In polarized epithelial cells, SNX27 regulates PDZ domain–directed trafficking of NHE3 from endosomes to the plasma membrane and increases the stability of brush border NHE3. This establishes SNX27 as an important regulator of polarized sorting in epithelial cells.
Abstract
Sorting nexin 27 (SNX27) contains a PDZ domain that is phylogenetically related to the PDZ domains of the NHERF proteins. Studies on nonepithelial cells have shown that this protein is located in endosomes, where it regulates trafficking of cargo proteins in a PDZ domain–dependent manner. However, the role of SNX27 in trafficking of cargo proteins in epithelial cells has not been adequately explored. Here we show that SNX27 directly interacts with NHE3 (C-terminus) primarily through the SNX27 PDZ domain. A combination of knockdown and reconstitution experiments with wild type and a PDZ domain mutant (GYGF → GAGA) of SNX27 demonstrate that the PDZ domain of SNX27 is required to maintain basal NHE3 activity and surface expression of NHE3 in polarized epithelial cells. Biotinylation-based recycling and degradation studies in intestinal epithelial cells show that SNX27 is required for the exocytosis (not endocytosis) of NHE3 from early endosome to plasma membrane. SNX27 is also required to regulate the retention of NHE3 on the plasma membrane. The findings of the present study extend our understanding of PDZ-mediated recycling of cargo proteins from endosome to plasma membrane in epithelial cells.
INTRODUCTION
In polarized epithelial cells, the representation of domain-specific integral membrane proteins, including transporters, involves trafficking machinery between intracellular organelles, including the trans-Golgi, endosomes, and lysosomes. The balance of endocytosis, exocytosis, plasma membrane stability, and sorting to breakdown and synthetic pathways determines plasma membrane residence. Protein–protein interactions are involved in multiple aspects of protein residence in intracellular and plasma membrane compartments, and a common motif involved is the postsynaptic density protein 95/discs large/zona occludens 1 (PDZ) domain. For instance, the single-PDZ-domain–containing protein cystic fibrosis transmembrane conductance regulator (CFTR)–associated ligand (CAL) contributes to setting the balance of CFTR in the synthetic compartment, lysosomes, and plasma membrane (Cheng et al., 2002). The involvement of PDZ domain–containing proteins in setting plasma membrane expression of trafficked proteins has also been demonstrated for G protein–coupled receptors, including the thyrotropin receptor, the somatostatin receptor subtype 5, CFTR, the β2-adrenoreceptor (β2AR), and Na+/H+ exchanger 3 (NHE3; B.C., unpublished data; Swiatecka-Urban et al., 2002; Lahuna et al., 2005; Wente et al., 2005; Lauffer et al., 2010). As another example, the endogenous intestinal and renal brush border Na+/H+ antiporter NHE3, which contributes to the majority of intestinal and renal sodium and water absorption, has a total half-life of ∼16 h, although it cycles between the plasma membrane and endosomal compartment (Cavet et al., 2001; Donowitz et al., 2005; Cha et al., 2006; Singh et al., 2014b). The balance between endocytosis and exocytosis of NHE3 is partially dependent on its binding PDZ domain–containing proteins. The identified brush border PDZ domain–containing proteins that affect NHE3 include the Na+/H+ exchange regulatory cofactor (NHERF) family of multi-PDZ-domain–containing scaffolding proteins (NHERF1–4) and the single-PDZ-domain–containing protein Shank2 (Donowitz et al., 2005; Han et al., 2006). The NHERF family was named because of its role in NHE3 regulation, including helping set the balance of NHE3 in the brush border versus the endosomal compartment. The NHERF proteins take part in multiple aspects of the regulated trafficking of NHE3; for instance, NHERF1–3 are involved in cAMP inhibition, whereas NHERF2 is necessary for stimulation of NHE3 by lysophosphatidic acid and d-glucose and inhibition by protein kinase C and cGMP/cGKII (Lee-Kwon et al., 2003; Cha et al., 2005, 2010; Cinar et al., 2007; Murtazina et al., 2007, 2011; Zachos et al., 2009; Lin et al., 2011a; Sarker et al., 2011). Nonetheless, how the NHERF proteins regulate the balance of NHE3 between the plasma membrane and endosomes has remained unclear, since the NHERFs are primarily localized to the plasma membrane, with no identified endosomal localization.
Insights into the possible roles that PDZ adapter proteins play in the endosomes come from the phylogenetic analysis of all mammalian proteins, which reveal that the NHERF-family PDZ domain sequences are related to the PDZ domain of sorting nexin 27(SNX27), the only known PDZ domain–containing protein identified in endosomes (M. Donowitz, A. Sharma, and C. Brett, unpublished results; Joubert et al., 2004). In fact, NHERF-family PDZ domain sequence homology was greater with SNX27 than with any other mammalian protein other than the homology among the four members of the NHERF family.
