Abstract
Interactions between recipient T cells and donor endothelial graft cells may be an important mechanism for both acute and chronic rejection of vascularized allografts. This finding provides a starting point for investigations to develop novel ways of inducing long-lasting immunologic tolerance to donor antigens.
The ultimate goal in clinical transplantation is to induce a state of donor-specific immunologic tolerance, where recipients can accept organs, tissues or cell grafts without the need of exogenous immunosuppression1. But to achieve this goal, we must first understand the exact cellular and molecular mechanisms of both acute and chronic graft rejection. In this issue, Kreisel et al. 2 highlight a potentially critical role of CD8+ T cell–endothelial cell interactions in mediating graft rejection. The study raises the important question: Where do T cells meet transplant antigens in the context of planning and initiating the alloimmune response that ultimately results in graft destruction?
Transplantation is the treatment of choice for end-stage failure of organs such as kidney, heart, lung, liver and pancreas. In the absence of exogenous immunosuppressive drugs, transplanted organs are almost invariably rejected. Even with potent immunosuppressive drugs, a significant proportion of transplanted organs undergo acute rejection (although the number has been declining with use of newer immunosuppressive strategies). However, the main problem in clinical transplantation, aside from organ shortage, has been the occurrence of slow, progressive organ dysfunction resulting in chronic attrition of grafts over time—a process often referred to as chronic rejection3.
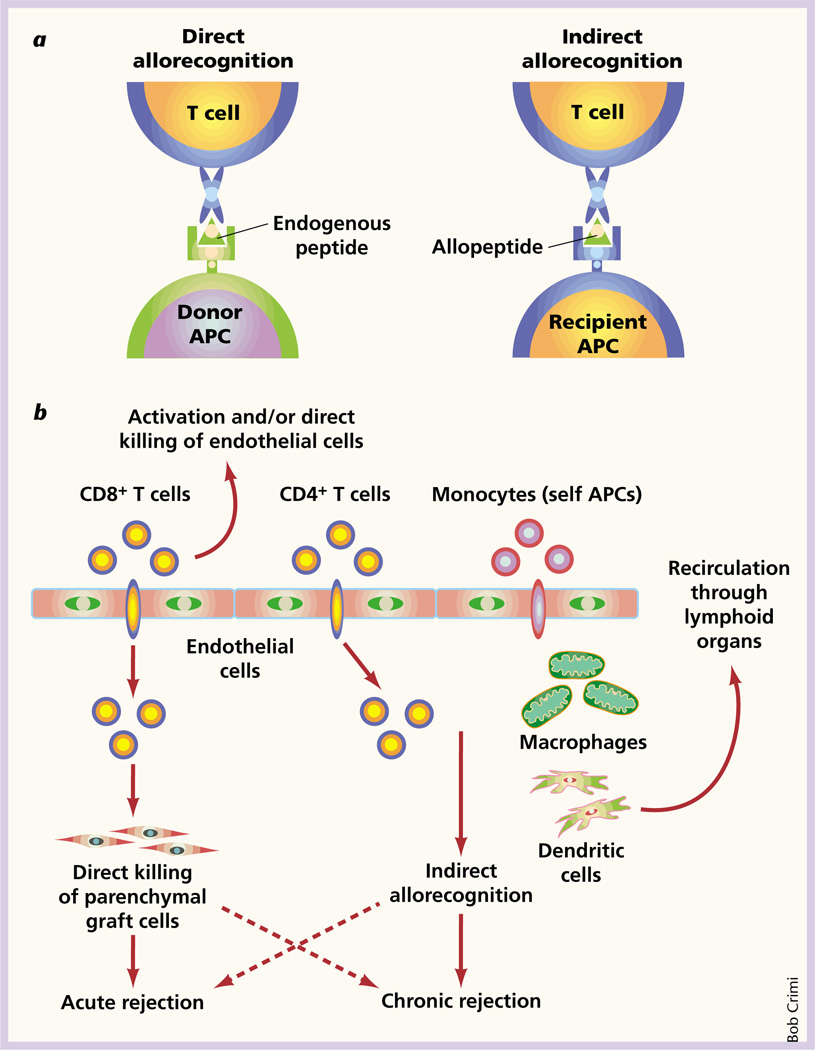
T cells are essential for both acute and chronic graft rejection3,4. The first step in an alloimmune response is a ‘rendezvous’ between T cells and transplant antigens where allorecognition occurs. There are two distinct though not necessarily mutually exclusive pathways of allorecognition3 (Fig. 1a). First, T cells can recognize intact major histocompatibility complex molecules on donor cells; this is, the ‘direct’ allorecognition pathway. T cells can also recognize processed alloantigens in the form of peptides presented by the recipient’s antigen-presenting cells. This ‘indirect’ pathway of allorecognition is analogous to that of physiologic antigen recognition. Indirect allorecognition has been shown to have a role in acute rejection5, but it also has been postulated to play a crucial part in the development and progression of chronic allograft rejection6.
