Abstract
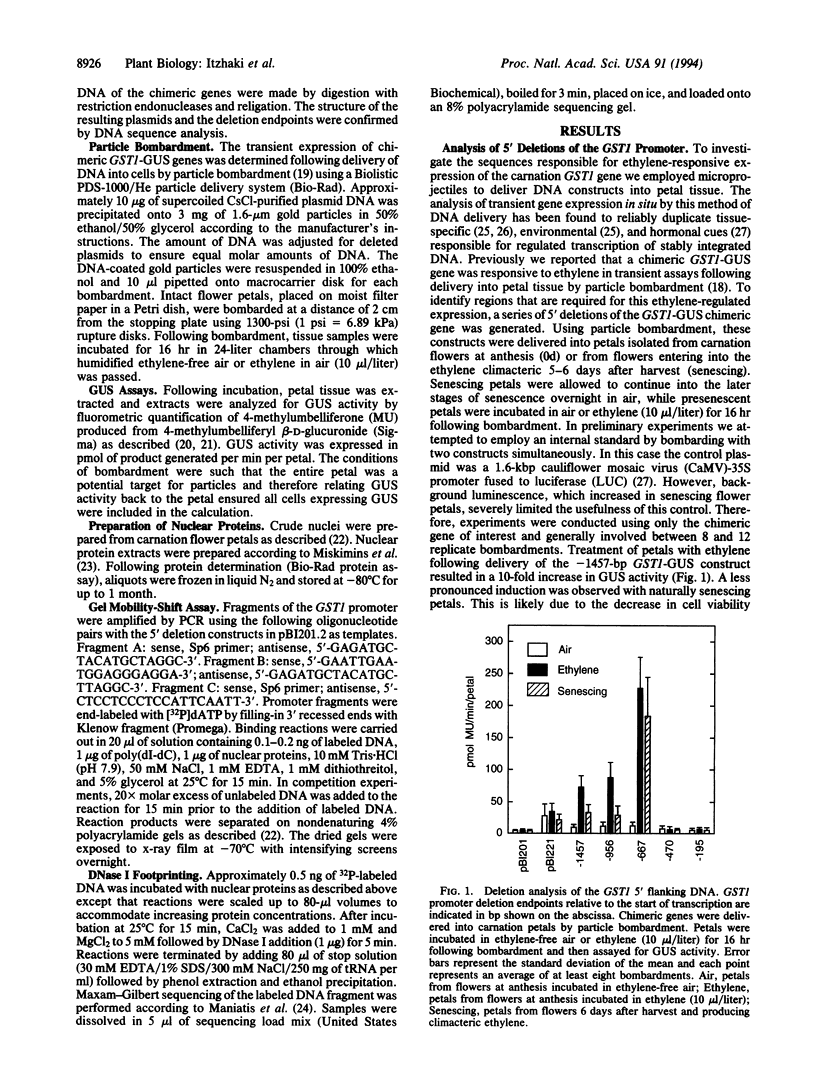
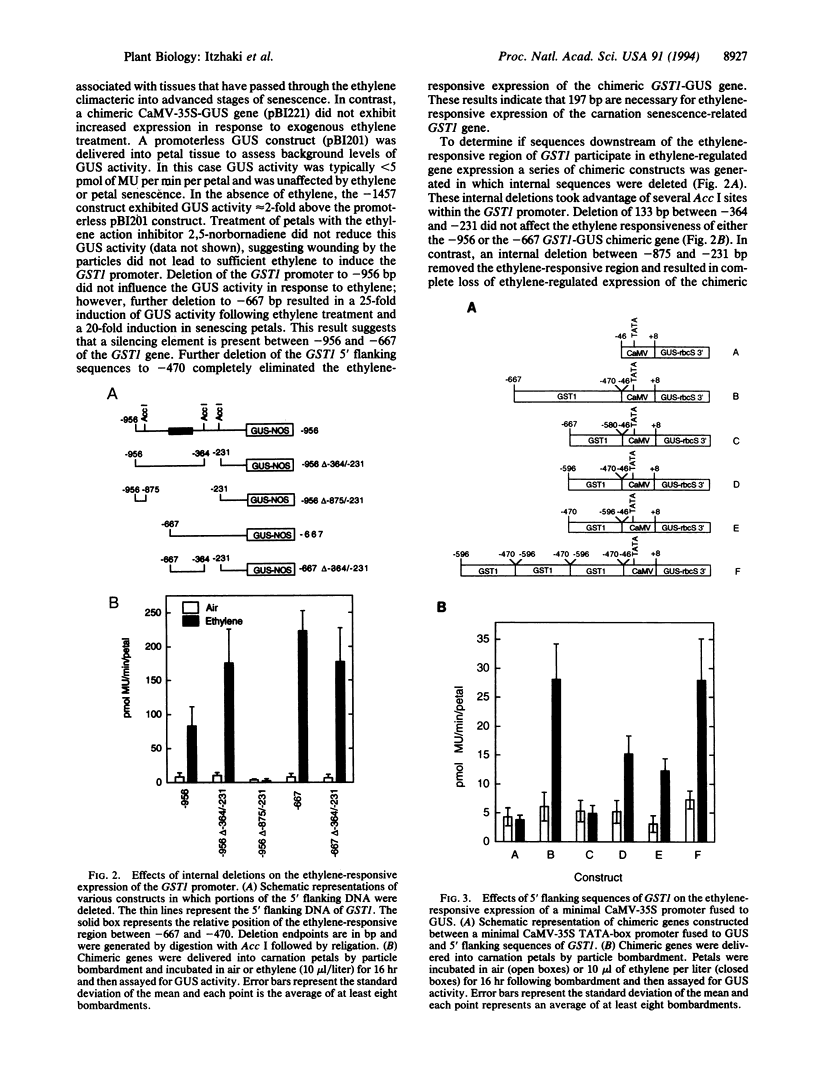
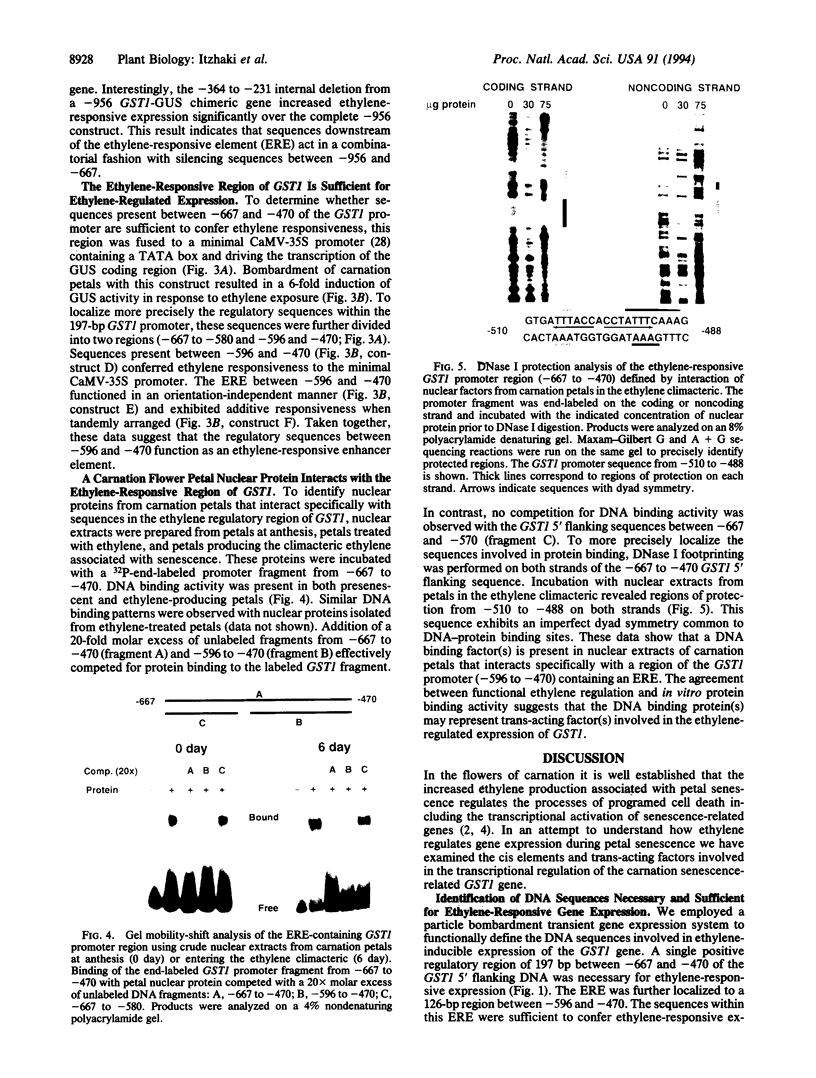
The increased production of ethylene during carnation petal senescence regulates the transcription of the GST1 gene encoding a subunit of glutathione-S-transferase. We have investigated the molecular basis for this ethylene-responsive transcription by examining the cis elements and trans-acting factors involved in the expression of the GST1 gene. Transient expression assays following delivery of GST1 5' flanking DNA fused to a beta-glucuronidase receptor gene were used to functionally define sequences responsible for ethylene-responsive expression. Deletion analysis of the 5' flanking sequences of GST1 identified a single positive regulatory element of 197 bp between -667 and -470 necessary for ethylene-responsive expression. The sequences within this ethylene-responsive region were further localized to 126 bp between -596 and -470. The ethylene-responsive element (ERE) within this region conferred ethylene-regulated expression upon a minimal cauliflower mosaic virus-35S TATA-box promoter in an orientation-independent manner. Gel electrophoresis mobility-shift assays and DNase I footprinting were used to identify proteins that bind to sequences within the ERE. Nuclear proteins from carnation petals were shown to specifically interact with the 126-bp ERE and the presence and binding of these proteins were independent of ethylene or petal senescence. DNase I footprinting defined DNA sequences between -510 and -488 within the ERE specifically protected by bound protein. An 8-bp sequence (ATTTCAAA) within the protected region shares significant homology with promoter sequences required for ethylene responsiveness from the tomato fruit-ripening E4 gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal K. C., Viret J. F., Haley J., Khan B. M., Schantz R., Bogorad L. Transient expression from cab-m1 and rbcS-m3 promoter sequences is different in mesophyll and bundle sheath cells in maize leaves. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3654–3658. doi: 10.1073/pnas.89.8.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfey P. N., Chua N. H. The Cauliflower Mosaic Virus 35S Promoter: Combinatorial Regulation of Transcription in Plants. Science. 1990 Nov 16;250(4983):959–966. doi: 10.1126/science.250.4983.959. [DOI] [PubMed] [Google Scholar]
- Bilang J., Macdonald H., King P. J., Sturm A. A soluble auxin-binding protein from Hyoscyamus muticus is a glutathione S-transferase. Plant Physiol. 1993 May;102(1):29–34. doi: 10.1104/pp.102.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie K. E., Biddle P., Cressman R., Broglie R. Functional analysis of DNA sequences responsible for ethylene regulation of a bean chitinase gene in transgenic tobacco. Plant Cell. 1989 Jun;1(6):599–607. doi: 10.1105/tpc.1.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass L. G., Kirven K. A., Christoffersen R. E. Isolation and characterization of a cellulase gene family member expressed during avocado fruit ripening. Mol Gen Genet. 1990 Aug;223(1):76–86. doi: 10.1007/BF00315799. [DOI] [PubMed] [Google Scholar]
- Daniel V. Glutathione S-transferases: gene structure and regulation of expression. Crit Rev Biochem Mol Biol. 1993;28(3):173–207. doi: 10.3109/10409239309086794. [DOI] [PubMed] [Google Scholar]
- Deikman J., Fischer R. L. Interaction of a DNA binding factor with the 5'-flanking region of an ethylene-responsive fruit ripening gene from tomato. EMBO J. 1988 Nov;7(11):3315–3320. doi: 10.1002/j.1460-2075.1988.tb03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droog F. N., Hooykaas P. J., Libbenga K. R., van der Zaal E. J. Proteins encoded by an auxin-regulated gene family of tobacco share limited but significant homology with glutathione S-transferases and one member indeed shows in vitro GST activity. Plant Mol Biol. 1993 Mar;21(6):965–972. doi: 10.1007/BF00023595. [DOI] [PubMed] [Google Scholar]
