Abstract
OBJECTIVE
To evaluate the trends in survival for infants with critical congenital heart defects (CCHDs) and to examine the potential impact of timing of diagnosis and other prognostic factors on survival.
METHODS
We performed a retrospective population-based cohort study in infants born with structural congenital heart defects (CHDs) between 1979 and 2005 and ascertained by the Metropolitan Atlanta Congenital Defects Program. We estimated Kaplan-Meier survival probabilities for 12 CCHD phenotypes by birth era and timing of diagnosis among infants without noncardiac defects or chromosomal disorders and used stratified Cox proportional hazards models to assess potential prognostic factors.
RESULTS
Of 1 056 541 births, there were 6965 infants with CHDs (1830 with CCHDs). One-year survival was 75.2% for those with CCHDs (n = 1336) vs 97.1% for those with noncritical CHDs (n = 3530; P < .001). One-year survival for infants with CCHDs improved from 67.4% for the 1979–1993 birth era to 82.5% for the 1994–2005 era (P < .001). One-year survival was 71.7% for infants with CCHDs diagnosed at ≤1 day of age (n = 890) vs 82.5% for those with CCHDs diagnosed at >1 day of age (n = 405; P < .001). There was a significantly higher risk of 1-year mortality for infants with an earlier birth era, earlier diagnosis, and low birth weight and whose mothers were <30 years old.
CONCLUSIONS
One-year survival for infants with CCHDs has been improving over time, yet mortality remains high. Later diagnosis is associated with improved 1-year survival. These benchmark data and identified prognostic factors may aid future evaluations of the impact of pulse oximetry screening on survival from CCHDs.
Keywords: epidemiology, congenital heart disease/defects, screening, neonatal, survival rate, pulse oximetry
Congenital heart defects (CHDs) occur in ~1 in every 110 births in the United States,1 with ~25% of cases comprising a group known as critical congenital heart defects (CCHDs).2 If not detected promptly, CCHDs may have catastrophic consequences.3,4 This delayed diagnosis has been shown to lead to significant mortality as well as substantial short- and long-term morbidity for survivors.5,6
Pulse oximetry is a simple, noninvasive bedside test that can accurately detect the percentage of hemoglobin saturated with oxygen; infants with CCHDs typically have a low percentage of saturation even before the onset of symptoms.7 In a recent review of 13 studies using pulse oximetry to screen for CCHDs, pulse oximetry screening (POS) was shown to have a sensitivity of 76.5% and a specificity of 99.9%.8 When screening is performed after 24 hours of age, there is an estimated very low false-positive rate of 0.05%. As such, POS is already in use in many parts of Europe7,9–11 and has recently been added to the Recommend Uniform Screening Panel for newborns in the United States.12,13
However, the impact that POS will have on outcomes for infants with CCHDs is unclear.14 To understand this potential impact in the United States, we used population-based data on survival for children with CCHDs to evaluate the trends in survival for infants with CCHDs and to examine the potential impact of timing of diagnosis and other prognostic factors on survival.
METHODS
Data Sources
Children with CHDs were ascertained by the Metropolitan Atlanta Congenital Defects Program (MACDP), an active, population-based surveillance system that has been previously well described. 15 Briefly, the MACDP was begun in 1967 to monitor the prevalence of congenital defects in metropolitan Atlanta, Georgia. The MACDP operates in collaboration with and on behalf of the Georgia Division of Public Health by the Georgia Department of Human Resources and has approval of the institutional review board of the Centers for Disease Control and Prevention. The monitoring program includes those children in whom a major birth defect is diagnosed before 6 years of age and whose mothers had a primary residence in the metropolitan Atlanta 5-county area at the time of delivery. The MACDP actively collects clinical and demographic information on all identified cases through review of medical records and autopsy reports. Survival status is determined via 3 possible methods: (1) review of available clinical records, (2) linkage with vital records from the state of Georgia, and (3) linkage with the National Death Index. For this project, National Death Index records were available from January 1, 1979, to December 31, 2006. With 1-year survival as the primary endpoint, the birth cohort was thus limited to those born from January 1, 1979, to December 31, 2005.
