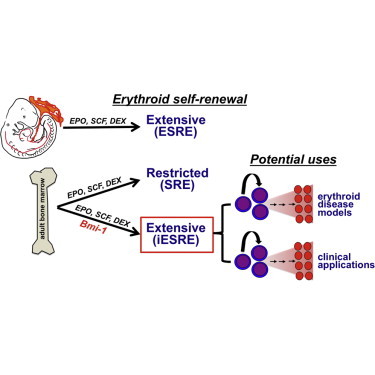
Summary
Red blood cells (RBCs), responsible for oxygen delivery and carbon dioxide exchange, are essential for our well-being. Alternative RBC sources are needed to meet the increased demand for RBC transfusions projected to occur as our population ages. We previously have discovered that erythroblasts derived from the early mouse embryo can self-renew extensively ex vivo for many months. To better understand the mechanisms regulating extensive erythroid self-renewal, global gene expression data sets from self-renewing and differentiating erythroblasts were analyzed and revealed the differential expression of Bmi-1. Bmi-1 overexpression conferred extensive self-renewal capacity upon adult bone-marrow-derived self-renewing erythroblasts, which normally have limited proliferative potential. Importantly, Bmi-1 transduction did not interfere with the ability of extensively self-renewing erythroblasts (ESREs) to terminally mature either in vitro or in vivo. Bmi-1-induced ESREs can serve to generate in vitro models of erythroid-intrinsic disorders and ultimately may serve as a source of cultured RBCs for transfusion therapy.
Graphical Abstract
Highlights
-
•
Bmi-1 promotes the extensive self-renewal of adult murine erythroblasts
-
•
Transfusion of iESRE leads to fully mature RBCs that circulate for 6–7 weeks in vivo
Palis and colleagues determined that Bmi-1 is preferentially expressed in self-renewing erythroblasts and that Bmi-1 promotes the extensive self-renewal of erythroblasts derived from adult murine bone marrow, which normally have limited self-renewal capacity. Importantly, Bmi-1-induced self-renewing erythroblasts maintain their capacity to terminally mature into enucleated red cells both in vitro and in vivo.
Introduction
Every year in the United States, 14 million units of red blood cells (RBCs) are used to treat more than 3.5 million severely anemic patients (Ansari and Szallasi, 2012). This great clinical need for RBCs is expected to increase as our population ages. The current reliance on blood donors is associated with infectious risks, high costs of screening, and supply bottlenecks for rare blood types and for alloimmunized patients requiring chronic transfusions. The in vitro production of RBCs is one potential solution to meet this growing need for blood (Migliaccio et al., 2012).
Adult humans synthesize more than two million RBCs every second to maintain steady-state circulating levels. These RBCs are derived from lineage-committed progenitors that give rise to maturing erythroblasts that ultimately enucleate. Erythropoiesis is regulated by several cytokines, particularly erythropoietin (EPO) and stem cell factor (SCF), which provide survival and proliferation signals to erythroid progenitors (Koury and Bondurant, 1990; Chui and Russell, 1974). Additionally, glucocorticoid signaling facilitates the rapid expansion of the erythron that occurs in response to acute anemia (Bauer et al., 1999).
Studies of viral-induced avian erythroleukemia led to the discovery that signaling pathways downstream of EPO, SCF, and glucocorticoids synergize to drive the in vitro self-renewal of mammalian erythroid cells (Beug et al., 1979; Panzenböck et al., 1998). Self-renewing erythroblasts (SREs) derived from postnatal sources undergo limited ex vivo proliferation when cultured with EPO, SCF, and the synthetic glucocorticoid dexamethasone (DEX) (von Lindern et al., 1999; England et al., 2011). We previously reported that erythroblasts derived from the yolk sac and early fetal liver of the mouse embryo or from differentiating mouse embryonic stem cells undergo an initial phase of limited self-renewal, which is followed by a subset of cells that undergo an extensive phase of self-renewal (England et al., 2011). Even after 1060-fold expansion in vitro, extensively self-renewing erythroblasts (ESREs) maintain the capacity to terminally mature into reticulocytes. While the continuous presence of EPO, SCF, and DEX is necessary both for the restricted and for the extensive phases of erythroblast proliferation, the regulation of SRE and ESRE self-renewal remains poorly understood.
To better understand the mechanisms underlying the self-renewal of erythroid lineage cells, we generated and compared global gene expression data sets from SREs and ESREs as well as primary proerythroblasts (ProEs). This analysis revealed that Bmi-1 and other polycomb repressive complex 1 (PRC1) components are upregulated in self-renewing erythroblasts. Importantly, overexpression of Bmi-1 conferred extensive self-renewal capacity upon erythroblasts derived from adult bone marrow without interfering with the ability of these Bmi-1-induced ESREs (iESREs) to terminally differentiate into enucleated RBCs both in vitro and in vivo. iESREs may ultimately provide an alternative source of cultured RBCs to meet the growing need for blood transfusions.
