Abstract
Background
The mechanisms by which volatile anaesthetics such as isoflurane alter neuronal function are poorly understood, in particular their presynaptic mechanisms. Presynaptic voltage-gated sodium channels (Nav) have been implicated as a target for anaesthetic inhibition of neurotransmitter release. We hypothesize that state-dependent interactions of isoflurane with Nav lead to increased inhibition of Na+ current (INa) during periods of high-frequency neuronal activity.
Methods
The electrophysiological effects of isoflurane, at concentrations equivalent to those used clinically, were measured on recombinant brain-type Nav1.2 expressed in ND7/23 neuroblastoma cells and on endogenous Nav in isolated rat neurohypophysial nerve terminals. Rate constants determined from experiments on the recombinant channel were used in a simple model of Nav gating.
Results
At resting membrane potentials, isoflurane depressed peak INa and shifted steady-state inactivation in a hyperpolarizing direction. After membrane depolarization, isoflurane accelerated entry (τcontrol=0.36 [0.03] ms compared with τisoflurane=0.33 [0.05] ms, P<0.05) and slowed recovery (τcontrol=6.9 [1.1] ms compared with τisoflurane=9.0 [1.9] ms, P<0.005) from apparent fast inactivation, resulting in enhanced depression of INa, during high-frequency stimulation of both recombinant and endogenous nerve terminal Nav. A simple model of Nav gating involving stabilisation of fast inactivation, accounts for this novel form of activity-dependent block.
Conclusions
Isoflurane stabilises the fast-inactivated state of neuronal Nav leading to greater depression of INa during high-frequency stimulation, consistent with enhanced inhibition of fast firing neurones.
Keywords: anaesthetics, general; isoflurane; presynaptic terminals; voltage-gated sodium channels
Editor's key points.
The mechanisms of the effects of isoflurane on neuronal function are unclear.
Isoflurane may inhibit neurotransmitter release through effects on presynaptic voltage gated sodium channels (Nav).
The effects of isoflurane were measured in Nav in neuroblastoma cells and in isolated rat nerve terminals.
Isoflurane stabilized the fast inactivated state of neuronal Nav.
This was consistent with increased inhibition of fast firing neurones.
General anaesthesia is a composite pharmacological state of amnesia, unconsciousness, and immobility. The molecular pharmacology of this state involves multiple target proteins, prominently including ligand-gated and voltage-gated ion channels.1–3 Optimization of anaesthetic drug design and clinical use requires detailed understanding of the roles of specific targets involved in the therapeutic (amnesia, unconsciousness, immobility) and undesirable (cardiovascular and respiratory depression, neurotoxic) effects of various anaesthetics.
A role for voltage-gated Na+ channels (Nav) in the modulation of neurotransmission by general anaesthetics, is supported by evidence that presynaptic blockade of Na+ current (INa) contributes to suppression of the release of multiple neurotransmitters, by volatile anaesthetics (VAs).4–6 The widely used anaesthetic isoflurane blocks multiple subtypes of Nav,7–11 and reduces action potential amplitude in rat hippocampal neurones12 and isolated rat neurohypophysial terminals.13 Moreover, intrathecal delivery of the highly specific Nav inhibitor tetrodotoxin (TTX) in adult rats enhances isoflurane potency in producing immobilization, a primarily spinal cord-mediated effect, whereas the Nav activator veratridine reduces isoflurane potency and antagonizes the effect of TTX.14
Inhibition of Nav by diverse compounds, including local anaesthetics, anti-arrhythmic drugs, antiepileptic drugs, and neurotoxins, is state-dependent15–17: their ability to interact with or bind to an ion channel is determined by the conformational state of the channel (resting - open - inactivated). The state of the channel is in part governed by membrane potential. State-dependent inhibition of Nav by isoflurane is supported by the observations that isoflurane inhibition is voltage-dependent, and is characterised by enhanced inactivation and delayed recovery from inactivation, which is consistent with stabilisation of fast inactivation.8,10,18 If isoflurane stabilises the fast-inactivated state, block of Nav should increase with repeated stimulation at frequencies high enough for fast-inactivated channels to accumulate, contributing to overall block of Nav. Block of Nav at clinical concentrations of general anaesthetics was previously considered too modest to be physiologically relevant.3 However, this conclusion was based on studies of tonic block which would be insensitive to possible ‘activity-dependent block’ and would therefore underestimate the magnitude of isoflurane effects on Nav at more physiologically relevant fast firing frequencies.
We examined activity-dependent block of isoflurane on Nav1.2, the principal neuronal Nav subtype, and endogenous rat neuronal Nav, using high-frequency stimulation protocols to elucidate the underlying kinetic mechanism. We show that isoflurane stabilises the fast-inactivated state of Nav, resulting in a novel form of activity-dependent anaesthetic block. This effect contributes significantly to overall block of INa and supports a role for Nav inhibition in presynaptic anaesthetic action, through reduction of presynaptic action potential amplitude and consequent neurotransmitter release.19
Methods
Anaesthetic solutions
External bath solutions were saturated with isoflurane (12–12.5 mM; Abbott Laboratories, North Chicago, IL USA) and diluted to final concentrations of 0.45–0.5 mM in gas-tight glass syringes. Isoflurane solutions were perfused using a pressure driven microperfusion system (ALA BPS-8; ALA Scientific, Westbury, NY USA), positioned 100–150 µm away from the cell. Concentrations of isoflurane sampled at the perfusion pipette tip were measured using a Shimadzu GC-2010 Plus gas chromatograph (Shimadzu, Tokyo, Japan), after extraction into octane (1:1 v/v), and reflected ∼10% loss from the syringe to the bath.11
Cell culture and Nav transfection of ND7/23 cells
Neuroblastoma ND7/23 cells (Sigma-Aldrich, St. Louis, MO USA) were plated on 12-mm glass coverslips and incubated in a humidified atmosphere at 37°C in 5% CO2, in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum, 2 mM L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (all reagents from Sigma-Aldrich unless specified).
Wild-type rat Nav1.2a (accession number NM_012647) was kindly provided by William Catterall (University of Washington, Seattle, USA). TTX-resistance was engineered into Nav1.2a by site-directed mutagenesis (F385S)20 (referred to as Nav1.2R) to allow isolation from endogenous channels and expression in a neuronal background, which is crucial to measuring the effect of isoflurane on Nav in heterologous expression systems.11 Cells were transiently transfected with Nav1.2R and pEGFP-N1 (Clontech, Mountain View, CA USA) cDNA using Lipofectamine LTX (Invitrogen, Carlsbad, CA USA) to allow identification of transfected cells by eGFP fluoresence imaging. Experiments were performed in the presence of 250 nM TTX (Sankyo Kasei, Tokyo, Japan) to block endogenous INa.