All cases in the MACDP with International Classification of Disease, Ninth Revision, modified British Pediatric Association codes for CHDs are reviewed and classified by experts in pediatric cardiology by using a standard clinical nomenclature adopted from the Society of Thoracic Surgeons and a schema based on current understanding of development morphogenesis. 16 All infants identified with a CHD in the MACDP were included in the baseline characteristics summary and the prevalence estimates, but those with either a chromosomal disorder or a noncardiac defect were excluded from the survival and proportional hazards analyses. For this project, the term CCHDs includes 12 CHDs that are likely to be detected by POS some or most of the time, including 7 primary and 5 secondary screening targets.6 Primary targets for screening are as follows: hypoplastic left heart syndrome, pulmonary atresia, tetralogy of Fallot, transposition of the great arteries, tricuspid atresia, truncus arteriosus, and total anomalous pulmonary venous return.6,13 Secondary targets are coarctation of the aorta, double outlet right ventricle, Ebstein’s anomaly, interrupted aortic arch, and single ventricle.6 Although severe cases of pulmonary stenosis and aortic stenosis are often considered critical,6 these defects were not considered as CCHDs for this analysis because disease severity is not routinely collected or classified by the MACDP.
Outcomes and Prognostic Factors
Among infants with isolated CHDs (those without chromosomal abnormalities or noncardiac defects), we first analyzed survival for infants with CCHDs versus those with noncritical CHDs. Within the CCHD cohort, we then assessed the following: (1) the difference in 1-year survival for primary versus secondary screening targets, (2) the trend in 1-year survival from 1979 to 2005 for all cases, and (3) the difference in 1-year survival based on timing of diagnosis (diagnosed at >1 day of age versus diagnosed at ≤1 day of age, including prenatal diagnoses). The 1-day of age cutoff for timing of diagnosis was chosen because this period most closely reflects the timing of potential diagnoses via the proposed POS algorithm in the United States.13 We did not assess diagnosis before versus after discharge from the newborn nursery, because the MACDP ascertains only day of diagnosis, not inpatient status at time of diagnosis.
As possible prognostic factors for mortality, we considered the following variables for which there was information available: birth era (1979–1993 vs 1994–2005), age at diagnosis (diagnosed at >1 day of age versus diagnosed at ≤1 day of age), race (white versus nonwhite), birth weight (<2500 vs ≥2500 g), gender, maternal age (<30 vs ≥30 years), and neighborhood poverty status (<20% in census tract living in poverty vs ≥20%).
Statistical Analyses
We used χ2 analyses to compare baseline characteristics between the CCHD cohort and the noncritical CHD cohort. Prevalence estimates were determined by using Poisson distribution. To compare survival, we estimated Kaplan-Meier survival probabilities and used the log-rank test to determine significance (P < .05). To evaluate potential risk factors for mortality among those with CCHDs, we first constructed univariate Cox proportional hazards models to determine the significance of the hazard ratio (HR) for each risk factor by using the Wald test. Those risk factors with a P < .20 were then included in a multivariate, stratified Cox proportional hazards model with the use of backward elimination to determine those factors meeting significance at the P = .05 level after adjustment. Because neighborhood poverty status did not satisfy the proportional hazards assumption, the final multivariate model was stratified on this variable to account for potential confounding. All analyses were performed with the use of SAS, version 9.3 (SAS Institute, Cary, NC).
RESULTS
Of the 1 056 541 live births in the 5-county metropolitan Atlanta region from 1979 to 2005, there were 6965 children identified with a CHD by the MACDP. Of these, 1830 (26.3%) had a CCHD, with 1204 (17.3%) having 1 of the 7 primary targets of CCHD screening and 626 (9.0%) having 1 of the 5 secondary targets. The overall estimated live birth prevalence was 65.9 per 10 000 live births for all CHDs, 17.3 per 10 000 live births for CCHDs, and 11.4 per 10 000 live births for the primary targets of CCHD screening. Compared with those with noncritical CHDs, those in the CCHD cohort were more likely to be male, to have noncardiac defects, and to be born to a mother under the age of 30 years, but they were less likely to be of low birth weight. There were no significant differences with respect to race, neighborhood poverty status, or presence of chromosomal syndromes. Among those without chromosomal or noncardiac defects, those with CCHDs were more likely to be diagnosed on or before 1 day of age than those with noncritical CHDs (Table 1). There was an increase in the detection of all CHDs from 1979 to 2005, increasing from 50.1 cases per 10 000 live births in 1979–1986 to 84.5 cases per 10 000 live births in 2000–2005 (Table 2).
TABLE 1.
