Abstract
Molecular signaling pathways delineating the induction of matrix metalloproteinases (MMPs) by ultraviolet radiation (UVR) are currently well-defined; however, the effects of UVR on epigenetic mechanisms of MMP induction are not as well understood. In this study, we examined solar simulated UVR (ssUVR)-induced gene expression changes and alterations to histone methylation in the promoters of MMP1 and MMP3 in primary human dermal fibroblasts (HDF). Gene expression changes, including the increased expression of MMP1 and MMP3, were observed using Affymetrix GeneChip arrays and confirmed by qRT-PCR. Using ChIP-PCR, we showed for the first time that in HDF irradiated with 12 J/cm2 ssUVR, the H3K4me3 transcriptional activating mark increased and the H3K9me2 transcriptional silencing mark decreased in abundance in promoters, correlating with the observed elevation of MMP1 and MMP3 mRNA levels following ssUVR exposure. Changes in mRNA levels due to a single exposure were transient and decreased 5 days after exposure.
Keywords: MMP1, MMP3, UVR, histone, epigenetic
Background
Photoaging is the premature aging of skin from overexposure to natural and/or artificial ultraviolet radiation (UVR). The phenotype of an aged cell, in part, results from a combination of DNA mutations and epigenetic reprogramming. In addition to direct DNA damage, the consequences of frequent UVR exposure to the skin are chronic inflammation and oxidative stress; these processes are thought to affect epigenetic regulators and alter gene expression patterns that contribute to aging (1). Epigenetic reprogramming (permanent alterations of covalent histone tail modifications or DNA methylation patterns) is sensitive to environmental factors, and can result in age-related differences in gene expression that contribute to an aged cell phenotype (2).
UVR-exposure induced DNA methylation pattern and histone tail modification alterations have been reported in in vivo and in vitro human studies (3, 4). The transcription of matrix metalloproteinases (MMPs), which are important enzymes secreted by dermal fibroblasts in response to UVR that mediate dermal remodeling, has been shown to be regulated by histone modifications. Histone 3 (H3) acetylation was found to be critical for the induction of MMP1 by combined UVA and UVB in human dermal fibroblasts (5). In a study of diabetic rats, MMP9 gene expression was found to be activated by the loss of H3K9me2 and the increase of H3K9ac at the NFκB binding site in the MMP9 promoter (6). The role of histone modification changes in regulating the response of dermal fibroblasts to UVR is not understood as well as the regulation of MMPs by UVR-induced signal transduction pathways and transcription factors (7). Epigenetic responses to UVR may contribute to the up regulation of MMPs, matrix remodeling, and photoaging.
Question Addressed
Are the UVR-induced changes in MMPs associated with H3 lysine methylation changes in the promoter of these genes?
Experimental Design
Results
Analysis of the Affymetrix GeneChip array data revealed that the expression of 306 genes significantly changed (fold-change ≥ 1.5, and a p-value ≤ 0.05) 24 hours post-irradiation with 12 J/cm2 ssUVR in HDF. A significant up regulation of MMP1 and MMP3 was observed and confirmed with qRT-PCR. In addition to MMP1 and MMP3, the altered expression of other genes related to the structure and function of the ECM were confirmed by qRT-PCR to validate the Affymetrix GeneChip array data set (Figure S). The direction of change for all of the cDNA tested matched the Affymetrix array data. Affymetrix GeneChip data analysis from a repeat experiment showed that less than 10% of the significantly altered genes remained changed 5 days after this single exposure. Therefore epigenetic alterations were not examined in samples from this later time. MMP1 and MMP3 mRNA levels were found to increase 1 day after the exposure in the irradiated cells relative to the sham, but then the MMP1 and MMP3 mRNA levels decreased in irradiated cells 5 days post-exposure relative to the sham (Figure 1). The overall amount of MMP1 and MMP3 mRNA significantly increased in the shams over the 5 days. These gene expression changes appeared to be a short-term response after a single ssUVR irradiation.
