Abstract
Exposure to war zone stressors is common, yet only a minority of soldiers experience clinically meaningful disturbance in psychological function. Identification of biomarkers that predict vulnerability to war zone stressors is critical for developing more effective treatment and prevention strategies not only in soldiers but also in civilians who are exposed to trauma. We investigated the role of the serotonin transporter linked polymorphic region (5-HTTLPR) genotype in predicting the emergence of post-traumatic stress disorder (PTSD), depressive and anxiety symptoms as a function of war zone stressors. A prospective cohort of 133 U.S. Army soldiers with no prior history of deployment to a war zone, who were scheduled to deploy to Iraq, was recruited. Multilevel regression models were used to investigate associations between 5-HTTLPR genotype, level of war zone stressors, and reported symptoms of PTSD, depression and anxiety while deployed to Iraq. Level of war zone stressors was associated with symptoms of PTSD, depression and anxiety. Consistent with its effects on stress responsiveness, 5-HTTLPR genotype moderated the relationship between level of war zone stressors and symptoms of emotional disturbance. Specifically, soldiers carrying one or two low functioning alleles (S or LG) reported heightened symptoms of PTSD, depression and anxiety in response to increased levels of exposure to war zone stressors, relative to soldiers homozygous for the high functioning allele (LA). These data suggest that 5-HTTLPR genotype moderates individual sensitivity to war zone stressors and the expression of emotional disturbance including PTSD symptoms. Replication of this association along with identification of other genetic moderators of risk can inform the development of biomarkers that can predict relative resilience vs. vulnerability to stress.
Keywords: 5-HTTLPR genotype, war zone stressors, PTSD, depression, anxiety, biomarkers, stress responsiveness
Although not without controversy (1), there is growing recognition that genetic factors in combination with exposure to stressful and/or life threatening situations contribute to the development of psychiatric disorders, such as post-traumatic stress disorder (PTSD) (2) and depression (3).
After controlling for baseline characteristics, deployed soldiers with exposure to combat stressors are three times more likely to develop PTSD symptoms relative to deployed soldiers with no combat stress exposure (4). However, exposure to war zone stressors does not impact all soldiers similarly. Some soldiers develop moderate to severe anxiety and depression symptoms following war zone stress exposure, whereas others do not (4). Similarly, epidemiologic data indicate that many Americans (60.7%) have been exposed to a traumatic stressor, yet only a small minority (8%) developed PTSD (5). Although a number of risk factors for PTSD and depression have been identified (6), genetic variation is believed to partially explain individual differences that occur in such dysfunctional responses to trauma (7).
Evidence from twin studies involving Vietnam War veterans first indicated that approximately 30% of the variance in PTSD can be attributed to shared genetic variance (8). Similar estimates for the genetic liability to depression and other anxiety disorders have been established (9). Subsequent candidate gene studies have provided limited evidence for specific genetic loci shaping risk for PTSD (10). Several promising gene-by-environment (GxE) interaction findings have recently been reported, involving genetic variants that putatively influence hypothalamic-pituitary-adrenal (HPA) axis function (i.e., FKBP5 and CRHR1) (11), GABA receptor functioning (i.e., GABRA2) (12), and G protein signaling (i.e., RGS2) (13).
Of particular interest in mapping the genetic risk for PTSD are studies of the functional variable number tandem repeat polymorphism in the proximal promoter of the serotonin transporter gene (i.e., the serotonin transporter linked polymorphic region or 5-HTTLPR). This interest reflects the importance of the serotonin transporter in mediating active clearance of extracellular serotonin and thereby influencing the duration and intensity of serotonin signaling. This signaling pathway is an important modulator of a cortico-limbic neural circuitry mediating behavioral and physiologic responses to stress and threat, including trauma (14,15).
The 5-HTTLPR is most commonly represented by two variants: a short (S) allele and a long (L) allele. The presence of one or two short alleles, rather than two copies of the long allele, may be associated with reduced transcriptional efficiency that putatively results in significant decreases (approximately 50%) in serotonin reuptake (16,17). This 5-HTTLPR effect may be modulated by a single nucleotide polymorphism (rs25531) comprised of an adenine (A) to guanine (G) substitution, most commonly occurring at the sixth nucleotide in the first of two extra 20 to 23 bp repeats of the L allele (18). Importantly, the L allele with guanine at the sixth nucleotide (LG) exhibits similar reductions in transcriptional activity to the S allele in comparison to the L allele with adenine at the sixth nucleotide (LA) (19). This has led most to adopt a “triallelic” classification scheme for the 5-HTTLPR, with the following functionally defined alleles: L’=LA and S’=S, LG, yielding the following functional genotypes: L’L’ (high activity), L’S’ (intermediate activity), and S’S’ (low activity).
Consistent with the resulting increases in synaptic serotonin (20), the S (or S’) allele has been associated with relatively increased neural, behavioral and physiologic reactivity to stress, threat and trauma (21). This profile of heightened sensitivity to environmental challenge translates into a well-documented GxE effect of increased risk for mood and anxiety disorders in the context of stressful life events in carriers of the S allele (3). Stratifying study samples by type of stressor has revealed a significant relationship between 5-HTTLPR genotype and depression for studies of childhood maltreatment, medical conditions, and life stress (21).