- Dudler R., Hertig C., Rebmann G., Bull J., Mauch F. A pathogen-induced wheat gene encodes a protein homologous to glutathione-S-transferases. Mol Plant Microbe Interact. 1991 Jan-Feb;4(1):14–18. doi: 10.1094/mpmi-4-014. [DOI] [PubMed] [Google Scholar]
- Eyal Y., Meller Y., Lev-Yadun S., Fluhr R. A basic-type PR-1 promoter directs ethylene responsiveness, vascular and abscission zone-specific expression. Plant J. 1993 Aug;4(2):225–234. doi: 10.1046/j.1365-313x.1993.04020225.x. [DOI] [PubMed] [Google Scholar]
- Friling R. S., Bensimon A., Tichauer Y., Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friling R. S., Bergelson S., Daniel V. Two adjacent AP-1-like binding sites form the electrophile-responsive element of the murine glutathione S-transferase Ya subunit gene. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):668–672. doi: 10.1073/pnas.89.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki H., Woodson W. R. Characterization of an ethylene-responsive glutathione S-transferase gene cluster in carnation. Plant Mol Biol. 1993 Apr;22(1):43–58. doi: 10.1007/BF00038994. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton K. A., Huang B., Goldsbrough P. B., Woodson W. R. Molecular cloning and characterization of senescence-related genes from carnation flower petals. Plant Physiol. 1989 Jun;90(2):690–696. doi: 10.1104/pp.90.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton K. A., Raghothama K. G., Goldsbrough P. B., Woodson W. R. Regulation of senescence-related gene expression in carnation flower petals by ethylene. Plant Physiol. 1990 Aug;93(4):1370–1375. doi: 10.1104/pp.93.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Hertig C., Rebmann G., Bull J., Dudler R. A wheat glutathione-S-transferase gene with transposon-like sequences in the promoter region. Plant Mol Biol. 1991 Jun;16(6):1089–1091. doi: 10.1007/BF00016083. [DOI] [PubMed] [Google Scholar]