Results
Bmi-1 Expression Is Upregulated in ESREs and Is Required for Erythroblast Self-Renewal
We previously determined that ESREs and primary ProEs share similar morphological and immunophenotypic characteristics, including high surface expression of KIT (CD117) and transferrin receptor (CD71) (England et al., 2011). Like ProEs, ESREs lie only 3–4 cell divisions upstream of reticulocytes; however, ESREs are blocked from maturing in vitro by DEX (England et al., 2011). To better understand the mechanisms regulating erythroid self-renewal, we compared global gene expression of self-renewing erythroblasts in the restricted and extensive phases of self-renewal (Figure 1A) with primary ProEs derived from adult bone marrow.
Figure 1.
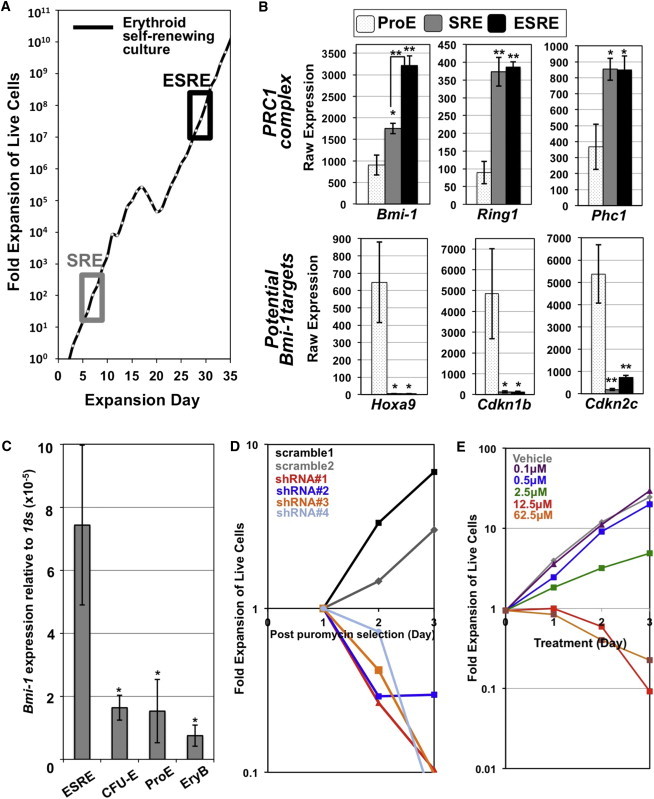
Bmi-1 Is Highly Expressed in ESREs and Is Required for Erythroblast Self-Renewal
(A) SREs and ESREs were isolated from restricted and extensive phases of self-renewal, respectively. One representative growth curve of four independent ESRE cultures is shown with timing of SRE and ESRE isolation boxed.
(B) Analysis of Affymetrix data sets revealed upregulation of genes associated with the PRC1 in ESREs and SREs compared to primary ProEs. Several known targets of Bmi-1 are significantly downregulated in ESREs/SREs compared to ProEs (mean ± SEM; N = 4 independent replicates for ESREs/SREs; N = 5 independent replicates for ProEs). p value was calculated using one-tailed Student’s t test. ∗p < 0.05; ∗∗p < 0.01.
(C) Bmi-1 transcripts are expressed at significantly higher levels in ESREs compared to primary CFU-Es, ProEs, and maturing erythroblasts (EryBs) (mean ± SEM; N = 3 independent replicates). p value was calculated using one-tailed Student’s t test. ∗p < 0.05.
See also Figure S1.
(D) shRNA-mediated knockdown of Bmi-1 rapidly decreased ESRE proliferation after puromycin selection (one representative culture of three independent experiments).
(E) PTC-209, a BMI-1 inhibitor, caused a dose-dependent inhibition of ESRE proliferation compared to vehicle (DMSO) control culture. One representative culture of six independent experiments is shown.
Analysis of differentially expressed genes (Figure S1A) revealed that several PRC1 components, including Bmi-1, Ring1, and Phc1, were expressed at significantly higher levels in self-renewing erythroblasts (Figure 1B). BMI-1 functions primarily as a repressor of multiple downstream target genes, including Hoxa9 (Abdouh et al., 2009; Zacharek et al., 2011; Biehs et al., 2013). Consistent with the differential expression of Bmi-1, the potential downstream target Hoxa9 was not expressed in ESREs/SREs but was highly expressed in primary ProEs (Figures 1B and S1C). BMI-1 also represses several cell-cycle inhibitors, including Cdkn1b (p27) and Cdkn2c (p18) (Leung et al., 2004; Abdouh et al., 2009; Zhang et al., 2010), which were also reduced in ESREs/SREs compared to ProEs (Figures 1B and S1C). The differential expression of Bmi-1 was validated in ESREs compared with primary late-stage erythroid progenitors (CFU-Es), ProEs, and maturing erythroblasts isolated from mouse bone marrow (Figures 1C and S1B).