SNX27 is a member of the sorting nexin family of proteins, which are a diverse group of cytoplasmic and membrane-associated proteins implicated in endocytosis and protein trafficking. This protein family is characterized by the presence of a phosphoinositide-binding PX domain, which targets proteins to phosphatidylinositol-3-monophosphate–rich membranes of the endosomal system (Carlton and Cullen 2005; Cullen 2008; van Weering et al., 2010). SNX27 is unique among the PX proteins in containing an N-terminal PDZ domain that is upstream of the PX domain. SNX27 has also been annotated to possess a Ras-association domain and an extended C-terminal region (Seet and Hong 2006; Cullen 2008). Relevant to NHE3, SNX27 has been implicated in mediating PDZ-directed sorting of proteins from endosomes to the plasma membrane (Joubert et al., 2004; Lunn et al., 2007; Lauffer et al., 2010; Cai et al., 2011; Valdes et al., 2011).
In this study, we identified SNX27 as an endosomal NHE3 binding partner and showed that it regulates NHE3 activity in polarized epithelial cells. Depletion of SNX27 in epithelial cells decreased basal and acutely stimulated NHE3 activity and reduced NHE3 surface expression. Further, we show that trafficking of NHE3 from endosomes to the plasma membranes requires a PDZ domain–dependent interaction between NHE3 and SNX27 and that SNX27 also is necessary for the brush border stability of NHE3. Therefore, these studies identify an endosomal PDZ domain–containing protein that helps set basal and stimulated trafficking of NHE3 to regulate intestinal Na+ absorption.
RESULTS
Identification of NHE3 as an SNX27 binding partner
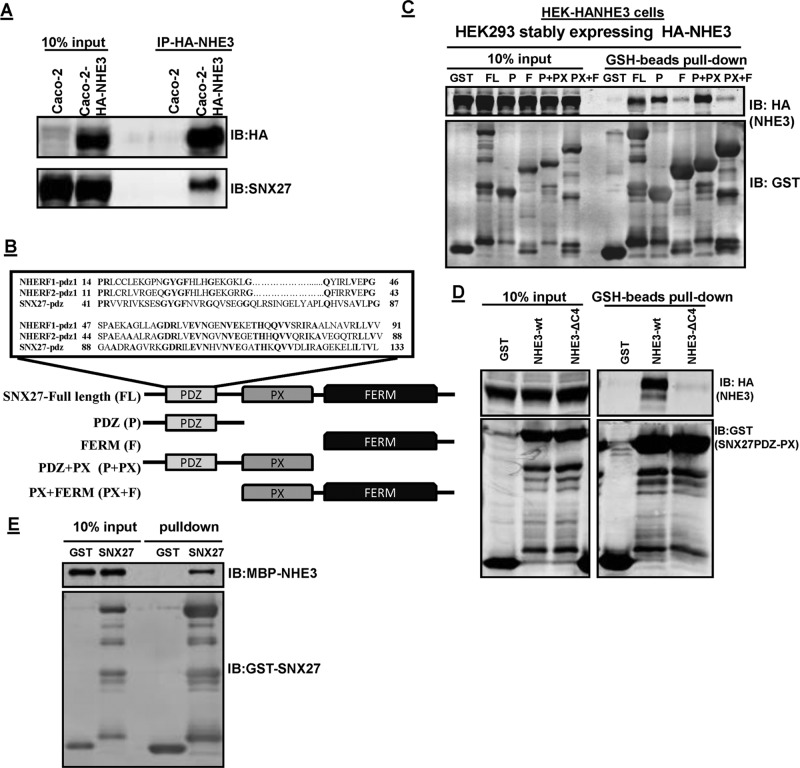
To expand the understanding of the role played by SNX27 as an adaptor in endocytic trafficking of NHE3, we used an immunoprecipitation (IP) approach to identify whether SNX27 associated with NHE3 under basal conditions. CoIP was performed with lysates prepared from the polarized intestinal Na+ absorptive epithelial cell line Caco-2BBe, which express endogenous SNX27 (Figure 1A). Because the endogenous expression of NHE3 was too low to perform biochemical studies, these cells were infected with adenovirus containing hemagglutinin (HA)-NHE3. As shown in Figure 1A, SNX27 coimmunoprecipitates with NHE3. This binding was further confirmed by in vitro pull-down assays. SNX27 belongs to the PX domain (Phox homology)–containing protein family but has a unique N-terminal PDZ domain upstream of the PX domain and an unusual band 4.1/ezrin/radixin/moesin (FERM)-like structure (Figure 1B). To determine more about the NHE3-SNX27 interaction, five glutathione S-transferase (GST)–SNX27 fusion proteins were purified: 1) GST-SNX27-FL (full length, amino acids [aa] 1–539); 2) PDZ (aa 1–156); 3) SNX27-F (FERM aa 272–539); 4) PDZ + PX (aa 1–266); and 5) PX + FERM (aa 158–539). These GST proteins were used to pull down NHE3 from cell lysate containing HA-NHE3. GST-SNX27 fusion proteins or GST control protein were incubated with HEK-HA-NHE3 cell lysates. After washing, proteins bound to GST beads were separated by SDS–PAGE and identified by anti-HA (NHE3) or anti-GST (SNX27) antibodies (Figure 1C). Based on the pull-down assays, in addition to SNX27-FL, NHE3 interacts with PDZ and PDZ + PX fusion proteins. It is important to note that further extension of the PDZ domain to the PX domain significantly increased the binding of NHE3 with the PDZ domain of SNX27 (Figure 1C). Weak but significant binding was also seen with FERM and PX-FERM domains of SNX27; however, further studies are needed to understand the physiological relevance of this weaker binding.
