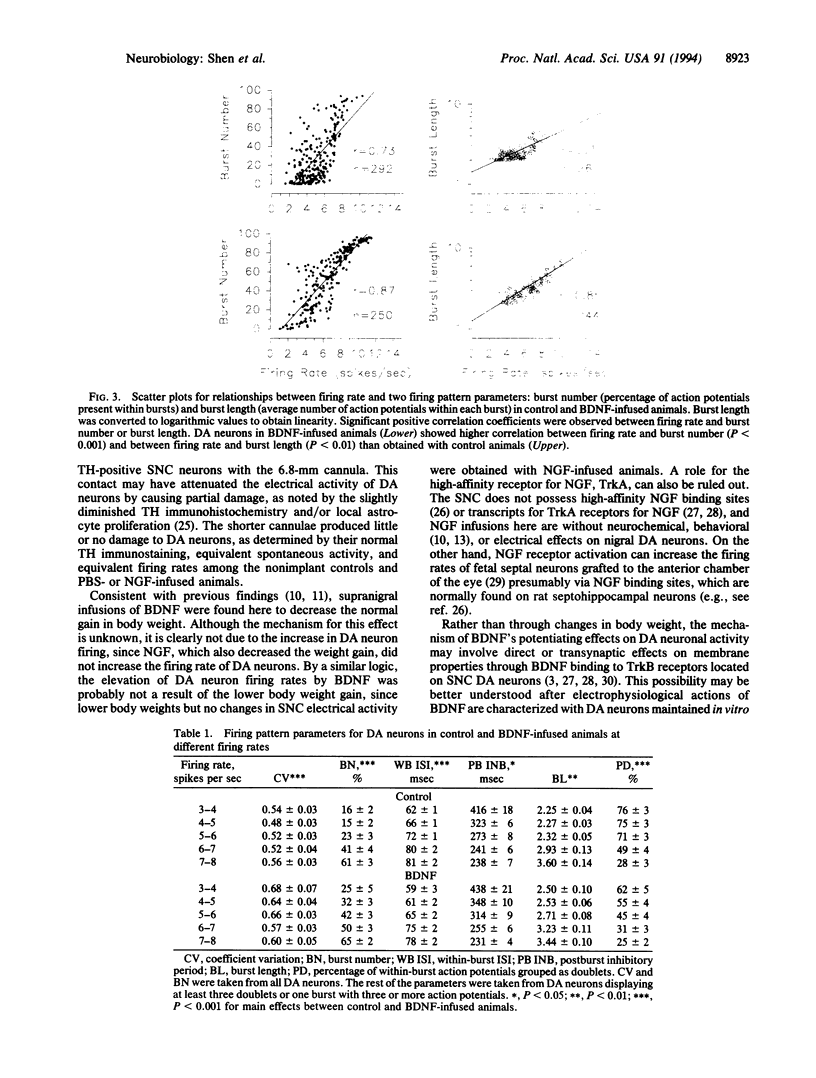
Abstract
Chronic infusions of brain-derived neurotrophic factor (BDNF) immediately above the substantia nigra augment spontaneous locomotion, rotational behavior, and striatal dopamine (DA) turnover, indicating that BDNF increases functions of the nigrostriatal DA system. Because the function of the nigrostriatal DA system is related to the electrical activity of DA neurons, we investigated the effect of BDNF on the electrical activity of DA neurons in the substantia nigra pars compacta in vivo. Chronic supranigral infusions of BDNF (12 micrograms/day), nerve growth factor (11 micrograms/day), or phosphate-buffered saline were started 2 weeks before the electrophysiological recordings. BDNF increased the number of spontaneously active DA neurons by 65-98%, increased the average firing rage by 32%, and increased the number of action potentials contained within bursts. Neither nerve growth factor nor phosphate-buffered saline infusions altered any of these properties relative to unoperated animals. In addition, extremely fast-firing DA neurons (> 10 spikes per sec) were commonly found only in the BDNF-infused animals. These results demonstrate neurotrophin effects on the electrical activity of intact central nervous system neurons in vivo and suggest that the increases in locomotor behavior and striatal dopamine turnover obtained during supranigral BDNF infusions may result from increases in the electrical activity of DA neurons.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acquas E., Carboni E., de Ree R. H., Da Prada M., Di Chiara G. Extracellular concentrations of dopamine and metabolites in the rat caudate after oral administration of a novel catechol-O-methyltransferase inhibitor Ro 40-7592. J Neurochem. 1992 Jul;59(1):326–330. doi: 10.1111/j.1471-4159.1992.tb08907.x. [DOI] [PubMed] [Google Scholar]
- Altar C. A., Boylan C. B., Jackson C., Hershenson S., Miller J., Wiegand S. J., Lindsay R. M., Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar C. A., Burton L. E., Bennett G. L., Dugich-Djordjevic M. Recombinant human nerve growth factor is biologically active and labels novel high-affinity binding sites in rat brain. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):281–285. doi: 10.1073/pnas.88.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck K. D., Knüsel B., Hefti F. The nature of the trophic action of brain-derived neurotrophic factor, des(1-3)-insulin-like growth factor-1, and basic fibroblast growth factor on mesencephalic dopaminergic neurons developing in culture. Neuroscience. 1993 Feb;52(4):855–866. doi: 10.1016/0306-4522(93)90534-m. [DOI] [PubMed] [Google Scholar]
- Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973 Dec;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Chiodo L. A., Bunney B. S. Population response of midbrain dopaminergic neurons to neuroleptics: further studies on time course and nondopaminergic neuronal influences. J Neurosci. 1987 Mar;7(3):629–633. doi: 10.1523/JNEUROSCI.07-03-00629.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo L. A., Bunney B. S. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983 Aug;3(8):1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo L. A. Dopamine-containing neurons in the mammalian central nervous system: electrophysiology and pharmacology. Neurosci Biobehav Rev. 1988 Spring;12(1):49–91. doi: 10.1016/s0149-7634(88)80073-3. [DOI] [PubMed] [Google Scholar]
- Davies J., Dray A. Substance P in the substantia nigra. Brain Res. 1976 May 14;107(3):623–627. doi: 10.1016/0006-8993(76)90150-5. [DOI] [PubMed] [Google Scholar]
- Gonon F. G., Buda M. J. Regulation of dopamine release by impulse flow and by autoreceptors as studied by in vivo voltammetry in the rat striatum. Neuroscience. 1985 Mar;14(3):765–774. doi: 10.1016/0306-4522(85)90141-1. [DOI] [PubMed] [Google Scholar]
- Grace A. A., Onn S. P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989 Oct;9(10):3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace A. A. Regulation of spontaneous activity and oscillatory spike firing in rat midbrain dopamine neurons recorded in vitro. Synapse. 1991 Mar;7(3):221–234. doi: 10.1002/syn.890070307. [DOI] [PubMed] [Google Scholar]
- Hommer D. W., Pert A. The actions of opiates in the rat substantia nigra: an electrophysiological analysis. Peptides. 1983 Sep-Oct;4(5):603–608. doi: 10.1016/0196-9781(83)90004-9. [DOI] [PubMed] [Google Scholar]
- Hyman C., Juhasz M., Jackson C., Wright P., Ip N. Y., Lindsay R. M. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J Neurosci. 1994 Jan;14(1):335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Aghajanian G. K. Pertussis toxin blocks autoreceptor-mediated inhibition of dopaminergic neurons in rat substantia nigra. Brain Res. 1987 May 12;411(1):139–143. doi: 10.1016/0006-8993(87)90690-1. [DOI] [PubMed] [Google Scholar]
- Knüsel B., Winslow J. W., Rosenthal A., Burton L. E., Seid D. P., Nikolics K., Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W., Felix D., Lienhart R., Hefti F. A quantitative correlation between single unit activity and fluorescence intensity of dopamine neurones in zona compacta of substantia nigra, as demonstrated under the influence of nicotine and physostigmine. Brain Res. 1976 Nov 19;117(1):85–103. doi: 10.1016/0006-8993(76)90558-8. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Wiegand S. J., Altar C. A., DiStefano P. S. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994 May;17(5):182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Lohof A. M., Ip N. Y., Poo M. M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993 May 27;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Maisonpierre P. C., Belluscio L., Squinto S., Ip N. Y., Furth M. E., Lindsay R. M., Yancopoulos G. D. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990 Mar 23;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson M. T., Todd K. G., Altar C. A. Brain-derived neurotrophic factor and neurotrophin-3 activate striatal dopamine and serotonin metabolism and related behaviors: interactions with amphetamine. J Neurosci. 1994 Mar;14(3 Pt 1):1262–1270. doi: 10.1523/JNEUROSCI.14-03-01262.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlio J. P., Ernfors P., Jaber M., Persson H. Molecular cloning of rat trkC and distribution of cells expressing messenger RNAs for members of the trk family in the rat central nervous system. Neuroscience. 1992 Dec;51(3):513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- Murrin L. C., Morgenroth V. H., 3rd, Roth R. H. Dopaminergic neurons: effects of electrical stimulation on tyrosine hydroxylase. Mol Pharmacol. 1976 Nov;12(6):1070–1081. [PubMed] [Google Scholar]
- Nowycky M. C., Walters J. R., Roth R. H. Dopaminergic neurons: effect of acute and chronic morphine administration on single cell activity and transmitter metabolism. J Neural Transm. 1978;42(2):99–116. doi: 10.1007/BF01675349. [DOI] [PubMed] [Google Scholar]
- Palmer M. R., Eriksdotter-Nilsson M., Henschen A., Ebendal T., Olson L. Nerve growth factor-induced excitation of selected neurons in the brain which is blocked by a low-affinity receptor antibody. Exp Brain Res. 1993;93(2):226–230. doi: 10.1007/BF00228389. [DOI] [PubMed] [Google Scholar]
- Sanghera M. K., Trulson M. E., German D. C. Electrophysiological properties of mouse dopamine neurons: in vivo and in vitro studies. Neuroscience. 1984 Jul;12(3):793–801. doi: 10.1016/0306-4522(84)90171-4. [DOI] [PubMed] [Google Scholar]
- Shepard P. D., German D. C. Electrophysiological and pharmacological evidence for the existence of distinct subpopulations of nigrostriatal dopaminergic neuron in the rat. Neuroscience. 1988 Nov;27(2):537–546. doi: 10.1016/0306-4522(88)90287-4. [DOI] [PubMed] [Google Scholar]
- Shults C. W., Matthews R. T., Altar C. A., Hill L. R., Langlais P. J. A single intramesencephalic injection of brain-derived neurotrophic factor induces persistent rotational asymmetry in rats. Exp Neurol. 1994 Feb;125(2):183–194. doi: 10.1006/exnr.1994.1023. [DOI] [PubMed] [Google Scholar]
- Snyder G. L., Zigmond M. J. The effects of L-dopa on in vitro dopamine release from striatum. Brain Res. 1990 Feb 5;508(2):181–187. doi: 10.1016/0006-8993(90)90394-q. [DOI] [PubMed] [Google Scholar]
- Spina M. B., Squinto S. P., Miller J., Lindsay R. M., Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J Neurochem. 1992 Jul;59(1):99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chiodo L. A., Freeman A. S. Electrophysiological effects of MK-801 on rat nigrostriatal and mesoaccumbal dopaminergic neurons. Brain Res. 1992 Sep 11;590(1-2):153–163. doi: 10.1016/0006-8993(92)91091-r. [DOI] [PubMed] [Google Scholar]
- Zigmond M. J., Abercrombie E. D., Berger T. W., Grace A. A., Stricker E. M. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990 Jul;13(7):290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]