We used a loss-of-function approach to test the hypothesis that Bmi-1 regulates erythroid self-renewal. ESREs transduced with shRNA targeting Bmi-1 rapidly died following puromycin selection, while ESREs transduced with a scrambled control vector continued to proliferate (Figure 1D). Furthermore, the BMI-1 inhibitor PTC-209 reduced ESRE proliferation in a dose-dependent manner (Figure 1E). Taken together, these data support the concept that Bmi-1 is required for in vitro erythroblast self-renewal.
Bmi-1 Is Sufficient to Induce Extensive Ex Vivo Self-Renewal of Adult Erythroblasts Cultured with EPO, SCF, and DEX
We next used a gain-of-function approach to determine whether Bmi-1 can extend the proliferative capacity of adult marrow-derived SREs, which normally proliferate for only 1–2 weeks ex vivo (England et al., 2011). Empty lentiviral vector-transduced adult SREs ceased proliferating within 2 weeks, consistent with their limited ex vivo self-renewal capacity. In contrast, erythroblasts from 10 of 11 bone-marrow-derived SRE cultures transduced with Bmi-1 proliferated at least 25 days (Figure 2A), while 3 cultures of Bmi-1-induced ESREs (iESREs) were maintained for more than 100 days (Figure 2B). iESREs were also generated from adult mice with hereditary hemolytic anemia due to Protein 4.1R deficiency (Figures S2A–S2D), providing a proof of principle that this experimental approach can be used to generate large numbers of mutant erythroblasts that can facilitate the study of red cell-intrinsic disorders.
Figure 2.
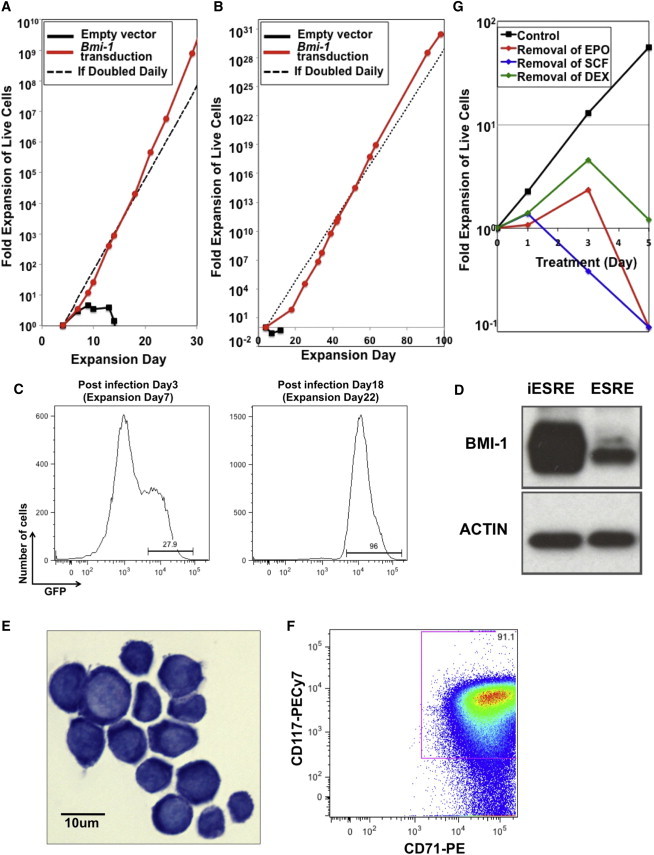
Bmi-1 Is Sufficient to Induce the Extensive Ex Vivo Self-Renewal of Adult Erythroblasts
(A) Lentiviral transduction of mouse Bmi-1 led to prolonged proliferation of bone-marrow-derived SREs grown in erythroid expansion media. Erythroid cells transduced with an empty vector proliferated for 2 weeks, while erythroid cells transduced with a Bmi-1 overexpression vector stably proliferated for more than a month (representative date from one of ten independent experiments). The dotted line represents expected cell proliferation if cells divide daily.
(B) Bmi-1-induced ESRE (iESRE) was maintained for 100 days, exhibiting more than 1030-fold total erythroblast expansion.
(C) The percentage of erythroblasts transduced with Bmi-1 (GFP+ cells) when analyzed 3 days and 18 days after infection. Representative data from one of two independent experiments are shown.