Baseline Characteristics of Infants With CCHDs Versus Noncritical CHDs in Metropolitan Atlanta, Georgia: 1979–2005
| CCHDs | Noncritical CHDs | P | |
|---|---|---|---|
| Gender | <.001 | ||
| Male | 1027 (56.1) | 2459 (47.9) | |
| Female | 802 (43.9) | 2671 (52.1) | |
| Maternal age | .01 | ||
| <20 years | 196 (10.7) | 473 (9.2) | |
| 20–24 years | 412 (22.5) | 1059 (20.6) | |
| 25–29 years | 492 (26.9) | 1354 (26.4) | |
| ≥30 years | 729 (39.9) | 2249 (43.8) | |
| Birth weight | <.001 | ||
| <2500 g | 415 (22.7) | 1368 (26.6) | |
| ≥2500 g | 1415 (77.3) | 3767 (73.4) | |
| Race | .19 | ||
| White | |||
| Nonwhite | 897 (49.0) | 2609 (50.8) | |
| Neighborhood poverty status | .85 | ||
| Neighborhood poverty <20% | 1495 (84.2) | 4172 (84.0) | |
| Neighborhood poverty ≥20% | 280 (15.8) | 793 (16.0) | |
| Presence of a chromosomal syndrome | |||
| No | 1596 (87.9) | 4236 (86.2) | |
| Yes | 220 (12.1) | 678 (13.8) | |
| Presence of noncardiac defects (without a syndrome) | |||
| No | 1557 (85.7) | 4339 (88.3) | |
| Yes | 259 (14.3) | 575 (11.7) | |
| Age at diagnosisa | <.001 | ||
| ≤ 1 day old | 890 (68.7) | 1776 (51.6) | |
| >1 day old | 405 (31.3) | 1666 (48.4) |
Data are n (%). CCHDs include primary targets (hypoplastic left heart syndrome, transposition of the great arteries, tricuspid atresia, truncus arteriosus, tetralogy of Fallot, total anomalous pulmonary venous return, and pulmonary atresia) and secondary targets (interrupted aortic arch, coarctation of the aorta, double outlet right ventricle, Ebstein’s anomaly, and single ventricle) of screening. Noncritical CHDs include all other structural congenital heart defects.
Excludes those with noncardiac defects and chromosomal syndromes.
TABLE 2.
Live Birth Prevalence of CHDs and CCHDs in Metropolitan Atlanta, Georgia: 1979–2005
| Period | Births | Cases per 10 000 Live Births (95% CI) |
||
|---|---|---|---|---|
| All CHDs | All CCHDs | CCHD Primary Targets | ||
| 1979–1986 | 228 566 | 50.1 (47.3–53.1) | 17.1 (15.5–18.9) | 11.9 (10.5–13.4) |
| 1987–1993 | 264 060 | 55.0 (52.3–57.9) | 16.4 (14.9–18.0) | 11.3 (10.1–12.6) |
| 1994–1999 | 255 847 | 68.9 (65.8–72.2) | 17.9 (16.4–19.7) | 11.3 (10.1–12.7) |
| 2000–2005 | 308 068 | 84.5 (81.3–87.8) | 17.8 (16.4–19.3) | 11.2 (10.1–12.5) |
| Total | 1 056 541 | 65.9 (64.4–67.5) | 17.3 (16.5–18.1) | 11.4 (10.8–12.1) |
CCHDs include primary targets (hypoplastic left heart syndrome, transposition of the great arteries, tricuspid atresia, truncus arteriosus, tetralogy of Fallot, total anomalous pulmonary venous return, and pulmonary atresia) and secondary targets (interrupted aortic arch, coarctation of the aorta, double outlet right ventricle, Ebstein’s anomaly, and single ventricle) of screening.
