Abstract
Early detection of psychosis is an important topic in psychiatry. Yet, there is limited information on the prevalence and clinical significance of high-risk symptoms in children and adolescents as compared to adults. We examined ultra-high-risk (UHR) symptoms and criteria in a sample of individuals aged 8-40 years from the general population of Canton Bern, Switzerland, enrolled from June 2011 to May 2014. The current presence of attenuated psychotic symptoms (APS) and brief intermittent psychotic symptoms (BLIPS) and the fulfillment of onset/worsening and frequency requirements for these symptoms in UHR criteria were assessed using the Structured Interview for Psychosis Risk Syndromes. Additionally, perceptive and non-perceptive APS were differentiated. Psychosocial functioning and current non-psychotic DSM-IV axis I disorders were also surveyed. Well-trained psychologists performed assessments. Altogether, 9.9% of subjects reported APS and none BLIPS, and 1.3% met all the UHR requirements for APS. APS were related to more current axis I disorders and impaired psychosocial functioning, indicating some clinical significance. A strong age effect was detected around age 16: compared to older individuals, 8-15-year olds reported more perceptive APS, that is, unusual perceptual experiences and attenuated hallucinations. Perceptive APS were generally less related to functional impairment, regardless of age. Conversely, non-perceptive APS were related to low functioning, although this relationship was weaker in those below age 16. Future studies should address the differential effects of perceptive and non-perceptive APS, and their interaction with age, also in terms of conversion to psychosis.
Keywords: Psychosis, ultra-high-risk symptoms, attenuated psychotic symptoms, brief intermittent psychotic symptoms, children and adolescents, general population
Early detection of psychosis has become an important topic in psychiatry. Yet, despite several efforts to define a clinical high risk of developing psychosis, limited attention has been specifically directed towards children and adolescents (<18 years) (1–4). Two early detection approaches, developed and evaluated predominately in adult or mixed-aged samples, currently prevail (3,5): the basic symptom (6) and the ultra-high-risk (UHR) approach (7). The latter mainly relies on attenuated psychotic symptoms (APS), that is, delusional and hallucinatory phenomena in which some insight is still maintained.
Within the debate on the attenuated psychosis syndrome, proposed for inclusion in the DSM-5, concern about pathologization of non-ill behaviors and experiences has been voiced (8). Such a concern might particularly apply to children and adolescents for several reasons. First, conversion rates in help-seeking UHR samples aged 12-18 years were lower than those observed in adult or mixed-age samples (3,4,9,10), which might indicate a lesser predictive accuracy of UHR criteria in this age group (3). Second, though not assessing the UHR criteria with specific instruments, community studies of children and adolescents found high prevalence rates of APS (11–14), particularly hallucinations, with a spontaneous remission in about three quarters of cases (14). Third, the association of both clinician-rated and self-rated APS with poorer socio-occupational functioning and psychiatric morbidity (12,15–17), and thus their clinical significance, seems to increase with age in community and help-seeking samples of children and adolescents, again especially in the case of hallucinatory phenomena. Thus, it has been recently argued that the validity of current risk criteria needs to be examined, and possibly adapted to this segment of the population (1–3,6).
Traditionally, an age threshold of 18 years is applied in psychiatry to distinguish between early and adult onset. However, it is currently unknown whether the postulated decrease in frequency and increase in clinical significance of APS with advancing age follows this traditional threshold, or rather corresponds to the transition from childhood to adolescence, around the age of 13, or from early to late adolescence, around the age of 16.
In this study, we explored the relationship between age and the prevalence and clinical significance of APS in a sample of individuals aged 8-40 years, who were randomly selected from the general population of Canton Bern, Switzerland.
METHODS
Study design and procedure
Stratified sampling by sex (1:1) was used to randomly select potential participants aged 8-17 years (in the Bi-national Evaluation of At-Risk Symptoms in Children and Adolescents, BEARS-Kid study) or 16-40 years (in the Bern Epidemiology At-Risk, BEAR study) from approximately 384,000 persons of these age groups included in the obligatory population register of Canton Bern, Switzerland.
In the BEARS-Kid study, subjects were assessed face-to-face, while in the BEAR study subjects were evaluated by telephone interviews that were supported by the computer-assisted telephone interviewing technique (18). Prior to the studies, excellent concordance rates (78-100%) were found for the telephone assessments of risk criteria/symptoms as compared to face-to-face assessments (19).
The ethics committee of the University of Bern approved both studies. Participation in the telephone interview was considered as provision of informed consent in the BEAR study. For the BEARS-Kid study, written informed assent/consent was secured from subjects and their parents.
Recruitment and assessments for the BEAR study were conducted over 14 months (from June 2011 to July 2012) and those for the BEARS-Kid study over 33 months (from September 2011 to May 2014).
In both studies, eligibility criteria included appropriate age, main residence in Canton Bern (i.e., a valid address and not being abroad during the assessment period), and an available telephone number. Interviews were discontinued if respondents had a lifetime diagnosis of psychosis or insufficient German, French or English language skills.
In the BEARS-Kid study, the participation rate was 41.5% of eligible children/adolescents. Those who participated did not differ from those who refused to participate in terms of age, gender and nationality. The main reasons for refusal to participate were lack of interest in the topic (49.6%), lack of time (33.8%), excessive length of the interview (16.5%), and expectation of an uncomfortable interview (11.0%). No reason was provided by 10.2% of those who refused to participate.
