Abstract
The term “synthetic cathinones” is fairly new; but, although the abuse of synthetic cathinones is a recent problem, research on cathinone analogs dates back >100 years. One structural element cathinone analogs have in common is an α-aminophenone moiety. Introduction of amine and/or aryl substituents affords a large number of agents. Today, >40 synthetic cathinones have been identified on the clandestine market and many have multiple “street names”. Many cathinone analogs, although not referred to as such until the late 1970s, were initially prepared as intermediates in the synthesis of ephedrine analogs. The cathinones do not represent a pharmacologically or mechanistically homogeneous class of agents. Currently abused synthetic cathinones are derived from earlier agents and seem to produce their actions primarily via the dopamine, norepinephrine, and/or serotonin transporter; that is, they either release and/or inhibit the reuptake of one or more of these neurotransmitters. The actions of these agents can resemble those of central stimulants such as methamphetamine, cocaine, and/or empathogens such as 1-(3,4-methylenedioxyphenyl)-2-aminopropane (MDMA; Ecstasy) and/or produce other effects. Side effects are primarily of a neurological and/or cardiovascular nature. The use of the “and/or” term is emphasized because synthetic cathinones represent a broad class of agents that produce a variety of actions; the agents cannot be viewed as being pharmacologically equivalent. Until valid structure-activity relationships are formulated for each behavioral/mechanistic action, individual synthetic cathinones remain to be evaluated on a case-by-case basis. Treatment of synthetic cathinone intoxication requires more “basic science” research. At this time, treatment is mostly palliative.
Keywords: α-Aminophenones, Amphetamine, Bath Salts, Cathinone, Dopamine Transporter, MDPV, Mephedrone, Methamphetamine, Methcathinone, Methylenedioxypyrovalerone, Methylone, Serotonin Transporter
Introduction
“Synthetic cathinones” represent an emerging drug-abuse problem. Relatively little seems to be known about these “mysterious” new agents and their pharmacology. But, more might be known than (or might be inferred from) what is commonly recognized or acknowledged. What are synthetic cathinones? Where did they come from? Do they really represent something new? Synthetic cathinones constitute a broad category of agents whose individual members produce similar, somewhat similar, dissimilar and, occasionally, unique effects; hence, their mechanisms of action cannot be identical. What should emerge from this review is the following: i) synthetic cathinones are structurally (i.e., chemically and stereochemically) derived from amphetamine (i.e., phenylisopropylamine) analogs, ii) the actions and mechanisms of action of synthetic cathinones are no more homogeneous than those of other “amphetamine-related” phenylisopropylamines, and iii) an understanding of amphetamine-related phenylisopropylamines (PIAs) and their structure-activity relationships (SARs) will provide a sound backdrop for understanding the synthetic cathinones.
Synthetic cathinones should not be viewed as an entirely novel class of drugs of abuse for which no previous literature or prior understanding is available. Actually, synthetic cathinones are derivatives of an agent (i.e., cathinone) that is the active stimulant component of a natural product (i.e., the khat plant). The use/abuse of khat is many centuries old; however, cathinone was not specifically identified as its active stimulant constituent until 1975 (UN Document, 1975). Much can be learned from an examination of earlier literature. In fact, many synthetic cathinones, although it should be recognized that they were not termed as such at the time of their initial discovery, have been around for decades; some have been known for 100 years. Unfortunately, complex and inconsistent chemical nomenclature has often obscured, or at least complicated, a proper understanding or appreciation of these agents.
Prior to just a few years ago, the scientific literature on cathinone, and cathinone-related analogs (α-aminopropiophenones and chain-extended derivatives thereof, now termed “synthetic cathinones”), was relatively meager, quite manageable, and presented an interesting (if somewhat incomplete) story. In the past, except for a brief period in the early 1980s – coincident with the identification of cathinone as a natural plant-derived product with abuse potential – there was relatively little scientific interest in cathinone or cathinone analogs. There was a second wave of interest in the early 1990s when methcathinone (N-methylcathinone or MCAT) was identified as a potential drug-abuse problem and scheduled by the US government as a Schedule I substance. Since then, the field has burgeoned tremendously. “Synthetic cathinones”, along with new “synthetic cannabinoids”, are two of the latest global drug abuse problems, and both seem to have had a nearly exponential growth rate. The two “problems” are not related from a structural or mechanistic perspective. Only the former will be discussed here.
Synthetic cathinones, synthetic cannabinoids, and other novel agents – including some recently-introduced LSD-like (i.e., lysergic acid diethylamide-like) hallucinogenic agents – have been termed “new psychoactive substances” or “NPSs” (UNODC, 2013). This is a “bucket” appellation that is appropriate and well-suited for legal purposes (perhaps the goal for which it was intended), and is a term suitable for the lay press, but it lends no understanding of the actions and mechanisms of action of the individual agents (or classes of agents) involved. Indeed, several NPSs already have been investigated and they are structurally diverse, produce different effects, and act by different mechanisms. Structurally, synthetic cathinones might be described as “nuevo amphetamines” or, better yet, as “nuevo phenylisopropylamine analogs” because their actions and, particularly, mechanisms of action, are not necessarily identical to that of amphetamine itself.
The roots of the “cathinone story” are >1,000 years old, but the bulk of what has been published on synthetic cathinones has occurred only in the past few years. By means of analogy, some parallels can be drawn from the current cocaine-abuse problem. Coca leaf (primarily Erythroxylon coca) was (and continues to be) chewed in certain parts of South America as an anti-fatigue agent. It was not until cocaine was identified as the major active stimulant constituent of coca leaf, and made widely available in pure form (i.e., in the form of cocaine and “crack”), that it became a major, worldwide drug-abuse problem. The same might be said about the khat plant – with cathinone now being considered it’s most potent central stimulant constituent – but with a twist. Cocaine analogs never became widely available. Why? Difficulty of synthesis of cocaine analogs? Indeed, there are some complex synthetic and stereochemistry problems here. The ready availability of cocaine? In contrast, many novel synthetic cathinone analogs are now flooding clandestine markets. The khat plant is not readily available outside its indigenous area, and it is the fresh plant that is desired (i.e., cathinone degrades as the harvested khat plant ages). Pure cathinone, unlike cocaine, has never been heavily trafficked. However, cathinone analogs are relatively easier to synthesize than cocaine analogs, they are generally more stable than cathinone (particularly in solution), and their synthetic precursors are readily available. Some synthetic cathinones are more potent than cathinone itself, can produce a different effect than cathinone, and possess different mechanisms of action (see below). Hence, they are fairly simple to synthesize, and a wide variety of analogs is possible. This might explain the rapid shift in market-available synthetic cathinone products, as time goes on, to circumvent legal restrictions.
Structurally, synthetic cathinones are, simply put, β-keto analogs of amphetamine-related structures. Cathinone, for example, is the β-keto analog of amphetamine (AMPH). In theory, each “AMPH analog” can have a synthetic cathinone counterpart. However, some synthetic cathinones represent novel entities whose parent AMPH has never been extensively (or at all) investigated (at least not in a systematic, scientific manner or in human subjects). So, it is not surprising that little is known about many of the new synthetic cathinones. It might appear that the synthetic cathinones are wholly novel and unexpected drug-abuse entities; but a retrospective analysis suggests that there is/was some seeming forethought behind the market introduction of the abused agents we now term synthetic cathinones.
Synthetic cathinones can be viewed from several perspectives. As mentioned above, they are β-keto analogs of AMPH (i.e., they are phenylisopropylamine) analogs. They can also be viewed as oxidation products of phenylpropanolamines (i.e., β-hydroxyphenylisopropylamines) such as ephedrine and norephedrine. Cathinone is the oxidized version of norephedrine where the β-hydroxyl group of norephedrine has been oxidized to a carbonyl group.
Phenylisopropylamines do not represent a pharmacologically or mechanistically homogeneous class of agents (Glennon & Young, 2011). Hence, there is no reason to assume that synthetic cathinones (or phenylpropanonamines) will be any more pharmacologically or mechanistically homogeneous than their phenylisopropylamine parents. These agents need to be investigated on a case-by-case basis (Dal Cason, Young & Glennon, 1997) until some general SARs can be identified. The pharmacology, SARs, and mechanism(s) of action of phenylisopropylamines and/or phenylpropanolamines have been the subject of scientific investigation for >100 years now. The alarming increase in the number of new phenylpropanonamines (i.e., synthetic cathinones) appearing on the clandestine market in the past few years will require a considerable catch-up effort by scientists, the medical community, and drug enforcement agencies so that intelligent treatment and legal decisions can be made.
Phenylisopropylamine analogs: Nomenclature and pharmacological assays
General nomenclature
Synthetic cathinones are best described as α-aminophenones (i.e., Ar-CO-CH(R3)-(NR1R2) where “Ar” is typically a phenyl or substituted phenyl ring, NR1R2 represents a primary, secondary or tertiary amine, and R3 is a carbon chain of 0 to several carbon atoms in length.
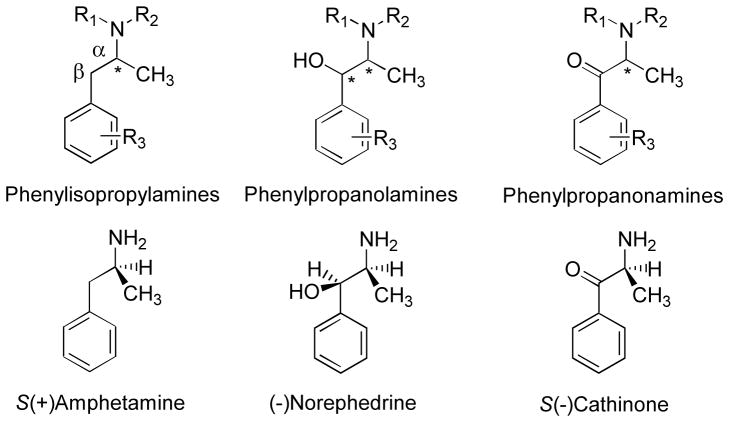
Amphetamine (1-phenyl-2-aminopropane or 1-phenyl-2-propanamine or AMPH) is not only the structural parent of a large class of agents referred to as phenylisopropylamines (PIAs), it is also known as phenylisopropylamine itself (i.e., from whence the class derives its name) (Figure 1). That is, the term phenylisopropylamine refers to a specific agent (i.e., AMPH), but also refers to a class of agents (i.e., the phenylisopropylamines) – all of which possess a similar structural skeleton. Substituted phenylisopropylamines are often referred to as substituted amphetamines. The latter (inaccurate) terminology should be resisted because it conjures up “AMPH-like” pharmacology. For example, the phenylisopropylamine DOM (i.e., 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane; see below) is frequently referred to as 1-(2,5-dimethoxy-4-methyl)amphetamine. The latter name suggests that, pharmacologically, DOM might be an “AMPH-like” agent. This is not the case. DOM is a potent classical hallucinogen that lacks central stimulant character, and acts via a mechanism that is entirely different from that of AMPH (Glennon & Young, 2011). Many other examples exist. Yet, both AMPH and DOM possess the same PIA backbone. Thus, the actions (and mechanisms of action) of substituted phenylisopropylamines are not homogeneous; action depends upon pendant substituents. In what follows, the term AMPH will be used to refer to amphetamine, phenylisopropylamines (or PIAs) to refer to the class as a whole, and AMPH-like action to represent a pharmacology consistent with that observed following administration of AMPH.
Figure 15.1.
General chemical structures of phenylisopropylamines, phenylpropanolamines, and phenylpropanonamines (top row; asterisks indicate chiral centers), and representative examples of such, respectively (bottom row).
Related PIAs with central stimulant character include β-hydroxyphenylisopropylamines and β-ketophenylisopropylamines. Phenylisopropylamine nomenclature is generic; that is, all three types of agents can be considered as being PIAs. All three possess the same structural skeleton. To distinguish amongst the three structural categories, a more specific nomenclature is employed here. That is, β-hydroxyphenylisopropylamines are more specifically synonymous with phenylpropanolamines, and β-ketophenylisopropylamines are more synonymous with phenylpropanonamines (or, now, more commonly referred to as β-ketoamphetamines, bk-amphetamines, bk-AMPHs, β-keto PIAs, bk-PIAs or, simply, “synthetic cathinones”). See Figure 1 for structural detail.
Stereochemistry
When PIAs possess a chiral center at the α-carbon atom (see Figure 1), two optical isomers are possible: (+) and (−). Their absolute configuration, or their three-dimensional structural arrangement, can be designated as R or S. For example, AMPH exists as S(+)AMPH and R(−)AMPH. An equal mixture of two optical isomers (the most frequently encountered form for many agents) is designated a racemate or the (±) form; if no stereochemical descriptor is provided, it must be assumed that the racemate is being referred to. Hence, (±)AMPH and AMPH (unless the term is being used in the most generic sense, such as in “AMPH-like agents” or “AMPH-like action”), refer, by definition, to racemic AMPH. When making stereochemical comparisons, it is the absolute configuration (i.e., R or S) that is to be compared; optical rotation (i.e., + and −) does not allow accurate structural comparisons to be made between agents. For greater detail, see Glennon and Young (2011).