Electrophysiological recording of ND7/23 cells
Whole-cell patch-clamp experiments were performed at room temperature (23–24°C) using an Axopatch 200B amplifier (Axon Instruments, Burlingame, CA USA), digitized via a Digidata 1321A interface, and analysed using pClamp 10.2 software (Axon Instruments). Whole-cell currents were sampled at 50 kHz and low-pass filtered at 5 kHz. Whole-cell seal resistance was 2–8 GΩ before patch rupture. Pipette resistance was 1.5–2.5 MΩ when filled with internal solution containing (in mM): 120 CsF, 10 NaCl, 10 HEPES, 10 EGTA, 10 TEA-Cl, 1 CaCl2, and 1 MgCl2 and adjusted to pH 7.3 (with CsOH) and 310 mOsm kg−1 H2O. External solution contained (in mM): 130 NaCl, 10 HEPES, 3.25 KCl, 2 MgCl2, 2 CaCl2, 20 TEA-Cl, 5 d-glucose, 0.00025 TTX and was adjusted to pH 7.4 with NaOH and 310 mOsm kg−1 H2O with sucrose. The liquid–junction potential (∼7.8 mV) was not corrected.
Only cells expressing 2–8 nA of peak current were analysed in order to minimize space clamp and series resistance errors. Capacitive transients were electronically cancelled and voltage error was minimized using 70–80% series resistance compensation. Series resistance was typically 2–4 MΩ and data were discarded if >10 MΩ. Experiments began 5 min after attaining whole-cell patch to allow equilibration of the pipette solution with the cytosol. Voltage protocols were applied from a holding potential (Vh) of −70 or −90 mV with 5-s intervals between sweeps. Protocols were applied in control solution and again after 5 min perfusion with isoflurane. Perfused cells showed stable responses (rundown <10%) for up to 5 min in control experiments (data not shown). Linear leak currents were subtracted using the P/4 method.21
Electrophysiological recording of isolated nerve terminals
Animal protocols were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College and relevant aspects of the ARRIVE guidelines and in the AVMA Guidelines for Euthanasia of Animals (https://www.avma.org/KB/Policies/Documents/euthanasia.pdf). Neurohypophysial nerve terminals were prepared as described9 with minor modifications. Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA USA) were anaesthetized by slowly replacing the air in their cage with regulated inflow from a tank of 80 CO2/20% O2 to avoid hypoxaemia and exposure to potent anaesthetics. Upon loss of righting reflex, animals were swiftly killed by decapitation. Animals showed no signs of distress with this technique. The neurohypophysis was removed and gently homogenized in 270 mM sucrose, 10 mM HEPES, and 0.01 mM K-EGTA, pH 7.25, using a 0.5-ml Teflon/glass homogenizer. The homogenate was pipetted into a plastic 35-mm Petri dish and allowed to settle for 5–8 min.
Dissociated nerve terminals were superfused with modified Locke's solution consisting of (in mM) 145 NaCl, 5 KCl, 2.2 CaCl2, 1 MgCl2, 10 HEPES, and 2 d-glucose, pH 7.3 with NaOH. Large terminals (11–16 µm diameter), identified by their bright refraction, were selected for study. An amphotericin B-perforated patch-clamp technique was used to reduce rundown of INa, that occurs with whole-terminal patch-clamp. Pipette tips were fire-polished and coated with SYLGARD (Dow Corning Corporation, Midland, MI USA) to reduce background noise and pipette capacitance. Pipette resistance was 3–7 MΩ, and seal resistance was 1–5 GΩ. Pipettes were filled with a solution containing (in mM) 10 NaCl, 135 Cs-glutamate, 2 CaCl2, 1 MgCl2, 10 HEPES, 5 d-glucose, 10 TEACl and 300–350 µg ml−1 amphotericin B, pH 7.25 with CsOH.
Gating model simulation and parameter estimation
A three-state Markov gating model was designed in MATLAB v7.5 (The MathWorks, Natick, MA USA) by solving the matrix equation X (t)=eQ(t) · X(0), where X(t) is a 3×1—state variable vector indicating the probability of resting (R), open (O), and the fast-inactivated (IF) states at time t, X(0) is the initial state vector at time 0, and Q(t)=the 3×3—state transition matrix of rate constants governing the transition rates between all connected states, given the R-O-IF gating scheme. Simulation paradigms involved two periods, or a repetitive sequence of these periods when addressing pulse trains, where membrane voltage was initially Vh followed by a depolarized pulse triggering channel activation. Parameter estimation used a least-squares method (MATLAB Optimization Toolbox 4.1; The MathWorks).
Statistical analysis
Data were analysed using Prism v6.05 (Graph-Pad Software Inc., San Diego, CA USA) and SigmaPlot 6.0 (SPSS Science Software Inc., Chicago, IL USA). Conductance (G) values were derived from the I−V relationship using the equation G=I/(V−Vrev), where I is the peak INa at a given voltage (V) and Vrev is the measured Na+ reversal potential. Voltages at half-maximal activation (V½act) were obtained from fitting the data for each cell to a Boltzmann equation of the form G/Gmax=1/[1+exp(V½act−V/k)], where G/Gmax is the normalized fractional conductance and k is the slope factor. The voltage at which fast inactivation is half-maximal (V½) was measured by fitting normalized steady-state INa values to a Boltzman function, of the form INa/INamax=1/[1+exp(V½−V/k)]. Time course data were fitted to the mono-exponential function Y=exp(−τ*n)+AP, where τ is the time constant, AP is the plateau and n is stimulus number. To determine the kinetics of macroscopic inactivation the decay phase of the current trace was fit with a bi-exponential equation, of the form A1 · exp(–t/τ1) +A2 · exp(−t/τ2)+B, where An is the nth component amplitude, B is the plateau, t is time and τn are time constants.
Data are expressed as mean and standard deviation (sd), and were analysed using two-tailed paired Student's t-test or ANOVA with post hoc testing as indicated, with statistical significance set as P=0.05.