FIGURE 1:
SNX27 and NHE3 directly interact with each other. (A) Interaction between endogenous SNX27 and NHE3 in Caco-2-HA-NHE3 cells. NHE3 (HA-NHE3) was immunoprecipitated (IP) from total lysate of Caco-2BBe cells using anti-HA antibody. Immunoprecipitated samples were subjected to Western blot analysis (IB) and probed with HA and SNX27 antibodies. Representative result from three independent experiments with similar results. (B) Schematic of SNX27 PDZ domain organization showing the PDZ domain, the PX domain, and the FERM domain of SNX27. Expanded box shows sequence comparison of the SNX27 PDZ domain with the first PDZ domain of NHERF1 and NHERF2. Conserved residues in the three PDZ domains are shown in bold. (C) HEK-HA-NHE3 cell extracts (1 mg) were incubated with GST or GST-fusion proteins: 1 nmol; SNX27-FL (aa 1–539), PDZ (P; aa 1–156), FERM (F; aa 272–539), PDZ+PX (P+PX; aa 1–266), PX +FERM (PX+F; aa 158–539). The presence of NHE3 in GST pull downs was detected by Western blotting (top). Equal GST loading was verified by Western blotting for GST. Representative results from three independent experiments. (D) HEK-HA-NHE3 or HA-NHE3∆C4 cell extracts (1 mg) were incubated with GST or GST-fusion proteins (1 nmol; SNX27- PDZ+PX) and then subjected to pull down with GSH resin. The presence of NHE3 in GST pull downs was detected by Western blotting (top). Representative results from three independent experiments. (E) GST or GST-SNX27-FL was mixed with MBP-NHE3-C-term (aa 642–832) and then subjected to pull down with GSH resin. Samples were analyzed by Western blot (IB) with antibodies against MBP and GST. The experiment was repeated three times, and one representative result is shown.
We also asked whether SNX27-PDZ interaction is via the PDZ motif at the C-terminus of NHE3 (-S-T-H-M). To determine this, we created a HA-NHE3∆C4 (-S-T-H-M) construct in HEK-293 cells and used GST-SNX27-PDZ-PX fusion proteins or GST control protein to pull down HEK-HA-NHE3 or HEK-HA-NHE3∆C4 as before. Unlike with HA-NHE3, the binding of SNX27-PDZ-PX was greatly reduced with deletion of the last four amino acids at the C-terminus of NHE3 (Figure 1D).
To confirm direct binding between NHE3 and SNX27, we also performed in vitro pull-down assays using the NHE3 C-terminal (aa 642–832) construct and SNX27-FL fusion proteins (Figure 1E). These results further showed that the interaction between NHE3 and SNX27 is direct. Together these results showed that NHE3 directly interacts with SNX27, which is primarily mediated by the PDZ domain of SNX27.
SNX27 regulates basal NHE3 activity and surface expression
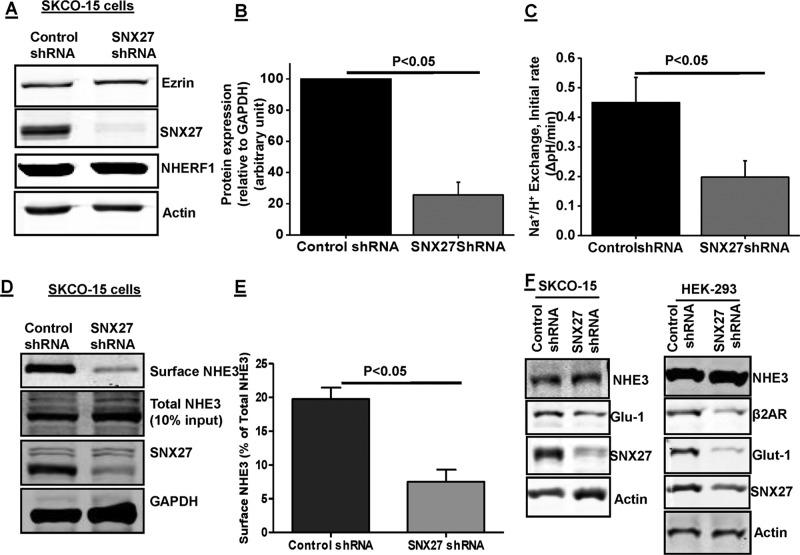
The functional role of the SNX27 binding to NHE3 was next evaluated in SK-CO15 cells, which are derived from a human adenocarcinoma of the colon. These cells form a polarized epithelial monolayer, with formation of microvilli (Yoo et al., 2012). These cells were selected for functional studies because they express both endogenous NHE3 and SNX27. A lentivirus-mediated delivery system was used to stably knock down SNX27 in these cells (Figure 2, A and B). The SNX27 protein expression was reduced by >80% in SNX27 short hairpin RNA (shRNA) lentivirus–infected cells (Figure 2B). Furthermore, this reduction was