In the BEAR study, the participation rate was 66.4% of eligible persons. Those who participated did not differ from those who refused to participate in terms of age, gender and nationality. The main reasons for refusal to participate were lack of interest in the topic (52.9%), lack of time (44.5%), expectation of the assessment of too intimate data (15.3%), and excessive length of the interview (13.2%). No reason was provided by 38.9% of those who refused to participate.
Where allowed by the subsample size, each child/adolescent (aged 8-17 years) was randomly matched by gender and highest educational level of parents to each of the four adult age groups (18-19, 20-24, 25-29, and 30-40 years). Our final sample comprised 535 adults and 154 children/adolescents.
Assessments
Assessments were performed by well-trained psychologists. The Structured Interview for Psychosis Risk Syndromes (SIPS, 20) was used to explore the current presence of APS and brief intermittent psychotic symptoms (BLIPS), and the fulfillment of onset/worsening and frequency requirements for these symptoms in UHR criteria. More specifically, the current presence of any APS (any SIPS item from P1 to P5 with a score between 3 and 5) and any BLIPS (any SIPS item from P1 to P5 with a score of 6) was assessed, as well as the fulfillment of the UHR requirements concerning onset/worsening (onset or worsening within the past 12 months for APS; level of psychotic intensity reached within the past 3 months for BLIPS) and frequency (at least weekly presence in the past month for APS; at least several minutes per day at a frequency of at least once per month for BLIPS). Non-perceptive (P1, P2, P3, and P5) and perceptive (P4) APS/BLIPS were also distinguished.
Symptom-independent current global level of psychosocial functioning was estimated using the Social and Occupational Functioning Assessment Scale (SOFAS, 21), in which a score of ≤70 was considered indicative of low functioning. The Mini-International Neuropsychiatric Interview (22) and its version for children (23) were used to assess current non-psychotic mental disorders according to DSM-IV criteria.
To ensure excellent data quality, interviewers received an intensive 3-month training prior to the start of both studies. Further, weekly supervision of symptom ratings was provided by two of the authors (F.S.-L. and C.M.), to avoid erroneous rating of vivid imaginations and fantasies or of experiences related to certain states of development as a UHR symptom, particularly within the BEARS-Kid study.
Statistical analyses
Using SPSS 21.0., frequencies and percentages were compared by chi-square tests, and non-normally distributed interval data and ordinal data were evaluated by the Mann-Whitney U tests.
Binary logistic regression analyses using “enter” were performed to assess effects of different age groups (8-12; 13-15; 16-17; 18-19; 25-29; 30-40) on UHR criteria and each of their requirements. The age group with a peak in the onset of first-episode psychosis (20-24 years) served as the reference group.
Logistic regression analyses were also used to assess the effects of UHR requirements and their interaction with age on low psychosocial functioning, and on the presence of any axis I disorder. To evaluate the potential additional effects of an age × requirement interaction, both the respective UHR requirements and their interaction with age were entered as independent variables, and the interaction with age was considered as relevant when both backward and forward logistic regression analyses equally selected the interaction term as a predictor. We expected small numbers of low functioning and axis I disorders per age group; therefore, age, rather than age group, was entered in the interaction analyses. Throughout, the goodness-of-fit (GoF) was estimated by the Omnibus test.
RESULTS
The current prevalence of any UHR symptom was 9.9%. As no BLIPS were detected, the UHR symptoms were exclusively APS. The prevalence of any perceptive APS was 4.9%; that of any non-perceptive APS was 6.1% (6.0% for any unusual thought content, 3.0% for any persecutory idea, 0.3% for any grandiosity, and 0.7% for any disorganized communication).
Subjects with APS were younger than those without APS, and more likely to have non-Swiss nationality, low psychosocial functioning, and any axis I diagnosis. The related effect sizes were small to medium. We did not find any significant difference in the frequency of APS with regard to participants’ gender or highest education of their parents (Table1).
Table 1.
Socio-demographic and clinical characteristics of subjects with and without ultra-high-risk (UHR) symptoms
| At least one UHR symptom | Total | Statistics | ||
|---|---|---|---|---|
| Yes (N=68; 9.9%) | No (N=621; 90.1%) | (N=689) | ||
| Male, n (%) | 26 (38.2) | 270 (43.5) | 296 (43.0) |
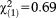 , p=0.407, V=0.032 , p=0.407, V=0.032 |
| Swiss nationality, n (%) | 58 (85.3) | 576 (92.8) | 634 (92.0) |
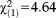 , p=0.031, V=0.082 , p=0.031, V=0.082 |
| Highest ISCED score of parents, median (quartiles) | 3 (3-5) | 3.5 (3-5) | 3 (3-5) | U=18354.0, p=0.059, r=0.072 |
| Age, median (quartiles) | 21.4 (16.1-28.4) | 23.5 (19.0-29.6) | 23.3 (18.5-29.5) | U=17920.5, p=0.040, r=0.078 |
| Age group, n (%) |
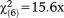 , p=0.016, V=0.151 , p=0.016, V=0.151 |
|||
| 8-12 years (n=45) | 10 (22.2) | 35 (77.8) | 45 (6.6) | |
| 13-15 years (n=31) | 7 (22.6) | 24 (77.4) | 31 (4.6) | |
| 16-17 years (n=78) | 8 (10.3) | 70 (89.7) | 78 (11.5) | |
| 18-19 years (n=81) | 6 (7.4) | 75 (92.6) | 81 (11.9) | |
| 20-24 years (n=155) | 12 (7.7) | 143 (92.3) | 155 (22.2) | |
| 25-29 years (n=144) | 11 (7.6) | 133 (92.4) | 144 (21.1) | |
| 30-40 years (n=155) | 14 (9.0) | 141 (91.0) | 155 (22.2) | |
| Any current axis I diagnosis, n (%) | 26 (38.2) | 68 (11.0) | 94 (13.6) |
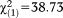 , p<0.001, V=0.237 , p<0.001, V=0.237 |
| First-degree relative with psychosis, n (%) | 0 | 6 (1.0) | 6 (0.9) |
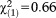 , p=0.415, V=0.031 , p=0.415, V=0.031 |
| SOFAS ≤ 70, n (%) | 12 (17.6) | 13 (2.1) | 25 (3.6) |
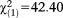 , p<0.001, V=0.248 , p<0.001, V=0.248 |
DSM-IV axis I diagnoses: n=57 (8.3%) mood disorders, mainly depression (n=28, 4.1%); n=34 (4.9%) anxiety disorders; n=6 (0.9%) obsessive-compulsive disorder; n=13 (1.9%) eating disorders; n=9 (1.3%) somatization disorders; only assessed in participants of the BEARS-Kid study (n=119): n=4 (3.4%) attention-deficit/hyperactivity disorder; n=1 (0.8%) conduct disorder
ISCED – International Standard Classification of Education, SOFAS – Social and Occupational Functioning Assessment Scale
In logistic regression analyses, the age group reliably distinguished between those with and without APS (GoF: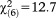 , p=0.049). Compared to persons aged 20-24 years, those aged 8-12 and 13-15 years were more likely to report APS, while all other age groups (i.e., 16-17, 18-19, 25-29, 30-40) were not (Table2).