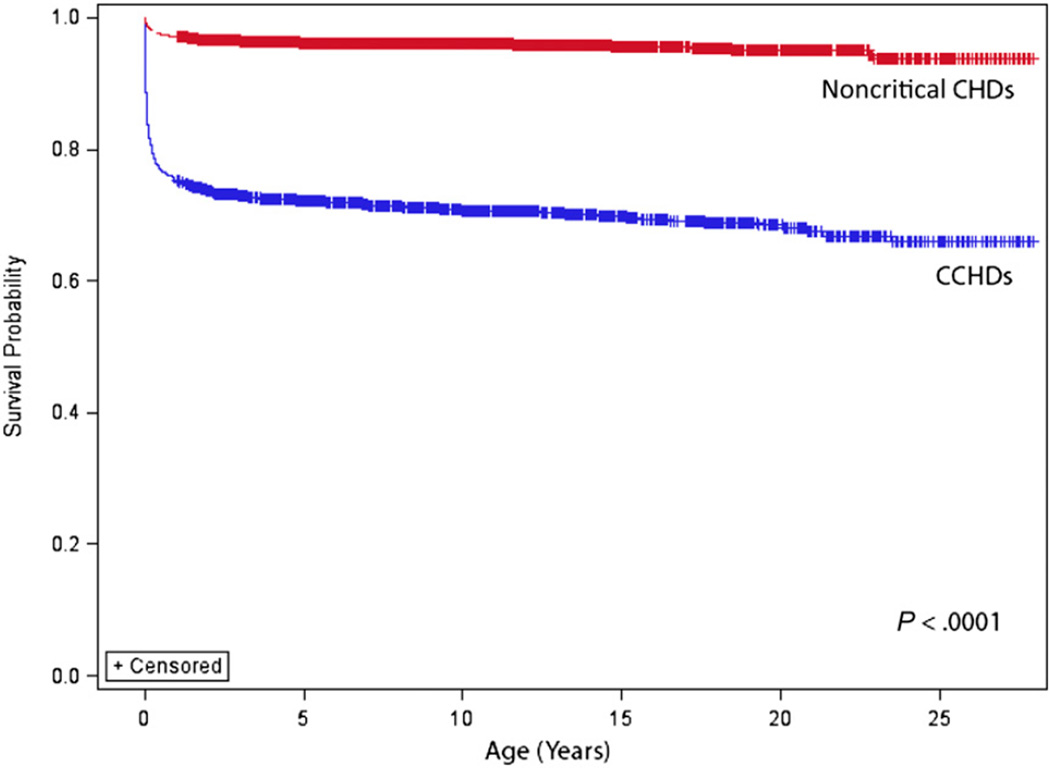
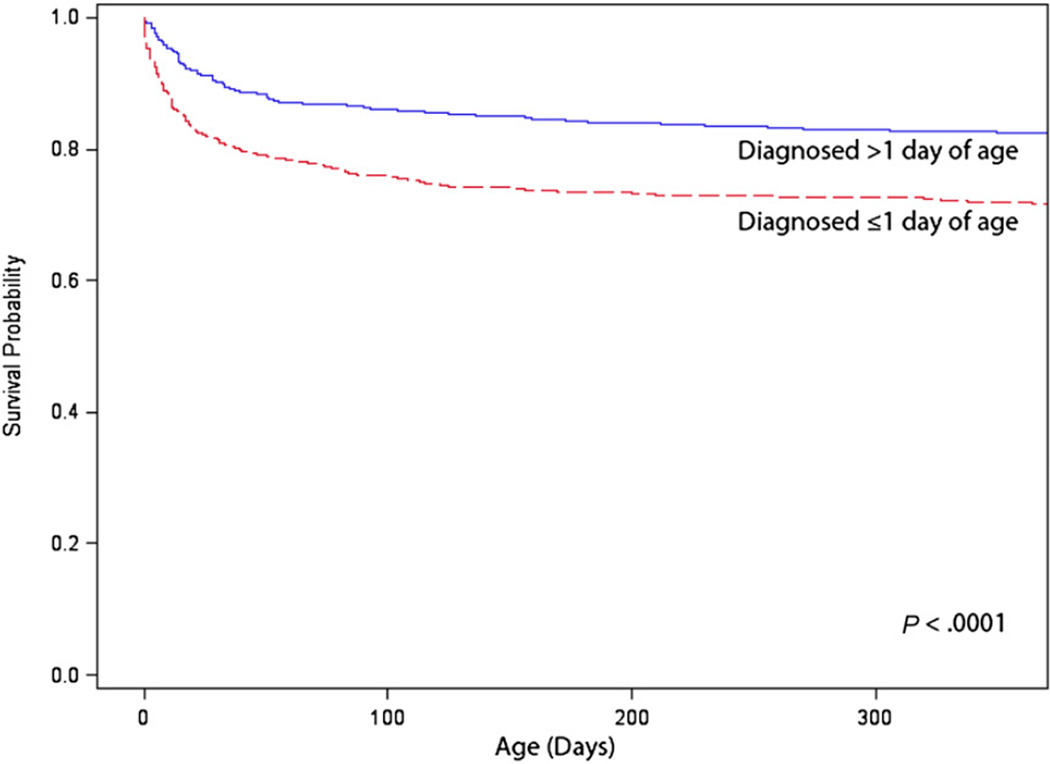
One-year survival was significantly greater for those with isolated noncritical CHDs (97.1%) compared with those with isolated CCHDs (75.2%; P < .001). Similarly, survival to adulthood (18 years of age) was significantly greater for those with isolated noncritical CHDs (95.4%) than for those with isolated CCHDs (68.8%; P < .001) (Fig 1). Among those with CCHDs, those with 1 of the 5 secondary targets of CCHD screening had better 1-year survival (85.1%) than those with 1 of the 7 primary targets of CCHD screening (70.2%; P <. 001). One-year survival for infants with CCHDs significantly improved over time, from 67.4% for the 1979–1993 birth era to 82.5% for the 1994–2005 era (P < .001). Those diagnosed with CCHDs on or before 1 day of age had poorer 1-year survival (71.7%) compared with those with CCHDs diagnosed after 1 day of age (82.5%; P < .001) (Fig 2).