Certain PIAs, specifically the phenylpropanolamines, can, depending upon their specific substituents, possess two chiral centers; hence, four optical isomers might be possible For example, the simplest phenylpropanolamines are norephedrine and pseudonorephedrine (a.k.a. Ψ-norephedrine); each has two possible isomers (Figure 2) (Glennon, 2008). Oxidation of the benzylic (or β−) alcohol of the phenylpropanolamines to a β-keto (i.e., carbonyl) group eliminates stereochemical considerations at this position and the resultant phenylpropanonamines exist only as a pair of (+) and (−) isomers. For example, the structure of S(−)cathinone, the oxidation product of both (−)norephedrine and (+)pseudonorephedrine, is shown in Figure 1.
Figure 15.2.
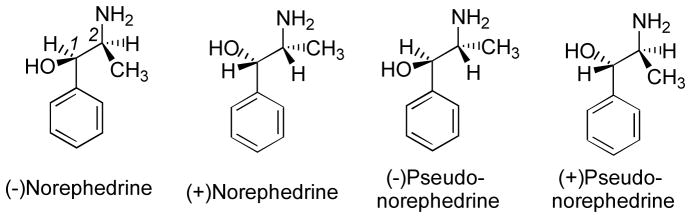
Structures of the four simplest phenylpropanolamines: (1R,2S)(−) norephedrine, (1S,2R)(+)norephedrine, (1R,2R)(−)pseudonorephedrine, and (1S,2S)(+)pseudonorephedrine or cathine. Introduction of an N-methyl group would afford the corresponding four optical isomers of ephedrine.
Pharmacological assays
A variety of animal assay have been employed to examine AMPH-like agents (and PIAs in general). The most common among them are i) drug discrimination, ii) self-administration, iii) locomotor activity, and iv) stereotypy. These assays are so widely used that they deserve a brief description here because they are quite pertinent to what follows. In drug discrimination studies, animals (typically rodents, but sometimes pigeons, monkeys or humans) are trained to respond (e.g. in a two-lever operant chamber – for rodents) to a given dose of a specific (i.e., training) agent; in subsequent tests of “stimulus generalization”, the subjects are administered doses of a novel agent. Stimulus generalization implies that the novel agent produces stimulus effects common to the training agent – though not necessarily via an identical mechanism of action (Glennon & Young, 2011). Stimulus generalization provides evidence for commonality (not identity) of effect. Self-administration is another type of operant behavior where the novel agent is the “reward”; a high frequency of self-administration suggests that a drug might be rewarding (i.e., possess abuse potential) (Negus & Banks, 2011). It is well known that AMPH-related stimulants produce hyperlocomotion (i.e., an increase in locomotor action) in rodents, and this is thought to be related to increased dopamine neurotransmission in the nucleus accumbens (Ljungberg & Ungerstedt, 1985). AMPH-like stimulants can also produce stereotypy in rodents; that is, AMPH-like agents produce an increase in rodent locomotor action but, at some higher dose(s), cause the animals to make certain repetitive behaviors (i.e., “stereotypy”) that result in decreased locomotor action. Certainly, other assays have been employed (and a few will be mentioned).
Cathinone Analogs: Historical perspectives
Ephedra and khat
Where did the synthetic cathinones come from? What follows is neither a comprehensive nor exhaustive treatment of the subject. Rather, it is meant to describe some early studies and put the subject in proper historical perspective; citations to some additional review articles are provided for those with greater interest.
Although the term “cathinone” is only about 40 years old, its lineage can be traced to two distinct shrubby parents: ephedra and khat. The ephedra plant (primarily Ephedra sinica, ma huang) has been used by the Chinese for thousands of years for its cardiovascular, bronchodilator, mild stimulant, and other effects (Lee, 2011). The major stimulant component of ephedra is (−)-ephedrine. Ephedra was a “wonder drug” of the late 1800s and early 1900s and, during this time, its chemistry and pharmacology were being investigated in many laboratories. There were synthetic and stereochemistry problems (ephedrine and norephedrine have two chiral centers and four isomeric phenylpropanolamines exist for each; see Figure 2). An apparent ephedra shortage in the 1920s spurred the chemical synthesis of ephedrine and novel ephedrine analogs; laboratories rose to the challenge and numerous patent applications were submitted. One such patent emanating from these studies was that for amphetamine. At the time, Chen and Kao (1926) wrote that “the success in the synthesis of ephedrine and pseudoephedrine marks one of the triumphs in the field of synthetic organic chemistry”.
According to Alles et al. (Alles, Fairchild & Jensen, 1961), the first written record concerning khat was in the 1300s, although khat use most certainly predates that time. The fresh leaves of the shrub Catha edulis are chewed in the Arabian peninsula and in certain regions of eastern Africa for their central stimulant effects. Occasionally, they are brewed as a tea. The leaves and preparations are known by nearly 100 different names – perhaps an indication of their popularity – including, for example, khat, k’at, kat, kath, gat, miraa, qat, tschat, Abyssinian tea, Arabian tea, Somali tea) (UN Document, 1979). The League of Nations considered the khat problem in 1935, and the United Nations/World Health Organization (WHO) considered it again in the 1960s and, later, in the 1970s (UN Document, 1979). Khat is still used on a regular basis, and the concept of “cultural drug dependence” has been introduced to explain its popularity and frequent use in certain geographic regions (Kennedy, Teague & Fairbanks, 1980). The khat literature has been reviewed (e.g. Al-Hebshi & Skaug, 2002; Anderson & Carrier, 2011; Fitzgerald, 2009; Halbach, 1972; Kalix & Braeden, 1985; Kennedy et al. 1980).
Cathine and cathinone
What is the active central stimulant component of khat? The norephedrine isomer (+)norpseudoephedrine (a.k.a. “cathine”) was first isolated from the khat plant in 1930 (Wolfes, 1930), and later by Alles et al. (1961) and Ristic and Thomas (1962). Alles et al. (1961) found cathine to possess central stimulant character. Given the popular interest in psychoactive substances at that time, khat and cathine were the subject of a major New York Times article (Fellows, 1967). But, soon after cathine was reported to be a central stimulant, it was found to less potent than fresh khat extract (Friebel & Brilla, 1963). This led to speculation that khat might contain other stimulant components. In a series of studies culminating in the eventual identification of “more than forty alkaloid [khat leaf] components” (UN Document, 1979), a UN working group isolated (−)α-aminopropiophenone from fresh khat leaves in 1975 and termed the substance “cathinone” (UN Document, 1975). (−)Cathinone (Schorno & Steinegger, 1978) as well as racemic or (±)cathinone and its optical antipode were synthesized (UN Document 1978), and the UN made samples available to various investigators.
(−)Cathinone was found to be a more potent AMPH-like locomotor stimulant in rodents than (±)cathinone, (+)cathinone and/or cathine (Glennon & Showalter, 1981; Kalix, 1980a; Knoll, 1979, Rosecrans, Campbell, Dewey & Harris, 1979; Yanagita, 1979), and produced AMPH-like stereotypic behavior in rats at high doses (Berardelli, Capocaccia, Pacitti, Tancredi, Quinteri & Elmi, 1980). Interestingly, van der Schoot et al. (van der Schoot, Ariens, van Rossum & Hurkmans, 1962) had found nearly twenty years earlier (i.e., prior to cathinone being identified as a constituent of khat, or before the term “cathinone” was introduced) in a random screen of a large number of PIA analogs that this aminopropiophenone produced locomotor stimulation in mice. As with its structural cousin AMPH, (−)cathinone also produced hyperthermia in rabbits that could be blocked by the dopamine antagonist haloperidol (Kalix, 1980b). Other studies (reviewed: Kalix & Braeden, 1985) also confirmed that cathinone is a potent AMPH-like substance; indeed, Kalix was probably the first to refer to (−)cathinone as “natural amphetamine” (Kalix, 1992). Furthermore, (−)cathinone was found, as was previously reported for (+)AMPH, to act as a dopamine (DA) releasing agent (e.g. Kalix, 1981; Kalix and Glennon, 1986).
In drug discrimination studies employing rats trained to discriminate (+)AMPH from vehicle, both isomers of cathinone substituted for training drug, with relative potencies of S(−)cathinone ≥ S(+)AMPH > (±)cathinone > R(+)cathinone (Glennon, Young, Hauck & McKenney, 1984), and stimulus generalization could be blocked by the dopamine receptor antagonist haloperidol (Glennon, 1986). Cathinone, itself, has been used as a training drug in drug discrimination studies with rats (Glennon, Schechter & Rosecrans, 1984; Schechter & Glennon, 1985). In these, and other, investigations S(−)cathinone was consistently found to be more potent than R(+)cathinone just as S(+)AMPH is more potent than R(−)AMPH (Glennon et al., 1995).
Methcathinone
In a structure-activity investigation, several analogs of cathinone were prepared and examined. One of these was N-monomethyl cathinone (termed “methcathinone” by analogy to the N-monomethyl analog of AMPH, METH) (Glennon, Yousif, Naiman & Kalix 1987). Methcathinone (MCAT) might be considered the first synthetic cathinone. MCAT was found to be at least as potent as METH as a locomotor stimulant, as a dopamine (DA) releasing agent, and in tests of stimulus generalization using rats trained to discriminate either (+)AMPH or cocaine from vehicle (Glennon, Young, Martin & Dal Cason, 1995; Young & Glennon, 1993). As expected, S(−)MCAT was found to be more potent than its R(+)enantiomer. Rats were subsequently trained to discriminate S(−)MCAT from vehicle and the stimulus was potently blocked by haloperidol (Young & Glennon, 1998). The S-isomer of MCAT was also more potent than its R-enantiomer as a locomotor stimulant in mice (Glennon et al., 1995). All evidence suggested that S(−)MCAT was a potent AMPH-like central stimulant. Methcathinone has now been found on the clandestine markets of various countries and is referred to as CAT, MCAT, and M-CAT.
As an aside, several phenylpropanonamines, including the substance now termed MCAT (as well as what is now known as cathinone), were initially synthesized by Eberhard in 1915 and again in 1920 (Eberhard, 1915, 1920) and by Fourneau and Kanao (1924) as synthetic intermediates in the preparation of ephedrine and norephedrine. Several other investigators repeated these syntheses (with slight modifications – and these synthetic routes are still employed today for the synthesis of synthetic cathinones), but the most commonly acknowledged synthesis is that by Roger Adams and his students in 1928 (Hyde, Browning & Adams, 1928) which is a replicate of the Eberhard (1920) synthesis. MCAT, using today’s terminology, has been around for 100 years, but, it was prepared as a precursor for ephedrine synthesis and as a potential cardiovascular agent – that is, its central stimulant properties were not evaluated at the time. This same substance, and both of its optical isomers, were also patented in Germany in 1936 as synthetic precursors for the preparation of ephedrine analogs (Bockmuhl & Gore, 1936). “N-Methyl-β-ketoamphetamine” (now termed MCAT) was later patented by Parke-Davis as an analeptic agent (L’Italien, Park & Rebstock, 1957). It was also shown, serendipitously, to be one of a number of several dozen PIA-related agents that act as locomotor stimulants in mice (van der Schoot et al., 1962). These studies never seemed to go any farther than to become historical footnotes.
In 1992, it was learned that what we had termed methcathinone was a very widely abused substance (under the name of ephedrone) in the former Soviet Union (personal written communication from Dr. I. Philippov, Lensoviet Technological Institute to R. A. Glennon dated August 18, 1992). A USSR Interior Ministry document released in 1989 (Savenko, Semkin, Sorokin & Kazankov, 1989) reported that “In our country, the most widely used amphetamine derivatives obtained from ephedrine are ephedron and pervitin [i.e., pervitin = methamphetamine]”. “The first pervitin synthesis for illegal distribution in the U.S.S.R. was in 1979 in Moscow and later in Leningrad”. It might be noted that the chemical reduction (i.e., hydrogenolysis) of ephedrine results in METH whereas the oxidation of ephedrine results in MCAT. Ephedrone (now recognized as being synonymous with MCAT) “surfaced in Leningrad for the first time in 1982” and was being prepared by the oxidation of ephedrine (Savenko et al., 1989). However, this report was not available until years later. The first mention of ephedrone in the Western literature was as a technical note in a forensic science journal (Zhingel, Dovensky, Crossman & Allen, 1991) and the agent was probably not immediately recognized by most, at the time, as being synonymous with methcathinone.
Methylone (MDMC)
Another early synthetic cathinone was methylenedioxymethcathinone (MDMC, methylone). The agent was independently prepared by two groups of investigators in the mid 1990s (Dal Cason et al, 1997; Jacob & Shulgin, 1996). Methylone is the β-keto analog of MDMA.