, p=0.049). Compared to persons aged 20-24 years, those aged 8-12 and 13-15 years were more likely to report APS, while all other age groups (i.e., 16-17, 18-19, 25-29, 30-40) were not (Table2).
Table 2.
Effects of age on prevalence and UHR onset/worsening and frequency requirements for APS
| Effect on APS prevalence (irrespective of other UHR requirements) | Effect on APS prevalence (considering the onset/worsening but not the frequency requirement) | Effect on APS prevalence (considering the frequency but not the onset/worsening requirement) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | Wald (df=1) | p | Exp (β) | 95% CI | β | SE | Wald (df=1) | p | Exp (β) | 95% CI | β | SE | Wald (df=1) | p | Exp (β) | 95% CI | ||
| Any APS | 8-12 yrs. | 1.23 | 0.47 | 6.86 | 0.009 | 3.41 | 1.36-8.52 | 2.25 | 0.63 | 12.74 | 0.000 | 9.44 | 2.75-32.37 | 1.34 | 0.61 | 4.91 | 0.027 | 3.82 | 1.17-12.50 |
| 13-15 yrs. | 1.25 | 0.52 | 5.65 | 0.017 | 3.48 | 1.22-9.71 | 1.98 | 0.70 | 7.94 | 0.005 | 7.26 | 1.83-28.83 | 0.98 | 0.74 | 1.77 | 0.184 | 2.66 | 0.63-11.27 | |
| 16-17 yrs. | 0.31 | 0.48 | 0.42 | 0.519 | 1.36 | 0.53-3.48 | 0.41 | 0.78 | 0.28 | 0.596 | 1.51 | 0.33-6.92 | −0.01 | 0.72 | 0.00 | 0.993 | 0.99 | 0.24-4.08 | |
| 18-19 yrs. | −0.05 | 0.52 | 0.01 | 0.927 | 0.95 | 0.34-2.64 | −0.05 | 0.88 | 0.00 | 0.959 | 0.96 | 0.17-5.33 | −0.46 | 0.83 | 0.31 | 0.575 | 0.63 | 0.12-3.19 | |
| 25-29 yrs. | −0.02 | 0.43 | 0.00 | 0.973 | 0.99 | 0.42-2.31 | −0.63 | 0.87 | 0.52 | 0.470 | 0.53 | 0.10-2.95 | −0.64 | 0.72 | 0.79 | 0.373 | 0.53 | 0.13-2.15 | |
| 30-40 yrs. | 0.17 | 0.41 | 0.17 | 0.682 | 1.18 | 0.53-2.65 | −1.41 | 1.12 | 1.57 | 0.211 | 0.25 | 0.03-2.22 | 0.54 | 0.53 | 1.03 | 0.309 | 1.71 | 0.61-4.83 | |
| Any perceptive APS | 8-12 yrs. | 2.02 | 0.59 | 11.75 | 0.001 | 7.50 | 2.37-23.74 | 2.47 | 0.84 | 8.70 | 0.003 | 11.77 | 2.29-60.58 | 2.47 | 0.84 | 8.70 | 0.003 | 11.77 | 2.29-60.58 |
| 13-15 yrs. | 1.97 | 0.64 | 9.43 | 0.002 | 7.20 | 2.04-25.39 | 2.43 | 0.89 | 7.43 | 0.006 | 11.33 | 1.98-64.96 | 0.94 | 1.24 | 0.57 | 0.451 | 2.55 | 0.22-29.03 | |
| 16-17 yrs. | −0.24 | 0.85 | 0.08 | 0.781 | 0.79 | 0.15-4.16 | −16.87 | 4551.0 | 0.00 | 0.997 | 0.00 | 0.00 | −0.01 | 1.23 | 0.00 | 0.996 | 0.99 | 0.09-11.13 | |
| 18-19 yrs. | 0.44 | 0.69 | 0.42 | 0.517 | 1.56 | 0.41-5.97 | −16.87 | 4465.9 | 0.00 | 0.997 | 0.00 | 0.00 | −0.05 | 1.23 | 0.00 | 0.971 | 0.96 | 0.09-10.71 | |
| 25-29 yrs. | −0.15 | 0.68 | 0.05 | 0.821 | 0.86 | 0.23-3.26 | −0.63 | 1.23 | 0.26 | 0.611 | 0.54 | 0.05-5.96 | −16.87 | 3349.4 | 0.00 | 0.996 | 0.00 | 0.00 | |
| 30-40 yrs. | −0.23 | 0.68 | 0.11 | 0.740 | 0.80 | 0.21-3.02 | −16.87 | 3228.4 | 0.00 | 0.996 | 0.00 | 0.00 | −0.70 | 1.23 | 0.32 | 0.569 | 0.50 | 0.05-5.54 | |
| Any non-perceptive APS | 8-12 yrs. | −0.16 | 0.81 | 0.034 | 0.846 | 0.86 | 0.18-4.18 | −16.87 | 5991.6 | 0.00 | 0.998 | 0.00 | 0.00 | −0.38 | 1.11 | 0.12 | 0.730 | 0.68 | 0.08-5.99 |
| 13-15 yrs. | 0.68 | 0.71 | 0.92 | 0.338 | 1.97 | 0.49-7.88 | −16.87 | 7218.9 | 0.00 | 0.998 | 0.00 | 0.00 | 1.17 | 0.76 | 2.37 | 0.124 | 3.21 | 0.73-14.22 | |
| 16-17 yrs. | 0.43 | 0.56 | 0.58 | 0.446 | 1.53 | 0.51-4.58 | −0.01 | 1.23 | 0.00 | 0.996 | 0.99 | 0.09-11.13 | −0.94 | 1.10 | 0.73 | 0.393 | 0.39 | 0.05-3.39 | |
| 18-19 yrs. | −0.35 | 0.69 | 0.25 | 0.616 | 0.71 | 0.18-2.74 | −0.05 | 1.23 | 0.00 | 0.971 | 0.96 | 0.09-10.71 | −0.28 | 0.85 | 0.11 | 0.746 | 0.76 | 0.14-4.00 | |
| 25-29 yrs. | −0.23 | 0.55 | 0.17 | 0.685 | 0.80 | 0.27-2.36 | −16.87 | 3349.4 | 0.00 | 0.996 | 0.00 | 0.00 | −0.45 | 0.74 | 0.37 | 0.544 | 0.64 | 0.15-2.72 | |