FIGURE 1.
Survival for persons with CCHDs versus noncritical CHDs: Atlanta, Georgia, 1979–2005. These data exclude persons with noncardiac defects and chromosomal syndromes.
FIGURE 2.
One-year survival for infants with CCHDs by age at diagnosis: Atlanta, Georgia, 1979–2005. These data exclude persons with noncardiac defects and chromosomal syndromes.
In the univariate analysis, risk factors associated with mortality at the P < .20 level included birth era, timing of diagnosis, gender, maternal age, and low birth weight. After including these variables in a multivariate model and performing backward elimination, factors with a significantly increased proportional hazards of mortality were earlier birth era (HR = 2.65; 95% confidence interval [CI]: 2.11–3.32) and low birth weight (HR = 1.73; 95% CI: 1.34–2.24); those factors with a significantly decreased hazards were diagnosis at >1 day of age (HR = 0.54; 95% CI: 0.42–0.69) and maternal age ≥30 years (HR = 0.77; 95% CI: 0.62–0.97) (Table 3).
TABLE 3.
Stratified Cox Proportional HRs for 1-Year Mortality in Infants Born With CCHDs: Atlana, Georgia, 1979–2005
| Variable | Referent Group | HRa | 95% CI | P |
|---|---|---|---|---|
| Born 1979–1993 | Born 1994–2005 | 2.65 | 2.11–3.32 | <.001 |
| Diagnosed at >1 day old | Diagnosed at ≤1 day old | 0.54 | 0.42–0.69 | <.001 |
| Maternal age ≥30 years | Maternal age <30 years | 0.77 | 0.62–0.97 | .02 |
| Birth weight <2500 g | Birth weight ≥2500 g | 1.73 | 1.34–2.24 | <.001 |
Infants with noncardiac defects and chromosomal syndromes were excluded.
Each HR was adjusted for the other variables in the table. The model was stratified on neighborhood poverty status because it violated the proportional hazards assumption.
DISCUSSION
As anticipated, our findings reveal that infants with CCHDs have poorer survival than those with noncritical CHDs, but that survival for the critical subset has been improving over time. Still, with only 82.5% 1-year survival in the most recent birth era, CCHDs remain a priority for public health action. Implementing POS is one such public health effort to improve survival. In our cohort, those with a later diagnosis had better survival than those with an earlier diagnosis. Importantly, there were several other significant prognostic factors that affected survival even after accounting for differences in timing of diagnosis, including birth era, maternal age, and birth weight. Future evaluations of the impact of POS should include these factors when assessing survival.
With regard to the temporal trends of birth prevalence of CHDs, there was an increase in the detection of all CHDs from 1979 to 2005, increasing from 50.1 cases per 10 000 live births in 1979–1986 to 84.5 cases per 10 000 live births in 2000–2005. This increase was primarily driven by the cases of noncritical CHDs, a phenomenon likely explained by increased ascertainment of those defects.2,17 The live birth prevalence of CCHDs (~17 per 10 000 live births) remained stable throughout the time course. These findings are similar to prevalence estimates from a recent evaluation of CHDs in Europe.18
Our findings with regard to the occurrence and outcomes of delayed diagnosis of CCHDs are similar to those of smaller European studies. In our study, 31.3% of isolated CCHD cases were diagnosed beyond the first day of life, a proportion comparable to the ~25% of cases diagnosed after discharge from the hospital in other studies.19–21 Similarly, our study is in agreement with smaller studies in the finding that infants with a later diagnosis of a CCHD had improved survival compared with those with a CCHD diagnosed earlier. In a review of 286 neonates (<1 month of age) undergoing cardiac surgery for CHDs in the United Kingdom in 1999–2002, infants with CHDs diagnosed after discharge from the hospital after birth had improved postoperative survival (survival to discharge from the hospital) compared with those with CHDs diagnosed prenatally; however, there was no difference in survival compared with those with CHDs diagnosed postnatally before discharge from the maternity unit.19 In a study in 259 infants with CCHDs in Sweden who underwent surgery before 2 months of age in 1993–2001, infants with CCHDs diagnosed after discharge from the maternity unit had better early postoperative survival (survival of >30 days after surgery) than those with CCHDs diagnosed before discharge.20 The precise explanation as to why those whose CCHDs are diagnosed earlier seem to have poorer survival is unclear. We believe that this observation is likely a reflection of the severity of disease, ie, those that have more severe disease are more likely to be diagnosed earlier and are also more likely to have poorer survival. Future studies are warranted to evaluate this hypothesis.