Results
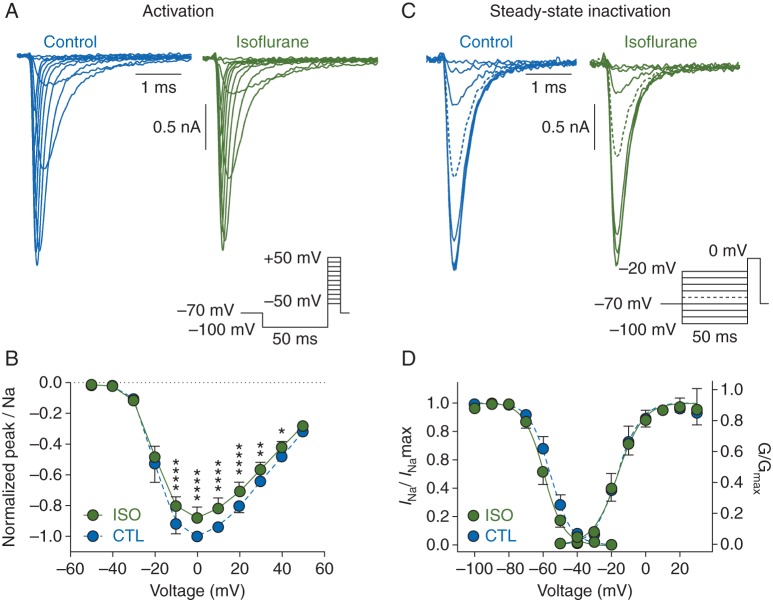
We first measured inhibition of peak Na+ current (INa) by a clinically relevant concentration of isoflurane. Fig. 1a shows whole-cell Nav1.2R currents recorded from ND7/23 cells in the absence or presence of isoflurane at a concentration that produces anaesthesia in rats [0.42 mM; equivalent to ∼1.5 times MAC (minimum alveolar concentration of anaesthetic required to abolish movement upon a painful stimulus in 50% of subjects)].22 Current was activated by a series of voltage steps from −50 to +50 mV preceded by a 50-ms prepulse to −100 mV to relieve channel inactivation. Isoflurane inhibited peak INa at test potentials of −10–+40 mV [n=7, *P<0.05, two-way (voltage x drug) ANOVA with Sidak's multiple comparisons test], without altering the current-voltage (I−V) relationship; maximum INa for both control and isoflurane conditions occurred at 0 mV (Fig. 1b).
Fig 1.
Effects of isoflurane on activation and inactivation properties of brain-type sodium channel Nav1.2R in a neuronal cell line. (a) Representative whole-cell Nav1.2R current traces in the absence (left) or presence (right) of 1.5 MAC isoflurane using the protocol in inset. (b) Normalized peak current (INa) plotted against test potential in the absence (CTL) or presence (ISO) of isoflurane [mean (sd), n=7, *P<0.05, **P<0.01, ****P<0.0001 compared with respective control value by two-way ANOVA]. (c) Representative current traces in the absence (left) or presence (right) of isoflurane using the protocol in inset to measure steady-state fast inactivation (prepulse to −60 mV indicated with a dashed line). (d) Steady-state fast inactivation (n=6), where INa at 0 mV was normalized to the maximum INa for each condition (INa/INamax) and normalized conductance (G/Gmax) values [mean (sd), n=7] were plotted against the voltage command and fit with a Boltzmann function.
The effect of isoflurane on steady-state inactivation was determined by eliciting currents at 0 mV after a 50-ms prepulse to voltages from −100 to −20 mV (Fig. 1c). Normalized INa/INamax values reflected the fraction of channels inactivated during the prepulse. As isoflurane alters the voltage-dependence of Nav1.2 gating,8–10 we plotted conductance (G) and steady-state inactivation against voltage (Fig. 1d). Isoflurane shifted the voltage-dependence of steady-state inactivation in a hyperpolarizing direction [V½ control= − 55.5 (2.1) mV; V½ isoflurane= − 59.3 (2.0) mV, n=6, P<0.001, by paired Student's t-test]. The voltage-dependence of activation was not affected [V½act control= − 15.3 (2.7) mV; V½act isoflurane= − 15.4 (2.0) mV, n=6, n.s.]. These data suggest that isoflurane inhibits peak INa by increasing the fraction of inactivated channels at normal resting membrane potentials.
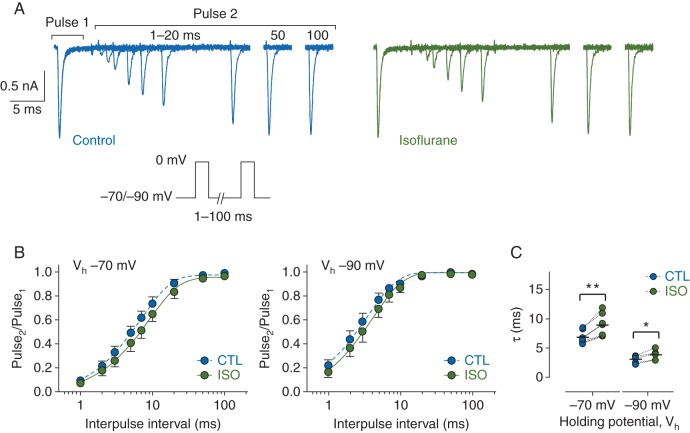
As neuronal firing frequency depends in part on how fast Nav can cycle through their various conformational states, we measured the time-course of recovery from inactivation (Fig. 2). Peak INa was recorded in response to two 5-ms pulses to 0 mV, where the duration between the two pulses was varied. Recovery time-courses were fit with a mono-exponential function in both control and isoflurane conditions, indicating that channels predominantly entered a single ‘fast’-inactivated state and not any ‘slow’-inactivated states. Isoflurane increased the time required for full channel recovery at a hyperpolarized Vh of −90 mV [τcontrol=3.1 (0.6) ms; τisoflurane=3.9 (0.8) ms, n=6, P<0.05 by paired Student's t-test]; this effect was enhanced at more physiological Vh [recovery at −70 mV, τcontrol=6.9 (1.1) ms; τisoflurane=9.0 (1.9) ms, n=7, P<0.005 by paired Student's t-test] (Fig. 2b and c).
Fig 2.