| 30-40 yrs. | 0.60 | 0.46 | 1.72 | 0.190 | 1.82 | 0.74-4.48 | −16.87 | 3228.4 | 0.00 | 0.996 | 0.00 | 0.00 | 0.62 | 0.57 | 1.16 | 0.281 | 1.85 | 0.61-5.65 | |
Binary logistic regression analyses with method “enter” and 20-24-year olds as reference age group. Significant predictors are in bold type
UHR – ultra-high-risk, APS – attenuated psychotic symptoms
The model became even more significant (GoF: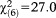 , p<0.001) when only perceptive APS were considered. Odds ratios in subjects aged 8-12 and 13-15 increased while, again, no effect emerged in the adult age groups and in the 16-17-year olds. Conversely, when only non-perceptive APS were considered, the model was non-significant (GoF:
, p<0.001) when only perceptive APS were considered. Odds ratios in subjects aged 8-12 and 13-15 increased while, again, no effect emerged in the adult age groups and in the 16-17-year olds. Conversely, when only non-perceptive APS were considered, the model was non-significant (GoF: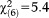 , p=0.490), indicating that persons across all age groups were equally likely to report non-perceptive APS (Table2).
, p=0.490), indicating that persons across all age groups were equally likely to report non-perceptive APS (Table2).
When the UHR onset/worsening requirement was considered, the age effects on the prevalence of APS increased (GoF: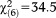 , p<0.001). Again, only persons aged 8-12 and 13-15 years were more likely to fulfill the requirement, as compared to the 20-24-year-olds. Separate analyses of the onset/worsening requirement for perceptive and non-perceptive APS again showed a stronger effect (GoF:
, p<0.001). Again, only persons aged 8-12 and 13-15 years were more likely to fulfill the requirement, as compared to the 20-24-year-olds. Separate analyses of the onset/worsening requirement for perceptive and non-perceptive APS again showed a stronger effect (GoF: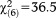 , p<0.001) of perceptive APS in persons aged 8-12 and 13-15 years, while an age effect for non-perceptive APS (GoF:
, p<0.001) of perceptive APS in persons aged 8-12 and 13-15 years, while an age effect for non-perceptive APS (GoF: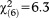 , p=0.389) was not observed (Table2).
, p=0.389) was not observed (Table2).
When the UHR frequency requirement was considered, age effects declined to a statistical trend level (GoF: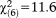 , p=0.071), where only persons aged 8-12 were significantly more likely to fulfill the requirement as compared to persons aged 20-24. Again, this age effect was maintained and even intensified for perceptive APS (GoF:
, p=0.071), where only persons aged 8-12 were significantly more likely to fulfill the requirement as compared to persons aged 20-24. Again, this age effect was maintained and even intensified for perceptive APS (GoF: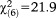 , p=0.001), while it was missing for non-perceptive APS (
, p=0.001), while it was missing for non-perceptive APS (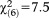 , p=0.277) (Table2).
, p=0.277) (Table2).
Nine persons (1.3%) fulfilled all the UHR requirements for APS (7 for perceptive and 2 for non-perceptive APS). Only persons aged 8-12 were more likely to meet all the requirements compared to those aged 20-24 (GoF: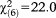 , p=0.001) (Table3). Due to the small sample size, no separate analyses were performed for perceptive and non-perceptive APS.