Of the other factors that we analyzed, our findings for low birth weight22 and earlier birth era23 are consistent with the findings of previous studies. Interestingly, although female infants24,25 and nonwhites26 have been shown to have increased in-hospital mortality after congenital heart surgery in previous studies, neither gender nor race was revealed to be a significant risk factor for 1-year survival in our CCHD cohort. Conversely, whereas maternal age was not shown to be associated with survival in a recent study in children with functional single ventricle defects, older maternal age was found to be a protective factor in our analysis.27
An important measure of the success of any screening program is its ability to improve outcomes for those being screened.28 As such, our findings underscore the notion that the goal of POS should not be to attain outcomes for those cases diagnosed through screening similar to the outcomes of those cases diagnosed earlier by other means (prenatal ultrasound, symptoms prompting evaluation). Rather, the goal should be to improve the survival for those cases currently experiencing delayed diagnosis, which might be a group with lower severity of disease. In our analysis, there were an estimated 17.3 cases of CCHDs per 10 000 live births, with 31.3% of cases (5.4 cases per 10 000 births) being diagnosed after 1 day of age. With a 1-year survival rate of 82.5%, there would be an expected 0.94 infant deaths per 10 000 live births for children with a late-diagnosed CCHD. In the United States, with 4 131 019 births in 2009,29 this would translate into ~390 deaths annually in infants with a CCHD diagnosed after 1 day of age.
It is unclear what proportion of deaths in children with CCHDs is directly attributable to a delayed diagnosis of a CCHD and thus would be potentially preventable with POS. Existing studies aimed at addressing this question have primarily focused on diagnoses made either postmortem or at the time of acute decompensation leading to death (not just delayed diagnoses), relied on multiple assumptions, and were limited by their amount of clinical data. Yet, they do help inform expectations of the impact of POS. In California it was estimated that there were 1.7 cases of missed CCHD diagnoses contributing to death per 100 000 live births from 1989 to 2004, although this number was markedly decreasing over time.30 In a previous analysis of MACDP data from 1990 to 2001, it was estimated that infant mortality from undetected CCHDs was 1.2 deaths per 100 000 live births.31 These estimates suggest that ~50 to 70 deaths annually in the United States might be due to missed diagnosis. Estimates as to the impact of delayed or missed CCHD diagnosis worldwide are not known.
This study has several strengths. First, a major strength of our study is the robust, active case ascertainment system of the MACDP. Instead of relying on passive reports of CHDs, abstractors regularly review birth records, prenatal records, pediatric records, genetic records, cardiology records at selected offices, laboratory reports, and autopsy reports in an effort to attain accurate reports of new diagnoses of birth defects. Second, the identified cases of cardiac birth defects undergo rigorous review by a team of pediatric cardiologists and are classified according to a state of the science classification and coding system, thus optimizing the accuracy of the data for surveillance and research.32 Finally, the data have been actively collected over 25 years on a population level, allowing us to monitor important public health trends as surgical and medical care of children with CHDs has advanced.
However, this study is not without its limitations. Whereas there are strong data on diagnosis, there are limited data on subsequent hospital course and surgical interventions, thus limiting our analyses. We were not able to control for severity of disease, a factor that we hypothesize contributes to both earlier diagnosis and poorer survival. In addition, as noted in the Methods section, without information on severity of disease and clinical course, we could not include cases of critical aortic stenosis or critical pulmonary stenosis in our CCHD case definition. Similarly, without adequate surgical data, we cannot determine which deaths may have been due to palliative care options nor can we assess trends in surgical mortality.