Surprisingly, relatively few studies have directly examined the moderating role of the 5-HTTLPR in the emergence of PTSD. Among female undergraduates who varied in their exposure to an on-campus shooting, the S’ allele was associated with significantly greater PTSD symptoms 2-4 weeks post-shooting (22). Similarly, the S’ allele was associated with increased risk for PTSD in individuals who lacked social support (23) or lived in regions with high unemployment and neighborhood crime (24) in the aftermath of Hurricane Katrina. Further, a cross-sectional study reported that the S allele was associated with increased risk for PTSD in individuals experiencing adult traumatic events and childhood adversity, and especially if they experienced both types of trauma (25). Among refugees from the Rwandan Civil War, the S allele was associated with increased risk for PTSD at relatively low levels of trauma; however, this differential risk for PTSD diminished as trauma exposure increased (26). In contrast, among people exposed to three or more traumas in a large epidemiological sample (N=3,045 adults from Pomerania, Germany), the L’ allele was associated with increased risk for PTSD (27).
Here we examine whether 5-HTTLPR genotype interacts with a stressful war zone environment to predict the development of PTSD, anxiety and depression symptoms among soldiers from the U.S. Army deployed to Iraq. The current prospective study is unique in several ways. First, exposure to war zone stressors was assessed during deployment in Iraq via web-based surveys in which soldiers provided monthly reports of recent war zone experiences. Most prior studies have been limited by retrospective recall of traumatic or stressful experiences over long periods of time (28,29). Second, the soldiers in the current study had not previously been deployed to a war zone, which helps minimize heterogeneity of our sample. Third, we rigorously assessed psychopathology at pre-deployment in order to account for its variability prior to exposure to war zone stress. Based on the documented neural, physiologic and behavioral effects of the 5-HTTLPR, we predicted that S’ carriers would be at greater risk than L’ homozygotes to develop PTSD, depressive and anxiety symptoms in response to increasing levels of exposure to war zone stressors.
METHODS
Participants
Participants were 133 U.S. Army soldiers with no prior war zone experience, who were scheduled to deploy to Iraq within 90 days. The principal investigator and the project director conducted briefing meetings for potential participants from eight combat and two combat support units at Fort Hood Texas. Of the 223 soldiers who attended the group orientation sessions, 184 (82%) provided informed consent and completed an extensive 8-hour pre-deployment assessment at the University of Texas at Austin. Six soldiers were not deployed and one soldier withdrew from the study. Of the 177 deployed soldiers, genetic data were unavailable for 31 soldiers, while 13 soldiers failed to complete any war-zone stress assessments while being deployed. Thus, the final sample included 133 soldiers who provided DNA samples prior to deployment and in-theater reports of war-zone stress experiences.
The study was approved by the Office of Research Support and Compliance at the University of Texas at Austin and the Brooks Army Medical Center Scientific and Human Use Review Committee. All study participants provided informed consent.
Assessments
Prior to deployment, groups of four to six soldiers arrived for study participation by 8.00 a.m., and were monitored by study personnel until dismissal approximately 8 to 9 hours later. After providing informed consent, participants provided a saliva sample for DNA isolation, completed a comprehensive stress risk assessment battery, and were interviewed to assess the presence of DSM-IV diagnoses by the Structured Clinical Interview for DSM-IV (SCID-I, (30).
Soldiers were deployed to Iraq approximately 60 to 90 days after the pre-deployment assessment, and reported war-zone stress experiences during deployment on a monthly basis using the Combat Experiences Log (CEL), a web-based system for prospectively assessing war-zone stress in theater (31). From a list of 18 well-validated war-zone stressors (e.g., received hostile incoming fire, been wounded or injured in combat, received bad news from home), they were asked to indicate stressors they experienced since their most recent in-theater CEL entry (or since deployment to the combat zone in the event of their very first response to the CEL system). These 18 stressors were drawn from a modified version of the Deployment Risk and Resilience Inventory (32). Further, the CEL allowed soldiers to record up to two unique stressors not covered by the 18 standard stressor items. The number of reported combat stressors was summed for each soldier to estimate the level of war-zone stress exposure for each CEL entry.
PTSD symptoms were assessed using the 4-item PTSD Checklist (PCL-Short) (33). Despite its brevity, the PCL-Short assesses each of the three core PTSD symptom clusters: re-experience (2 items), avoidance (1 item), and hyperarousal (1 item), with a diagnostic accuracy estimate equivalent to that of the original 17-item PCL (33). For the current sample, the internal consistency (Cronbach's alpha) computed from soldiers’ first in-theater entry was .79.
In-theater depression symptoms were assessed using the brief 10-item version of the Center for Epidemiologic Studies Depression Scale (CES-D) (34). The CES-D was developed to screen general populations for the presence of depressive symptoms, and thus the content of its items is designed to be understandable and emotionally accessible to all individuals irrespective of their clinical status. Moreover, the CES-D has shown excellent psychometric properties, and has been widely administered in various measurement modes, including web-based assessment (35). The 10-item version is strongly associated with scores from the full 20-item version (kappa=.97, p<0.001) (34). For the current sample, the internal consistency computed from soldiers’ first in-theater entry was .73.