(D) BMI-1 protein expression is increased in erythroblasts transduced with FUGW-Bmi1. Actin served as an internal control. Representative data from one of three for ESREs and one of four for iESREs independent experiments are shown.
(E) Bmi-1-induced iESREs exhibited a high nuclear to cytoplasmic ratio and basophilic cytoplasm resembling proerythroblasts.
(F) iESREs express both KIT (CD117) and transferrin receptor (CD71) on their cell surface. Representative data from one of three independent experiments are shown.
(G) iESREs remain dependent on the continued presence of EPO, SCF, and DEX for ex vivo self-renewal. Withdrawal of individual factors resulted in the rapid loss of cell proliferation. One representative culture of five independent experiments is shown.
See also Figure S2.
Since the Bmi-1 expression vector contains GFP, we analyzed the percentage of Bmi-1-transduced erythroblasts over time in culture. While 20%–30% of erythroblasts were initially GFP+ following transduction, almost all of the erythroblasts in the extensive phase of proliferation were GFP+ (Figure 2C), consistent with the notion that Bmi-1 expression facilitates erythroblast self-renewal. Indeed, BMI-1 is overexpressed in these transduced iESREs compared to ESREs derived from fetal sources (Figure 2D).
SREs transduced with Bmi-1 display a high nuclear to cytoplasmic ratio and a basophilic cytoplasm, similar to the morphology of primary ProEs and to ESREs derived from embryonic sources (Figure 2E). Like their embryo-derived counterparts, iESREs expressed KIT and transferrin receptor (Figure 2F). Similar to embryo-derived ESREs, which remain highly dependent on EPO, SCF, and DEX (England et al., 2011), Bmi-1-induced adult iESREs are also dependent on the presence of each of these cytokines to maintain ex vivo self-renewal (Figure 2G).
iESREs Maintain the Potential to Terminally Mature into Reticulocytes In Vitro
A mandatory feature of self-renewal is the generation of daughter cells with the same identity as the parent cell, which for ESREs include the maintenance of their capacity to terminally differentiate into reticulocytes. Transfer of iESREs and ESREs into maturation media resulted in similar kinetics of differentiation into reticulocytes as evidenced by the rapid accumulation of hemoglobin (Figure 3A) and by their transition at day 3 into enucleated reticulocytes (Figure 3B; England et al., 2011). Consistent with normal erythroblast maturation, iESREs also progressively downregulated the cell surface expression of KIT and transferrin receptor (Figure 3C). Taken together, these data indicate that iESREs are immature erythroid precursors poised to terminally mature in vitro.
Figure 3.
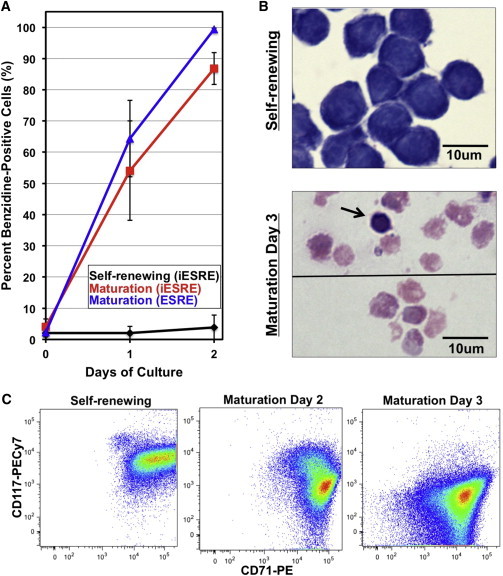
iESREs Terminally Mature into Reticulocytes In Vitro
(A) Almost all of the Bmi-1-induced ESREs (iESREs) in expansion cultures lack detectable hemoglobin (black line). Transfer of iESREs and ESREs into maturation media on day 0 resulted in rapid accumulation of hemoglobin (red and blue lines, respectively), quantified by benzidine staining. (Mean ± SEM; N = 6 independent replicates for iESRE self-renewing cultures; N = 7 and 3 independent replicates for iESRE and ESRE maturation cultures, respectively.)
(B) Morphology of self-renewing (upper panel) and maturing (lower panels) iESREs stained with Wright-Giemsa. iESREs differentiated in vitro into reticulocytes and pyrenocytes (arrow). One representative image of more than three independent experiments is shown.