Newer synthetic cathinones and “Bath Salts”
Although investigations with cathinone-related analogs occasionally appeared in the scientific literature, there was relatively little scientific interest in synthetic cathinones until Iversen (2010) prepared a report for the UK Home Office entitled “Consideration of the Cathinones”. About two dozen synthetic cathinones were identified as becoming an abuse problem in the European community. One preparation receiving particular attention at the time was “bath salts” which, presumably, included mephedrone, methylenedioxypyrolovalerone (MDPV), and/or methylone (MDMC).1
Mephedrone is the para-methyl analog of MCAT or the beta-keto analog of pTAP (see below); methylone was described above. MDPV was, seemingly, something novel. The first report on the possible abuse of MDPV appeared in 2007 (Fuwa, Fukumori, Tanaka, Kubo, Ogata, Uehara, et al., 2007). Today, dozens of synthetic cathinones are available, they are sold under the general names of, for example, bath salts, plant food, stain removers, insect repellants, glass cleaners, room deodorizers, and are usually labeled “not for human consumption” (e.g. Kelley, 2011; UNODC, 2013).
Amphetamine (i.e., phenylisopropylamine; PIA) analogs
Because synthetic cathinones or β-ketoamphetamines are structurally related to AMPH-like structures, a very brief overview on some simple phenylisopropylamines, or AMPH analogs, will provide a backdrop on what is to come in the subsequent section. Furthermore, it might be noted that certain AMPH analogs (sometimes, even long-known AMPH analogs) are now appearing on the clandestine market as “new” designer drugs. Indeed, although AMPH-analogs and synthetic cathinones do not necessarily produce identical effects, they are inextricably linked. An appreciation of AMPH analogs will assist the understanding of the synthetic cathinones, and will also provide some understanding of their structural evolution.
Structural modifications
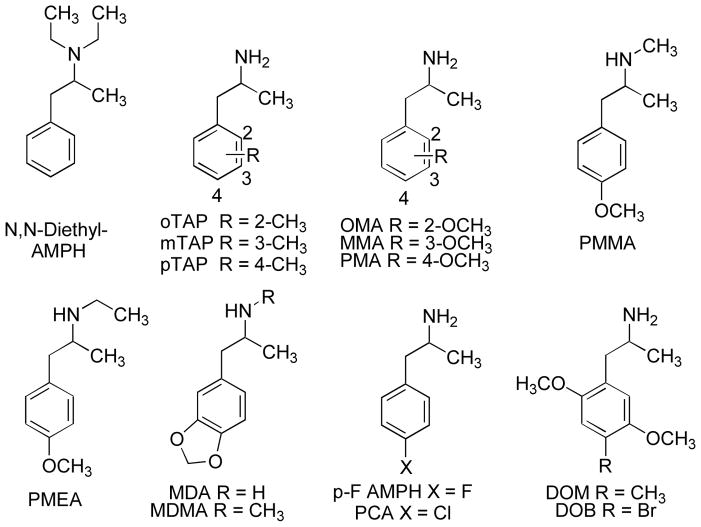
AMPH and its N-monomethyl analog, METH, are well-established central stimulants with an extensive history. But, what happens when minor structural alterations are made to these structures? The simplest structural modification of AMPH analogs involves introduction of a single new substituent. For example, N,N-dimethylamphetamine (DiMe AMPH; Figure 3), the N-methyl analog of METH, has been found on the clandestine market since the early 1990s, but its abuse has never been particularly widespread. At one time it was thought that DiMe AMPH was merely an impurity in the clandestine synthesis of METH. DiMe AMPH has been examined in drug discrimination studies and in self-administration studies employing monkeys. In general, DiMe AMPH seems to be an AMPH-like agent but is at least 10-fold less potent than AMPH or METH (Dal Cason et al., 1997; Katz et al., 1992, Young & Glennon 1986), and S(+)DiMe AMPH is the more potent of the two isomers.
Figure 15.3.

The structures of several basic (i.e., AMPH-like) phenylisopropylamines (top row) and their respective β-ketophenylisopropylamine (i.e., synthetic cathinone) counterparts (bottom row) demonstrating their structural similarity.
S(+)DiMe AMPH preferentially undergoes demethylation to S(+)METH (Lee, Yoo, In, Jin, Kim, 2013); hence, some of the actions of DiMe AMPH might be attributed to the formation of this metabolite. Where investigated, this seems to be a common theme; that is, N,N-dimethyl PIAs generally undergo demethylation to their N-monomethyl products and/or their primary amines (though other routes of metabolism are also possible). In fact, for a series of N-substituted and N,N-disubstituted AMPH-derivatives, it was found that, given the same lipophilicity, i) tertiary amines are excreted faster that secondary amines, which are, in turn, excreted faster than primary amines, and ii) the rate of N-dealkylation increases with the lipophilicity of the agent, but decreases with increasing bulk of the leaving N-substituent (Testa & Salvesen, 1980). In other words, tertiary amine analogs of AMPH can be converted to their secondary amine counterparts, and this is most true when the N,N-dialkyl AMPH analog possesses small, sterically unhindered alkyl groups (such as a methyl or ethyl group). The metabolism of PIAs has been extensively investigated (reviewed: Kraemer & Maurer, 2002). Typical pathways involve N-demethylation or N-dealkylation of N-substituted AMPHs (at least of N-methyl and N-ethyl AMPHs), and O-demethylation of methoxy or methylenedioxy-substituted AMPHs. Depending upon structure, the O-demethylated AMPHs can undergo further O-methylation by catecholamine O-methyltransferase. Some of these metabolites retain psychoactive character (see specific cases) (Kraemer & Maurer, 2002).
Other N,N-disubstituted AMPH analogs are known, but are uncommon. It is recognized that homologation (i.e., extension) of the N-methyl group of METH to an ethyl, n-propyl, or n-butyl group results in a progressive decrease in potency/action as determined in self-administration studies with rhesus monkeys (Woolverton, Shybut & Johanson, 1980). Given that conversion of METH to its tertiary-amine DiMe AMPH counterpart results in at least a 10-fold potency decrease in AMPH-like action, it is perhaps not surprising that there is relatively little literature on N,N-diethyl AMPH or its higher N,N-di-substituted homologs, or agents with very bulky terminal amine substituents.
Conjoining the termini of the two ethyl groups of N,N-diethyl AMPH by a carbon-carbon single-bond results in the pyrrolidine derivative MPEP (compare the structure of N,N-diethyl AMPH in Figure 4 with the structure of MPEP in Figure 3). MPEP possesses central stimulant action. Aminoketones, including what is now termed α-PPP (Figure 3 – discussed later), were prepared as intermediates or synthetic precursors en route to the preparation of their corresponding phenylpropanolamine analogs that were being explored at the time as ephedrine-like (i.e., as sympathomimetic) agents. Reduction of the phenylpropanonamines provided the desired phenylpropanolamines. Over-reduction (i.e., hydrogenolysis of the resulting phenylpropanolamines – not a particularly desired consequence of these studies) resulted in PIAs (including, for example, MPEP) (Heinzelman & Aspergren, 1953). Shortly thereafter, several of these PIA analogs (including MPEP) were patented for their central stimulant actions (Thomae, 1959).
Figure 15.4.
Some phenylisopropylamines described in this section.
Extension of the α-methyl group of MPEP to an n-propyl group results in prolintane (Figure 3). The agent, patented in 1959 (Thomae, 1959), has seen clinical application as a stimulant (although not in the U.S.) for the treatment of, for example, fatigue. Several studies have demonstrated the AMPH-like central stimulant character of prolintane (Hollister & Gillespie, 1970; Kuitunen, Kärkkäinen & Ylitalo, 1984; Nicholson, Stone & Jones, 1980). Now, there are recent reports of prolintane abuse (Gaulier, Canal, Pradeille, Marquet & Lachâtre, 2002; Kyle & Daley, 2007; Payá, Guisado, Vaz & Crespo-Facorro, 2002).
Up to this point, discussion has been focused on the terminal amine and the α-alkyl group of the PIAs. Certainly, other amine substituents can be introduced, and the length and nature of the α-alkyl chain can be varied (i.e., shortened, lengthened, branched). Indeed, a wide variety of such analogs was patented more than 50 years ago (e.g. Thomae, 1959).
AMPH-like agents can also possess substituents on the aromatic ring. For example, there are three monomethyl analogs of AMPH, known as ortho-tolylaminopropane (oTAP), meta-tolylaminopropane (mTAP) and para-tolylaminopropane (pTAP) (Figure 4). In drug discrimination studies, only oTAP fully substituted in (+)AMPH-trained rats and was about 10-fold less potent than (+)AMPH itself (Higgs & Glennon, 1990); mTAP and pTAP disrupted the animals’ behavior (i.e., no conclusions could be drawn). However, both mTAP and pTAP (oTAP was not examined) were self-administered by rhesus monkeys (Wee, Anderson, Baumann, Rothman, Blough & Woolverton, 2005) indicating at least some potential for abuse liability. There are three monomethoxy analogs of AMPH: (the ortho-methoxy analog OMA, the meta-methoxy analog MMA, and the para-methoxy analog PMA) (Figure 4); all three substituted in rats trained to discriminate (+)AMPH from vehicle in tests of stimulus generalization (Glennon, Young & Hauck, 1985). That is, these agents were able to produce AMPH-like stimulus effects in animals. Dimethoxy and trimethoxy analogs failed to substitute. PMA and its N-monomethyl analog, para-methoxymethamphetamine (PMMA) (Figure 4), have been abused and are responsible for a number of deaths over the years (see: Zaitsu, Katagi, Kamata, Kamata, Shima, Tsuchihashi, et al., 2008), and now a homolog of PMMA, para-methoxy-N-ethylamphetamine (PMEA) (Figure 4) has appeared as a new designer drug (Zaitsu et al., 2008). Methylenedioxy analogs of AMPH have been known for some time. A rather Interesting PIA is 1-(3,4-methylenedioxyphenyl)-2-aminopropane or MDA (Figure 4). Its S(+)-isomer behaves as a central stimulant whereas its R(−)-isomer acts more like a classical hallucinogen (Young & Glennon, 1996). Its N-monomethyl homolog is the well-known empathogen MDMA (Figure 4).
A variety of halogenated AMPH analogs has been studied. For example, the meta-fluoro and para-fluoro (i.e., p-F AMPH; Figure 4) analogs of AMPH were self-administered in rhesus monkeys (Wee et al., 2005) and both compounds, including the para-fluoro analog (as well as the para-fluoro analogs of METH, N-ethylamphetamine, and α-ethylamphetamine) have been encountered on the clandestine market (Rösner, Quednow, Girreser & Junge, 2005). The para-chloro analog of AMPH (i.e., PCA; Figure 4) has also appeared on the illicit market (Lin, Lin & Lua, 2011) as has the 5-fluoro analog of OMA and the 3-fluoro analog of PMA (Rösner et al., 2005). Numerous combinations and permutations of aryl-substituted, N-substituted phenylisopropylamines are possible. Many have been examined (e.g. Shulgin and Shulgin, 1991), quite a few have been encountered on the clandestine market and, undoubtedly, more are likely to appear.
The number of potential psychoactive PIAs, or chain-extended PIAs, is staggering. Certainly, many PIA analogs lack AMPH-like stimulant properties. This does not necessarily mean they are inactive. For example, depending on the number and type of substituents, certain PIAs are classical hallucinogens (Glennon, 1996); these types of agents, typified by DOM and DOB (Figure 4), will not be described here. The above discussion was simply meant to be a sampling of the types of PIAs that have been investigated, and nearly all have been found on the clandestine market. This discussion serves as an introduction to the synthetic cathinones; recall that introduction of a β-keto group to a PIA converts it to a phenylpropnanonamine. In the section that follows, many of the same substitution patterns described above will be re-encountered.