- Meyer R. C., Jr, Goldsbrough P. B., Woodson W. R. An ethylene-responsive flower senescence-related gene from carnation encodes a protein homologous to glutathione S-transferases. Plant Mol Biol. 1991 Aug;17(2):277–281. doi: 10.1007/BF00039505. [DOI] [PubMed] [Google Scholar]
- Miskimins W. K., Roberts M. P., McClelland A., Ruddle F. H. Use of a protein-blotting procedure and a specific DNA probe to identify nuclear proteins that recognize the promoter region of the transferrin receptor gene. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6741–6744. doi: 10.1073/pnas.82.20.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J., Goldman S., Deikman J., Margossian L., Fischer R. L. Identification of an ethylene-responsive region in the promoter of a fruit ripening gene. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5939–5943. doi: 10.1073/pnas.90.13.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer T. J., Tiemeier D. C., Jaworski E. G. Purification and characterization of corn glutathione S-transferase. Biochemistry. 1983 Mar 1;22(5):1068–1072. doi: 10.1021/bi00274a011. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Lu A. Y. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- Raghothama K. G., Lawton K. A., Goldsbrough P. B., Woodson W. R. Characterization of an ethylene-regulated flower senescence-related gene from carnation. Plant Mol Biol. 1991 Jul;17(1):61–71. doi: 10.1007/BF00036806. [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., Pickett C. B. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990 Aug 25;265(24):14648–14653. [PubMed] [Google Scholar]
- Twell D., Klein T. M., Fromm M. E., McCormick S. Transient expression of chimeric genes delivered into pollen by microprojectile bombardment. Plant Physiol. 1989 Dec;91(4):1270–1274. doi: 10.1104/pp.91.4.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Goldsbrough P. B. An Arabidopsis gene with homology to glutathione S-transferases is regulated by ethylene. Plant Mol Biol. 1993 Jun;22(3):517–523. doi: 10.1007/BF00015980. [DOI] [PubMed] [Google Scholar]