Effects of isoflurane on recovery of brain-type sodium channel Nav1.2R from fast inactivation. (a) Families of whole-cell Nav1.2R current traces recorded in the absence (left) or presence (right) of 1.5 MAC isoflurane and evoked from a Vh of −70 mV using a paired-pulse protocol where the duration between two 5-ms pulses to 0 mV was varied from 1–100 ms (protocol in inset). (b) Normalized peak current (Pulse2/Pulse1) was plotted against duration of the interpulse interval for control (CTL) and isoflurane (ISO) from a Vh of −70 mV (n=7) or −90 mV (n=6). Data are presented as mean [sd] and fit with a mono-exponential function. (c) Time constants (τ) determined from mono-exponential fits of data from individual cells in B in the absence (CTL) or presence (ISO) of isoflurane from a Vh of −70 (left) or −90 mV (right) (*P<0.05, **P<0.005; by two-tailed, paired Student's t-test).
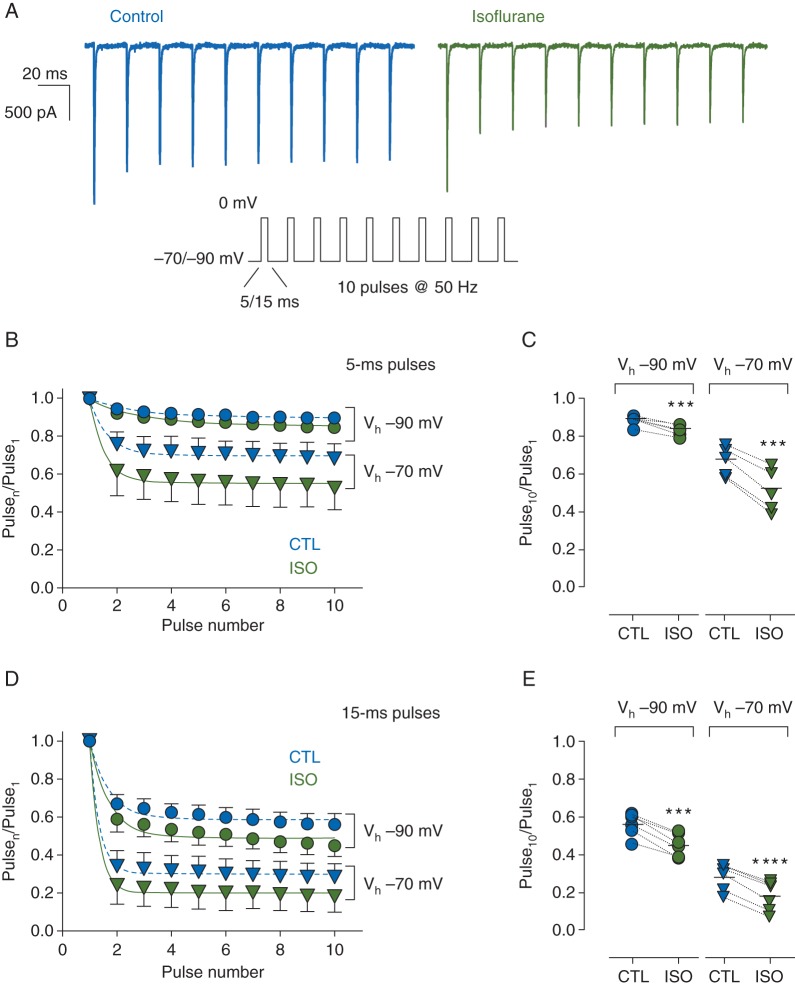
We posited that this delay in recovery from inactivation after membrane depolarization would lead to progressive inhibition of INa during trains of action potentials. We tested this by administering depolarizing stimuli at 50 Hz (Fig. 3a), normalizing INa of each pulse to that of the first pulse (Pulsen/Pulse1) to remove the effect of resting block by isoflurane (Fig. 3b and d). The reduced INa at the 10th pulse was thus ‘activity-dependent’ as a result of repeated membrane depolarization. For 5-ms pulses delivered at 50 Hz, from a Vh of −90 mV, isoflurane reduced the fraction of current at Pulse10 (Pulse10/Pulse1) from 0.90 [0.03] to 0.85 [0.03] (n=6, P<0.001). From a Vh of −70 mV, isoflurane reduced Pulse10/Pulse1 from 0.68 [0.08] to 0.53 [0.11] (n=5, P<0.0005) (Fig. 3c). For 15-ms pulses delivered at 50 Hz from a Vh of −90 mV, isoflurane reduced Pulse10/Pulse1 from 0.56 [0.06] to 0.45 [0.06] (n=6, P<0.0005) and from a Vh of −70 mV, isoflurane reduced Pulse10/Pulse1 from 0.28 [0.07] to 0.18 [0.08] (n=6, P<0.0001) (Fig. 3e). Activity-dependent block was not seen in time control experiments, which used a mock perfusion to ensure the reduction in INa was as a result of isoflurane block and not experimental time (data not shown).
Fig 3.
Effects of isoflurane on brain-type sodium channel Nav1.2R expressed in a neuronal cell line stimulated at high-frequency. (a) Representative current traces in response to a 50 Hz train of 5-ms depolarizing pulses to 0 mV from a Vh of −70 mV in the absence (left) or presence (right) of 1.5 MAC isoflurane (protocol in inset). (b) Peak INa elicited by a 50 Hz train of 5-ms pulses in the absence (CTL) or presence (ISO) of isoflurane from a Vh of −70 mV (triangles, n=5) or −90 mV (circles, n=6) normalized to the first pulse of the train (Pulsen/Pulse1). Mean [sd] data were fit to a mono-exponential function. (c) Normalized INa at Pulse10 for each cell in b. (d) Similar experiments as B but with 15-ms depolarizing pulses from a Vh of −70 mV (n=6) or −90 mV (n=6). (e) Normalized INa at Pulse10 for each cell in d. Dotted lines denote paired data. ***P<0.001, ****P<0.0001 by two-tailed, paired Student's t-test.
Block was increased by more depolarized Vh and shorter recovery intervals, conditions that promote inactivation, implicating stabilisation of fast-inactivation as the mechanism of activity-dependent block. This mechanism predicts that higher frequency stimulation will enhance channel block. To test this prediction we examined the pulse train responses with 100 Hz frequency (Vh= − 90 mV, 5-ms pulses, data not shown), in control and isoflurane which we compared with those at the 50 Hz frequency (Fig. 3b). To focus on the fractional block of channels available for opening in control at near steady-state we applied the following calculation: [fractional block=(1− (ISO P10)/(CTL P10))]. Increasing the stimulation frequency from 50 to 100 Hz more than doubled the fractional block by isoflurane [50 Hz, 0.058 (0.018); 100 Hz, 0.137 (0.034), n=6, P<0.001, data not shown]. The results provide additional support for the activity-dependent block mechanism.