Fig. 1.
Allorecognition pathways and graft rejection. a, Pathways of allorecognition. In the ‘direct’ pathway, T cells recognize intact major histocompatibility molecules on donor antigen-presenting cells (left). In the ‘indirect’ pathway, T cells recognize processed alloantigen in the form of peptides presented by recipient antigen-presenting cells (right). b, Interactions among endothelial cells, T cells and recipient antigen-presenting cells in allograft rejection. Recipient monocytes are recruited by endothelial cells to the graft tissue. They are also transformed to become highly efficient antigen-presenting dendritic cells that may need to recirculate to peripheral lymphoid organs for maturation. The dendritic cells and intragraft macrophages present donor peptides via the indirect pathway to recruited CD4+ T cells. CD8+ T cells, on the other hand, are activated by donor endothelial cells and can either directly kill endothelial cells or traverse the endothelium and kill parenchymal graft cells.
Endothelial cells of donor origin are uniquely located at the interface between the recipient’s blood and the allograft, and they have been implicated in graft rejection7. Endothelial cells may promote indirect allorecognition by a crosstalk mechanism, which involves the recruitment and transformation of recipient monocytes by endothelial cells into highly efficient antigen-presenting dendritic cells8,9. These dendritic cells may recirculate to peripheral lymphoid organs for maturation and, in turn, present alloantigen via the indirect pathway to memory T cells in the periphery or in the transplanted tissue (Fig. 1b).
Graft endothelial cells also express major histocompatibility complex class I and II molecules and have been long suspected to directly stimulate T cells, although in vivo evidence was lacking until now. Graft endothelial cells can induce proliferation of, and cytokine production by, allogeneic T cells in vitro10, and induce and regulate cytolytic T-cell differentiation11. The data suggest that graft endothelial cells could promote direct allorecognition by either serving as antigen-presenting cells and/or as targets for T cell–mediated cytotoxicity.
To address this question, Kreisel et al. used an elegant transgenic animal model system to make two important and novel observations. First, CD8+ T cells can recognize alloantigen on graft endothelial cells in vivo. Second, T cell–endothelial cell interactions, presumably via direct allorecognition, can result in allograft rejection even in the absence of CD4+ T-cell help and professional hematopoietic antigen-presenting cells.
On the surface, the latter finding appears to challenge the observations of Lakkis et al.12. They showed that peripheral lymphoid organs are required for allograft rejection, which implies that unprimed T cells must encounter antigens first outside the graft, in the guise of donor antigen-presenting cells that have migrated to host lymph nodes or processed allopeptides presented by self-antigen presenting cells. However, it is also possible that T cells, after recognizing antigens on graft endothelium, migrate to peripheral lymphoid organs for further maturation and differentiation before they are recruited back to the graft to effect tissue destruction.
Thus, taking the current study and earlier findings together, one can envision that graft endothelial cells that are present for the lifetime of the organ can be important players in promoting both direct and indirect allorecognition leading to acute and/or chronic rejection (Fig. 1b).