, p=0.001) (Table3). Due to the small sample size, no separate analyses were performed for perceptive and non-perceptive APS.
Table 3.
Effect of age on current presence of any APS
| Age group | β | SE | Wald (df=1) | p | Exp(β) | 95% CI |
|---|---|---|---|---|---|---|
| 8-12 yrs. | 2.01 | 0.88 | 5.17 | 0.023 | 7.46 | 1.32-42.18 |
| 13-15 yrs. | 1.66 | 1.02 | 2.66 | 0.103 | 5.28 | 0.71-38.97 |
| 16-17 yrs. | −16.87 | 4550.96 | 0.00 | 0.997 | 0.00 | 0.00 |
| 18-19 yrs. | −0.05 | 1.23 | 0.00 | 0.971 | 0.96 | 0.09-10.71 |
| 25-29 yrs. | −16.87 | 3349.41 | 0.00 | 0.996 | 0.00 | 0.00 |
| 30-40 yrs. | −16.87 | 3228.38 | 0.00 | 0.996 | 0.00 | 0.00 |
Binary logistic regression analysis with method “enter” and 20-24-year olds as reference age group. Significant predictors are in bold type
APS – attenuated psychotic symptoms
Low psychosocial functioning was predicted by all APS requirements, and APS frequency was found to be the strongest predictor (Table4). For perceptive APS, the effect on functioning was less pronounced: only sufficiently frequent APS predicted low functioning. On the contrary, all non-perceptive APS requirements were highly predictive of low functioning (Table4).
Table 4.
Prediction of low psychosocial functioning (SOFAS score ≤ 70) by APS requirements and estimation of interaction with age effects
| Prediction by APS requirements* | β | SE | Wald (df=1) | p | Exp (β) | 95% CI | Omnibus test | Interaction with age effects** | β | SE | Wald (df=1) | p | Exp (β) | 95% CI | Omnibus test | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occurrence of APS irrespective of onset/worsening and frequency requirements | ||||||||||||||||
| Any APS | 2.31 | 0.42 | 29.55 | <0.001 | 10.02 | 4.37-23.01 |
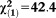 , p<0.001 , p<0.001
|
Any APS × age | 0.09 | 0.02 | 33.99 | <0.001 | 1.09 | 1.06-1.12 |
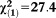 , p<0.001 , p<0.001
|
|
| Any perceptive APS | 1.02 | 0.64 | 2.541 | 0.111 | 2.78 | 0.79-9.81 |
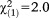 , p=0.154 , p=0.154 |
Only perceptive APS × age | No interaction effect | |||||||
| Any non-perceptive APS | 2.58 | 0.45 | 33.31 | <0.001 | 13.17 | 5.49-31.60 |
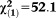 , p<0.001 , p<0.001
|
Only non-perceptive APS × age | No stable model | |||||||
| Occurrence of APS considering the onset/worsening but not the frequency requirement | ||||||||||||||||
| Any APS | 1.72 | 0.59 | 8.54 | 0.003 | 5.56 | 1.76-17.57 |
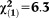 , p=0.012 , p=0.012
|
Any APS × age | 0.10 | 0.03 | 11.94 | 0.001 | 1.10 | 1.04-1.16 |
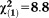 , p=0.003 , p=0.003
|
|
| Any perceptive APS | 0.82 | 1.06 | 0.59 | 0.441 | 2.26 | 0.28-18.13 |
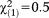 , p=0.488 , p=0.488 |
Only perceptive APS × age | No interaction effect | |||||||
| Any non-perceptive APS | 3.36 | 1.02 | 10.80 | 0.001 | 28.78 | 3.88-213.44 |
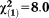 , p=0.05 , p=0.05
|
Only non-perceptive APS × age | 0.17 | 0.05 | 10.75 | 0.001 | 1.18 | 1.07-1.30 |
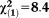 , p=0.004 , p=0.004
|
|
| Occurrence of APS considering the frequency but not the onset/worsening requirement | ||||||||||||||||
| Any APS | 2.92 | 0.46 | 40.33 | <0.001 | 18.58 | 7.54-45.78 |
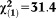 , p<0.001 , p<0.001
|
Any APS × age | No stable model | |||||||
| Any perceptive APS | 1.74 | 0.80 | 4.68 | 0.030 | 5.69 | 1.18-27.45 |
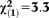 , p=0.069 , p=0.069
|
Only perceptive APS × age | No interaction effect | |||||||
| Any non-perceptive APS | 3.19 | 0.49 | 42.14 | <0.001 | 24.34 | 9.28-63.81 |
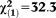 , p<0.001 , p<0.001
|
Only non-perceptive APS × age | No stable model | |||||||
Binary logistic regression analyses with method “enter”, ** binary logistic regression analyses with method “backward” and “forward” using the respective requirement and its interaction with age as independent variables. Significant predictors are in bold type
APS – attenuated psychotic symptoms, SOFAS – Social and Occupational Functioning Assessment Scale
Significant age × requirement interactions in the prediction of low psychosocial functioning indicated that APS occurrence and recency were more predictive of low functioning in the older sample, but frequency requirements were not (Table4). A similar, slightly stronger effect was detected for non-perceptive APS onset requirement (Table4). On the contrary, all interactions between age and perceptive APS requirements on low psychosocial functioning were non-significant (Table4).
Psychiatric morbidity was predicted by all APS requirements (Table5). Again, APS frequency was found to be the strongest predictor. For both perceptive and non-perceptive APS, only occurrence and frequency were predictive, but not the onset/worsening requirement (Table5). An interaction with age was detected only for the onset/worsening requirement (Table5), indicating that recent onset or worsening of APS had a stronger association with psychiatric morbidity in the older age group. No specific age-interaction effects were detected regarding the impact of psychiatric morbidity on perceptive or non-perceptive APS (Table5).