CONCLUSIONS
One-year survival for infants with CCHDs has been improving over time, yet mortality remains high. As such, improving survival from CCHDs remains an opportunity for public health action. Evaluations of POS or other screening modalities for CCHDs should include assessments of improvements in outcomes, particularly long-term survival. Our findings of trends in and prognostic factors for survival of children with CCHDs may aid such evaluations.
WHAT’S KNOWN ON THIS SUBJECT
Pulse oximetry testing in newborns can detect asymptomatic cases of critical congenital heart defects and has been added to the US Recommended Uniform Screening Panel. However, the impact that earlier diagnosis may have on survival in this population is unclear.
WHAT THIS STUDY ADDS
One-year survival for infants with critical congenital heart defects has been improving over time, yet mortality remains high. Survival has been greatest for those diagnosed after 1 day of age and may increase more with screening using pulse oximetry.
ACKNOWLEDGMENTS
We thank the Metropolitan Atlanta Congenital Defects Program abstractors for their conscientious and skilled data collection efforts.
ABBREVIATIONS
- CCHD
critical congenital heart defect
- CHD
congenital heart defect
- CI
confidence interval
- HR
hazard ratio
- MACDP
Metropolitan Atlanta Congenital Defects Program
- POS
pulse oximetry screening
Footnotes
Dr Oster provided substantial contributions to the study concept and design, analyzed and interpreted data, drafted the manuscript, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published; Mr Lee provided substantial contributions to the analysis and interpretation of data, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published; and Drs Honein, Riehle-Colarusso, Shin, and Correa contributed substantially to the study concept and design and interpretation of data, revised the manuscript critically for important intellectual content, and provided final approval of the version to be published.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: No external funding.
REFERENCES
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153(6):807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botto LD, Correa A, Erickson JD. Racial and temporal variations in the prevalence of heart defects. Pediatrics. 2001;107(3):E32. doi: 10.1542/peds.107.3.e32. Available at: www.pediatrics.org/cgi/content/full/107/3/e32. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D, Coltri A, Butera G, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99(7):916–918. doi: 10.1161/01.cir.99.7.916. [DOI] [PubMed] [Google Scholar]
- 4.Soongswang J, Adatia I, Newman C, Smallhorn JF, Williams WG, Freedom RM. Mortality in potential arterial switch candidates with transposition of the great arteries. J Am Coll Cardiol. 1998;32(3):753–757. doi: 10.1016/s0735-1097(98)00310-6. [DOI] [PubMed] [Google Scholar]
- 5.Kuehl KS, Loffredo CA, Ferencz C. Failure to diagnose congenital heart disease in infancy. Pediatrics. 1999;103(4 Pt 1):743–747. doi: 10.1542/peds.103.4.743. [DOI] [PubMed] [Google Scholar]
- 6.Mahle WT, Newburger JW, Matherne GP, et al. American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research; American Academy of Pediatrics Section on Cardiology and Cardiac Surgery and Committee on Fetus and Newborn. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Circulation. 2009;120(5):447–458. doi: 10.1161/CIRCULATIONAHA.109.192576. [DOI] [PubMed] [Google Scholar]
- 7.Ewer AK, Middleton LJ, Furmston AT, et al. PulseOx Study Group. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): a test accuracy study. Lancet. 2011;378(9793):785–794. doi: 10.1016/S0140-6736(11)60753-8. [DOI] [PubMed] [Google Scholar]
- 8.Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: a systematic review and meta-analysis. Lancet. 2012;379(9835):2459–2464. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]
- 9.Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine—results from a prospective multicenter study. Eur J Pediatr. 2010;169(8):975–981. doi: 10.1007/s00431-010-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de-Wahl Granelli A, Wennergren M, Sandberg K, et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ. 2009;338:a3037. doi: 10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuelling B, Arlettaz Mieth R, Bauersfeld U, Balmer C. Pulse oximetry screening for congenital heart defects in Switzerland: most but not all maternity units screen their neonates. Swiss Med Wkly. 2009;139(47–48):699–704. doi: 10.4414/smw.2009.12880. [DOI] [PubMed] [Google Scholar]
- 12.Sebelius K. [Accessed January 30, 2012];Letter to the Secretary’s Advisory Council on Hereditary Diseases of Newborn and Children. Available at: www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/recommendations/correspondence/cyanoticheartsecre09212011.pdf.