Figure 1.
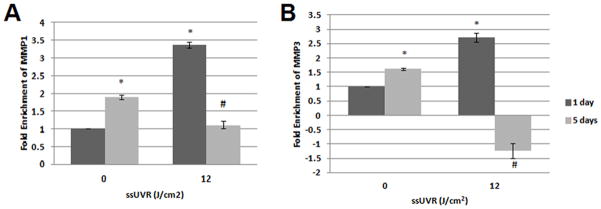
QRT-PCR validation of MMP1 and MMP3 gene expression changes 1 and 5 days after irradiation with 12 J/cm2 ssUVR. A) MMP1 B) MMP3 (*) = p-value ≤ 0.05 relative to sham exposed cells on 1 day of recovery. (#) = p-value ≤ 0.05 relative to sham exposed cells at 5 days of recovery. Each value is the average of four data points run in two experiments and similar changes were observed in other experiments.
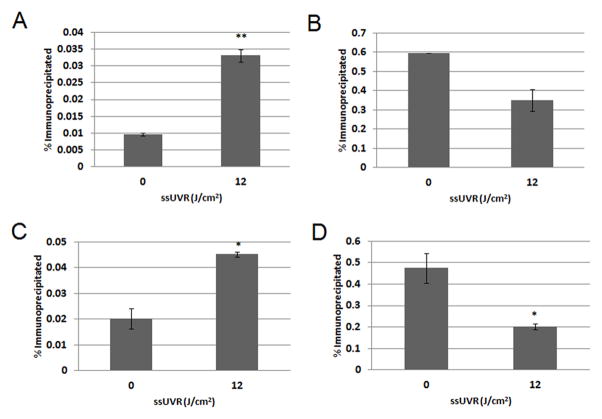
To determine if the gene expression increase of MMP1 and MMP3 was associated with specific histone modification changes, the relative levels of H3K4 and H3K9 methylation in the MMP1 and MMP3 gene promoters was assayed by ChIP-PCR 16 hours after a single irradiation. ChIP was conducted with antibodies towards H3K4me3 and H3K9me2, and the resulting immunoprecipitated DNA was used for qRT-PCR with primers specific to the gene promoters of MMP1 and MMP3. A significant increase in the amount of H3K4me3 (p=0.003) was seen in the promoter of MMP1 (Figure 2a) in response to ssUVR. H3K9me2 appeared to decrease in the MMP1 promoter, but this reduction was not statistically significant (Figure 2b). In the MMP3 promoter, H3K4me3 significantly increased (p=0.012) (Figure 2c), and H3K9me2 was significantly decreased (p=0.032) (Figure 2d). The observed increase of the H3K4me3 transcriptional activating mark and decrease of H3K9me2 transcriptional silencing mark correlates with the ssUVR-induced elevation of MMP1 and MMP3 mRNA levels.
Figure 2.
Histone methylation changes in the MMP1 and MMP3 gene promoters of cells exposed to 12 J/cm2 ssUVR and sham exposed cells 16 hours post-exposure. A) H3K4me3 was significantly increased (p=0.003) in the promoter of MMP1. B) H3K9me2 (p=0.104) in the MMP1 promoter. C) H3K4me3 abundance significantly increased (p=0.012) in the promoter of MMP3. D) H3K9me2 significantly decreased (p=0.032) in the promoter of MMP3. Each value is the average of two data points from one experiment and similar changes were observed in other experiments.
Conclusions
We showed for the first time in HDF that a single irradiation with 12 J/cm2 ssUVR induced histone methylation pattern changes that resulted in the transcriptional activation of genes strongly associated with photoaging, MMP1 and MMP3. H3K4me3 increased and H3K9me2 decreased in the promoters of MMP1 and MMP3 in response to ssUVR, providing a potential epigenetic mechanism behind UVR-induction of MMPs and photoaging. Since MMPs are induced temporally, and their mRNA levels were found to shift in the opposite direction 5 days after irradiation, we predict that the histone methylation changes could also be temporal and change in the presence or absence of environmental and molecular cues.