Table 5.
Prediction of presence of any axis I disorder by APS requirements and estimation of interaction with age effects
| Prediction by APS requirements* | β | SE | Wald (df=1) | p | Exp (β) | 95% CI | Omnibus test | Interaction with age effects** | β | SE | Wald (df=1) | p | Exp (β) | 95% CI | Omnibus test | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occurrence of APS irrespective of onset/worsening and frequency requirements | ||||||||||||||||
| Any APS | 1.62 | 0.28 | 33.16 | <0.001 | 5.03 | 2.90-8.73 |
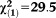 , p<0.001 , p<0.001
|
Any APS × age | No interaction effect | |||||||
| Any perceptive APS | 1.48 | 0.37 | 15.77 | <0.001 | 4.39 | 2.12-9.10 |
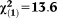 , p<0.001 , p<0.001
|
Only perceptive APS × age | No stable model | |||||||
| Any non-perceptive APS | 1.50 | 0.34 | 19.52 | <0.001 | 4.49 | 2.31-8.74 |
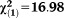 , p=0.044 , p=0.044
|
Only non-perceptive APS × age | No interaction effect | |||||||
| Occurrence of APS considering the onset/worsening but not the frequency requirement | ||||||||||||||||
| Any APS | 1.80 | 0.41 | 19.29 | <0.001 | 6.07 | 2.72-13.58 |
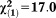 , p<0.001 , p<0.001
|
Any APS × age | 0.10 | 0.02 | 18.97 | <0.001 | 1.11 | 1.06-1.16 |
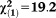 , p<0.001 , p<0.001
|
|
| Any perceptive APS | 1.06 | 0.61 | 3.02 | 0.082 | 2.89 | 0.87-9.59 |
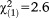 , p=0.108 , p=0.108 |
Only perceptive APS × age | No interaction effect | |||||||
| Any non-perceptive APS | 1.86 | 1.01 | 3.43 | 0.064 | 6.45 | 0.90-46.32 |
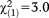 , p=0.081 , p=0.081 |
Only non-perceptive APS × age | No interaction effect | |||||||
| Occurrence of APS considering the frequency but not the onset/worsening requirement | ||||||||||||||||
| Any APS | 2.08 | 0.37 | 31.75 | <0.001 | 7.99 | 3.88-16.46 |
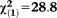 , p<0.001 , p<0.001
|
Any APS × age | No interaction effect | |||||||
| Any perceptive APS | 1.55 | 0.60 | 6.77 | 0.009 | 4.72 | 1.47-15.19 |
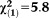 , p=0.016 , p=0.016
|
Only perceptive APS × age | No interaction effect | |||||||
| Any non-perceptive APS | 2.14 | 0.43 | 25.24 | <0.001 | 8.52 | 3.69-19.66 |
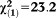 , p<0.001 , p<0.001
|
Only non-perceptive APS × age | No interaction effect | |||||||
Binary logistic regression analyses with method “enter”, ** binary logistic regression analyses with method “backward” and “forward” using the respective requirement and its interaction with age as independent variables. Significant predictors are in bold type
APS – attenuated psychotic symptoms
The age effect on the occurrence of APS, particularly perceptive APS, indicated an age threshold around age 16. To confirm this, and to test for additional age effects within the two age groups below (8-15) and above (16-40) this cut-off, we re-ran logistic regression analyses on occurrence, onset and frequency requirements, as well as the APS criterion separately, within these two age groups. In support of a single 16-year threshold, all results were non-significant.
Next, we used the 16-year cut-off to re-explore the interactions between the two age groups and APS requirements on psychosocial functioning and axis I diagnosis. Results supported the age threshold with regard to the interaction effects on psychosocial functioning. The interactions again indicated a stronger association between lower psychosocial functioning and presence (GoF: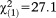 , p<0.001) or recency of APS (GoF:
, p<0.001) or recency of APS (GoF: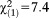 , p=0.007), in particular non-perceptive ones (GoF:
, p=0.007), in particular non-perceptive ones (GoF: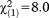 , p=0.005) and in the older group (as indicated by significant standardized residuals in chi-square tests, i.e., 3.7-6.4). The age × onset interaction effect on psychiatric morbidity, however, was not replicated by using the 16-year cut-off.
, p=0.005) and in the older group (as indicated by significant standardized residuals in chi-square tests, i.e., 3.7-6.4). The age × onset interaction effect on psychiatric morbidity, however, was not replicated by using the 16-year cut-off.
DISCUSSION
Within our community sample of never-psychotic 8-40-year olds, 9.9% reported UHR symptoms in the clinical interviews carried out by well-trained clinical psychologists, using the SIPS. UHR symptoms were exclusively rated as APS. All the UHR requirements for APS were fulfilled by only 1.3% of the sample, or 13.2% of those with APS. Indicating some clinical significance of APS, their presence was related to more frequent current DSM-IV axis I disorders and/or functional impairment.
The results strongly indicated an age effect, with a significant shift in both prevalence and clinical significance of APS and their UHR requirements from early to late adolescence, i.e., around age 16 years. The age effect on prevalence was exclusive to perceptive APS, i.e., unusual perceptual experiences and attenuated hallucinations. As compared to 16-40-year olds, subjects aged 8-15 were more likely to report perceptive APS, and were more likely to do so with an onset or worsening of symptoms within the year prior to the interview. This is in line with earlier studies on hallucinations in community samples of children/adolescents, which indicated a high prevalence, though little persistence over time, particularly of infrequent, less than weekly hallucinations (24).