maintained in culture medium containing puromycin. Quantitative PCR confirmed that the reduced NHE3 activity in SNX27-depleted cells was not caused by transcriptional down-regulation of NHE3 (no change in mRNA; unpublished data). These cells were further used to determine the effect of knockdown on NHE3 basal activity and surface expression. SNX27-knockdown cells had ∼55% reduced basal NHE3 activity (Figure 2C); cell surface biotinylation showed that SNX27-KD caused a ∼60% reduced NHE3 surface expression (Figure 2, D and E); however, total NHE3 expression was not changed with SNX27 knockdown (Figure 2D). Similar effects of SNX27 knockdown (Figure 2, A and B) were observed in HEK-293-HA-NHE3 cells (Figure 3, C and E). Taken together, these results show that in intestinal epithelial cells, SNX27 is required to maintain normal NHE3 basal activity and cell surface expression. There was no effect on the expression levels of NHERF1 or ezrin in these knockdown cells (Figure 2A), suggesting that the SNX27-mediated regulation of NHE3 is not mediated by changes in the expression of some of the other major proteins involved in NHE3 regulation. The lack of enhanced degradation for total NHE3 is interesting and specific to NHE3, as the other cargo proteins known to be regulated by SNX27, such as Glut-1 and β2AR, showed less protein expression in SNX27-KD HEK-293 and SK-CO15 cells (Figure 2F).
FIGURE 2:
SNX27 regulates NHE3 activity and surface expression in SK-CO15 cells. (A) Western blot analysis of total cell lysate prepared from SK-CO15 cells infected with lentivirus control shRNA or SNX27 shRNA. β-Actin was used as loading control. Ezrin and NHERF1 were used as internal controls. (B) Densitometric analysis of total protein expression from control shRNA and SNX27 shRNA–infected lysates showed that SNX27 protein expression was significantly reduced in SK-CO15 cells containing puromycin as selection marker (n = 4). (C) Na+/H+ exchange was measured in SK-CO15 cells expressing control shRNA or SNX27 shRNA using the pH-sensitive dye BCECF. Results are means ± SE of four separate studies. p values are comparison between control shRNA and SNX27 shRNA. Initial rates are shown. (D) Single study of NHE3 surface expression using biotinylation in SK-CO15 cells. Three dilutions of total cell lysate (10, 20, 40 μl) and avidin precipitate/surface (10, 20, 40 μl) were loaded on SDS–PAGE, and NHE3, SNX27, and GAPDH were identified by immunoblotting; percentage of surface to total was calculated as explained in Materials and Methods. To illustrate the results, in this example, we show results of the loading of one equal amount of total cell lysate (40 μl) and avidin precipitate/surface (40 μl) on SDS–PAGE with NHE3, SNX27, and GAPDH identified by immunoblotting. (E) Quantification presents surface NHE3 normalized to total NHE3 for control shRNA and SNX27 shRNA. Results are means ± SE of four separate studies. p values are comparison between control shRNA and SNX27 shRNA. (F) Western blot analysis of total cell lysate prepared from SK-CO15 and HEK293 cells infected with SNX27 shRNA or control shRNA. Expression of NHE3, Glut-1, and β2AR is shown. β-Actin was used as loading control. A representative result from three independent experiments is shown.