The CEL also measures anxiety reactions during deployment using 18 items constructed to address common anxiety symptoms across three major domains: cognitive (e.g., fear of losing control), emotional (e.g., feeling scared), and somatic (e.g., tension in muscles). Each symptom was rated on a 5-point scale (1=not at all to 5=extremely). Internal consistency computed from soldiers’ first in-theater entry was .92 for the current sample (31).
DNA collection and genotyping
Saliva was collected with the Oragene DNA self-collection kit following the manufacturer's instructions. Participants rubbed their tongues around the inside of their mouth for about 15 sec and then deposited approximately 2 ml of saliva into the collection cup. Participants secured the cup firmly by screwing it clockwise until snug which released a solution from the lower compartment that mixed with the saliva. This started the initial phase of DNA isolation and stabilized the saliva sample for long-term storage at room temperature (36). Saliva samples were shipped to the University of Pittsburgh for DNA extraction and genotyping.
A triplex polymerase chain reaction (PCR) protocol followed by double restriction endonuclease digestion was used to identify the 5-HTTLPR and rs25531 variants: S, LA and LG (18). In a total volume of 20 l, 25 ng of genomic DNA were amplified in 1 Multiplex master mix (Qiagen, Valencia, CA) primers at final concentrations of 200 nM. The primer sequences were the following: forward, 5’-TCCTCCGCTTTGGCGCCTCTTCC-3’, and reverse, 5’-TGGGGGTTGCAGGGGAGATCCTG-3’. Thermal cycling involved 15 min of initial denaturation at 95°C followed by 35 cycles at 94°C for 30 sec, 62°C for 90 sec, and 72°C for 60 sec. This was followed by thermal cycling at 72°C for 10 min. To distinguish the A/G single nucleotide polymorphism of the rs25531, we extracted 7 ul of the PCR product for digestion by 5 U HpaII (an isoschizomer of MspI) or 10 U MspI, for a total reaction of 17 ul. These were loaded side by side on 2.5-3.0% agarose gel.
These methods produced allele frequencies of S, n=114 (42.86%); LA, n=143 (53.76%); and LG, n=9 (3.38%), and a genotype distribution of SS, n=22 (16.54%); SLG, n=3 (2.26%); LGLG, n=0 (0%); SLA, n=67 (50.38%); LGLA, n=6 (4.51%); and LALA, n=35 (26.32%). Genotype distribution of the 5-HTTLPR across all participants was in Hardy-Weinberg equilibrium, χ2 (3)=0.67, p=0.85. Consistent with previous research (19,37), the S and LG alleles were treated as functionally equivalent for purposes of analysis, which employed the following genotype groups: L’L’=35, L’S’=73, and S’S’=25.
Statistical analysis
Multilevel, mixed-effects random coefficient regression models (MRMs) were used to analyze the data. Our dependent variables were PTSD symptoms, depressive symptoms and anxiety symptoms (together referred to as war zone stress reactions), measured monthly during deployment. In order to examine the relation between stressors and war zone stress reactions over and above the mutual effect of “time since deployment” (referred to as “time”) on both, we controlled for the effects of time in the MRM models. The effects of time were modeled as a quadratic function, since the relation between time and war zone stress reactions has been shown to be curvilinear (38).
With respect to 5-HTTLPR genotype, we performed a series of preliminary analyses testing for allele load effects by comparing L’S’ vs. S’S’ genotypes. These analyses revealed no significant load effects (all p values >0.43). Thus we followed the recommendation of Hariri et al (39) and modeled genotype as a two-level variable (S’ carriers vs. L’ homozygotes). The predictors of war zone stress reactions in the MRM models included time (months since deployment), time2, gender, war zone stressors (assessed monthly during deployment), 5-HTTLPR genotype (S’ carrier vs. L’L’), and the interaction between 5-HTTLPR genotype and level of war zone stressor exposure.
Following Hedeker and Gibbons (40), we decomposed the monthly measure of war zone stressors into a between-soldier effect (the average level of stressors reported over the deployment period) and a within-soldier effect (the deviation from the “average level” of stressors for each soldier at each point in time, referred to as “change in war zone stressors”). Failing to decompose these effects would confound the between- and within-soldier effects, resulting in potentially misleading results (22).
All variables were centered at their grand mean so that results for every main effect would represent the average effect for the sample. Gender was included as a covariate in all the analyses, because of the observed linkage between gender and both depressive and anxiety symptoms.
RESULTS
Participants
The analyses were conducted on the data from 133 soldiers, who provided a total of 926 monthly assessments (mean number of assessments 6.96±5.62, median 5.0, range 1-18). The mean age of the final sample was 23.5±6.0 years. The large majority of the sample (85.7%) was male, and participants were predominantly Caucasian (72.9%), of which 24 (18% of the total sample) were Hispanic. Other ethnic/racial groups included African-Americans (9.8%), American Indians (12.8%), and Asian/Pacific Islanders (4.5%).
At pre-deployment, 20 (15.0%) participants met criteria for one or more current Axis I diagnoses, including substance use disorder (n=7, 5.3%), anxiety disorder (n=9, 6.8%), mood disorder (n=6, 4.5%), and adjustment disorder (n=5, 3.8%).