With regard to clinical significance, perceptive APS were mainly unrelated to low current psychosocial functioning, except when frequent. This pattern persisted when age was considered. Actually, although functional impairment has been related to clinician-assessed APS-like symptoms in other samples of children and adolescents (12,25,26), those studies did not separately examine perceptive and non-perceptive symptoms. Indeed, we found a positive association between the occurrence of any APS and low psychosocial functioning, increasing with age. Yet, this association, particularly in older age groups, relied heavily on non-perceptive APS, mainly unusual thought content or persecutory ideas. These consistent, though different, interaction patterns of perceptive and non-perceptive APS with functional deficits and age suggest that attenuated delusional ideas, but not attenuated hallucinatory experiences, co-occur with functional deficits. It also indicates that this co-occurrence is more likely when the onset or worsening of attenuated delusional ideas is recent, or their frequency high, and when the person has already entered late adolescence or adulthood.
Compared to the interaction between APS and psychosocial functioning, that between psychiatric morbidity and APS was more general, and not moderated by age, except for the onset/worsening requirement. Thus, the increase in the association between APS-like symptoms and psychiatric morbidity with advancing age, suggested by a descriptive comparison of results of two separate samples of 11-13 and 13-15-year olds (13), was not confirmed in our sample. Only with respect to the onset/worsening requirement, a similar interaction was observed: a recent onset or worsening of APS was more strongly linked to psychiatric morbidity in older age groups. In terms of a schizotypy model (27), this stronger association between recently developed APS and non-psychotic psychiatric morbidity in the older age groups might indicate that APS developing in childhood or adolescence might be subject to a certain mental stabilization and adjustment across early adulthood, being therefore less linked to psychiatric morbidity. Yet, more research is clearly required to examine this interaction and its potential moderators.
As our results might have significant clinical implications, they call for replication in larger samples. In particular, the age groups below age 16 years were rather small in the present study, thus potentially limiting the power of our analyses. A strength of the study, however, was the broad age range, allowing for data-driven comparisons of age effects, rather than comparisons of rates reported in separate samples in the literature. The minimum age of 8 years was chosen because the source monitoring of perception necessary for distinguishing between hallucinations and products of fantasy might not have been completely developed before that age (28). The maximum age of 40 years was chosen on the basis of the highest reported intake age in clinical UHR samples (18). Yet, in particular with regard to the second onset peak of psychosis in women (29,30), possible gender-related age effects on the prevalence and clinical significance of APS in samples older than age 40 still warrant examination. However, our chosen maximum age should sufficiently ensure the absence of brain processes related to old age that might result in APS-like phenomena, possibly not identifiable in telephone interviews.
The participation rate of eligible children/adolescents in the BEARS-Kid study was within the reported range of other epidemiological studies on children and adolescents (30). The participation rate of eligible persons in the BEAR study was excellent (18). Both samples were sufficiently representative of the general population of the Canton Bern, although the eligible BEARS-Kid sample was slightly older (small effect), while the eligible BEAR sample was slightly biased against 26-30-year olds and towards 36-40-year olds (18). However, this bias was unlikely to have influenced our findings, as no age effect within the adult age groups was detected.
One possible limitation in terms of assessments was that interviews were conducted face-to-face in children/adolescents and via telephone in adults. Yet, prior to commencing the study, we had found that both face-to-face and telephone-interviews enabled a reliable assessment of APS across age groups (18,19). Nevertheless, the use of risk criteria identical to those adopted in clinical settings and the assessment of symptoms by an established interview for attenuated and frank psychotic symptoms, conducted by trained and closely supervised clinical psychologists, is a strength that ensured the high quality of the data.
In conclusion, as the early detection of psychosis is increasingly moving into younger age groups, the issue of validity and clinical significance of current UHR symptoms and criteria in children/adolescents is becoming increasingly pressing (1–3,6). Indeed, our findings clearly ask for further studies of APS in relation to different age groups, in order to avoid misinterpretation of their psychopathological nature. Thereby, the higher prevalence of perceptive APS in children and young adolescents below age 16 calls for their re-appraisal in this age group in both the early detection of psychosis and the diagnosis of attenuated psychosis syndrome, if it is introduced into the DSM-5.1.
Of interest for all age groups, perceptive APS seem to be less related to low psychosocial functioning than non-perceptive APS in the general population, unless they are frequent. These findings ask for replication and the differential study of perceptive and non-perceptive APS and their interaction with age, in order to better distinguish ill from non-ill experiences in the general population, and in children and young adolescents in particular.
Acknowledgments
This work was supported by two independent project grants from the Swiss National Science Foundation (320030L_144100 and 32003B_135381).