- 13.Kemper AR, Mahle WT, Martin GR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5):e1259–e1267. doi: 10.1542/peds.2011-1317. Available at: www.pediatrics.org/cgi/content/full/128/5/e1259. [DOI] [PubMed] [Google Scholar]
- 14.Mahle WT, Martin GR, Beekman RH, III, Morrow WR Section on Cardiology and Cardiac Surgery Executive Committee. Endorsement of Health and Human Services recommendation for pulse oximetry screening for critical congenital heart disease. Pediatrics. 2012;129(1):190–192. doi: 10.1542/peds.2011-3211. [DOI] [PubMed] [Google Scholar]
- 15.Correa A, Cragan JD, Kucik JE, et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol. 2007;79(2):65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 16.Riehle-Colarusso T, Strickland MJ, Reller MD, et al. Improving the quality of surveillance data on congenital heart defects in the metropolitan Atlanta congenital defects program. Birth Defects Res A Clin Mol Teratol. 2007;79(11):743–753. doi: 10.1002/bdra.20412. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 18.Dolk H, Loane M, Garne E European Surveillance of Congenital Anomalies (EUROCAT) Working Group. Congenital heart defects in Europe: prevalence and perinatal mortality, 2000 to 2005. Circulation. 2011;123(8):841–849. doi: 10.1161/CIRCULATIONAHA.110.958405. [DOI] [PubMed] [Google Scholar]
- 19.Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart. 2006;92(9):1298–1302. doi: 10.1136/hrt.2005.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mellander M, Sunnegårdh J. Failure to diagnose critical heart malformations in newborns before discharge—an increasing problem? Acta Paediatr. 2006;95(4):407–413. doi: 10.1080/08035250500541910. [DOI] [PubMed] [Google Scholar]
- 21.Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed. 2008;93(1):F33–F35. doi: 10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 22.Curzon CL, Milford-Beland S, Li JS, et al. Cardiac surgery in infants with low birth weight is associated with increased mortality: analysis of the Society of Thoracic Surgeons Congenital Heart Database. J Thorac Cardiovasc Surg. 2008;135(3):546–551. doi: 10.1016/j.jtcvs.2007.09.068. [DOI] [PubMed] [Google Scholar]
- 23.Wren C, Irving CA, Griffiths JA, et al. Mortality in infants with cardiovascular malformations. Eur J Pediatr. 2012;171(2):281–287. doi: 10.1007/s00431-011-1525-3. [DOI] [PubMed] [Google Scholar]
- 24.Marelli A, Gauvreau K, Landzberg M, Jenkins K. Sex differences in mortality in children undergoing congenital heart disease surgery: a United States population-based study. Circulation. 2010;122(11 suppl):S234–S240. doi: 10.1161/CIRCULATIONAHA.109.928325. [DOI] [PubMed] [Google Scholar]
- 25.Seifert HA, Howard DL, Silber JH, Jobes DR. Female gender increases the risk of death during hospitalization for pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2007;133(3):668–675. doi: 10.1016/j.jtcvs.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Oster ME, Strickland MJ, Mahle WT. Racial and ethnic disparities in post-operative mortality following congenital heart surgery. J Pediatr. 2011;159(2):222–226. doi: 10.1016/j.jpeds.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 27.Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation. 2010;121(5):644–650. doi: 10.1161/CIRCULATIONAHA.109.881904. [DOI] [PubMed] [Google Scholar]
- 28.Harris RP, Helfand M, Woolf SH, et al. Methods Work Group, Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med. 2001;20(3 suppl):21–35. doi: 10.1016/s0749-3797(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 29.Sutton PD, Hamilton BE, Mathews TJ. Recent decline in births in the United States, 2007–2009. NCHS data brief, no. 60. 2011. [PubMed] [Google Scholar]
- 30.Chang RK, Gurvitz M, Rodriguez S. Missed diagnosis of critical congenital heart disease. Arch Pediatr Adolesc Med. 2008;162(10):969–974. doi: 10.1001/archpedi.162.10.969. [DOI] [PubMed] [Google Scholar]
- 31.Riehle-Colarusso T, Mahle WT, Siffel C, Correa A. Deaths among undiagnosed cases of congenital heart disease in a metropolitan area, 1990–2001: a population-based study [abstract] Congenit Heart Dis. 2007;2(5):363–382. [Google Scholar]
- 32.Strickland MJ, Riehle-Colarusso TJ, Jacobs JP, et al. The importance of nomenclature for congenital cardiac disease: implications for research and evaluation. Cardiol Young. 2008;18(suppl 2):92–100. doi: 10.1017/S1047951108002515. [DOI] [PMC free article] [PubMed] [Google Scholar]