(C) In vitro maturation of iESREs is characterized by the progressive downregulation of KIT (CD117) and transferrin receptor (CD71). Representative data from one of three independent experiments are shown.
iESREs Are Capable of Terminal Erythroid Maturation In Vivo
We next asked whether iESREs also have the capacity to mature in vivo. To track erythrocytes after transfusion, iESREs were generated from the marrow of adult ubiquitin C (UBC)-GFP mice that express GFP driven by the ubiquitin C promoter. 5–10 × 107 iESREs were injected intravenously into NOD scid gamma (NSG) or C57BL/6J mice treated with 1.5 Gy total body irradiation (TBI) to transiently suppress endogenous erythropoiesis. While most ESREs were localized to the spleen and bone marrow 1 day after transfusion (Figure S3B), a small percentage of GFP+ reticulocytes were detected in the circulation, likely derived from the small number of spontaneously maturing (benzidine+) cells present in the self-renewing cultures (Figure 3A). A large, transient wave of reticulocytes entered the bloodstream between 4 and 8 days after iESRE transfusion into irradiated mice (Figures 4A and S3C). At day 6, 40%–90% of the reticulocytes in the bloodstream were GFP+ (Figures 4A and S3C).
Figure 4.
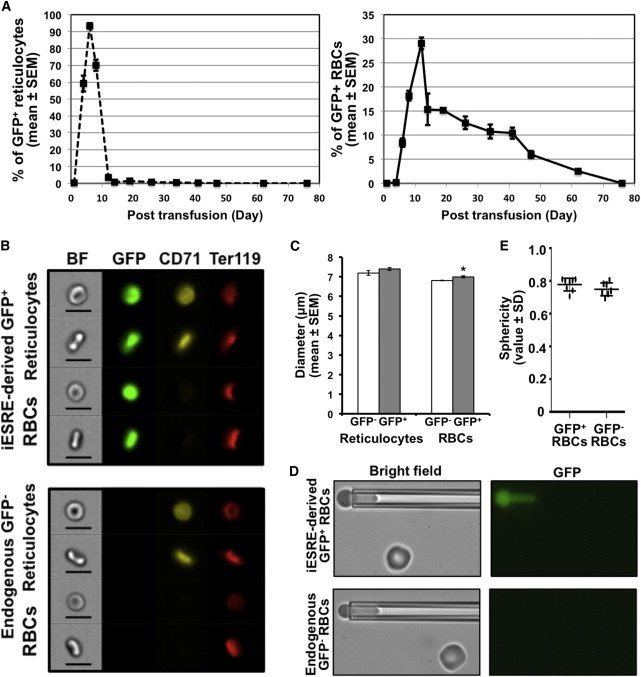
Intravenous Transfusion of iESREs Results in a Transient Wave of Reticulocytes and the Circulation of iESRE-Derived RBCs for Several Weeks
(A) Kinetics of GFP+/CD71+ reticulocytes (left panel) and GFP+/CD71− mature RBCs derived from 10 × 107 GFP+ iESREs transfused on day 0 into recipient mice. The percentages of iESRE-derived reticulocytes and RBCs were calculated as the number of GFP+ cells divided by the total cell number in the parent gate (mean ± SEM; N = 3 mice).
(B) The morphology of circulating mature RBCs (Ter119+/CD71−/DRAQ5−), was visualized by imaging flow cytometry on day 6 post-GFP+-iESRE transfusion. Reticulocytes and RBCs derived from iESREs (GFP+, upper panel) showed similar biconcave shape as endogenous (GFP−, lower panel) RBCs. Representative data from one of three mice are shown (size bar represents 10 μm).
(C) The size of iESRE-derived (GFP+) and endogenous (GFP−) circulating reticulocytes and RBCs on day 6 post-transfusion were compared by imaging flow cytometry. Reticulocytes were similar in size (mean ± SEM; N = 3 independent experiments; p = 0.22; left panel), while iESRE-derived RBCs were significantly larger than endogenous RBCs (mean ± SEM; N = 3 independent experiments; right panel). p value was calculated using two-tailed Student’s t test. ∗p < 0.05.
(D) Fluorescence-imaged microdeformation analysis of iESRE-derived (GFP+, upper panel) and endogenous (GFP−, lower panel) RBCs.
(E) Sphericity of iESRE-derived (GFP+) and endogenous (GFP−) RBCs on day 26 post-transfusion, based on micropipette measurements of surface area and volume measurements (individual value ± SD; p value = 0.177). p value was calculated using two-tailed Student’s t test. Representative data from one of three mice are shown.
See also Figure S3.