The mean duration of deployment was 393.0±67.8 days. The number of in-theater war zone stressors reported by a soldier in a given month ranged from 0 to 18 (mean±SD 2.01±2.40). The average level of stressors reported by each soldier over the course of deployment ranged from 0 to 14 (mean±SD 3.08±2.75). The month-to-month changes in war zone stressors for a soldier ranged from −9 to 9 (mean±SD 0.00±1.47).
Growth curve analyses of war zone stressors and war zone stress reactions over time
Initial MRM analyses were performed to examine the linear and quadratic effects of time for the three indices of war zone stress reactions. Results revealed that the quadratic trend over time was significant for all three measures: b=−.02, t (147)=−5.08, p<0.001 for PTSD symptoms; b=−.04, t (44)=−4.77, p<0.001 for depressive symptoms; and b=−.06, t (49)=−3.97, p<0.001 for anxiety symptoms. On the contrary, for all measures, the linear effect of time was not significant (p values >0.43). These results indicate that, subsequent to deployment, symptoms steadily increased, with a peak about 8 months after deployment, followed by a gradual return to initial levels around month 16.
The soldiers' exposure to war zone stressors over time followed a different pattern. Neither the linear nor quadratic trends were significant (p values >0.26), indicating that stressors remained relatively constant over the term of the soldiers' deployment.
Main effects of war zone stressors, 5-HTTLPR genotype and gender on war zone stress reactions
Results from the MRM analyses revealed that higher average levels of war zone stressors were significantly related to greater war zone stress reactions on all three measures: b=.22, t (137)=4.01, p<0.001 for PTSD symptoms; b=.49, t (135)=2.28, p<0.05 for depressive symptoms; and b=1.12, t (141)=3.22, p<0.01 for anxiety symptoms. Similarly, month-to-month changes in war zone stressors were positively related to concurrent levels of depressive symptoms (b=.62, t (60)=3.63, p=0.001) and anxiety symptoms (b=1.21, t (59)=4.14, p<0.001), but not PTSD symptoms (p>0.23). Females, in comparison to males, reported higher levels of both depressive symptoms (b=2.86, t (82)=2.12, p<0.05) and anxiety symptoms (b=5.47, t (92)=2.42, p<0.05). There was no significant main effect of 5-HTTLPR genotype on any of the three indices of war zone stress reactions (p values >0.55) (Table1).
Table 1.
Regression coefficients for each class of war zone stress reaction
| Predictor | War zone stress reactions | ||
|---|---|---|---|
| PTSD symptoms | Depressive symptoms | Anxiety symptoms | |
| Average level of stressors | .22*** | .49* | 1.12** |
| Change in level of stressors | .07 | .62*** | 1.21*** |
| 5-HTTLPR genotype | −.18 | −.14 | −.23 |
| Average stress x 5-HTTLPR genotype | .21* | .83* | 1.36* |
| Change in stress x HTTLPR genotype | −.14 | −.31 | .20 |
| Time (months since deployment) | .00 | .03 | −.06 |
| Time2 | −.02*** | −.04*** | −.06*** |
| Gender (male=0; female=1) | .50 | 2.86* | 5.47* |
PTSD – post-traumatic stress disorder,
p<0.05,
p<0.01,
p≤0.001
For war zone stressors, females tended to report slightly fewer stressors than males (b=−1.21, t (93)=1.93, p=0.057), perhaps reflecting different war zone assignments. There were no differences between the numbers of stressors experienced by S’ allele carriers vs. L’ homozygotes (p=0.97).
Effects of the interaction of war zone stressors by 5-HTTLPR genotype on war zone stress reactions
MRM analyses revealed significant interactions between 5-HTTLPR genotype and average level of war zone stressors during deployment for all three measures of war zone stress reactions: b=.21, t (121)=2.08, p<0.05 for PTSD symptoms; b=.83, t (126)=2.13, p<0.05 for depressive symptoms; and b=1.36, t (134)=2.12, p<0.05 for anxiety symptoms. However, none of the interactions between the 5-HTTLPR genotype and month-to-month changes in war zone stressors was significant (p values >0.28) (Table1).
To examine the nature of these interactions between 5-HTTLPR genotype and average level of war zone stressors, we followed the recommendations formulated by Aiken and West (41). Using their approach, we calculated the relation between soldiers’ average stress levels and their stress reactions separately for S’ carriers and their L’ homozygote counterparts (this approach uses the entire sample to calculate each simple slope, but computes these effects for each group of soldiers separately). For L’ homozygotes, higher levels of average stress reported in the field did not predict higher symptoms: b=.07, t (85)=.86, p>0.39 for PTSD symptoms; b=−.12, t (108)=−.43, p>0.66 for depressive symptoms; and b=.11, t (116)=.24, p>0.81 for anxiety symptoms. In contrast, and consistent with our hypothesis, S’ carriers responded to higher levels of average stress with higher levels of PTSD symptoms (b=.28, t (134)=4.01, p<0.0001); depressive symptoms (b=.71, t (138)=2.62, p=0.01), and anxiety symptoms (b=1.48, t (142)=3.39, p=0.001) (see Figures 1, 2 and 3).
Figure 1.