Synthetic cathinones: Specific agents
Simple structural modifications
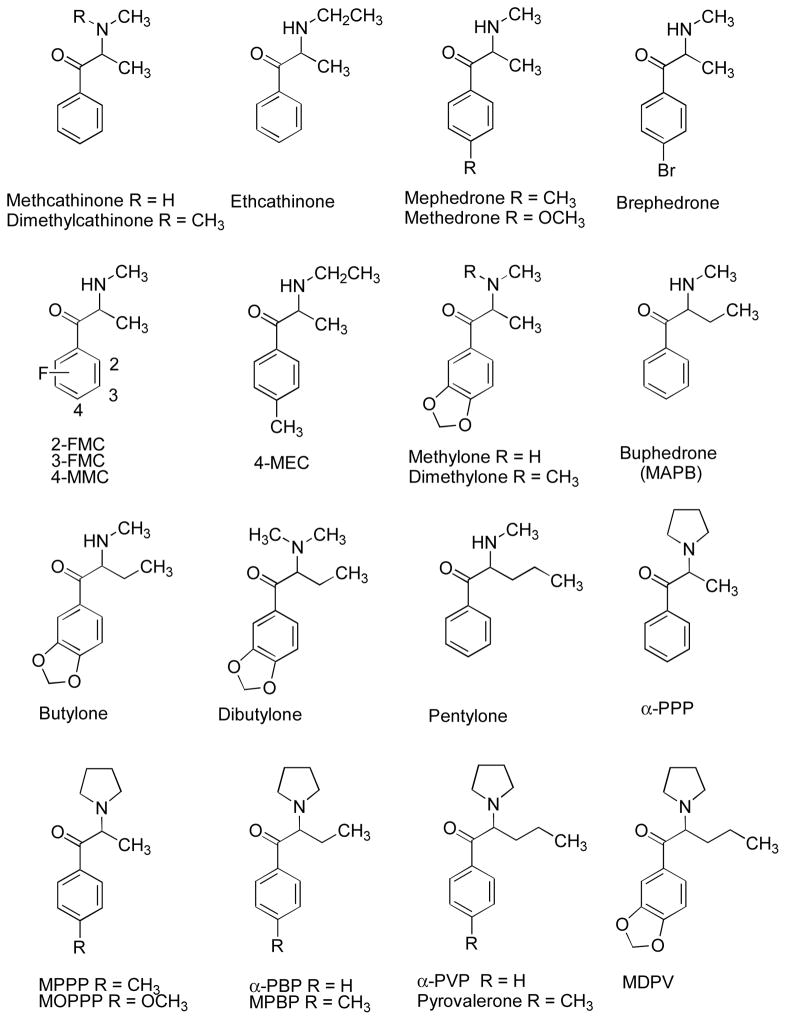
The simplest modified cathinone or MCAT analog is dimethylcathinone, or the β-keto analog of DiMe AMPH. The agent, synthesized in 1954 (Iwao, Kowaki & Rakemi, 1954) and later patented as an anorectic agent together with diethylpropion and several related structures in 1961 (Schütte, 1961), is known by a number of names including dimethylpropion, dimepropion, and metamfepramone. There are some anecdotal reports of its abuse. It has also been identified by the United Nations Office of Drugs and Crime (UNODC, 2013). Dimethylcathinone is metabolized to MCAT and methylpseudoephedrine (Markantonis, Kyroudis & Beckett, 1989). This has led to recent efforts to differentiate among the three substances using instrumental methods (Thevis, Sigmund, Thomas, Gougoulidis, Rodchenkov & Schanzer, 2009). [It might be noted that most of the terminology and acronyms used herein are those used by the UNODC (2013); for the most part, these were not those originally used to describe the agents when they were first reported in the scientific or patent literature.]
Another simple structural modification of MCAT is homologation of the N-methyl substituent to an N-ethyl group (i.e., ethcathinone; Figure 5). Aromatic substituents, similar to those described above for the AMPH analogs, have been incorporated into cathinone and MCAT. For example, the 4-methyl analog of MCAT is known as mephedrone (4-methylmethcathinone or 4-MMC) whereas its corresponding 4-methoxy analog is termed methedrone (4-methoxymethcathinone or PMMC, by analogy to para-methoxymethamphetamine or PMMA). Mephedrone is the most widely seized synthetic cathinone by European law enforcement officials (UNODC, 2013). Other aryl-substituted cathinone analogs include 4-bromomethcathinone (brephedrone, 4-BMC), all three positional isomers of fluromethcathinone (2-FMC, 3-FMC, 4-FMC), and the 3,4-methylenedioxy analog of MCAT (methylone, MDMC) (Figure 5).
Figure 15.5.
Structure of some synthetic cathinones described in this section. All (including about two dozen others) have been reported by the United Nations Office of Drugs and Crime (UNODC, 2013).
Complex structural modifications
Terminal amine and aryl modifications can appear in the same agent. For example, the 4-methyl counterpart of ethcathinone is 4-methylethcathinone or 4-MEC, whereas the 3,4-methylenedioxy counterpart of dimethylcathinone is dimethylone. The latter agent (Iwao et al., 1954), as well as 3,4-dimethoxy- and 4-methoxydimethylcathinone were first investigated in the 1950s as sympathomimetic agents, but their central stimulant properties were not examined (Shapiro, 1950).
A common molecular modification among the synthetic cathinones is homologation of the α-methyl group. Perhaps the first “extended” cathinone analog is what is now termed buphedrone (MAPB) (Hyde et al., 1928). Buphedrone-related agents include butylone and dibutylone. Further extension of the chain results in pentylone (Hyde et al., 1928) (Figure 5). Here, too, their stimulant character was not a subject of investigation at the time.
Constraint of the ethyl substituents of N,N-diethyl AMPH (Figure 4) by conversion to a pyrrolidine ring afforded MPEP (Figure 3). A similar strategy in the cathinone series results in α-pyrrolidinopropiophenone (α-PPP). α-PPP, along with its 4-methoxy analog (now known as MOPPP) (Figure 5), were first prepared by Heinzelman and Aspergren (1953) as precursors for the synthesis of sympathomimetic amines. Replacement of the pyrrolidine ring of α-PPP with a piperidine ring affords its piperidinyl counterpart (Iwao et al. 1954). Several years later, α-PPP was one of a number of agents (including 1-piperidynyl, morpholinyl, and N-methylpiperazinyl derivatives) patented as anorectic agents (Schütte, 1961). Extension of the α-PPP side chain to an ethyl group results in α-pyrrolidinobutyrophenone (α-PBP), and further extension to an n-propyl group results in α-pyrrolidinovalerophenone (α-PVP) (Figure 5); both agents (including 4-methyl, 4-methoxy, and 4-chloro α-PVP) were patented in 1964 as central stimulants (Seeger, 1964). The 4-methyl analog of α-PVP, pyrovalerone, and a number of related analogs have been recently prepared (Meltzer, Butler, Deschamps & Madras, 2006). α-PVP and its 4-methyl, 4-methoxy, and 4-chloro derivatives were patented earlier as central stimulants (Wander, 1963), but no pharmacological data were provided. Methylenedioxypyrovalerone (MDPV), an occasional component of “bath salts” was patented as a central stimulant in 1969 (Boehringer Ingelheim, 1969; Köppe, Ludwig & Zeile, 1969).
Metabolism
It is rather remarkable that many agents now termed synthetic cathinones were initially examined as anorectic agents or central stimulants (patented, primarily, by the pharmaceutical industry) in the 1960s or earlier. It might also be noted that novel synthetic cathinones (and phenylpropanolamines) can result from the metabolism of known synthetic cathinones and become a potential source of new drugs of abuse. For example, one of the metabolites of MCAT is cathinone (Beyer, Peters, Kraemer & Maurer, 2007; Paul & Cole, 2001), and cathinone is pharmacologically active as a central stimulant. In addition, cathinone can be further metabolized to norephedrine or cathine, depending upon the cathinone isomer ingested, and cathine, too, is a known (although weak) central stimulant. The pharmacology (and toxicology) of most synthetic cathinone metabolites has yet to be studied.
Typically, cathinones undergo N-dealkylation (as mentioned above for AMPH-related agents), and reduction of the carbonyl group to an alcohol (i.e., a phenylpropanolamine). When a 3,4-methylenedioxy group is present, it undergoes ring-opening to afford a dihydroxy intermediate that is eventually converted to its corresponding 3-hydroxy-4-methoxy and/or 4-hydroxy-3-methoxy counterparts; with pyrrolidine-containing compounds, the pyrrolidine ring can either be oxidized to a lactam (that, in some cases, undergoes ring opening) or is converted to an iminium salt that is subsequently hydrolyzed to the corresponding ketone. The metabolism of many of the synthetic cathinones shown in Figure 5 has been examined, including: cathinone (Beyer et al., 2007; Brenneisen, Geisshüsler & Schorno, 1986), MCAT (Beyer et al., 2007; Paul & Cole, 2001), methylone (Kamata, Shima, Zaitsu, Kamata, Miki, Nishikawa, et al., 2006). 3-FMC (Pawlik, Plässer, Mahler & Daldrup, 2012), butylone (Zaitsu, Katagi, Kamata, Kamata, Shima, Miki et al., 2009), α-PPP (Springer, Fritschi & Maurer, 2003b; Meyer, Du, Schuster & Maurer, 2010) and its 3,4-methylenedioxy counterpart (Springer, Fritschi & Maurer, 2003a), MPPP (Springer, Peters, Fritschi & Maurer, 2002; Springer et al., 2003b; Springer, Paul, Staack, Kraemer & Maurer, 2003c), MOPPP (Springer, Staack, Paul, Kraemer & Maurer 2003d), MPBP (Westphal, Junge, Rösner, Fritschi, Klein & Girreser, 2007), α-PVP (Springer et al., 2003d), and MDPV (Meyer, Du, Schuster & Maurer, 2010; Strano-Rossi, Cadwallader, de la Torre & Botrè, 2010). Ammanna et al. (Ammanna, McLaren, Gerostamoulos & Beyer, 2012) have also examined 25 synthetic cathinone analogs in an attempt to develop instrumental assays that can differentiate amongst them. More such studies are required to separate and/or identify newer cathinones and cathinone metabolites.
Synthetic Cathinones: Mechanisms of Action and Behavioral Studies
Many of the “new” synthetic cathinones have not been extensively investigated and only very recently has attention been focused on these agents. Hence, potency comparisons are elusive (and, for reasons to be discussed below, are often difficult to make). Mechanistic data are scant. That is, although there is some new information on what a few specific synthetic cathinone analogs might “do” (transporter-wise, receptor-wise, and behaviorally), for the most part their behavioral actions (in the few cases where such data are available) have not been specifically related to specific mechanisms of action.
Transporter studies
Cathinone and MCAT were shown quite some time ago to cause release of DA (Glennon et al., 1987). Subsequently, S(−)MCAT was found to act at NET, DAT and SERT and displayed potencies similar to S(+)METH (i.e., NET ≈ DAT > SERT) (Rothman, Vu, Partilla, Roth, Hufeisen, Compton-Toth, et al., 2003). Notable is that reduction of the keto group of S(−)MCAT, to afford (−)ephedrine and (+)pseudoephedrine, resulted in decreased potency at NET and DAT, and loss of activity at SERT. Others have since found that MCAT is nearly equipotent as a DA releasing agent and reuptake inhibitor, whereas cathinone was several-fold more potent as a releasing agent (Simmler, Buser, Donzelli, Schramm, Dieu, Huwyler et al., 2012). Both agents were more potent at releasing NE than DA, and neither agent had a significant effect at SERT. MCAT and methylone were substantially less potent as inhibitors of the vesicular monoamine transporter (bovine VMAT2) than at inhibiting transmembrane reuptake by serotonin (human platelets), DAT and NET (expressed in human glial cells) (Cozzi, Sievert, Shulgin, Jacob III & Ruoho, 1999).
Amongst the newer synthetic cathinones, two of the first to be examined were mephedrone and MDPV. Using a frog oocyte preparation transfected with hDAT, mephedrone produced DA-like depolarization whereas MDPV produced cocaine-like hyperpolarization (Cameron, Kolanos, Solis, Glennon & De Felice, 2013a; Cameron, Kolanos, Vekariya, De Felice & Glennon, 2013b; Kolanos, Cameron, Vekariya, De Felice & Glennon 2011). These are signatures of a releasing agent and a reuptake inhibitor, respectively. Simmler et al. (2012) found mephedrone to be nearly equipotent as an inhibitor and releaser of DA and 5-HT; it was substantially more potent as an inhibitor of NET. In contrast, MDPV was a potent inhibitor of DAT and NET, a very weak inhibitor of SERT, but neither released DA or 5-HT (Simmler et al., 2012). Others (Eshleman, Wofrum, Hatfield, Johnson, Murphy & Janowsky, 2013) reported comparable results. A study of butylone, methylone, ethylone, flephedrone pyrovalerone, MDPV, and several other agents concluded that all of the cathinone analogs were inhibitors of the three monoamine transporters, but with varying selectivities; most of the compounds (with the exception of methylone, pyrovalerone, and MDPV) were substrate releasers (Simmler et al., 2012). These same agents displayed low affinity for 5-HT1A, 5-HT2A, 5-HT2C, D1, D2, D3, H1 histamine receptors, α1A- and α2A-adrenoceptors (Simmler at al., 2012). Pyrovalerone and several related agents had been found earlier to act as DAT/NET inhibitors, to have little effect at SERT, and to lack affinity for 5-HT1A, 5-HT1B, 5-HT2C, D1, D2, or D3 receptors (Meltzer et al., 2006). Mephedrone displayed low micromolar affinity 5-HT2 receptors and even lower affinity for DA receptors (Martínez-Clemente, Escubedo, Pubill & Camarasa, 2012). An examination of a series of cathinone analogs revealed that 4-FMC, mephedrone, and methylone, but not butylone or MDPV, generally induced release of neurotransmitter from DAT, NET, and SERT; these agents were also shown bind with low (i.e. μM) affinity at 5-HT1A, 5-HT2A, and 5-HT2C receptors, with little to no affinity for DA receptors (Eshleman et al., 2013). Iversen et al. (Iversen, Gibbons, Treble, Setola, Huang & Roth, 2013) examined the binding of several synthetic cathinones (including mephedrone, 4-MEC, and four others not discussed here) at 49 receptors and transporters. Except for the transporters, and a modest affinity (pKi = 6.1) for mephedrone at 5-HT2B receptors, the agents typically displayed, at best, micromolar affinity. Synthetic cathinones currently being abused seem to produce their actions primarily at the DA, norepinephrine (NE), and/or serotonin (5-HT) transporter; that is, they either release and/or block the reuptake of one or more of these neurotransmitters. Simmler et al. (2012) suggested a classification of various cathinone analogs, based on their transporter profiles, as i) cocaine-MDMA mixed cathinones, ii) metamphetamine-like cathinones, and iii) pyrovalerone cathinones. Additional agents will need to be examined, and careful SAR studies need to be performed, but transporter profiles will certainly be a key to unraveling the behavioral (and other) actions of these agents. In a recent study, for example, it was demonstrated that both the extended chain and the pyrrolidine moiety of pyrovalerone- or MDPV-type agents need not be present for the agents to function as hyperpolarizing agents at DAT expressed in frog oocytes; for example dimethylone, 3,4-methylenedioxy-α-PPP, and N-methyl-3,4-methylenedioxypentylone all produced MDPV-like hyperpolarization (i.e., cocaine-like DAT inhibition) (Kolanos, Solis, Sakloth, De Felice & Glennon, 2013).