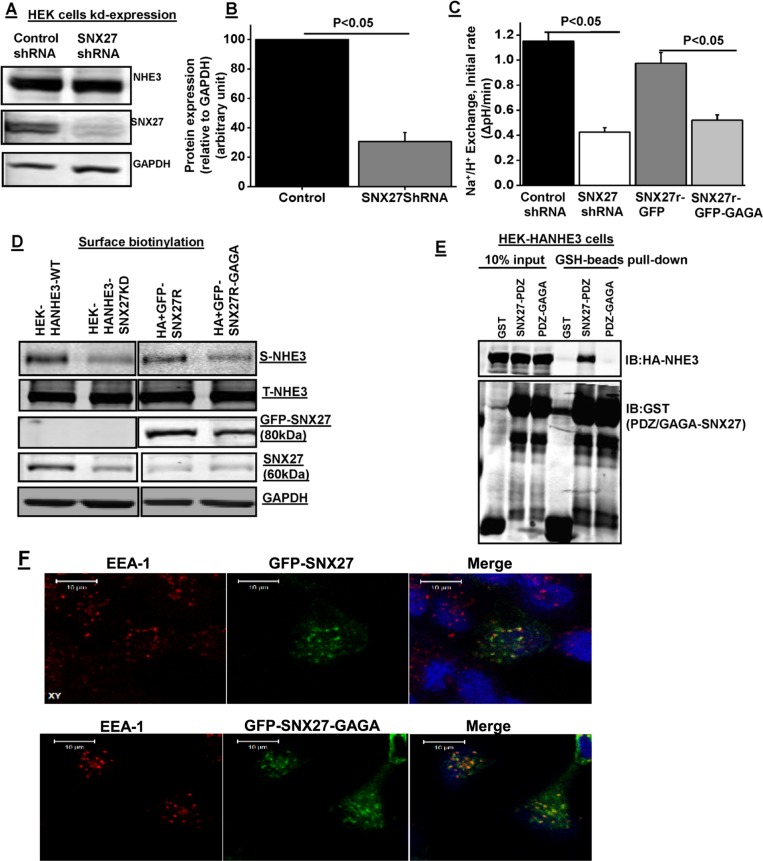
FIGURE 3:
PDZ domain–mediated interaction of SNX27 maintains plasma membrane localization and activity of NHE3. (A) Western blot analysis of total cell lysate prepared from HEK293-HA-NHE3 cells infected with lentivirus control shRNA or SNX27 shRNA. GAPDH was used as internal control. (B) Densitometric analysis of total protein expression from control shRNA and SNX27 shRNA–infected lysates showed that SNX27 protein expression was significantly reduced in HEK293-HA-NHE3 cells. Results are means ± SE of four separate studies. p values are comparison between control shRNA and SNX27 shRNA. (C) Na+/H+ exchange was measured in HEK293-HA-NHE3 cells expressing SNX27 shRNA or control shRNA with or without reconstitution of SNX27 by cotransfection of rat SNX27-GFP or PDZ mutant SNX27-GFP-GAGA. Results are means ± SE of four separate studies. p values are comparison between control shRNA and SNX27 shRNA or GFP-SNX27 and GFP-SNX27-GAGA. (D) Western blot analysis of NHE3 surface expression using biotinylation in HEK-HA-NHE3 cells expressing SNX27 shRNA or control shRNA with or without replacement by cotransfection of rat SNX27-GFP or its PDZ mutant SNX27-GFP-GAGA. A representative blot from three independent experiments with similar results. (E) GST, GST-SNX27-PDZ, or GST-SNX27-PDZ-GAGA was mixed with HEK-HA-NHE3 cell lysate and then subjected to pull down with GSH resin. Samples were analyzed by Western blot (IB) with antibodies against HA and GST. The experiment was repeated three times, and one representative result is shown. (F) Representative examples of fluorescence localization patterns of rat GFP-SNX27 or its PDZ mutant (GYGF → GAGA) versions of SNX27 relative to EEA1, verifying that its PDZ domain is not required for early endosomal localization of SNX27. Bar, 10 μm. Representative images.
PDZ domain of SNX27 maintains plasma membrane localization and activity of NHE3
To examine the specificity of the SNX27-knockdown effect, we investigated the rescue using rat-derived SNX27b constructs not targeted by the human-specific shRNA lentivirus. Selective knockdown (KD) and replacement were done in HEK-293-HA-NHE3 cells (easier to transfect than with SK-CO15 cells). KD was confirmed by anti-SNX27 immunoblotting (Figure 3, A and B). The SNX27-knockdown HEK-293-HA-NHE3 cells that contained rat-derived SNX27–green fluorescent protein (GFP) effectively rescued NHE3 basal activity and surface expression (Figure 3, C and E).
We next determined whether the regulation of NHE3 activity by SNX27 requires its ability to bind the single SNX27 PDZ domain. PDZ domains generally bind to their ligands through a groove formed by the sequence GYGF (Doyle et al., 1996; Jemth and Gianni 2007). The binding ability of PDZ domains to most of their ligands is abolished in GYGF (aa 52–55) mutants (Karthikeyan et al., 2002; Weinman et al., 2003). Therefore the GYGF sequence was mutated to GAGA, and mutant GFP-SNX27-PDZ-GAGA was studied for its effect on NHE3 activity and cell surface expression. By immunolocalization, SNX27-GFP colocalizes prominently with EEA1-containing endosomes, as shown previously (Lauffer et al., 2010). Similar to wild type, GAGA mutant also localized to the EEA1 compartment (Figure 3F). Wild-type rat-GFP-SNX27 fully rescued basal NHE3 activity; cell surface expression of NHE3 was also similar to wild-type SNX27 in control cells, whereas the PDZ mutant reconstituted neither wild-type NHE3 activity nor cell surface expression (Figure 3, C and D). In addition, the PDZ mutant fusion protein (GST-PDZ-GAGA+PX) was unable to pull down NHE3 from the HEK293-HA-NHE3 cell line (Figure 3E). Together these results indicate that NHE3 basal activity and cell surface expression depend on the interaction with the SNX27 PDZ domain.