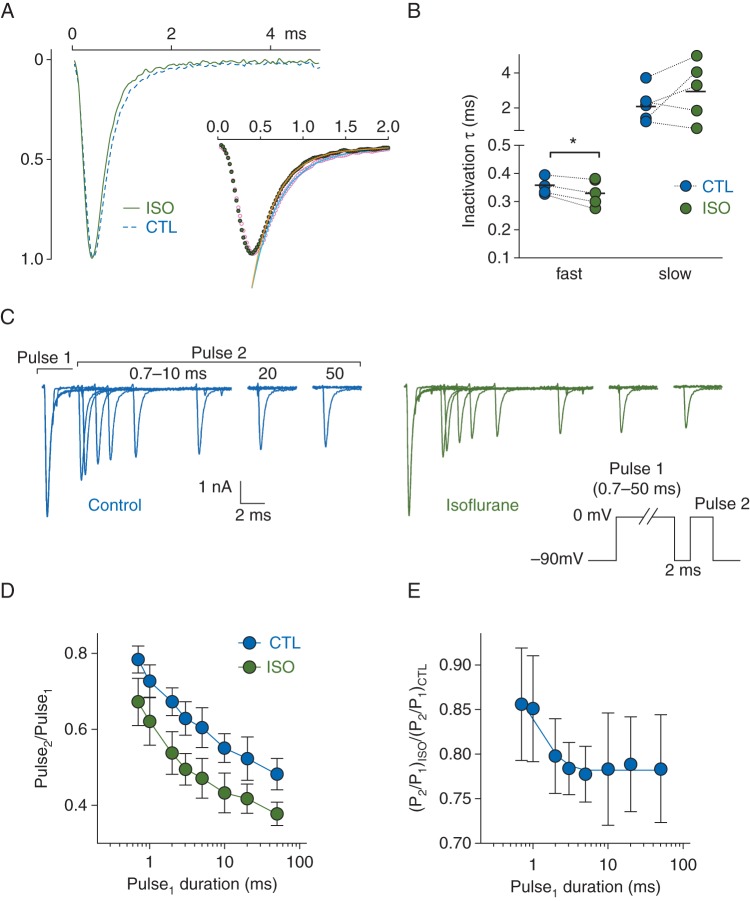
We measured the effect of isoflurane on the kinetics of inactivation by comparing the rates of macroscopic INa decay. We normalized current traces to peak INa of paired control and isoflurane experiments and fit the decay phases with a double exponential function (Fig. 4a, inset). The majority of the decay phase (∼95%) could be described by the fast time constant (τ). Isoflurane reduced the fast τ from 0.36 [0.03] ms in control to 0.33 [0.05] ms with isoflurane (n=5, P<0.05, paired Student's t-test). Isoflurane had little effect on the slow time constant (2.11 [0.99] for control; 2.93 [1.67] for isoflurane, n=5, n.s.) (Fig. 4b). These results are summarized in Supplementary Table S1 and are consistent with isoflurane enhancing fast inactivation or promoting a unique inhibited state with entry rates similar to that of fast inactivation.
Fig 4.
Acceleration by isoflurane of inactivation in brain-type sodium channel Nav1.2R expressed in a neuronal cell line. (a) Representative responses to a 5-ms stimulus pulse to 0 mV from a Vh of −70 mV for control (CTL) or 1.5 MAC isoflurane (ISO) treated cells normalized to the peak of CTL. Inset shows a bi-exponential fit of the decay phase for CTL (blue line) and ISO (orange line). (b) Slow and fast τ derived from bi-exponential fitting of individual cells in the absence (CTL) or presence (ISO) of 1.5 MAC isoflurane. Dotted lines denote paired data. (*P<0.05 by two-tailed, paired Student's t-test). (c) Representative current traces recorded in the absence (left) or presence (right) of 1.5 MAC isoflurane using a two-pulse protocol (inset) to investigate onset of isoflurane inhibition. From a Vh of −90 mV, cells were stimulated with two pulses to 0 mV with the duration of the first pulse varied from 0.7–50 ms. (d) Peak INa of the test pulse (Pulse2) was normalized to that of the conditioning pulse (Pulse1) to yield fractional INa (Pulse2/Pulse1). Mean [sd] fractional INa (n=8) plotted vs Pulse1 duration. (e) Fractional INa [mean (sd), n=8] for ISO normalized to that of CTL to determine the onset of isoflurane inhibition fitted with a mono-exponential function.
We investigated the onset of isoflurane inhibition using a double-pulse protocol (Fig. 4c). Conditioning pulse durations (Pulse 1) greater than 3 ms were used, which typically produce complete channel inactivation. Consequently, the protocol briefly (2 ms) returns to Vh to allow partial recovery of fast-inactivated and inhibited channels, which inversely reflect the degree of inactivation and inhibition induced by Pulse 1. In control conditions, longer Pulse 1 durations reduced fractional INa (Pulse 2 normalized to Pulse 1), reflecting a growing population of inactivated channels that fail to fully recover between pulses. Isoflurane depressed fractional INa for all Pulse 1 durations relative to control (Fig. 4d). To investigate the time course of inhibition onset, we normalized the isoflurane response to that of control (Fig. 4e). The response reached an apparent plateau at around 10 ms and was well fit by a mono-exponential function with kinetics [τ=2.43 (1.62) ms, AP=0.76 (0.04), n=8], comparable with fast inactivation. These findings further support the involvement of fast inactivation in isoflurane action.