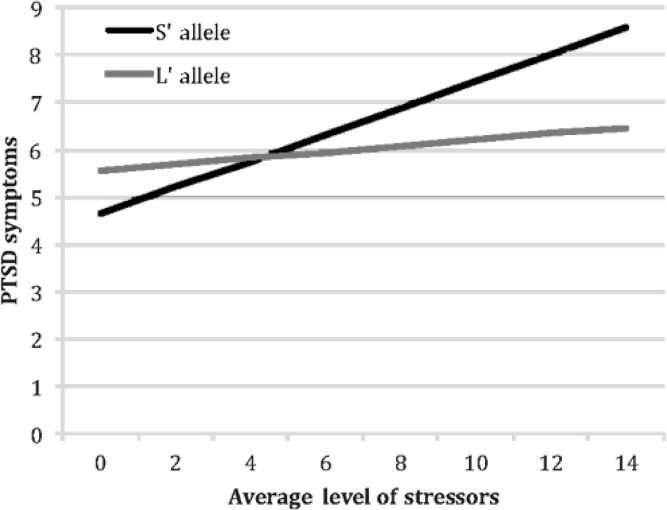
Moderating effects of 5-HTTLPR genotype on the emergence of post-traumatic stress disorder (PTSD) symptoms in response to increasing levels of war zone stressors
Figure 2.
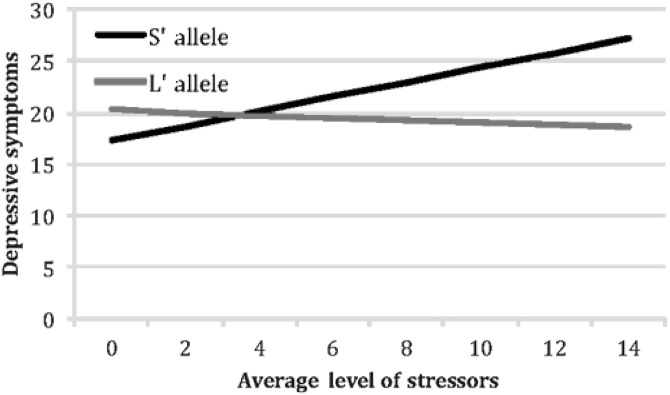
Moderating effects of 5-HTTLPR genotype on the emergence of symptoms of depression in response to increasing levels of war zone stressors
Figure 3.
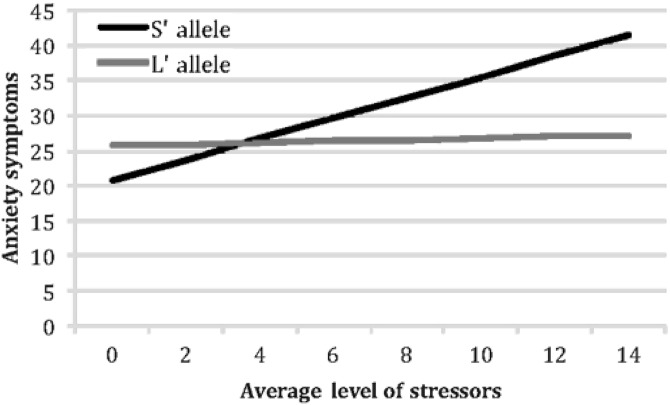
Moderating effects of 5-HTTLPR genotype on the emergence of symptoms of anxiety in response to increasing levels of war zone stressors
To investigate whether these findings were due to pre-existing psychopathology, we repeated these analyses controlling for lifetime history of an Axis I disorder (0 = no disorder, 1 = one or more Axis I disorders). Although a history of Axis I disorder was generally related to greater war zone stress reactions (b=.49, t (123)=1.85, p<0.07 for PTSD symptoms; b=2.48, t (103)=2.59, p=0.01 for depressive symptoms; and b=4.54, t (113)=2.89, p=0.005 for anxiety symptoms), all the significant effects reported above (including the interactions between genotype and level of stressors) were still significant after controlling for that history.
Effects of race in moderating the interactive effects
Because differences in the genetic backgrounds of individuals as represented by race can possibly confound the effects of specific genetic polymorphisms (42), MLM analyses were performed to examine the effects of race on the observed interaction effects of war zone stressors x 5-HTTLPR genotype for each of the three war zone stress reactions. Race was coded as white (n=97), African-American (n=13), and other (n=23), and was represented by two dummy variables. Interactions were formed between these two dummy variables and all of the terms predicting outcome in the MRM analyses.
The resulting analyses were consistent in showing no significant effects for race. Comparing models with and without race as a variable revealed no significant differences in their deviance scores (–2 log likelihoods): χ2 (12)=6.18, p=0.91 for PTSD symptoms, χ2 (12)=11.19, p=0.51 for depressive symptoms, and χ2 (12)=10.88, p=0.54 for anxiety symptoms. These results indicate that race did not have a significant overall effect on any of the war zone reactions. Further, none of the race x war zone stressors x 5-HTTLPR genotype interactions for PTSD, depression or anxiety symptoms were significant (all p values ranged between 0.17 and 0.47), indicating that the observed war zone stressors x 5-HTTLPR genotype interactions were not influenced by soldiers’ race.