NHE3 colocalizes with SNX27 on early endosomes
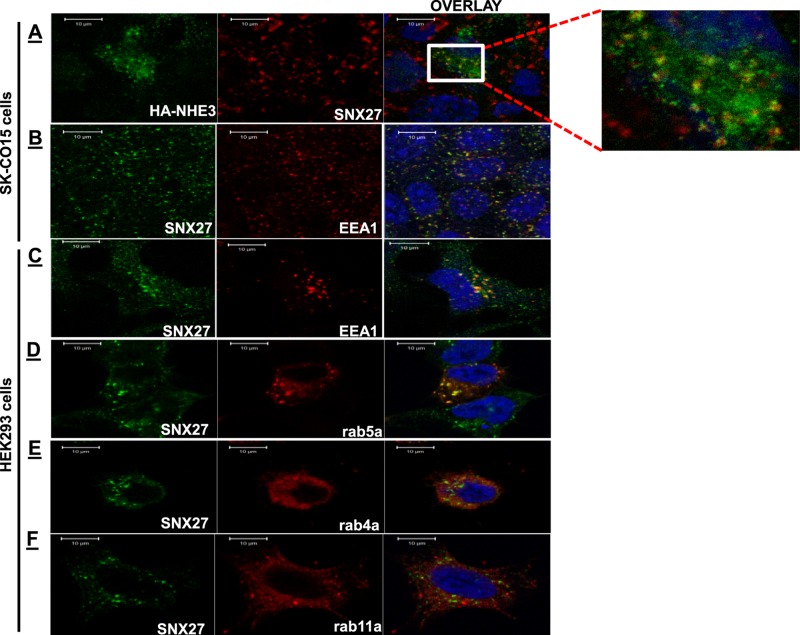
To further understand the mechanism of SNX27's effect on NHE3, their intracellular localization was determined. Immunofluorescence analysis was performed using SK-CO15 cells. In the basal state, there was very little colocalization of SNX27 and NHE3. Then we examined localization of NHE3 that had been on the plasma membrane and was subsequently endocytosed. Surface NHE3 was labeled at 4°C with HA antibody and allowed to internalize at 37°C. After 1 h of incubation at 37°C, internalized antibody (NHE3) accumulated in an internal vesicle population. NHE3 (green) colocalized with SNX27 (red) in these vesicles (Figure 4A, inset). The endosomal location of SNX27 was confirmed by costaining with EEA1 or GFP-Rab5a (Figure 4, B and D), early endosome markers. As is evident in Figure 4, B–D, the majority of the SNX27 (green)-containing vesicles are EEA1 (red) or Rab-5a (red) positive. Furthermore, we examined whether SNX27 was expressed in recycling endosomes. HEK-293 cells were transfected with GFP-Rab4a (Grant and Donaldson 2009) or GFP-Rab11a (Mohrmann and van der Sluijs 1999; markers for early and late recycling endosomes, respectively). Figure 4, E and F, shows that SNX27 (green) was not found in these populations of recycling endosomes where Rab4a or Rab11a (red) is expressed. Therefore these results are consistent with a role of SNX27 in recycling from early endosomes (Wang et al., 2013; Hussain et al., 2014; Munoz and Slesinger 2014).
FIGURE 4:
Internalized NHE3 colocalizes with SNX27 in EEA-1– and Rab5-containing vesicles near the plasma membrane. (A) Biochemical internalization assay of HA-NHE3 in SK-CO15 cells. Representative examples of fluorescence localization pattern of endogenous SNX27 (red) and internalized NHE3 (green). The inset shows a magnified image of the boxed area. (B, C). Cellular localization of endogenous SNX27 (green) and EEA1 (red) in SK-CO15 and HEK293 cells, respectively. (D) HEK293 cells were transfected with early endosome marker GFP-rab5a or (E) recycling marker GFP-rab4a or (F) GFP-rab11a. The colocalization of endogenous SNX27 (green) and endosomal marker (red) was determined with anti-SNX27 antibody and anti-GFP antibodies. Scale bar, 10 μm. Representative images from six or seven independent experiments with similar results.