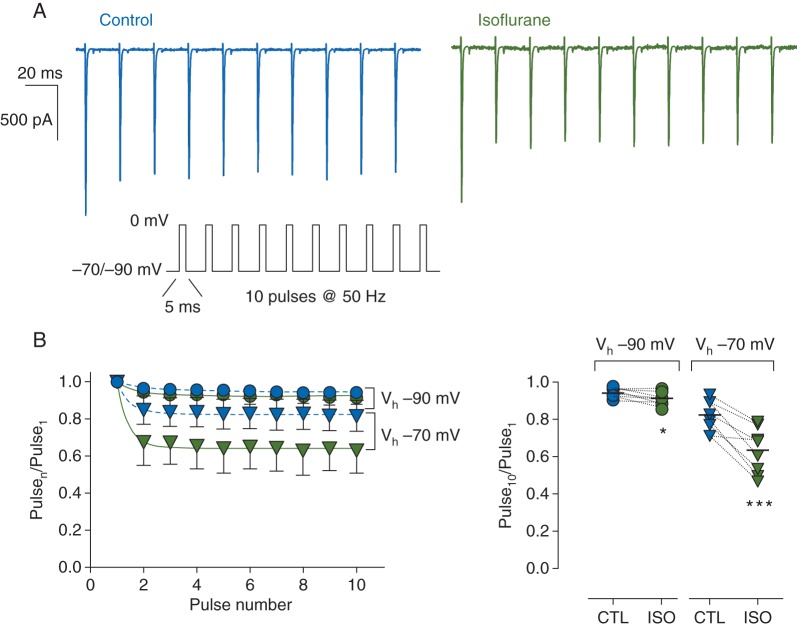
We examined whether activity-dependent block by isoflurane occurs in isolated rat neurohypophysial nerve terminals, an intact preparation that contains endogenous nerve terminal Nav channel complexes. Isoflurane produced activity-dependent block of normalized peak INa at a stimulation frequency (50 Hz) and pulse duration (5 ms) that produced minimal decrements in control (Fig. 5a). Isoflurane produced rapidly developing activity-dependent block, evident in enhanced reduction in plateau amplitude of normalized peak INa (Fig. 5b). At a Vh of −90 mV, the fractional peak of the last pulse of the train was reduced from 0.94 [0.02] in control to 0.91 [0.03] with isoflurane (n=8, P<0.05), and at a Vh of −70 mV, from 0.82 [0.08] for control to 0.63 [0.12] for isoflurane (n=8, P<0.001). Combined with resting block, activity-dependent block significantly enhanced overall inhibition of INa by isoflurane with repetitive stimulation.
Fig 5.
Effects of isoflurane on peak Na+ current (INa) in isolated rat neurohypophysial nerve terminals during high-frequency stimulation. (a) Representative current traces for a 50 Hz train of 5-ms depolarizing pulses to 0 mV from Vh of −70 mV in the absence (left) or presence (right) of 1.5 MAC isoflurane (protocol in inset). (b) Left panel, shows normalized peak INa [mean (sd), n=8] in the absence (blue symbols) or presence (green symbols) of isoflurane from Vh of −70 mV (triangles) or −90 mV (circles). Peak amplitude of each pulse was normalized to the first pulse (Pulsen/Pulse1) and fitted with a mono-exponential function. Right panel, individual normalized responses for the last pulse (Pulse10/Pulse1) in the absence (blue symbols) or presence (green symbols) of isoflurane. Dotted lines denote paired data. *P< 0.05, ***P<0.001 by two-tailed, paired Student's t-test.
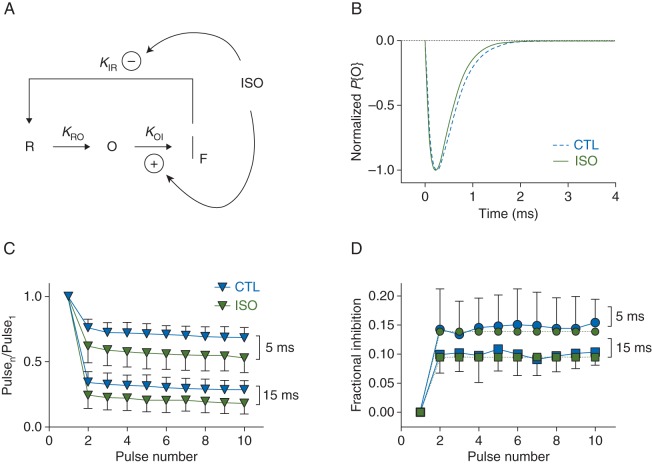
We constructed a simple gating model to examine whether stabilisation of fast inactivation is sufficient to quantitatively account for our experimental observations. Figure 6a shows a 3-state (R-O-IF) Markov model, in which isoflurane accelerates transitions from open to fast-inactivated states and slows recovery from fast-inactivated states upon repolarization. Rate constants kRO (3.6 ms−1) and kOI (control, 2.77 ms−1; isoflurane, 3.03 ms−1) were derived from mono-exponential fits to activation (not shown) and fast-inactivation (Fig. 4a) time courses. Model parameters derived from empirical responses, demonstrate that the model accounts for changes in macroscopic current time course during single depolarizations (Fig. 6b). The derived empirical time course of isoflurane inhibition during pulse trains (Fig. 6c and d) was well accounted for by the R-O-IF model, using estimated values of kIR (control, 89.4 s−1; isoflurane, 59.7 s−1) comparable with those observed experimentally (control, 137 s−1; isoflurane, 100 s−1, see Fig. 2b). We conclude that stabilisation of the fast-inactivated state is sufficient to quantitatively account for our experimental observations of activity-dependent block of INa by isoflurane.
Fig 6.
Gating model for isoflurane inhibition of NaV1.2R involving stabilisation of fast inactivation. (a) Simple Markov gating model of isoflurane inhibition involving primary resting (R), open (O) and fast inactivated (IF) states (R-O-IF model). Transitions between states are indicated by arrows shown with associated rate constants. During activation, channels transition from R to O to IF, and upon repolarization channels recover to availability by transitioning from IF to R. It is assumed that transitions from O to R are negligible and IF is an absorbing state during activation. Curved arrows identify transitions modulated by isoflurane (ISO). Isoflurane enhances (+) transitions from O to IF and slows (−) transitions from IF to R, which together represents IF stabilisation. (b) Simulated responses to single depolarizations (5 ms, 0 mV, Vh= − 70 mV). Normalized probability of state O (P{O}), proportional to current, plotted against time for control (CTL) and isoflurane (ISO). (c) Empirical pulse trains replotted from Fig. 3b and d (Vh= − 70 mV), with pulse durations as indicated. (d) Time course of isoflurane inhibition during a pulse train determined by plotting the fraction of channels inhibited (Fractional Inhibition; 1 − Pulsen/Pulse1) against pulse number. Empirical responses were derived from the pulse trains of panel C shown as blue symbols. Simulated responses are shown as green symbols, Data shown as mean [sd], with pulse durations indicated.