DISCUSSION
Our in-theater web-based assessment allowed us to examine prospectively the main and interactive effects of 5-HTTLPR genotype and exposure to war zone stressors in predicting psychological dysfunction as they occur over the course of soldiers’ deployment. The results provide novel evidence for an association between 5-HTTLPR genotype, level of exposure to war zone stressors, and symptoms of PTSD, depression and anxiety among soldiers deployed to a war zone. Our approach offers significant advantages over static, retrospective assessments used in previous combat stress risk studies (31).
The changes in war zone stress reactions over time were more complex than expected on the basis of previous reports of a positive association between length of deployment and war zone stress reactions (43,44). Each of the three targeted indices of war zone stress reactions – PTSD symptoms, depressive symptoms and anxiety symptoms – showed a significant inverted U-pattern in their respective growth curves over time. Stress reactions increased during the first eight months of deployment but then decreased to their earlier levels over the final eight months. This finding may reflect the effects of simple habituation or an increase in soldiers’ sense of mastery in response to repeated confrontation with similar war zone stressors.
We found no evidence for a main effect of 5-HTTLPR genotype on any of the three war zone stress reactions. This finding is consistent with previous longitudinal studies showing that 5-HTTLPR moderates, but does not predict as a main effect, the impact of stress on risk for depression (45–48) and anxiety (28). Because it has been suggested that 5-HTTLPR genotype may influence one's risk of exposure to stressors via gene-environment correlation (49), we tested whether S’ carriers were more likely to report heightened levels of exposure to war zone stressors relative to L’ homozygotes. We found no such association between 5-HTTLPR genotype and war zone stressor severity. This finding is not surprising, since the potential threats (stressors) facing soldiers in a war zone are often not under the control of the individual soldier, whereas in non-military contexts, individual variables such as genetic factors and personality traits are more likely to influence the situations people face.
Consistent with previous studies (31,50), soldiers reporting more severe war zone stressors also reported higher levels of PTSD, depressive and anxiety symptoms. However, this main effect of war zone stressors was moderated by 5-HTTLPR genotype. Specifically, S’ carriers responded to increasing levels of war zone stressors with increasingly greater war zone stress reactions across all three symptom domains. In contrast, there was no relationship between war zone stressors and the emergence of psychological symptoms for L’ homozygotes. This finding is quite consistent with a diathesis-stress formulation of combat stress (51), and with 5-HTTLPR S’ allele moderating risk for psychosocial dysfunction specifically in the wake of stressful life events (5).
Our observed GxE effect is likely mediated through the shaping of behavioral and neural responses to stress by the 5-HTTLPR. As described by Caspi et al (21), the 5-HTTLPR constitutes a genetic substrate for the personality trait of negative emotionality, which has been conceptualized as the propensity to experience aversive emotional states under conditions of stress (47,52,53). This expression of the S’ allele on negative emotionality reflects the polymorphism's influence on serotonin signaling and, in turn, the development and functioning of a distributed cortico-limbic circuitry mediating behavioral and physiologic responses to stress, threat and trauma (14,15). Specifically, the S’ (or S) allele of the 5-HTTLPR is associated with increased threat-related reactivity of the amygdala, which is critical for the expression of fear conditioning and anxiety (54).
Consistent with the greater attentional bias to threat observed in individuals with high negative emotionality and the importance of the amygdala in driving this bias, our group has recently shown, in a subset of these soldiers, that the S’ allele is associated with pre-deployment attentional bias for aversive stimuli (55) as well as pre- to post-deployment shifts in gaze bias toward negative facial stimuli (56). We are now actively exploring the links between 5-HTTLPR genotype, threat-related amygdala reactivity, physiologic and behavioral indices of negative emotionality (38), and the emergence of war zone stress reactions.
Several design features of the study merit comment. First, we chose to employ a triallelic classification of the 5-HTTLPR accounting for rs25531 genotype. Among our sample, 3.4% of the L alleles were functionally reclassified as low expressing based on rs25531 status (i.e., LG). These alleles would have been misclassified as high expressing alleles had we used the standard biallelic classification system. Although the different classification schemes did not affect the results of the current study, this misclassification issue may be one of the factors accounting for the discrepancies in findings across studies. Second, our prospective design offers advantages over case only, case control and cross-sectional designs, by minimizing reporting biases associated with the retrospective assessment of exposure to the stressor. Third, our repeated assessment of war zone stressors and symptoms of PTSD, depression and anxiety allows us to examine patterns of change in symptoms as a result of repeated exposure to stressors. Converging evidence from research with rodents, primates and humans implicates repeated exposure to a stressor as a critical dimension in determining the emergence of psychopathology (21).