Locomotor studies
Like AMPH, METH and cathinone (vide supra), some synthetic cathinones can increase rodent locomotor actvity. Racemic MCAT produced locomotor stimulation in mice similar to that produced by cathinone itself (Glennon et al., 1987; van der Schoot et al., 1962); the rank order of potency of its optical isomers was S(−)MCAT > S(+)AMPH ≥ R(+)MCAT (Glennon et al., 1995). Likewise, mephedrone and methylone produced hyperlocomotion in rats (Baumann, Ayestas, Partilla, Sink, Shulgin, Daley et al., 2012; Kehr, Ichinose, Yoshitake, Goiny, Sievertsson, Nyberg, et al., 2011; Marusich, Grant, Blough & Wiley, 2012; Motbey, Hunt, Bowen, Artiss & McGregor, 2011) and mephedrone produced a similar effect in mice (Angoa-Perez, Kane, Fracescutti, Sykes, Shah, Mohamed, et al., 2012). Mephedrone, methylone, and butylone produced hyperlocomotion in mice (potency: METH > butylone > methylone ≥ mephedrone) (López-Arnau, Martinez-Clemente, Pubill, Escubedo & Camarasa, 2012), and mephedrone increased wheel-running activity in rats (Huang, Aarde, Angrish, Houseknecht, Dickerson & Taffe, 2012). Mepherdone and MDMA induced the release both of DA and 5-HT in rat nucleus accumbens that was accompanied by increased locomotor activity (Kehr et al., 2011).
MDPV was reported to “exhibit extraordinarily powerful central nervous system stimulating activities in warm-blooded animals” (Köppe et al., 1969) but no data were provided, and oral administration of a single dose of MDPV increased mouse locomotor activity (Fuwa et al., 2007). Huang et al. (Huang, Aarde, Angrish, Houseknecht, Dickerson & Taffe, 2012) found that MDPV behaved in a manner similar to that of (+)METH in a wheel-turning locomotor assay, but differently than that of mephedone, suggesting that different mechanisms of action might be involved. Others have found that MDPV is a locomotor stimulant in mice, and that its effects are potentiated by warm ambient temperatures (Fanttegrossi et al., 2013).
Marusich et al. (2012) compared the hyperlocomotor actions of six synthetic cathinones (including mephedrone, methylone, methedrone, MDPV, 3-FMC, and 4-FMC) in rats; all were locomotor stimulants with MDPV being among the most potent and methedrone being the least potent. MDPV was found to be comparable in potency to (+)METH as a locomotor stimulant (Aarde, Huang, Creehan, Dickerson & Taffe, 2013). Another mouse locomotor study examined six synthetic cathinones and resulted in the following order of potency: S(+)METH > MDPV ≅ mephedrone > methylone > flephedrone > butylone > cocaine > naphylone; the stimulant actions of MDPV were long-lasting (i.e., 250 to 300 min depending upon dose) (Gatch, Taylor & Forster, 2013).
AMPH-related stimulants produce hyperlocomotion in rodents that is related to increased DA transmission (Ljungberg & Ungerstedt, 1985). Although weaker than AMPH, PMA, the para-methoxy analog of AMPH, is a locomotor stimulant; its actions seem to be mediated through a serotonergic rather than dopaminergic mechanism (Loh & Tseng, 1978). N-Monomethylation of PMA to PMMA results in a loss in locomotor stimulant action (Glennon, Ismaiel, Martin, Poff & Sutton, 1988). However, introduction of a β-keto group, converting PMMA to methedrone, reintroduces hyperlocomotor character. It has been reported that 5-HT (i.e., 5-HT2A) receptors play a role in DA release and locomotor responses to AMPH (Auclair, Drouin, Cotecchia, Glowinski & Tassin, 2004). Indeed, pretreatment of mice with the 5-HT2 antagonist ketanserin or the DA antagonist haloperidol blocked the hyperlocomotor actions of methylone and butylone and partially inhibited the actions of mephedrone; pretreatment with a 5-HT1B antagonist reduced the actions of butylone but failed to inhibit the locomotor actions of methylone or butylone (López-Arnau et al. 2012).
Bupropion, the N-tert-butyl analog of 3-chlorocathinone, is a clinically employed antidepressant, In a comparison of rat locomotor action, several cathinone analogs produced hyperlocomotor effects with relative potencies of MCAT > 3-bromomethcathinone (3-BMC) > bupropion (Foley & Cozzi, 2003). The 4-bromo positional isomer of 3-BMC, 4-BMC, was inactive at the highest dose evaluated, but the des-chloro analog of bupropion produced an effect comparable to that of a similar dose of bupropion (Foley & Cozzi, 2003). 4-(Trifluoromethyl)methcathinone failed to increase rat horizontal motor action (Cozzi, Brandt, Daley, Partilla, Rothman, et al., 2013). Compared to methcathinone, all three possible trifluoromethyl analogs were less potent at releasing or blocking the reuptake of DA, NE, and 5-HT, but introduction of a ring substituent at the 3- or 4-positions increased their potency at SERT and decreased potency at DAT and NET resulting in agents with enhanced SERT-selectivity (Cozzi et al., 2013). In another recent study, the hyperlocomotor potencies of six cathinone analogs were found to correlate with their binding at VMAT2 and inhibition of NE uptake by VMAT2 (Gatch et al., 2013).
Drug discrimination studies
Few cathinone analogs have been examined in drug discrimination studies, and even fewer have been used as training drugs. In (+)AMPH-trained rats, cathinone and its individual optical isomers substituted (S-cathinone was more potent than R-cathinone), but α-desmethylcathinone failed to generalize (Glennon et al., 1984; Kalix & Glennon, 1986). Lacking an α-methyl group, α-desmethylcathinone might not readily penetrate the blood-brain barrier. S(−)Cathinone (the R-isomer was not examined) also substituted in cocaine-trained rats (Woolverton, 1991). Racemic MCAT and both of its optical isomers substituted for (+)AMPH (Glennon et al., 1995; Glennon et al., 1987) and for cocaine (Young & Glennon, 1993). α-Desmethylcathinone and β-aminopropiophenone failed to substitute (Kalix & Glennon, 1986). S(−)Dimethylcathinone, (±)dimethylcathinone, ethcathinone, N-n-propylcathinone, and methylone (listed in decreasing order of potency) substituted in (+)AMPH-trained rats, but 3,4-methylenedioxycathinone (i.e., the N-desmethyl counterpart of methylone) did not (Dal Cason et al., 1997). S(−)Methcathinone, but not S(+)METH, substituted in ()ephedrine-trained rats (Bondareva, Young & Glennon, 2002). With racemic cathinone as the training drug, stimulus generalization occurred to both cathinone optical isomers (S > R), cathine, (+)AMPH, METH, and cocaine, but not to α-desmethylcathinone, 4-hydroxycathinone, 4-methoxycathinone, or 4-chlorocathinone (Glennon et al., 1984; Schechter & Glennon, 1985), nor 4-fluorocathinone (unpublished data). Likewise, (+)AMPH, cocaine, cathine, but not α-desmethylcathinone, substituted in cathinone-trained rats (Goudie, Atkinson & West, 1986). S(−)MCAT-trained rats recognized (±)MCAT, S(+)METH, cathinone, R(−)MCAT, cocaine and several other central stimulants (Young & Glennon, 1998). Clearly, cathinone and MCAT produce stimulus effects similar to those of other central stimulants, and alteration of structure influences their potency and actions. Furthermore, the S(−)MCAT stimulus was potently antagonized by the DA antagonist haloperidol (Young & Glennon, 1998). However, MDMA substituted in rats trained to discriminate mephedrone, but full substitution failed to occur with METH or cocaine; furthermore, the mephedrone stimulus could not be antagonized by pretreatment of the animals with haloperidol (Varner, Daigle, Weed, Lewis, Mahne, Sankaranarayanan et al., 2012). It was recently demonstrated, using mice trained to discriminate MDPV from saline, that substitution occurred following administration of (±)METH and (±)MDMA (Fantegrossi et al., 2013) suggesting similarities amongst the stimulus actions of the three agents. In rats trained to discriminate S(+)METH from vehicle, each of the following agents was found to substitute, with relative potencies of S(+)METH > MDPV > mephedrone > butylone ≅ methylone ≅ flephedrone ≅ naphylone, whereas in cocaine-trained rats their relative potencies were MDPV > mephedrone ≅ methylone > naphylone ≅ cocaine ≅ flephedrone > butylone (Gatch et al., 2013). Clearly, additional studies are required to better understand the complex stimulus properties of the synthetic cathinones. Nevertheless, it would appear that these agents do not represent a behaviorally homogeneous class.
Other studies
MDPV, but not mephedrone, produced stereotypic behavior in rats (Aarde, et al., 2013; Huang et al., 2012), and MDPV produced stereotypy in mice (Fanttegrossi et al., 2013). Mephedrone was self-administered and increased core body temperature (rats) (Hadlock, Webb, McFadden, Chu, Ellis, Allen et al., 2011). MDPV had only a negligible effect on body temperature (Aarde et al., 2013). In mice, hyperthermia following MDPV administration was observed only at warm ambient temperatures (Fantegrossi et al., 2013). MDPV was also self-administered by rats (Aarde et al., 2013; Watterson, Kufahl, Nemirovsky, Sewalia, Grabenauer, Thomas, et al. 2013) and was more potent and efficacious than S(+)METH. MCAT, methylone, mephedrone and MDPV facilitated intracranial self-stimulation (ICSS) in rats; MCAT displayed the highest efficacy and mephedrone the lowest (Bonano, Glennon, De Felice, Banks & Negus (2013). Several studies have examined the “binge-like” actions of methedrone by administration of multiple doses (Angoa-Perez et al., 2012; Hadlock et al (2011). In one such study, it was shown that mephedrone enhanced the hyperthmic action of (+)METH and enhanced the neurotoxic actions of (+)AMPH and MDMA on DA nerve endings (Angoa-Perez et al., 2012).
Synthetic Cathinones: Human Studies
The desired effects of synthetic cathinones apparently include euphoria, mental alertness, talkativeness, sexual arousal, a focused mind, and overall positive feelings; the effects generally occur within 30 to 45 minutes following administration and last from 1 to 3 hours (Marinetti & Antonides, 2013). The undesirable effects, primarily neurological and cardiovascular, can last for hours to days (Marinetti & Antonides, 2013). This is probably a fairly accurate, if not somewhat generalized, statement. No controlled clinical studies have been performed with synthetic cathinones. What makes descriptions of the human pharmacology of specific synthetic cathinones particularly difficult is that i) the various preparations are known by dozens of names, ii) some preparations contain multiple constituents – up to as many as 10 (Gil, Adamowicz, Skulska, Tokarczyk & Stanaszek, 2013), iii) the constituents are constantly changing (even if a “brand name” doesn’t), and iv) individuals presenting at emergency departments typically are unaware of specifically what they have taken. Another confounding factor is the route of administration. For example, synthetic cathinone preparations can be administered orally, rectally, intramuscularly, intravenously, or by inhalation (Prosser & Nelson, 2012); route of administration will likely influence potency, rate of onset, duration of action, and metabolism. Self-reported doses range from a few milligrams to >1 gram (and, of course, certain synthetic cathinones are more potent than others given a common route of administration). Because users cannot be certain of the contents or purity of the drug, self-reporting results can be highly variable (Prosser & Nelson, 2012).