This robust wave of GFP+ reticulocytes was followed by the circulation of GFP+ mature RBCs. iESRE-derived RBCs persistently constituted 10%–15% of the circulating red cell mass for 5 weeks (Figure 4A). iESRE-derived (GFP+) reticulocytes and RBCs displayed a similar biconcave shape when compared to their endogenous (GFP−) counterparts (Figure 4B). iESRE-derived reticulocytes were similar in size to endogenous reticulocytes, while iESRE-derived mature RBCs were significantly larger than endogenous RBCs, consistent with their recent maturation as a neocyte cohort on day 6 posttransfusion (Figure 4C). The circulation of this large cohort of GFP+ RBCs for several weeks suggests that they can deform normally to survive the microcirculation. Indeed, pipette aspiration measurements revealed that iESRE-derived RBCs had normal surface area to volume ratios (sphericity) (Figures 4D and 4E). Furthermore, RBCs-derived from iESREs had similar hemoglobin levels compared with endogenous RBCs (Figure S3D). Taken together, these data indicate that iESREs mature synchronously into reticulocytes both in vitro and in vivo and can become fully mature RBCs in vivo with a functional cytoskeleton that facilitates their persistent circulation for weeks in the bloodstream.
Discussion
The self-renewal capacity of erythroid progenitors is an important component of current protocols to generate cultured RBCs for potential therapeutic purposes. However, a major obstacle in generating the 2.5 × 1012 RBCs that constitutes a single unit of blood is the limited capacity of erythroblasts derived from neonatal and adult sources to self-renew in vitro. Understanding the mechanisms promoting extensive erythroid self-renewal could lead to improved generation of cultured RBCs. Here, we have discovered that Bmi-1 is preferentially expressed in self-renewing compared to differentiating erythroblasts. Importantly, we determined that overexpression of Bmi-1 is sufficient to induce the extensive ex vivo self-renewal of adult erythroblasts that normally are only capable of limited self-renewal. These iESREs have a similar morphology, cell surface phenotype, and cytokine dependence compared to ESREs derived from embryonic sources.
Bmi-1 regulates the self-renewal of several adult stem cell populations, including hematopoietic, neural, and cancer stem cells, often through repression of p16Ink4a/p19Arf (Jacobs et al., 1999; Park et al., 2003; Molofsky et al., 2003; Kreso et al., 2014). While we did not detect Cdkn2a in erythroblasts (Kingsley et al., 2013), we did identify several other potential BMI-1 target genes that are differentially expressed in ESREs/iESREs, studies suggesting that BMI-1 may function independently of p16Ink4a/p19Arf repression in ESREs (Bruggeman et al., 2007; Abdouh et al., 2009). The function of Bmi-1 in ESRE self-renewal may be independent of its role in erythroid maturation, where it was recently shown to positively regulate ribosomal protein genes (Gao et al., 2015).
Bmi-1 transduction of self-renewing erythroblasts did not interfere with terminal in vitro maturation of iESREs into reticulocytes. The generation of iESREs from adult Protein 4.1R null mice indicates that this experimental approach can be used to generate large numbers of mutant erythroblasts for the study of red-cell-intrinsic disorders, particularly diseases that affect terminal stages of erythroid maturation such as disorders of the membrane cytoskeleton or of globin gene expression.
Intravenous transfusion of iESREs resulted in the emergence of a large wave of reticulocytes 4–8 days later. The timing and the transient nature of this reticulocyte emergence indicate that iESREs rapidly switched in vivo from a self-renewal to a maturation program. This transient wave of iESRE-derived reticulocytes generated a population of mature RBCs that constituted 10%–15% of the total circulating RBC mass from week 2 to week 6 following iESRE transfusion. Consistent with a near normal life span, iESRE-derived RBCs displayed normal shape and deformability when compared to co-circulating endogenous RBCs. Transfusion of 10 × 107 iESREs resulted in the stable circulation of approximately 1.6 × 109 RBCs, suggesting that each iESRE generated 16–32 RBCs, consistent with the differentiation potential of normal ProE/CFU-E. Taken together, these findings indicate that Bmi-1-induced ESREs are not immortalized but rather constitute committed erythroid precursors that rapidly mature, not only in vitro but also in vivo. These findings also distinguish iESREs from immortalized erythroid cell lines generated by genetic perturbation of multiple transcription regulators, including C-MYC, TP53, E6/E7, and SOX2, some of which must be exogenously extinguished before terminal maturation can proceed (Huang et al., 2014; Hirose et al., 2013; Kurita et al., 2013).
Our data indicate that Bmi-1 promotes the extensive erythroid self-renewal of adult erythroblasts cultured ex vivo with EPO, SCF, and DEX. iESREs may ultimately provide an alternative source of cultured RBCs to meet the challenge of generating the extremely large numbers of RBCs needed for transfusion therapy. Interestingly, iESREs transfused in vivo result in large numbers of fully mature RBCs, obviating the need to generate and fully mature RBCs in vitro. Importantly, no GFP+ cells in peripheral blood or tumors were detected in recipients 6 months after transfusion (data not shown). However, host conditioning and safety concerns must be evaluated before iESREs can be considered as a potential transfusion product.