Several limitations of our study should also be noted. First, the sample size limits the power and stability of our findings across subgroups such as racial/ethnic minority participants. Second, although participants were recruited from ten different army units, we cannot rule out the possibility that our findings may not generalize to soldiers from outside Fort Hood. Replication with a larger sample across multiple army bases is warranted. Third, we chose to include only soldiers who had no history of prior deployment to a war zone, in order to eliminate the inferential ambiguity associated with prior exposure. However, this design decision precluded the investigation of 5-HTTLPR x prior deployment interaction effects. Fourth, while our evaluations of war zone stress reactions are based on validated self-report symptom measures, which offer the advantage of providing a convenient means for modeling change in psychological symptoms during deployment and for testing the main and interactive effects of genetic and environmental influences on those changes, they do not assess threshold diagnoses of PTSD, depression or other anxiety disorders. It should be noted that, upon their return from deployment, soldiers were administered several diagnostic interviews (i.e., the SCID and the Clinician-Administered PTSD Scale) by a trained clinician. Consistent with previous reports using stringent diagnostic criteria (4,57), only a small percentage (15%) met full criteria for a threshold mental disorder. The small numbers of threshold diagnoses precluded formal analyses of this outcome variable. However, the importance of assessing the full dimensionality of psychopathology, especially that of mood and anxiety, has emerged as a critical factor in advancing treatment and prevention (58). Thus, our focus on continuous measures of symptoms is likely an advantage in mapping the genetic and environmental substrates of risk for psychopathology.
Despite the emerging GxE literature on the importance of the 5-HTTLPR in moderating stress sensitivity and the surrounding debates (1,3,21,22), this is the first investigation to test the 5-HTTLPR x stress exposure interaction among soldiers deployed to a war zone. The current results serve to both replicate and extend the positive findings of previous studies in several important ways.
First, it has been suggested that both the type of stressor (specific vs. non-specific) as well as the method of stressor assessment (interview vs. self-report) may account for the discrepancy in findings across studies. Specifically, Caspi et al (21) assert that studies employing interview as opposed to self-report stressor assessments are more likely to show support for the 5-HTTLPR x stress interaction. Our study, which employed measures of war zone stressors via web-based self-reports, suggests otherwise. This difference may reflect the repeated nature of our self-report assessments, which may have led to more accurate reporting. Alternatively, it could be a result of the MRM analysis used in the current study, which increased power by including all subjects, regardless of missing data, and a large number of data points from repeated assessments.
Second, our findings point to the importance of stressor severity in moderating the impact of the 5-HTTLPR on soldiers’ risk of experiencing psychological dysfunction while deployed. S’ carriers showed equivalent levels of PTSD, depressive and anxiety symptoms relative to L’ homozygotes when specific war zone stressors were low, but showed greater symptoms in all three dimensions as exposure to war zone stressors increased.
Thus, our data support a specific role for the 5-HTTLPR as a genetic vulnerability factor that potentiates the effects of war zone stress on the psychological well-being of deployed soldiers. More generally, they further the potential utility of this polymorphism, especially when combined with other genetic moderators of risk, to inform the development of biomarkers that predict relative resilience and vulnerability to stress broadly.
Acknowledgments
This work was funded by the U.S. Army Research, Development, and Engineering Command Acquisition Center, Natick Contracting Division, and the U.S. Defense Advanced Research Projects Agency under contract W911QY-07-C-0002 (to M.J. Telch). The sponsors were not involved in the design or conduct of the study; collection, analysis, management or interpretation of the data; and preparation or approval of the manuscript. The views expressed in this publication are those of the authors and may not necessarily be endorsed by the U.S. Army.
References
- 1.Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–9. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutter M, Moffitt TE. Gene-environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry. 2006;47:226–61. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 3.Karg K, Burmeister M, Shedden K, et al. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TC, Ryan MA, Wingard DL, et al. New onset and persistent symptoms of post-traumatic stress disorder self reported after deployment and combat exposures: prospective population based US military cohort study. BMJ. 2008;336:366–71. doi: 10.1136/bmj.39430.638241.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 6.Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748–66. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- 7.Mehta D, Binder EB. Gene x environment vulnerability factors for PTSD: the HPA axis. Neuropharmacology. 2012;62:654–62. doi: 10.1016/j.neuropharm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 8.True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–64. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, Kessler RC, Walters EE, et al. Stressful life events, genetic liability, and onset of an episode of major depression in women. Am J Psychiatry. 1995;152:833–42. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis MC, Nugent NR, Amstadter AB, et al. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Curr Psychiatry Rep. 2010;12:313–26. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder EB, Bradley RG, Liu W, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson EC, Agrawal A, Pergadia ML, et al. Association of childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Mol Psychiatry. 2009;14:234–5. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amstadter AB, Koenen KC, Ruggiero KJ, et al. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. J Anxiety Disord. 2009;23:369–73. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes A, Hariri AR. The serotonin transporter gene-linked polymorphism and negative emotionality: placing single gene effects in the context of genetic background and environment. Genes Brain Behav. 2003;2:332–5. doi: 10.1046/j.1601-1848.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 15.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10:182–91. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Heinz A, Jones DW, Mazzanti C, et al. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol Psychiatry. 2000;47:643–9. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- 17.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 18.Wendland JR, Martin BJ, Kruse MR, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–6. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Oroszi G, Chun J, et al. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes RA, Murthy NV, Dresner MA, et al. Human 5-HT transporter availability predicts amygdala reactivity in vivo. J Neurosci. 2007;27:9233–7. doi: 10.1523/JNEUROSCI.1175-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caspi A, Hariri AR, Holmes A, et al. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167:509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercer KB, Orcutt HK, Quinn JF, et al. Acute and posttraumatic stress symptoms in a prospective gene x environment study of a university campus shooting. Arch Gen Psychiatry. 2012;69:89–97. doi: 10.1001/archgenpsychiatry.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilpatrick DG, Koenen KC, Ruggiero KJ, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–9. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- 24.Koenen KC, Aiello AE, Bakshis E, et al. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169:704–11. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie P, Kranzler HR, Poling J, et al. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–9. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolassa I, Ertl V, Eckart C, et al. Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry. 2010;71:543–7. doi: 10.4088/JCP.08m04787blu. [DOI] [PubMed] [Google Scholar]
- 27.Grabe HJ, Spitzer C, Schwahn C, et al. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am J Psychiatry. 2009;166:926–33. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- 28.Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 29.Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems and barriers to care. US Army Med Dep J. 2008:7–17. [PubMed] [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 31.Lee HJ, Goudarzi K, Baldwin B, et al. The Combat Experience Log: a web-based system for the in theater assessment of war zone stress. J Anxiety Disord. 2011;25:794–800. doi: 10.1016/j.janxdis.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 32.King DW, King LA, Voight DS. Manual for the Deployment Risk and Resilience Inventory (DRRI): a collection of measures for studying deployment related experiences of military veterans. Boston: National Center for PTSD; 2003. [Google Scholar]
- 33.Bliese PD, Wright KM, Adler AB, et al. Validating the primary care posttraumatic stress disorder screen and the posttraumatic stress disorder checklist with soldiers returning from combat. J Consult Clin Psychol. 2008;76:272–81. doi: 10.1037/0022-006X.76.2.272. [DOI] [PubMed] [Google Scholar]
- 34.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 35.Eaton W, Smith C, Ybarra M. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R) In: Maruish M, editor. The use of psychological testing for treatment planning and outcomes assessment. 3rd. Mahwah: Lawrence Erlbaum; 2004. pp. 63–77. 3. [Google Scholar]
- 36.Rogers NL, Cole SA, Lan HC, et al. New saliva DNA collection method compared to buccal cell collection techniques for epidemiological studies. Am J Hum Biol. 2007;19:319–26. doi: 10.1002/ajhb.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zalsman G, Huang Y-Y, Oquendo MA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–93. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 38.Telch MJ, Rosenfield D, Lee H, et al. Emotional reactivity to a single inhalation of 35% carbon dioxide and its association with later symptoms of post-traumatic stress and anxiety in soldiers deployed to Iraq. Arch Gen Psychiatry. 2012;69:1161–8. doi: 10.1001/archgenpsychiatry.2012.8. [DOI] [PubMed] [Google Scholar]
- 39.Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 40.Hedeker D, Gibbon RD. Longitudinal data analysis. Hoboken: Wiley-Interscience; 2006. [Google Scholar]
- 41.Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park: Sage; 1991. [Google Scholar]
- 42.Holmes A, Lit Q, Murphy DL, et al. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–80. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 43.Rona RJ, Fear NT, Hull L, et al. Mental health consequences of overstretch in the UK armed forces: first phase of a cohort study. BMJ. 2007;335:603. doi: 10.1136/bmj.39274.585752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen YC, Arkes J, Pilgrim J. The effects of deployment intensity on post-traumatic stress disorder: 2002-2006. Mil Med. 2009;174:217–23. doi: 10.7205/milmed-d-03-4307. [DOI] [PubMed] [Google Scholar]
- 45.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 46.Kendler KS, Kuhn JW, Vittum J, et al. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs N, Kenis G, Peeters F, et al. Stress-related negative affectivity and genetically altered serotonin transporter function: evidence of synergism in shaping risk of depression. Arch Gen Psychiatry. 2006;63:989–96. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- 48.Wilhelm K, Mitchell PB, Niven H, et al. Life events, first depression onset and the serotonin transporter gene. Br J Psychiatry. 2006;188:210–5. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- 49.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12:432–42. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolkow TT, Spira JL, Morse JS, et al. Post-traumatic stress disorder and depression in health care providers returning from deployment to Iraq and Afghanistan. Mil Med. 2007;172:451–5. doi: 10.7205/milmed.172.5.451. [DOI] [PubMed] [Google Scholar]
- 51.McKeever VM, Huff ME. A diathesis-stress model of posttraumatic stress disorder: ecological, biological, and residual stress pathways. Rev Gen Psychol. 2003;7:237. [Google Scholar]
- 52.Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–90. [PubMed] [Google Scholar]
- 53.Lesch K-P, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 54.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 55.Beevers CG, Marti CN, Lee HJ, et al. Associations between serotonin transporter gene promoter region (5-HTTLPR) polymorphism and gaze bias for emotional information. J Abnorm Psychol. 2011;120:187–97. doi: 10.1037/a0022125. [DOI] [PubMed] [Google Scholar]
- 56.Disner SG, Beevers CG, Lee H-J, et al. War zone stress interacts with the 5-HTTLPR polymorphism to predict the development of sustained attention for negative emotion stimuli in soldiers returning from Iraq. Clin Psychol Sci. 2013;1:413–25. [Google Scholar]
- 57.Engelhard IM, van den Hout MA, Weerts J, et al. Deployment-related stress and trauma in Dutch soldiers returning from Iraq. Prospective study. Br J Psychiatry. 2007;191:140–5. doi: 10.1192/bjp.bp.106.034884. [DOI] [PubMed] [Google Scholar]
- 58.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]