References
- 1.Schimmelmann BG, Schultze-Lutter F. Early detection and intervention of psychosis in children and adolescents: urgent need for studies. Eur Child Adolesc Psychiatry. 2012;21:239–41. doi: 10.1007/s00787-012-0271-z. [DOI] [PubMed] [Google Scholar]
- 2.Schimmelmann BG, Walger P, Schultze-Lutter F. The significance of at-risk symptoms for psychosis in children and adolescents. Can J Psychiatry. 2013;58:32–40. doi: 10.1177/070674371305800107. [DOI] [PubMed] [Google Scholar]
- 3.Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30:405–16. doi: 10.1016/j.eurpsy.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Welsh P, Tiffin PA. The ‘at-risk mental state’ for psychosis in adolescents: clinical presentation, transition and remission. Child Psychiatry Hum Dev. 2014;45:90–8. doi: 10.1007/s10578-013-0380-z. [DOI] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–20. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultze-Lutter F, Ruhrmann S, Fusar-Poli P, et al. Basic symptoms and the prediction of first-episode psychosis. Curr Pharm Des. 2012;18:351–7. doi: 10.2174/138161212799316064. [DOI] [PubMed] [Google Scholar]
- 7.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry. 1998;172(Suppl. 33):14–20. [PubMed] [Google Scholar]
- 8.Carpenter WT. Attenuated psychosis syndrome: need for debate on a new disorder. Psychopathology. 2014;47:287–91. doi: 10.1159/000365221. [DOI] [PubMed] [Google Scholar]
- 9.Walder DJ, Mittal V, Trotman HD, et al. Neurocognition and conversion to psychosis in adolescents at high-risk. Schizophr Res. 2008;101:161–8. doi: 10.1016/j.schres.2007.12.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziermans TB, Schothorst PF, Sprong M, et al. Transition and remission in adolescents at ultra-high risk for psychosis. Schizophr Res. 2011;126:58–64. doi: 10.1016/j.schres.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Rubio JM, Sanjuán J, Flórez-Salamanca L, et al. Examining the course of hallucinatory experiences in children and adolescents: a systematic review. Schizophr Res. 2012;138:248–54. doi: 10.1016/j.schres.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher I, Murtagh A, Molloy C, et al. Identification and characterization of prodromal risk syndromes in young adolescents in the community: a population-based clinical interview study. Schizophr Bull. 2012;38:239–46. doi: 10.1093/schbul/sbr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelleher I, Keeley H, Corcoran P, et al. Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry. 2012;201:26–32. doi: 10.1192/bjp.bp.111.101543. [DOI] [PubMed] [Google Scholar]
- 14.Bartels-Velthuis AA, van de Willige G, Jenner JA, et al. Course of auditory vocal hallucinations in childhood: 5-year follow-up study. Br J Psychiatry. 2011;199:296–302. doi: 10.1192/bjp.bp.110.086918. [DOI] [PubMed] [Google Scholar]
- 15.Brandizzi M, Schultze-Lutter F, Masillo A, et al. Self-reported attenuated psychotic-like experiences in help-seeking adolescents and their association with age, functioning and psychopathology. Schizophr Res. 2014;160:110–7. doi: 10.1016/j.schres.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher I, Devlin N, Wigman JT, et al. Psychotic experiences in a mental health clinic sample: implications for suicidality, multimorbidity and functioning. Psychol Med. 2014;44:1615–24. doi: 10.1017/S0033291713002122. [DOI] [PubMed] [Google Scholar]
- 17.Laurens KR, Hobbs MJ, Sunderland M, et al. Psychotic-like experiences in a community sample of 8000 children aged 9 to 11 years: an item response theory analysis. Psychol Med. 2012;42:1495–506. doi: 10.1017/S0033291711002108. [DOI] [PubMed] [Google Scholar]
- 18.Schultze-Lutter F, Michel C, Ruhrmann S, et al. Prevalence and clinical significance of DSM-5-attenuated psychosis syndrome in adolescents and young adults in the general population: the Bern Epidemiological At-Risk (BEAR) Study. Schizophr Bull. 2014;40:1499–508. doi: 10.1093/schbul/sbt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel C, Schimmelmann BG, Kupferschmid S, et al. Reliability of telephone assessments of at-risk criteria of psychosis: a comparison to face-to-face interviews. Schizophr Res. 2014;153:251–53. doi: 10.1016/j.schres.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 20.McGlashan T, Walsh B, Woods S. The psychosis-risk syndrome. Handbook for diagnosis and follow-up. New York: Oxford University Press; 2010. [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–34. [PubMed] [Google Scholar]
- 23.Sheehan DV, Sheehan KH, Shytle RD, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71:313–26. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 24.Escher S, Romme M, Buiks A, et al. Independent course of childhood auditory hallucinations: a sequential 3-year follow-up study. Br J Psychiatry. 2002;181:10–8. doi: 10.1192/bjp.181.43.s10. [DOI] [PubMed] [Google Scholar]
- 25.Asher L, Zammit S, Sullivan S, et al. The relationship between psychotic symptoms and social functioning in a non-clinical population of 12 year olds. Schizophr Res. 2013;150:404–9. doi: 10.1016/j.schres.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Calkins ME, Moore TM, Merikangas KR, et al. The psychosis spectrum in a young U.S. community sample: findings from the Philadelphia Neurodevelopmental Cohort. World Psychiatry. 2014;13:296–305. doi: 10.1002/wps.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debbané M, Eliez S, Badoud D, et al. Developing psychosis and its risk states through the lens of schizotypy. Schizophr Bull. 2015;41(Suppl. 2):S396–407. doi: 10.1093/schbul/sbu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts KP. Children's ability to distinguish between memories from multiple sources: implications for the quality of eyewitness statements. Dev Rev. 2002;22:403–35. [Google Scholar]
- 29.Kirkbride JB, Errazuriz A, Croudace TJ, et al. Incidence of schizophrenia and other psychoses in England, 1950-2009: a systematic review and meta-analyses. PLoS One. 2012;7:e31660. doi: 10.1371/journal.pone.0031660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saha S, Whiteford H, McGrath J. Modelling the incidence and mortality of psychotic disorders: data from the second Australian national survey of psychosis. Aust N Zeal J Psychiatry. 2014;48:352–9. doi: 10.1177/0004867413513341. [DOI] [PubMed] [Google Scholar]