Experimental Procedures
Mice and Tissues
All experiments were approved by the University of Rochester’s Committee on Animal Resources. Mouse strains included outbred ICR mice (Taconic Farms), C57BL/6J mice (Jackson Laboratory), C57BL/6-Tg(UBC-GFP)30Scha/J (Jackson Laboratory), and Protein 4.1R knockout mice (kindly provided by Dr. John Conboy). Tissues from adult male bone marrow were processed as previously described to derive iESREs (England et al., 2011). iESREs were transfused into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (NSG; Jackson Laboratory) or C57BL/6J mice.
Erythroid Cell Evaluation
Cultured cells were stained with Wright-Giemsa or benzidine as previously described (Palis et al., 1995). Images were acquired and processed as previously published (England et al., 2011) using Photoshop CS5 (Adobe). The immunophenotype of self-renewing and maturing erythroid cells was analyzed with CD117 (phycoerythrin [PE]-indocyanine [Cy] 7), CD71 (PE), and Ter119 (allophycocyanin [APC] antibodies) using an LSR-II (BD Bioscience) flow cytometer and FlowJo software (version 8.8.7). Additional details about antibodies are in Supplemental Experimental Procedures.
Imaging Flow Cytometry Analysis
Circulating reticulocytes and RBCs were visualized with live DAPI−, Ter119hi(PE-Cy7), CD71+/−(PE), DRAQ5−, and GFP+/− using an imaging flow cytometer (ImageStream GenX; Amnis) and analyzed with IDEAS software (version 4.0; Amnis). The size of each population was measured after gating with live, single, and focused cells using the “height” feature.
Affymetrix Gene Expression Analysis
Erythroblasts from restricted and extensive phases of self-renewal were isolated from two independent cultures initiated each from E9.5 yolk sacs and E12.5 livers using FACS for Propidium Iodide− (PI), CD117hi(PE-Cy5), and CD71hi(PE). Primary ProEs from adult bone marrow were isolated by FACS as described (Kingsley et al., 2013). Gene expression data sets were generated using Affymetrix Mouse Genome 430_2.0 GeneChips and analyzed as described (Kingsley et al., 2013), including the identification of genes differentially expressed between ESREs and primary ProEs. Microarray data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-3112.
Transfusion of Self-Renewing Erythroblasts
5–10 × 107 iESREs, generated from UBC-GFP mice, were transfused intravenously into each of three NSG mice and two C57BL/6J mice irradiated with 1.5 Gy TBI 6 hr previously, as well as an unirradiated C57BL/6J mouse. RBCs were gated as single live (DAPI−), Ter119+(APC), CD71−(PE) cells, and reticulocytes were gated as Ter119+(APC), CD71+(PE) cells. Cells derived from transfused iESREs were identified as GFP+ within each gate (Figure S3A). RBCs collected from iESRE-transfused NSG recipient mice were analyzed for surface area and volume (sphericity) as described (Waugh et al., 2013).
Statistical Analysis
Statistical significance was calculated by Student’s t test. All data are presented with mean ± SEM, except sphericity and relative hemoglobin levels of RBCs, which are shown as value ± SD.
Author Contributions
A.R.K. designed and performed experiments, analyzed data, and wrote the manuscript. J.L.O, S.J.E., Y.-S.H., K.H.F., and L.F.D. performed experiments. P.D.K. performed experiments and analyzed data. K.E.M. and R.E.W. analyzed data. J.P. designed experiments, analyzed data, and wrote the manuscript.
Acknowledgments
The authors thank Leah Vit, Seana Catherman, Anne Koniski, and URMC Flow Cytometry Core Facility for assistance with experiments. The authors also thank Dr. Sally Temple for providing the FUWG-Bmi1 overexpression vector (Addgene). This research was supported by grants from the New York State Stem Cell Science (NYSTEM; N08G-33G), the NIH National Center for Advanced Translational Sciences (UL1TR000042), and the NIH/NHLBI (U01HL099656) and by the Michael Napoleone Memorial Foundation.
Published: May 28, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Accession Numbers
Microarray data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-3112.