To illustrate the complexity of the problem, a few examples are provided. Samples of 24 products sold as Energy (e.g. NRG-1, NRG-2) in the UK were analyzed and 70% contained mixtures of cathinones including mephedrone, butylone, flephedrone, and MDPV (Brandt, Sumnall, Measham & Cole, 2010). A follow-up study additionally identified pentylone, MPPP, and MDPBP (i.e., the 3,4-methylenedioxy analog of α-PBP) (Brandt, Freeman, Sumnall, Measham & Cole, 2011). A similar study conducted in the US on 15 “brand name” products identified single-component preparations (e.g. mephedrone, MDPV, methylone) and mixtures of synthetic cathinones (e.g. mephedrone + MDPV + methylone, MDPV + methylone); in two instances, products occurring with the same “brand name” and packaging consisted of different synthetic cathinones (Spiller, Ryan, Weston & Jansen, 2011).
A retrospective analysis of 236 poison center records for “bath salts” exposure revealed 39 separate “brand names” from patient histories; of these, the two most frequently cited were Cloud 9 and White Lightening (Spiller et al., 2011). The most common symptoms included agitation, violent behavior, tachycardia, hallucinations, and paranoia. Perhaps the first study to report analytically-confirmed mephedrone intoxication was that by Wood et al. (Wood, Davies, Greene, Button, Hollt, Ramsey et al., 2010) who found that the clinical features were consistent with an acute sympathomimetic toxidrome (e.g. hypertension, tachycardia, and agitation). An analysis of 32 cases of presumed synthetic cathinone use in Ohio, including 23 postmortem studes, quantitatively identified MDPV, methylone, pentylone, pyrovalerone, α-PVP, and methedrone (Marinetti & Antonides, 2013).
In a case series it was suggested that a consumed “bath salt” product consisted of mephedrone (Kasick, McKnight & Klisovic, 2012); in another study it was argued that because MDPV, but not mephedrone, can produce a false positive for phencyclidine (a finding common to both studies), the substance consumed in the first study might have been MDPV (Penders, Gestring & Vilensky, 2012). Both studies found that the agents produced an extreme degree of psychomotor agitation and violent behavior (referred to as excited delerium syndrome or ExDS). In another case study describing similar symptoms, both MDPV and flephedrone were identified in blood and urine, and an analysis of the actual powdered material showed a nearly equal mixture of the two agents (Thornton, Gerona & Tomaszewski, 2012). A clearer case study described a patient exhibiting unusual behavior, severe agitation, altered mental status, tachycardia, hypertension, and ultimately multi-organ failure, who tested positive for MDPV and negative for about three dozen other drugs of abuse including mephedrone (Borek & Holstege, 2012).
A case series identified 4-MEC in powdered form, and in two of the three cases, the subjects’ blood and/or urine 4-MEC levels were measured; in two cases, subjects had a high blood-alcohol level whereas in the third case, resulting in death, AMPH, PMA, PMMA were also identified (Gil et al., 2013).
Some studies on the acute clinical/subjective effects (with route of administration and duration of action) of mephedrone, methylone, MDPV, methedrone, and butylone have been reviewed (Karila & Reynaud, 2010; Kelley, 2011; Prosser & Nelson, 2012).
Treatment for patients with exposure to synthetic cathinones is primarily supportive and consists of benzodiazepines to control agitation and siezures (e.g. Borek & Holstege, 2012; Prosser & Nelson, 2012). Haloperidol (Kasick et al., 2012) and droperidol (Thornton et al, 2012) also have been used. The health risks of mephedrone (Dybdal, Holder, Ottoson, Sweeney & Williams, 2013) and MDPV (Coppola & Mondola, 2012) have been reviewed. Specific treatments have also been recently reviewed (Zawilska & Wojcieszak, 2013).
A problem, likely restricted to MCAT abuse alone, due to a method of preparation – oxidation of ephedrine with potassium permanganate – is manganism caused by manganese in impure samples. The parkinsonian-like extrapyramidal syndrome of manganism is irreversible and unresponsive to treatment with levodopa (e.g. Sikk, Haldre, Aquilonius, Asser, Paris, Roose, et al, 2013).
Conclusions
Synthetic cathinones are either β-keto analogs of known PIAs, or are chain-extended derivatives thereof. Many so-called “synthetic cathinones” have been known for quite some time in the scientific or patent literature (although they were never termed such), and some are simply derived from the application of AMPH-like or PIA-like SAR to cathinone or MCAT. PIAs do not represent a functional or mechanistically homogeneous class of agents! Thus, there is no reason to suspect that phenylpropanonamines (i.e., synthetic cathinones) will be any more homogeneous in their actions or mechanisms of action, We indicated, more than 15 years ago, that cathinone analogs will need to be examined on a case-by-case basis (Dal Cason et al., 1997). The results presented above now echo this sentiment. Much can be learned about synthetic cathinones by examining their corresponding AMPH-like counterparts. But, the addition of a β-keto group can influence function in unexpected ways. Some synthetic cathinones (e.g. MCAT) function as might be expected – that is, the resulting agent is simply a more selective and potent stimulant (and DA releasing agent) than its corresponding AMPH-counterpart – METH. Other synthetic cathinones, due to minor tweaks in their ability to release or block the reuptake of 5-HT, DA, and/or NE, possess different qualities. Some inroads have been made, but much more needs to be done to understand this large, and growing, class of agents. For the most part, these agents either block and/or release the reuptake of DA, 5-HT, and/or NE. Selectivity profiles remain to be fully investigated. And such studies are ongoing. None of the agents, thus far, show any significant affinity for the various neurotransmitter receptors at which they have been examined (at least, no trend has yet been identified), except for their affinity at the various transporters.
To conclude, synthetic cathinones represent a heterogeneous class of psychoactive agents that likely act, primarily, at one or more of three major neurotransmitter transporters, by release and or reuptake, or both, and require much more investigation on a case-by-case basis.
Acknowledgments
Work from our laboratories described above was supported by DA01642 and DA033930.
Abbreviations
- AMPH
amphetamine
- 3-BMC
3-bromomethcathinone
- 4-BMC
4-bromomethcathinone
- DA
dopamine
- DAT
dopamine transporter
- DiMe AMPH
N,N-dimethylamphetamine
- DMC
N,N-dimethylcathinone
- DOM
1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane
- 2-FMC
2-fluoromethcathinone
- 3-FMC
3-fluoromethcathinone
- 4-FMC
4-fluoromethcathinone
- MAPB
buphedrone
- MCAT
methcathinone
- MDA
1-(3,4-methylenedioxyphenyl)-2-aminopropane
- MDMA
N-methyl-1-(3,4-methylenedioxyphenyl)-2-aminopropame
- MDMC
methylone
- MDPBP
3,4-methylenedioxy-α-PBP
- MDPV
methylenedioxypyrovalerone
- 4-MEC
4-methylethcathinone
- METH
methamphetamine
- MMA
3-methoxyamphetamine
- MOPPP
4-methoxy-α-PPP
- MPEP
1-phenyl-2-pyrrolidin-1-yl)propane
- NET
norepinephrine transporter
- NPS
new psychoactive substance
- OMA
2-methoxyamphetamine
- PCA
4-chloroamphetamine
- PIA(s)
phenylisopropylamine(s)
- PMA
4-methoxyamphetamine
- PMEA
3-methoxy-N-ethylamphetamine
- PMMA
4-methoxymethamphetamine
- α-PBP
α-pyrrolidinobutyrophenone
- α-PPP
α-(pyrrolidin-1-yl)propiophenone
- α-PVP
α-(pyrrolidin-1-yl)valerophenone
- SAR
structure-activity relationships
- SERT
serotonin transporter
- mTAP
meta-tolylaminopropane
- oTAP
ortho-tolylaminopropane
- pTAP
para-tolylaminopropane
- UNODC
United Nations Office of Drugs and Crime
- VMAT
vesicular monoamine transporter
Footnotes
There is anecdotal information that “bath salts” initially contained one, two, or more of these agents (perhaps in combination with other agents). Three synthetic cathinones were scheduled (US Schedule I) in 2011 with the statement that: “Mephedrone, methylone, and MDPV are falsely marketed as … ‘bath salts’.” (Federal Register, 2011); the exact composition of “bath salts” was not specified. Spiller et al. (2011) have used the term collectively to refer to individual synthetic cathinones as well as to combinations of these agents. Indeed, the term “bath salts” has morphed into a generic term that now encompasses nearly any synthetic cathinone, alone, or in combination with other agents. The term “bath salts” does not refer to a specific agent or an unvarying combination of agents.
Conflict of Interest: The author has no conflicts of interest to declare.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hebshi NN, Skaug N. Khat (Catha edulis) – an updated review. Addiction Biology. 2002;10:299–307. doi: 10.1080/13556210500353020. [DOI] [PubMed] [Google Scholar]
- Alles GA, Fairchild MD, Jensen M. Chemical pharmacology of Catha edulis. Journal of Medicinal and Pharmaceutical Chemistry. 1961;3:323–352. doi: 10.1021/jm50015a010. [DOI] [PubMed] [Google Scholar]
- Ammanna D, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: part 2 – designer cathinones. Journal of Analytical Toxicology. 2012;36:381–389. doi: 10.1093/jat/bks049. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Carrier NCM. U K Home Office Report. 2011. Khat: Social harms and legislation. A literature review. [Google Scholar]
- Angoa-Perez M, Kane MJ, Fracescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn D. Mephedrone, and abused psychoactive component of “bath salts” and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. Journal of Neurochemistry. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and α1B adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. European Journal of Neuroscience. 2004;20:3073–3084. doi: 10.1111/j.1460-9568.2004.03805.x. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Journal of Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardelli A, Capocaccia L, Pacitti C, Tancredi V, Quinteri F, Elmi A. Behavioural and EEG effects induced by an amphetamine-like substance (cathinone) in rats. Pharmacological Research Communications. 1980;12:959–964. doi: 10.1016/s0031-6989(80)80163-9. [DOI] [PubMed] [Google Scholar]
- Beyer J, Peters FT, Kraemer T, Maurer HH. Detection and validated quantification of nine herbal phenalkylamines and methcathinone in human blood plasma by LC-MS/MS with electrospray ionization. Journal of Mass Spectrometry. 2007;42:150–160. doi: 10.1002/jms.1132. [DOI] [PubMed] [Google Scholar]
- Bockmuhl M, Gorr G. Verfahren zur Darstellung von optisch active 1-aryl-2-amino-1-propanolen. German patent. 1936 Nov 28;639:126. [Google Scholar]
- Boehringer Ingelheim. α-Substituted-ketones and processes for their preparation. British patent. 1969 Apr 23;1:149, 366. [Google Scholar]
- Bonano J, Glennon RA, De Felice LJ, Banks M, Negus SS. Abuse-related and abuse-limiting effects of methcathinone, and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3223-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondareva TS, Young R, Glennon RA. Central stimulants as discriminative stimuli: Asymmetric generalization between (−)ephedrine and (S+)methamphetamine. Pharmacology, Biochemistry, and Behavior. 2002;74:157–162. doi: 10.1016/s0091-3057(02)00963-2. [DOI] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Annals of Emergency Medicine. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Sumnall HR, Measham F, Cole J. Analyses of second-generation ‘legal highs’ in the UK: Initial findings. Drug Testing and Analysis. 2010;2:377–382. doi: 10.1002/dta.155. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Freeman S, Sumnall HR, Measham F, Cole J. Analysis of NRG ‘legal highs’ in the UK: identification and formation of novel cathinones. Drug Testing and Analysis. 2011;3:569–575. doi: 10.1002/dta.204. [DOI] [PubMed] [Google Scholar]
- Brenneisen R, Geisshüsler S, Schorno X. Metabolism of cathinone to (−)-norephedrine and (−)-norpseudoephedrine. Journal of Pharmacy Pharmacology. 1986;38:298–300. doi: 10.1111/j.2042-7158.1986.tb04571.x. [DOI] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. British Journal of Pharmacology. 2013;68:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron K, Kolanos R, Vekariya R, De Felice L, Glennon RA. Mephedrone and methylenedioxypyrovalerone (MDPV), major constituents of “bath salts,” produce opposite effects at the human dopamine transporter. Psychopharmacology. 2013b;227:493–499. doi: 10.1007/s00213-013-2967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KK, Kao CH. Ephedrine and pseudoephedrine, their isolation, constitution, isomerism, properties, derivatives, and synthesis. Journal of the American Pharmaceutical Association. 1926;15:625–639. [Google Scholar]
- Coppola M, Mondola R. 3.4-Methylenedioxypyrovalerone (MDPV): Chemistry, pharmacology and toxicology of a new designer drug of abuse marketed online. Toxicology Letters. 2012;208:12–15. doi: 10.1016/j.toxlet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, III, Ruoho AE. Inhibition of plasma membrane monoamine transporters by β-ketoamphetamines. European Journal of Pharmacology. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Brandt SD, Daley PF, Partilla JS, Rothman RB, Tulzer A, Sitte HA, Baumann MH. Pharmacological examination of trifluoromethyl ring-substituted methcathinone analogs. European Journal of Pharmacology. 2013;699:180–187. doi: 10.1016/j.ejphar.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. Cathinone: An investigation of several N-alkyl and methylenedioxy analogs. Pharmacology, Biochemistry, and Behavior. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- Dybdal NF, Holder ND, Ottoson PE, Sweeney MD, Williams T. Mephedrone: public health risk, mechanisms of action, and behavioral effects. European Journal of Pharmacology. 2013 doi: 10.1016/j.ejphar.2013.05.024. http://dx.doi.org/10.1016/j.ejphar.2013.05.024. [DOI] [PubMed]
- Eberhard A. Ueber das Ephedrine und verwante Verbindugen. Archivs die Pharmazie. 1915;253:62–91. [Google Scholar]
- Eberhard A. Ueber die Synthese des inaktiven Ephedrine bez. Pseudoephedrins. Archivs die Pharmazie. 1920;258:97–129. [Google Scholar]
- Eshleman AJ, Wofrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochemical Pharmacology. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: Drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Register. Schedules of Controlled Substances: Temporary placement of three synthetic cathinones into Schedule I. Federal Register. 2011 Oct 21;76:65371–65375. [PubMed] [Google Scholar]
- Fellows L. East Africa turns on with khat. New York Times, July. 1967;9:1967. [Google Scholar]
- Fitzgerald J. Khat: a literature review. 2009 http://www.ceh.org.au/downloads/khat_-report_final.pdf.