SNX27 regulates NHE3 recycling in polarized epithelial cells
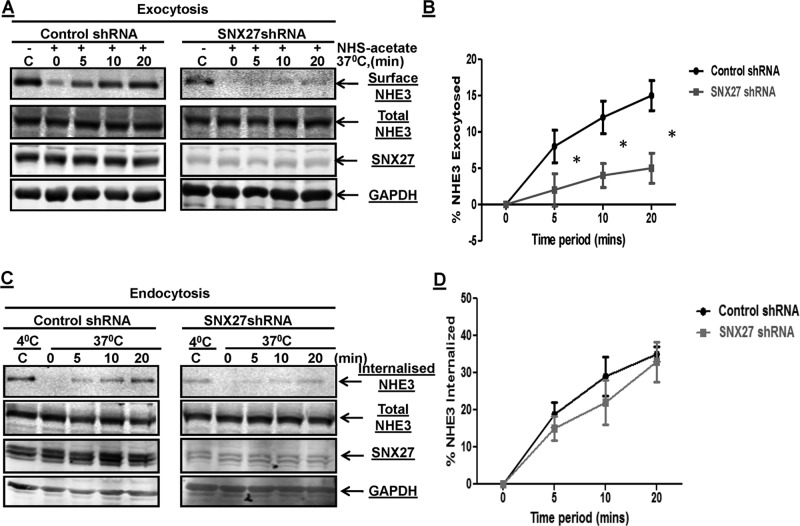
That the SNX27 knockdown reduced basal NHE3 activity and surface expression without a change in total cellular expression and that the intracellular pool of NHE3 partly overlaps with SNX27 in endosomes support that SNX27 plays a role in NHE3 trafficking. Mechanistic studies were undertaken to define this role of SNX27 in NHE3 regulation. Because the surface amount of NHE3 is also dependent on the balance of exocytic insertion and endocytic internalization, we further examined whether these two processes are affected in SNX27-knockdown SK-CO15 cells. Cell surface biotinylation–based methods were used to determine the rate of exocytic insertion and endocytic internalization separately. The rate of exocytic insertion of NHE3 was measured for 5, 10, and 20 min in SNX27-KD SK-CO15 cells, as shown in Figure 5, A and B. The exocytic insertion of NHE3 was ∼50% less than (p < 0.05) that of the control cells at all the time points tested. Whereas the rates of exocytosis were significantly reduced in SNX27-KD cells, the rate of endocytosis in control and knockdown cells was not significantly different (Figure 5, C and D). The amount of NHE3 internalized at 5, 10, and 20 min was normalized to the surface NHE3 that was measured at 4°C and was not treated with glutathione (GSH; control). Taken together, the rate of NHE3 endocytosis was not significantly different between SNX27-control and SNX27-KD cells, showing that basal endocytosis was not altered by SNX27-KD in SK-CO15 cells; however, exocytic insertion of NHE3 to the plasma membrane was significantly reduced in the absence of SNX27. These studies suggest that SNX27 is required for delivery of NHE3 from endocytic vesicles to the plasma membrane.
FIGURE 5:
SNX27-KD decreases basal exocytosis but not endocytosis of NHE3 in SK-CO15 cells. (A) Confluent serum-starved SK-CO15 cells were incubated with NHS-acetate. Control shRNA and SNX27 shRNA SK-CO15 cells were incubated for 0, 5, 10, and 20 min at 37°C to allow exocytosis of NHE3. Cells were then chilled to 4°C, and newly inserted NHE3 (exocytosis of NHE3) was biotinylated and detected by Western blotting. GAPDH levels were measured in the surface biotinylated fraction as a negative control to ensure the absence of intracellular protein biotinylation. A representative blot from three independent experiments showed that exocytosis of NHE3 was less in SNX27-KD cells than with control at all time points. (B) The surface amount of NHE3 exocytosed in A was measured by densitometry with signal at 0 min (background) subtracted from all time points. Error bar indicates the SE of three experiments. *p < 0.05 values are comparison between control and SNX27-depleted cells. (C) Rate of endocytosis of NHE3 in control shRNA and SNX27 shRNA SK-CO15 cells. Biotinylated cells were incubated at 37°C for 0, 5, 10, and 20 min. The amount of endocytosis of NHE3 (internalization of surface NHE3) at the indicated time points was determined by a GSH-resistant endocytosis assay as described in Materials and Methods. A representative blot from three independent experiments. (D) Quantitative analysis of endocytosis of NHE3 by densitometry from experiments described in C. The amount of internalized NHE3 via endocytosis at each time point was calculated as the percentage of surface NHE3 of corresponding control group, which was always kept at 4°C and never exposed to GSH. Error bars represent the SE of three experiments. *p < 0.05 values are comparison between control and SNX27-depleted cells.