Supplemental Information
References
- Abdouh M., Facchino S., Chatoo W., Balasingam V., Ferreira J., Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J. Neurosci. 2009;29:8884–8896. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari S., Szallasi A. Blood management by transfusion triggers: when less is more. Blood Transfus. 2012;10:28–33. doi: 10.2450/2011.0108-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Tronche F., Wessely O., Kellendonk C., Reichardt H.M., Steinlein P., Schütz G., Beug H. The glucocorticoid receptor is required for stress erythropoiesis. Genes Dev. 1999;13:2996–3002. doi: 10.1101/gad.13.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J.F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979;18:375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Biehs B., Hu J.K., Strauli N.B., Sangiorgi E., Jung H., Heber R.P., Ho S., Goodwin A.F., Dasen J.S., Capecchi M.R., Klein O.D. BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nat. Cell Biol. 2013;15:846–852. doi: 10.1038/ncb2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggeman S.W., Hulsman D., Tanger E., Buckle T., Blom M., Zevenhoven J., van Tellingen O., van Lohuizen M. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell. 2007;12:328–341. doi: 10.1016/j.ccr.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Chui D.H., Russell E.S. Fetal erythropoiesis in steel mutant mice. I. A morphological study of erythroid cell development in fetal liver. Dev. Biol. 1974;40:256–269. doi: 10.1016/0012-1606(74)90128-6. [DOI] [PubMed] [Google Scholar]
- England S.J., McGrath K.E., Frame J.M., Palis J. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117:2708–2717. doi: 10.1182/blood-2010-07-299743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Chen S., Kobayashi M., Yu H., Zhang Y., Wan Y., Young S.K., Soltis A., Yu M., Vemula S. Bmi1 promotes erythroid development through regulating ribosome biogenesis. Stem Cells. 2015;33:925–938. doi: 10.1002/stem.1896. Published online November 11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Takayama N., Nakamura S., Nagasawa K., Ochi K., Hirata S., Yamazaki S., Yamaguchi T., Otsu M., Sano S. Immortalization of erythroblasts by c-MYC and BCL-XL enables large-scale erythrocyte production from human pluripotent stem cells. Stem Cell Reports. 2013;1:499–508. doi: 10.1016/j.stemcr.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Shah S., Wang J., Ye Z., Dowey S.N., Tsang K.M., Mendelsohn L.G., Kato G.J., Kickler T.S., Cheng L. Extensive ex vivo expansion of functional human erythroid precursors established from umbilical cord blood cells by defined factors. Mol. Ther. 2014;22:451–463. doi: 10.1038/mt.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.J., Kieboom K., Marino S., DePinho R.A., van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- Kingsley P.D., Greenfest-Allen E., Frame J.M., Bushnell T.P., Malik J., McGrath K.E., Stoeckert C.J., Palis J. Ontogeny of erythroid gene expression. Blood. 2013;121:e5–e13. doi: 10.1182/blood-2012-04-422394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury M.J., Bondurant M.C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990;248:378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Kreso A., van Galen P., Pedley N.M., Lima-Fernandes E., Frelin C., Davis T., Cao L., Baiazitov R., Du W., Sydorenko N. Self-renewal as a therapeutic target in human colorectal cancer. Nat. Med. 2014;20:29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- Kurita R., Suda N., Sudo K., Miharada K., Hiroyama T., Miyoshi H., Tani K., Nakamura Y. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS ONE. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C., Lingbeek M., Shakhova O., Liu J., Tanger E., Saremaslani P., Van Lohuizen M., Marino S. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428:337–341. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- Migliaccio A.R., Whitsett C., Papayannopoulou T., Sadelain M. The potential of stem cells as an in vitro source of red blood cells for transfusion. Cell Stem Cell. 2012;10:115–119. doi: 10.1016/j.stem.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A.V., Pardal R., Iwashita T., Park I.K., Clarke M.F., Morrison S.J. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palis J., McGrath K.E., Kingsley P.D. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- Panzenböck B., Bartunek P., Mapara M.Y., Zenke M. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood. 1998;92:3658–3668. [PubMed] [Google Scholar]
- Park I.K., Qian D., Kiel M., Becker M.W., Pihalja M., Weissman I.L., Morrison S.J., Clarke M.F. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- von Lindern M., Zauner W., Mellitzer G., Steinlein P., Fritsch G., Huber K., Löwenberg B., Beug H. The glucocorticoid receptor cooperates with the erythropoietin receptor and c-Kit to enhance and sustain proliferation of erythroid progenitors in vitro. Blood. 1999;94:550–559. [PubMed] [Google Scholar]
- Waugh R.E., Huang Y.S., Arif B.J., Bauserman R., Palis J. Development of membrane mechanical function during terminal stages of primitive erythropoiesis in mice. Exp. Hematol. 2013;41:398–408, e2. doi: 10.1016/j.exphem.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek S.J., Fillmore C.M., Lau A.N., Gludish D.W., Chou A., Ho J.W., Zamponi R., Gazit R., Bock C., Jäger N. Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell. 2011;9:272–281. doi: 10.1016/j.stem.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.W., Ding J., Jin J.L., Guo J., Liu J.N., Karaplis A., Goltzman D., Miao D. Defects in mesenchymal stem cell self-renewal and cell fate determination lead to an osteopenic phenotype in Bmi-1 null mice. J. Bone Miner. Res. 2010;25:640–652. doi: 10.1359/jbmr.090812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