Discussion
We show here that isoflurane enhances activity-dependent depression of INa in both brain-type Nav1.2 and endogenous nerve terminal sodium channels. This novel anaesthetic effect on Nav contributes significantly to overall block during high-frequency stimulation. This should lead to greater sensitivity to isoflurane of fast firing neuronal networks, including depression of presynaptic excitability and reduced neurotransmitter release. Use of both a neuronal expression system and neuronal tissue, allowed us to demonstrate activity-dependent block of the major neuronal Nav subtype and of endogenous nerve terminal Nav subtypes in situ, in a physiological context as heterologous expression could influence anaesthetic effects.10–11,23 We further demonstrate that this novel form of Nav block involves stabilisation of the fast-inactivated state.
Neuronal signals are transmitted via trains of action potentials, so activity-dependent block of INa is a potentially important mechanism of volatile anaesthetic action on neuronal networks. Previous assessments of the role of Nav inhibition by general anaesthetics in vitro have not considered the impact of activity-dependent block, which significantly enhances the efficacy of isoflurane inhibition of neuronal Nav, under physiological conditions. For example, the magnitude of tonic INa inhibition by clinical concentrations of isoflurane is relatively modest: we observed ∼10% reduction of peak INa, comparable with previous reports.8,10 In comparison, we observed an additional ∼20% block at 50 Hz stimulation, from a physiological Vh of −70 mV. Even modest block of Nav can strongly affect neuronal transmission, as small reductions in peak INa alter both frequency of action potential firing24 and neurotransmitter release.5 Small reductions in INa produce significant effects on oscillatory activity in neuronal networks,25 which is relevant to systems level mechanisms of general anaesthesia. By analogy with use-dependent block by local anaesthetics, volatile anaesthetics would preferentially inhibit more active neurones to selectively suppress fast firing networks.26,27 Neurohypophysial nerve terminals express Nav in high density,9 comparable with hippocampal mossy fibre boutons, in which Nav amplify presynaptic action potential amplitude and enhance Ca2+ influx coupled to transmitter release.28 This high concentration of Nav at the bouton could explain how small changes in INa lead to substantial inhibition of synaptic vesicle exocytosis.
To our knowledge this is the first report of activity-dependent decay of INa in intact nerve terminals. This preparation has the advantage over recombinant Nav of reflecting the gating properties of native nerve terminal Nav in situ. Electrophysiology is limited in its ability to measure ionic currents at the synapse, where volatile anaesthetics exert their most potent effects.1–3 We show that isoflurane inhibits endogenous neurohypophysial nerve terminal Nav,29 which are coupled to neurotransmitter release by depolarizing the membrane and thus lead to activation of voltage-gated Ca2+ channels, Ca2+ influx and exocytosis. Magnocellular neurones of the supraoptic nucleus that innervate the neurohypophysis, express both Nav1.2 and Nav1.6,30 which are likely to mediate INa in these central nervous system nerve terminals. Heterologously expressed Nav1.2 and Nav1.6 are inhibited by isoflurane and other volatile anaesthetics at clinically relevant concentrations.8,18 Interestingly, heterologously expressed Nav1.6 currents exhibit use-dependent potentiation compared with Nav1.2 currents, which show use-dependent inhibition.31 As neurohypophysial nerve terminals exhibited rapid activity-dependent reductions in peak INa, amplitude with repetitive stimulation comparable with recombinant Nav1.2R, the dominant Nav isoform in neurohypophysial terminals is most likely Nav1.2.
Our mechanistic analysis of Nav1.2R revealing stabilisation of fast inactivation, is relevant to the intrinsic neurophysiological behaviour of presynaptic Nav, and has important implications for the anaesthetic sensitivity of nerve terminals at high firing frequencies. Although the molecular details underlying our proposal that isoflurane stabilises the fast-inactivated state are beyond the scope of the current investigation, the mechanism could involve isoflurane binding to a freely accessible receptor, that then allosterically modulates free energy profiles to stabilise the fast-inactivated state. Isoflurane can participate in hydrogen bonding by forming dipoles, and has been shown to bind hydrophobic macromolecules.32 Consistent with this possibility, anaesthetic binding has been demonstrated in prokaryotic voltage-gated ion channels,33,34 and molecular dynamics simulations of a homology model of NaChBac35 revealed a possible pathway for isoflurane to enter the pore via hydrophobic side fenestrations.36 Further structural and molecular dynamics studies are necessary to determine where isoflurane binds and what residues are involved in stabilizing the bound state of the channel.
In summary, we show that isoflurane stabilises the fast-inactivated state of neuronal Nav such that recovery from fast inactivation is delayed and entry into fast inactivation is accelerated, resulting in activity-dependent inhibition. This enhanced inactivation leads to progressive inhibition of INa, with high-frequency stimulation and contributes significantly to overall inhibition of INa by isoflurane, through activity-dependent inhibition compared with tonic inhibition. At high stimulus frequencies, Nav inhibition by isoflurane and probably other anaesthetics that exhibit state-dependent inhibition, will be greater than that suggested by previous studies of resting block alone.
Authors' contributions
H.C.H. contributed to the study design and writing the paper. K.P. contributed to the data collection, data analysis and writing the paper. K.J.G., and W.O. contributed to the data collection and data analysis. K.F.H. contributed to the study design and data analysis. All of the authors read, revised and approved the final manuscript.
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Declaration of interests
H.C.H. is an Editor of the BJA and of Anesthesiology. K.P., K.J.G., W.O. and K.F.H. declare no interests.
Funding
Supported by NIH grant GM58055 and GM58055S1.