Our study expands upon current literature by showing that histone methylation is changed specifically at the promoters of MMP1 and MMP3 in response to ssUVR. Our findings are in agreement with existing data on epigenetic regulation of MMP transcription, which show that histone modifications are crucial for the regulation of MMP gene expression. Furthermore, there exists a connection between UVR and the establishment or erasure of histone modifications. UV irradiation causes a significant reduction of histone deacetylase activity and increase of histone acetyltransferase activity (5), and UV-induced oxidative stress can impact the activity of histone methyltransferases (8) and histone demethylases (2, 9). The combination of oxidative stress, DNA damage, and inflammation caused by UVR is likely involved in triggering epigenetic changes in skin cells. In addition to photoaging, the impact of UV induced epigenetic reprogramming could contribute to skin cancer (10). Understanding how the altered abundance of histone methylation in the promoter of MMP1 and MMP3 up regulates these enzymes in response to UVR could open doors for potential therapies whereby the epigenetic responses to UVR could be reversed to reduce MMP expression.
Supplementary Material
Acknowledgments
This research was supported by NIH center grant number P01 ES000260, and by The Estee Lauder Companies, Inc.
Footnotes
Author Contributions
LG performed the research, analyzed and interpreted the data, and wrote the manuscript. TK assisted with performing the research. MM and MC were responsible for conceptualizing the study design and critically revised the manuscript. All authors approved the submitted manuscript version.
Conflict of interests
The authors do not have any conflicts of interests to disclose.
References
- 1.Katiyar SK, Singh T, Prasad R, et al. Epigenetic alterations in ultraviolet radiation-induced skin carcinogenesis: interaction of bioactive dietary components on epigenetic targets. Photochemistry and photobiology. 2012;88:1066–1074. doi: 10.1111/j.1751-1097.2011.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rando TA, Chang HY. Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock. Cell. 2012;148:46–57. doi: 10.1016/j.cell.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronniger E, Weber B, Heil O, et al. Aging and chronic sun exposure cause distinct epigenetic changes in human skin. PLoS genetics. 2010;6:e1000971. doi: 10.1371/journal.pgen.1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollack BP, Sapkota B, Boss JM. Ultraviolet radiation-induced transcription is associated with gene-specific histone acetylation. Photochemistry and photobiology. 2009;85:652–662. doi: 10.1111/j.1751-1097.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim MK, Shin JM, Eun HC, et al. The role of p300 histone acetyltransferase in UV-induced histone modifications and MMP-1 gene transcription. PloS one. 2009;4:e4864. doi: 10.1371/journal.pone.0004864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhong Q, Kowluru RA. Regulation of matrix metalloproteinase-9 by epigenetic modifications and the development of diabetic retinopathy. Diabetes. 2013;62:2559–2568. doi: 10.2337/db12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang BM, Noh EM, Kim JS, et al. Curcumin inhibits UVB-induced matrix metalloproteinase-1/3 expression by suppressing the MAPK-p38/JNK pathways in human dermal fibroblasts. Experimental dermatology. 2013;22:371–374. doi: 10.1111/exd.12137. [DOI] [PubMed] [Google Scholar]
- 8.Goswami SK. Cellular redox, epigenetics and diseases. Sub-cellular biochemistry. 2013;61:527–542. doi: 10.1007/978-94-007-4525-4_23. [DOI] [PubMed] [Google Scholar]
- 9.Chervona Y, Costa M. The control of histone methylation and gene expression by oxidative stress, hypoxia, and metals. Free radical biology & medicine. 2012;53:1041–1047. doi: 10.1016/j.freeradbiomed.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kostaki M, Manona AD, Stavraka I, et al. High-frequency p16(INK) (4A) promoter methylation is associated with histone methyltransferase SETDB1 expression in sporadic cutaneous melanoma. Experimental dermatology. 2014;23:332–338. doi: 10.1111/exd.12398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.