The unique property of graft endothelial cells to promote direct allorecognition may be critical for activation of alloreactive CD8+ T cells, especially in situations where CD4+ T-cell activation has been inhibited, such as with T-cell costimulatory blockade13. What is unknown, however, is whether endothelial cells in vivo function to present antigen and activate CD8+ T cells that in turn kill graft cells, whether they merely serve as targets for CD8+ T cell–mediated cytotoxicity, or both.
Several other questions remain to be answered. First, what is the physiologic relevance of the reported observations in normal non-transgenic animals and ultimately humans? Second, are there unique T-cell costimulatory pathways that provide second signals to T cells activated by endothelial cells14? Third, what is the relative contribution of this unique allorecognition mechanism to acute and chronic rejection? Fourth, how can these data be reconciled with the observations of Lechler’s group suggesting that activated endothelial cells can in fact tolerize T cells activated via the direct pathway of allorecognition15,16? And are these differences related to the tissue source of endothelial cells? Finally, what is the role of this unique allorecognition mechanism in resistance to tolerance? These clinically relevant questions require further investigation.
The hope is that the results of these investigations will give rise to the development of novel and reproducible strategies to induce donor-specific immunologic tolerance that can be translated effectively from rodent models to primates and ultimately humans1.
References
- 1.Salama AD, Remuzzi G, Harmon WE, Sayegh MH. Challenges to achieving clinical transplantation tolerance. J. Clin. Invest. 2001;108:943–948. doi: 10.1172/JCI14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreisel D, et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nature Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 3.Sayegh MH, Turka LA. The role of T cell costimulatory activation in transplant rejection. N. Engl. J. Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 4.Krensky A, Weiss A, Crabtree G, Davis M, Parham P. T-lymphocyte-antigen interactions in transplant rejection. N. Engl. J. Med. 1990;322:510–517. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- 5.Auchincloss HJ, et al. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc. Natl. Acad. Sci. USA. 1993;90:3373–3377. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee RS, et al. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc. Natl. Acad. Sci. USA. 2001;98:3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briscoe DM, Alexander SI, Lichtman AH. Interactions between T lymphocytes and endothelial cells in allograft rejection. Curr. Opin. Immunol. 1998;10:525–531. doi: 10.1016/s0952-7915(98)80218-5. [DOI] [PubMed] [Google Scholar]
- 8.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 9.Denton MD, Geehan CS, Alexander SI, Sayegh MH, Briscoe DM. Endothelial cells modify the costimulatory capacity of transmigrating leukocytes and promote CD28-mediated CD4+ T cell alloactivation. J. Exp. Med. 1999;190:555–566. doi: 10.1084/jem.190.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epperson DE, Pober JS. Antigen-presenting function of human endothelial cells. Direct activation of resting CD8 T cells. J. Immunol. 1994;153:5402–5412. [PubMed] [Google Scholar]
- 11.Biedermann BC, Pober JS. Human endothelial cells induce and regulate cytolytic T cell differentiation. J. Immunol. 1998;161:4679–4687. [PubMed] [Google Scholar]
- 12.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nature Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 13.Trambley J, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada A, Salama AD, Sayegh MH. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J. Am. Soc. Nephrol. 2002;13:559–575. doi: 10.1681/ASN.V132559. [DOI] [PubMed] [Google Scholar]
- 15.Marelli-Berg FM, et al. Major histocompatibility complex class II-expressing endothelial cells induce allospecific nonresponsiveness in naive T cells. J. Exp. Med. 1996;183:1603–1612. doi: 10.1084/jem.183.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marelli-Berg FM, et al. Activated murine endothelial cells have reduced immunogenicity for CD8+ T cells: a mechanism of immunoregulation? J. Immunol. 2000;165:4182–4189. doi: 10.4049/jimmunol.165.8.4182. [DOI] [PubMed] [Google Scholar]