- Foley KF, Cozzi NV. Novel aminopropiophenones as potential antidepressants. Drug Development Research. 2003;60:252–260. [Google Scholar]
- Fourneau E, Kanao S. Sur la synthese l’ephedrine. Bulletin de la Sociétée chimique de France. 1924;35:614–625. [Google Scholar]
- Friebel H, Brilla R. Über den zentralerregenden Wirkstoff der frischen Blätter und Zweigspitzen von Catha edulis Forskal. Naturwissenschaften. 1963;50:354–355. [Google Scholar]
- Fuwa T, Fukumori N, Tanaka T, Kubo Y, Ogata A, Uehara S, Honda Y, Kodama T. Microdialysis study of drug effects on central nervous system: Changes of dopamine levels in mice striatum after oral administration of methylenedioxypyrovalerone. Annual Report Tokyo Metropolitan Institute of Public Health. 2007;58:287–292. [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of “bath salt” cathinones. Behavioral Pharmacology. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D, Adamowicz P, Skulska A, Tokarczyk B, Stanaszek R. Analysis of 4-MEC in biological and non-biological material-three case reports. Forensic Science International. 2013;228:e11–e15. doi: 10.1016/j.forsciint.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Discriminative stimulus properties of phenylisopropylamine derivatives. Drug and Alcohol Dependence. 1986;17:119–134. doi: 10.1016/0376-8716(86)90003-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Classical hallucinogens. In: Schuster CR, Kuhar MJ, editors. Handbook of Experimental Pharmacology: Pharmacological Aspects of Drug Dependence. Springer Verlag; Basel, Switzerland: 1996. pp. 343–371. [Google Scholar]
- Glennon RA. Hallucinogens, stimulants, and related drugs of abuse. In: Williams DA, Lempke TL, Roche VF, Zito SW, editors. Foye’s Principles of Medicinal Chemistry. 5. Lippincott, Williams & Wilkins; Baltimore (MD): 2008. pp. 631–651. [Google Scholar]
- Glennon RA, Showalter D. The effect of cathinone and several related derivatives on locomotor activity. Research Communications on Substance Abuse. 1981;2:186–192. [Google Scholar]
- Glennon RA, Young R. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. John Wiley and Sons, Inc; Hoboken: 2011. [Google Scholar]
- Glennon RA, Schechter MD, Rosecrans JA. Discriminative stimulus properties of S(−)- and R(+)-cathinone, (+)-cathine and several structural modifications. Pharmacology, Biochemistry, and Behavior. 1984;21:1–3. doi: 10.1016/0091-3057(84)90121-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Hauck AE. Structure-activity studies on methoxy-substituted phenylisopropylamines using drug discrimination methodology. Pharmacology, Biochemistry, and Behavior. 1985;22:723–729. doi: 10.1016/0091-3057(85)90520-9. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Hauck AE, McKenney JD. Structure-activity studies of amphetamine analogs using drug discrimination methodology. Pharmacology, Biochemistry, and Behavior. 1984;21:895–901. doi: 10.1016/s0091-3057(84)80071-4. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Martin BR, Dal Cason TA. Methcathinone (“Cat”): An enantiomeric potency comparison. Pharmacology, Biochemistry, and Behavior. 1995;50:601–606. doi: 10.1016/0091-3057(94)00348-3. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, Naiman NA, Kalix P. Methcathinone: A new and potent amphetamine-like agent. Pharmacology, Biochemistry, and Behavior. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Ismaiel AM, Martin B, Poff D, Sutton M. A Preliminary Behavioral Investigation of PMMA, the 4-Methoxy Analog of Methamphetamine. Pharmacology, Biochemistry, and Behavior. 1988;31:9–13. doi: 10.1016/0091-3057(88)90303-6. [DOI] [PubMed] [Google Scholar]
- Gaulier JM, Canal M, Pradeille JL, Marquet P, Lachâtre G. New Drugs at “rave parties”: ketamine and prolintane. Acta Clinica Belgica. Supplementum. 2002:41–46. [PubMed] [Google Scholar]
- Goudie AJ, Atkinson J, West CR. Discriminative properties of the psychostimulant dl-cathinone in a two lever operant task. Lack of evidence for dopaminergic mediation. Neuropharmacology. 1986;25:85–94. doi: 10.1016/0028-3908(86)90063-8. [DOI] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of Pharmacology and Experimental Therapeutics. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbach H. Medical aspects of the chewing of khat leaves. Bulletin of the World Health Organization. 1972;47:21–29. [PMC free article] [PubMed] [Google Scholar]
- Heinzelman RV, Aspergren DB. Compounds containing the pyrrolidine ring: Analogs of sympathomimetic amines. Journal of the American Chemical Society. 1953;75:3409–3413. [Google Scholar]
- Higgs RA, Glennon RA. Stimulus properties of ring-methyl amphetamine analogs. Pharmacology, Biochemistry and Behavior. 1990;37:835–837. doi: 10.1016/0091-3057(90)90571-x. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Gillespie HK. A new stimulant, prolintane hydrochloride, compared with dextroamphetamine in fatigued volunteers. The Journal of Clinical Pharmacology. 1970;10:103–109. doi: 10.1177/009127007001000205. [DOI] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug and Alcohol Dependence. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JF, Browning E, Adams R. Synthetic homologs of d,l-ephedrine. Journal of the American Chemical Society. 1928;50:2287–2292. [Google Scholar]
- Iversen LE. ‘Consideration of the cathinones’. Advisory Council on the Misuse of Drugs. A report submitted to the Home Secretary of the UK (March. 2010;31:2010. [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL. Neurochemical profiles of some novel psychoactive substances. European Journal of Pharmacology. 2013;700:147–151. doi: 10.1016/j.ejphar.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao J, Kowaki C, Rakemi H. Studies on alkanolamines. II. Synthesis of N-methylephedrone and its derivatives. Application of the Viogt Reaction. Yakugaku Zasshi. 1954;74:551–553. [Google Scholar]
- Jacob P, Shulgin AT. Novel N-substituted 2-amino-3′,4′-methylenedioxy-propiophenones. 9639133. WO patent. 1996 Dec 12;
- Kalix P. Hypermotility of the amphetamine type induced by a constituent of khat leaves. British Journal of Pharmacology. 1980a;68:11–13. doi: 10.1111/j.1476-5381.1980.tb10690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalix P. Hyperthermic response to (−)cathinone, an alkaloid of Catha edulis (khat) Journal of Pharmacy and Pharmacology. 1980b;32:662–663. doi: 10.1111/j.2042-7158.1980.tb13031.x. [DOI] [PubMed] [Google Scholar]
- Kalix P. Cathinone, an alkaloid from khat leaves with an amphetamine-like releasing effect. Psychopharmacology. 1981;74:269–270. doi: 10.1007/BF00427108. [DOI] [PubMed] [Google Scholar]
- Kalix P. Cathinone, a natural amphetamine. Pharmacology and Toxicology. 1992;70:77–86. doi: 10.1111/j.1600-0773.1992.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Kalix P, Braeden O. Pharmacological aspects of the chewing of khat leaves. Pharmacological Reviews. 1985;37:149–164. [PubMed] [Google Scholar]
- Kalix P, Glennon RA. Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochemical Pharmacology. 1986;35:3015–3019. doi: 10.1016/0006-2952(86)90380-1. [DOI] [PubMed] [Google Scholar]
- Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, Katagi M, Tsuchihashi H. Metabolism of the recently encountered designer drug, methylone, in humans and rats. Xenobiotica. 2006;36:709–723. doi: 10.1080/00498250600780191. [DOI] [PubMed] [Google Scholar]
- Karila L, Reynaud M. GHB and synthetic cathinones: clinical effects and potential consequences. Drug Testing and Analysis. 2010;3:552–559. doi: 10.1002/dta.210. [DOI] [PubMed] [Google Scholar]
- Kasick DP, McKnight CA, Kilsovic E. “Bath salt” ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. Clinical Toxicology. 2012;38:176–180. doi: 10.3109/00952990.2011.643999. [DOI] [PubMed] [Google Scholar]
- Katz JL, Ricaurte GA, Witkin JM. Reinforcing effects of enantiomers of N,N-dimethylamphetamine in squirrel monkeys. Psychopharmacology. 1992;107:315–318. doi: 10.1007/BF02245154. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. British Journal of Pharmacology. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Testing and Analysis. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- Kennedy JG, Teague J, Fairbanks L. Qat use in North Yemen and the problem of addiction: A study in medical anthropology. Culture, Medicine and Psychiatry. 1980;4:311–344. doi: 10.1007/BF00051810. [DOI] [PubMed] [Google Scholar]
- Knoll J. Studies on the central effects of (−)cathinone. NIDA Research Monograph. 1979;27:322–323. [PubMed] [Google Scholar]
- Kolanos R, Cameron KN, Vekariya RH, De Felice LJ, Glennon RA. “Bath salts”: An imitation of methamphetamine plus cocaine?. Southeast Regional Meeting of American Chemical Society (SERMACS); Richmond VA. October 26–29.2011. [Google Scholar]
- Kolanos R, Solis E, Sakloth F, De Felice LJ, Glennon RA. ‘Deconstruction’ of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. 2013. Unpublished data, manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppe H. 1-(3′4′-Methylenedioxy-phenyl)-2-pyrrolidino-alkanones-(1) 3,478,050. U S Patent. 1969 Nov 11;
- Kraemer T, Maurer HH. Toxicokinetics of amphetamines: Metabolism and toxicokinetic data of designer drugs, amphetamine, methamphetamine, and their N-alkyl derivatives. Therapeutic Drug Monitoring. 2002;24:277–289. doi: 10.1097/00007691-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Kuitunen T, Kärkkäinen S, Ylitalo P. Comparison of the acute physical and mental effects of ephedrine, fenfluramine, phentermine and prolintane. Methods and Findings in Experimental and Clinical Pharmacology. 1984;6:265–270. [PubMed] [Google Scholar]
- Kyle PB, Daley WP. Domestic abuse of the European rave drug prolintane. Journal of Analytical Toxicology. 2007;31:415–418. doi: 10.1093/jat/31.7.415. [DOI] [PubMed] [Google Scholar]
- Lee MR. The history of ephedra (ma-huang) The Journal of the Royal College of Physicians of Edinburg. 2011;41:78–84. doi: 10.4997/JRCPE.2011.116. [DOI] [PubMed] [Google Scholar]
- Lee S, Yoo HH, In MK, Jin C, Kim DH. Stereoselectivity in the cytochrome P450-dependent N-demethylation and flavin monooxygenase-dependent N-oxidation of N,N-dimethylamphetamine. Archives of Pharmacal Research. 2013 doi: 10.1007/s12272-013-0137-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lin TC, Lin DL, Lua AC. Detection of p-chloroamphetamine in urine samples with mass spectrometry. Journal of Analytical Toxicology. 2011;35:205–210. doi: 10.1093/anatox/35.4.205. [DOI] [PubMed] [Google Scholar]
- L’Italien YJ, Park H, Rebstock LC. Methylaminopropiophenone compounds and methods for producing the same. 2,802,865. US patent. 1957 Aug 13;
- Ljungberg T, Ungerstedt U. A rapid and simple behavioural screening method for simultaneous assessment of limbic and striatal blocking effects of neuroleptic drugs. Pharmacology, Biochemistry, and Behavior. 1985;23:479–485. doi: 10.1016/0091-3057(85)90025-5. [DOI] [PubMed] [Google Scholar]
- Loh HH, Tseng L-F. Role of biogenic amines in the actions of methoxyamphetamines. In: Stillman RC, Willette RE, editors. The Psychopharmacology of Hallucinogens. Pergamon Press; New York: 1978. pp. 13–22. [Google Scholar]
- López-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. British Journal of Pharmacology. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markantonis SL, Kyroudis A, Beckett AH. The in vitro reduction of dimethylpropion. Biochemical Medicine and Metabolic Biology. 1989;42:1–8. doi: 10.1016/0885-4505(89)90035-2. [DOI] [PubMed] [Google Scholar]
- Marinetti LJ, Antonides HM. Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. Journal of Analytical Toxicology. 2013;37:135–146. doi: 10.1093/jat/bks136. [DOI] [PubMed] [Google Scholar]