Supplementary Material
References
- 1.Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging mechanisms of general anesthetic action. Trends Pharmacol Sci 2005; 26: 503–10 [DOI] [PubMed] [Google Scholar]
- 2.Eger EI, 2nd, Raines DE, Shafer SL, Hemmings HC, Jr, Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg 2008; 107: 832–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature 1994; 367: 607–14 [DOI] [PubMed] [Google Scholar]
- 4.Westphalen RI, Hemmings HC., Jr Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Therap 2003; 304: 1188–96 [DOI] [PubMed] [Google Scholar]
- 5.Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology 2004; 100: 663–70 [DOI] [PubMed] [Google Scholar]
- 6.Hemmings HC, Jr, Yan W, Westphalen RI, Ryan TA. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol 2005; 67: 1591–9 [DOI] [PubMed] [Google Scholar]
- 7.Urban BW. Differential effects of gaseous and volatile anaesthetics on sodium and potassium channels. Br J Anaesth 1993; 71: 25–38 [DOI] [PubMed] [Google Scholar]
- 8.Rehberg B, Xiao YH, Duch DS. Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology 1996; 84: 1223–33 [DOI] [PubMed] [Google Scholar]
- 9.Ouyang W, Wang G, Hemmings HC., Jr Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Mol Pharmacol 2003; 64: 373–81 [DOI] [PubMed] [Google Scholar]
- 10.Shiraishi M, Harris RA. Effects of alcohols and anesthetics on recombinant voltage-gated Na channels. J Pharmacol Exp Ther 2004; 309: 987–94 [DOI] [PubMed] [Google Scholar]
- 11.Herold KF, Nau C, Ouyang W, Hemmings HC., Jr Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology 2009; 111: 591–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujiwara N, Higashi H, Nishi S, Shimoji K, Sugita S, Yoshimura M. Changes in the spontaneous firing patterns of rat hippocampal neurones induced by volatile anaesthetics. J Physiol 1988; 402: 155–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang W, Hemmings HC., Jr Depression by isoflurane of the action potential and underlying voltage-gated ion currents in isolated rat neurohypophysial nerve terminals. J Pharmacol Exp Ther 2005; 312: 801–8 [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Guzinski M, Eger EI, 2nd, et al. Bidirectional modulation of isoflurane potency by intrathecal tetrodotoxin and veratridine in rats. Br J Pharmacol 2010; 159: 872–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze V, Stoetzer C, O'Reilly AO, et al. The opioid methadone induces a local anaesthetic-like inhibition of the cardiac Na+ channel, Na(v)1.5. Br J Pharmacol 2014; 171: 427–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ragsdale DS, Scheuer T, Catterall WA. Frequency and voltage-dependent inhibition of type IIA Na+ channels, expressed in a mammalian cell line, by local anesthetic, antiarrhythmic, and anticonvulsant drugs. Mol Pharmacol 1991; 40: 756–65 [PubMed] [Google Scholar]
- 17.Sheets MF, Fozzard HA, Lipkind GM, Hanck DA. Sodium channel molecular conformations and antiarrhythmic drug affinity. Trends Cardiovasc Med 2010; 20: 16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouyang W, Hemmings HC., Jr Isoform-selective effects of isoflurane on voltage-gated Na+ channels. Anesthesiology 2007; 107: 91–8 [DOI] [PubMed] [Google Scholar]
- 19.Hemmings HC., Jr Sodium channels and the synaptic mechanisms of inhaled anaesthetics. Br J Anesth 2009; 103: 61–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leffler A, Herzog RI, Dib-Hajj SD, Waxman SG, Cummins TR. Pharmacological properties of neuronal TTX-resistant sodium channels and the role of a critical serine pore residue. Pflugers Arch 2005; 451: 454–63 [DOI] [PubMed] [Google Scholar]
- 21.Benzanilla F, Armstrong CM. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol 1977; 70: 549–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taheri S, Halsey MJ, Liu J, Eger EI, 2nd, Koblin DD, Laster MJ. What solvent best represents the site of action of inhaled anesthetics in humans, rats, and dogs? Anesth Analg 1991; 72: 627–34 [DOI] [PubMed] [Google Scholar]
- 23.John V, Main M, Powell A, et al. Heterologous expression and functional analysis of rat NaV1.8 (SNS) voltage-gated sodium channels in the dorsal root ganglion neuroblastoma cell line ND7–23. Neuropharmacology 2004; 46: 425–38 [DOI] [PubMed] [Google Scholar]
- 24.Scholz A, Kuboyama N, Hempelmann G, Vogel W. Complex blockade of TTX-resistant Na+ currents by lidocaine and bupivicaine reduce firing frequency in DRG neurons. J Neurophysiol 1998; 79: 1746–54 [DOI] [PubMed] [Google Scholar]
- 25.Århem P, Klement G, Nilsson J. Mechanisms of Anesthesia: Towards Integrating Network, Cellular, and Molecular Level Modeling. Neuropsychopharmacology 2003; 28: S40–7 [DOI] [PubMed] [Google Scholar]
- 26.Stewart A, Lambert DH, Concepcion MA, et al. Decreased incidence of tourniquet pain during spinal anesthesia with bupivicaine. Anesth Analg 1988; 67: 833–7 [PubMed] [Google Scholar]
- 27.Wildsmith JAW, Brown DT, Paul D, Johnson S. Structure-activity relationships in differential nerve block at high and low frequency stimulation. Br J Anaesth 1989; 63: 444–52 [DOI] [PubMed] [Google Scholar]
- 28.Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 2005; 45: 405–17 [DOI] [PubMed] [Google Scholar]
- 29.Westphalen RI, Yu J, Krivitski M, Jih TY, Hemmings HC., Jr Regional differences in nerve terminal Na+ channel subtype expression and Na+ channel-dependent glutamate and GABA release in rat CNS. J Neurochem 2010; 113: 1611–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka M, Cummins TR, Ishikawa K, Black JA, Ibata Y, Waxman SG. Molecular and functional remodeling of electrogenic membrane of hypothalamic neurons in response to changes in their input. Proc Natl Acad Sci 1999; 96: 1088–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou W, Goldin AL. Use-dependent potentiation of the Nav1.6 sodium channel. Biophys J 2004; 87: 3862–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Johansson JS. An isothermal titration calorimetry study on the binding of four volatile general anesthetics to the hydrophobic core of a four-alpha-helix bundle protein. Biophys J 2003; 85: 3279–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nury H, Van Renterghem C, Weng Y, et al. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature 2011; 469: 428–31 [DOI] [PubMed] [Google Scholar]
- 34.Spurny R, Billen B, Howard RJ, et al. Multisite binding of a general anesthetic to the prokaryotic pentameric Erwinia chrysanthemi ligand-gated ion channel (ELIC). J Biol Chem 2013; 288: 8355–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raju SG, Barber AF, LeBard DN, Klein ML, Carnevale V. Exploring volatile general anesthetic binding to a closed membrane-bound bacterial voltage-gated sodium channel via computation. PLoS Comput Biol 2013; 9: e1003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature 2011; 475: 353–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.