- Martínez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. European Neuropsychopharmacology. 2012 doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. Neuro Toxicology. 2012;33:1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (pyrovalerone) analogs. A promising class of monoamine uptake inhibitors. Journal of Medicinal Chemistry. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Du P, Schuster F, Maurer HH. Studies on the metabolism of the α-pyrrolidinophenone designer drug methylenedioxy-pyrovalerone (MDPV) in rat and human urine and human liver microsomes using GC-MS and LC-high-resolution MS and its detectability in urine by GC-MS. Journal of Mass Spectrometry. 2010;45:1426–1442. doi: 10.1002/jms.1859. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addiction Biology. 2011;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Negus SS, Banks ML. Making the right choice: Lessons from drug discrimination, for research on drug reinforcement and drug self-administration. In: Glennon RA, Young R, editors. Drug Discrimination: Applications to Medicinal Chemistry and Drug Studies. John Wiley and Sons, Inc; Hoboken: 2011. pp. 361–388. [Google Scholar]
- Nicholson AN, Stone BM, Jones MM. Wakefulness and reduced rapid eye movement sleep: Studies with prolintane and pemoline. British Journal of Clinical Pharmacology. 1980;10:465–472. doi: 10.1111/j.1365-2125.1980.tb01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, Cole KA. Cathinone (Khat) and methcathinone (CAT) in urine specimens: A gas chromatographic-mass spectrometric detection procedure. Journal of Analytical Toxicology. 2001;25:525–530. doi: 10.1093/jat/25.7.525. [DOI] [PubMed] [Google Scholar]
- Pawlik E, Plässer G, Mahler H, Daldrup T. Studies on the phase I metabolism of the new designer drug 3-fluoromethcathinone using rabbit liver slices. International Journal of Legal Medicine. 2012;126:231–240. doi: 10.1007/s00414-011-0601-6. [DOI] [PubMed] [Google Scholar]
- Payá B, Guisado JA, Vaz FJ, Crespo-Facorro B. Visual hallucinations induced by the combination of prolintane and diphenhydramine. Pharmacopsychiatry. 2002;35:24–25. doi: 10.1055/s-2002-19829. [DOI] [PubMed] [Google Scholar]
- Penders TM, Gestring RE, Vilensky DA. Intoxication delirium following use of synthetic cathinone derivatives. Clinical Toxicology. 2012;38:616–617. doi: 10.3109/00952990.2012.694535. [DOI] [PubMed] [Google Scholar]
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. Journal of Medical Toxicology. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic S, Thomas A. On the constituents of Catha edulis. Archives die Pharmazie. 1962;295:524–525. doi: 10.1002/ardp.19622950709. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Campbell OL, Dewey WL, Harris LS. Discriminative stimulus and neurochemical mechanism of cathinone: A preliminary study. NIDA Research Monograph. 1979;27:328–329. [PubMed] [Google Scholar]
- Rösner P, Quednow B, Girreser U, Junge T. Isomeric fluoro-methoxy-phenylalkylamines: a new series of controlled-substance analogues (designer drugs) Forensic Science International. 2005;148:143–156. doi: 10.1016/j.forsciint.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. The Journal of Pharmacology and Experimental Therapeutics. 2003;307:138–145. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- Savenko VG, Semkin EP, Sorokin VI, Kazankov SP. Ministry of the Interior, All-Union Scientific Research Institute Report. 1989. Expert examination of narcotic substances obtained from ephedrine; pp. 1–22. [Google Scholar]
- Schechter MD, Glennon RA. Cathinone, cocaine and methamphetamine: Similarity of behavioral effects. Pharmacology, Biochemistry, and Behavior. 1985;22:913–916. doi: 10.1016/0091-3057(85)90295-3. [DOI] [PubMed] [Google Scholar]
- Schorno X, Steinegger E. United Nations Laboratory document MNAR/7/1978, GE. 1978. The phenylalkylamines of Catha edulis Forsk. The absolute configuration of cathinone; pp. 78–3956. [Google Scholar]
- Schütte J. Anorexigenic propiophenones. 3, 001,910. U S patent. 1961 Sep 26;
- Seeger E. Verfahren zur Herstellung von α-Pyrrolidinoketonen und deren Salzen. 1,161,274. German patent. 1964 Jan 16;
- Shapiro D. Benzoisoquinoline studies. Part I. Open-ring models of 4-benzylisoquinolines. Journal of Organic Chemistry. 1950;15:1027–1036. [Google Scholar]
- Shulgin A, Shulgin A. Pihkal. Transform Press; Berkeley, CA: 1991. [Google Scholar]
- Sikk K, Haldre S, Aquilonius SM, Asser A, Paris M, Roose A, Petterson J, Eriksson SL, Bergquist J, Taba P. Manganese-induced parkinsonism in methcathinone abusers: bio-markers of exposure and follow-up. European Journal of Neurology. 2013;20:915–920. doi: 10.1111/ene.12088. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. British Journal of Pharmacology. 2012;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clinical Toxicology. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Springer D, Peters FT, Fritschi G, Maurer HH. Studies on the metabolism and toxicological detection of the new designer drug 4-methyl-α-pyrrolidinopropiophenone in urine using gas chromatography-mass spectrometry. Journal of Chromatography B. 2002;773:25–33. doi: 10.1016/s1570-0232(01)00578-5. [DOI] [PubMed] [Google Scholar]
- Springer D, Fritschi G, Maurer HH. Metabolism and toxicological detection of the new designer drug 3,4-methylenedioxy-α-pyrrolidinopropiophenone studied in urine using gas chromatography-mass spectrometry. Journal of Chromatography B. 2003a;793:377–388. doi: 10.1016/s1570-0232(03)00350-7. [DOI] [PubMed] [Google Scholar]
- Springer D, Fritschi G, Maurer HH. Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2003b;796:253–266. doi: 10.1016/j.jchromb.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Springer D, Paul LD, Staack RF, Kraemer T, Maurer HH. Identification of cytochrome P450 enzymes involved in the metabolism of 4-methyl-α-pyrrolidinopropiophenone, a novel scheduled designer drug, in human liver microsomes. The American Society for Pharmacology and Experimental Therapeutics. 2003c;31:979–982. doi: 10.1124/dmd.31.8.979. [DOI] [PubMed] [Google Scholar]
- Springer D, Staack RF, Paul LD, Kraemer T, Maurer HH. Identification of cytochrome P450 enzymes involved in the metabolism of 4′-methoxy-α-pyrrolidinopropiophenone (MOPPP), a designer drug, in human liver microsomes. Xenobiotica. 2003d;33:989–998. doi: 10.1080/00498250310001602775. [DOI] [PubMed] [Google Scholar]
- Strano-Rossi S, Cadwallader AB, de la Torre X, Botrè F. Toxicological determination and in vitro metabolism of the designer drug methylenedioxypyrovalerone (MPDV) by gas chromatography/mass spectrometry and liquid chromatography/quadrupole time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2010;24:2706–2714. doi: 10.1002/rcm.4692. [DOI] [PubMed] [Google Scholar]
- Testa B, Salvesen B. Quantitative structure-activity relationships in drug metabolism and disposition: Pharmacokinetics of N-substituted amphetamines in humans. Journal of Pharmaceutical Sciences. 1980;69:497–501. doi: 10.1002/jps.2600690505. [DOI] [PubMed] [Google Scholar]
- Thevis M, Sigmund G, Thomas A, Gougoulidis V, Rodchenkov G, Schanzer W. Doping control analysis of metamfepramone and two major metabolites using liquid chromatography-tandem mass spectrometry. European Journal of Mass Spectrometry. 2009;15:507–515. doi: 10.1255/ejms.1010. [DOI] [PubMed] [Google Scholar]
- Thomae K. Improvements in or relating to tertiary amines and their salts and the production thereof. GB814153. UK patent. 1959 May 27;
- Thornton SL, Gerona RR. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. Journal of Medical Toxicology. 2012;8:310–313. doi: 10.1007/s13181-012-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN Document. United Nations Laboratory document MNAR/11/75, GE. 1975. Studies on the chemical composition of khat. III. Investigations on the phenylalkylamine fraction; pp. 75–1264. [Google Scholar]
- UN Document. United Nations Laboratory document MNAR/3/1978, GE. 1978. Note on the synthesis of cathinone and its “dimer” (3,6-dimethyl-2,5-diphenylpyrazine) pp. 78–1836. [Google Scholar]
- UN Document. United Nations Laboratory document MNAR/3/1979, GE. 1979. The botany and chemistry of khat; pp. 79–10365. [Google Scholar]
- UNODC. A report from the United Nations Office on Drugs and Crime. Vienna, Austria: 2013. The challenge of new psychoactive substances; pp. 1–56. [Google Scholar]
- van der Schoot JB, Ariens EJ, van Rossum JM, Hurkmans JATM. Phenylisopropylamine derivatives, structure and action. Arznemittel-Forschung. 1962;12:902–907. [PubMed] [Google Scholar]
- Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, Winsauer PJ. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology (Berlin) 2012;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wander A. α-Pyrrolidino-valerophenones. 927,475. British patent. 1963 May 29;
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Foster Olive M. Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV) Addiction Biology. 2013 doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. The Journal of Pharmacology and Experimental Therapeutics. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Westphal F, Junge T, Rösner P, Fritschi G, Klein B, Girreser U. Mass spectral and NMR spectral data of two new designer drugs with an α-aminophenone structure: 4′-methyl-α-pyrrolidinohexanophenone and 4′-methyl-α-pyrrolidinobutyrophenone. Forensic Science International. 2007;169:32–42. doi: 10.1016/j.forsciint.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Wolfes O. Über das Vorkommen von d-nor-iso-Ephedrin in Catha edulis. Archives die Pharmazie. 1930;268:81–83. [Google Scholar]
- Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, Dargan PL. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clinical Toxicology. 2010;48:924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- Woolverton WL. Discriminative stimulus effects of cocaine. In: Glennon RA, Jarbe TUC, Frankenheim J, editors. Drug Discrimination: Applications to Drug Abuse Research. National Institute on Drug Abuse; Rockville (MD): 1991. pp. 61–74. [Google Scholar]
- Woolverton WL, Shybut G, Johanson CE. Structure-activity relationships among some d-N-alkylated amphetamines. Pharmacology, Biochemistry, and Behavior. 1980;13:869–876. doi: 10.1016/0091-3057(80)90221-x. [DOI] [PubMed] [Google Scholar]
- Yanagita T. Studies on cathinones: Cardiovascular and behavioral effects in rats and self-administration experiment in Rhesus monkeys. NIDA Research Monograph. 1979;27:326–327. [PubMed] [Google Scholar]
- Young R, Glennon RA. Discriminative stimulus properties of amphetamine and structurally related phenalkylamines. Medicinal Research Reviews. 1986;6:99–130. doi: 10.1002/med.2610060105. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. Cocaine-stimulus generalization to two new designer drugs: Methcathinone and 4-methylaminorex. Pharmacology, Biochemistry, and Behavior. 1993;45:229–231. doi: 10.1016/0091-3057(93)90110-f. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA. A three-lever operant procedure differentiates the stimulus effects of R(−)-MDA from S(+)-MDA. Journal of Pharmacology and Experimental Therapeutics. 1996;276:594–601. [PubMed] [Google Scholar]
- Young R, Glennon RA. Discriminative stimulus effects of S(−)-methcathinone (CAT): A potent stimulant drug of abuse. Psychopharmacology. 1998;140:250–256. doi: 10.1007/s002130050765. [DOI] [PubMed] [Google Scholar]
- Zaitsu K, Katagi M, Kamata T, Kamata H, Shima N, Tsuchihashi H, Hayashi T, Kuroki H, Matoba R. Determination of a newly encountered designer drug “p-methoxyethylamphetamine” and its metabolites in human urine and blood. Forensic Science International. 2008;177:77–84. doi: 10.1016/j.forsciint.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Zaitsu K, Katagi M, Kamata HT, Kamata T, Shima N, Miki A, Tsuchihashi H, Mori Y. Determination of the metabolites of the new designer drugs bk-MBDB and bk-MDEA in human urine. Forensic Science International. 2009;188:131–139. doi: 10.1016/j.forsciint.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Zawilska JB, Wojcieszak J. Designer cathinones-an emerging class of novel recreational drugs. Forensic Science International. 2013;231:42–53. doi: 10.1016/j.forsciint.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Zhingel KY, Dovensky W, Crossman A, Allen A. Ephedrone: 2-Methylamino-1-phenylpropan-1-one. Journal of Forensic Sciences. 1991;36:915–920. [Google Scholar]