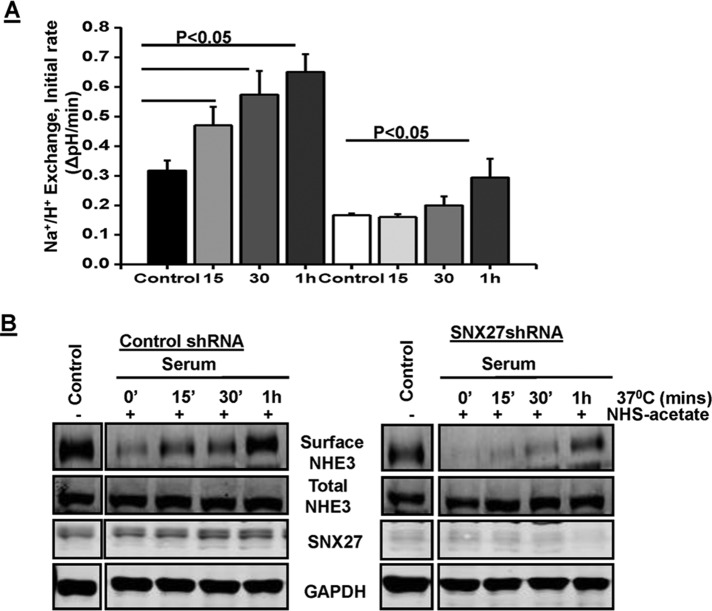
Acute stimulation of NHE3 activity by serum is mediated by SNX27
Most of the acute stimulation of NHE3 involves an increase in surface expression, which is a result of an increase in exocytotic trafficking (Yang et al., 2000; Peng et al., 2001; Bobulescu et al., 2005; Zachos et al., 2005; Donowitz et al., 2009). Because exocytosis, but not endocytosis, was affected by KD of SNX27 in SK-CO15 cells, we next investigated whether the acute stimulation of NHE3 by dialyzed serum, which involves an increase in surface expression, was also affected in SNX27-knockdown cells. Therefore, Na+/H+ exchange activity and cell surface amount were measured in response to serum for different time periods (15, 30, and 60 min) in SK-CO15 control and SNX27-KD cells. The basal rate of Na+-dependent pH change by NHE3 was measured in the presence of the NHE1 and NHE2 inhibitor Hoe-694 (50 μM). Serum treatment in control cells increased NHE3 activity by ∼47, 78, and 103% at 15, 30, and 60 min, respectively (Figure 6A). In contrast, the loss of SNX27 significantly inhibited the effect of serum on NHE3 activity, resulting in no significant stimulation at 15- and 30-min time points, whereas there was significant stimulation (∼80%) at 60 min (Figure 6A). These findings showed that SNX27 plays a significant role in, but is not obligatory for, serum-mediated acute stimulation of NHE3 activity. On the basis of the foregoing finding, we hypothesized that NHE3 requires SNX27 in order to maintain the rapid rate of trafficking to the plasma membrane. Therefore we next investigated the rate of exocytotic trafficking of NHE3 at different time points (15, 30, and 60 min) in response to serum in polarized SK-CO15 WT and SNX27-KD cells. In control cells, the exocytosis of NHE3 in response to serum starts as early as 15 min and gradually increases approximately twofold by 60 min (Figure 6B). In contrast, in SNX27-KD cells, the exocytosis of NHE3 in response to serum was slower than with control cells; the only significant amount of exocytosed NHE3 was at 60 min (Figure 6B). However, the percentage increase in NHE3 activity and surface amount at 60 min was similar in control and SNX27-KD cells. Taken together, these results demonstrate that the presence of SNX27 is required to maintain the rate of exocytotic trafficking of NHE3 in response to serum.
FIGURE 6:
SNX27 is necessary for serum to rapidly stimulate NHE3 activity. (A) SKCO-15 cells expressing control shRNA or SNX27 shRNA were treated with 10% dialyzed fetal bovine serum for t = 0, 15, 30, or 60 min, followed by determination of Na+/H+ exchange activity as described. Data shown are means ± SE of three separate experiments. p < 0.05 values are compared with untreated control. (B) Postconfluent, serum-starved control shRNA and SNX27 shRNA SKCO-15 cells were incubated with NHS-acetate. Cells were then incubated with 10% dialyzed fetal bovine serum for 0, 15, 30, and 60 min at 37°C to allow exocytosis of NHE3 in response to serum. Cells were then chilled to 4°C, and newly inserted NHE3 (exocytosis of NHE3) was biotinylated and detected by Western blotting as described. A representative blot from three independent experiments with similar results.
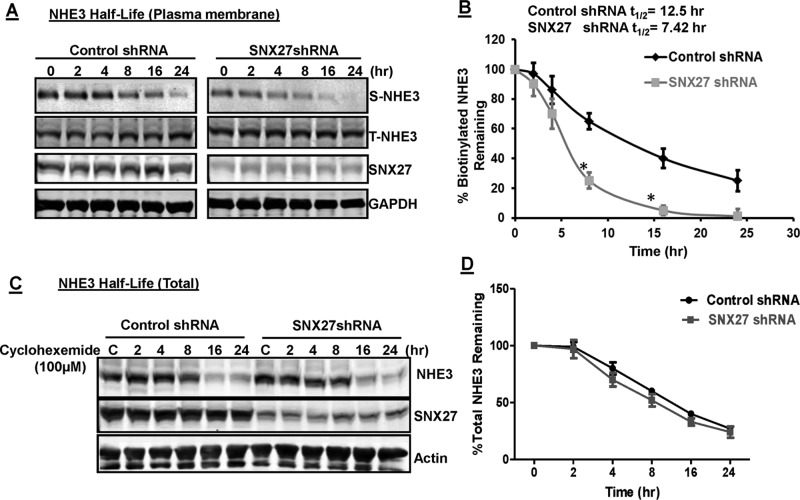
Half-life of plasma membrane but not total NHE3 depends on SNX27 in polarized epithelial cells
The phenotype of SNX27 depletion reduced NHE3 surface expression, as assessed by cell surface biotinylation. Plasma membrane retention of protein depends on the balance between the rates of endocytosis and exocytosis. Biochemical internalization and recycling assays revealed that although NHE3 was internalized at similar relative rates in SNX27-depleted cells, the return to the cell surface was severely inhibited (Figure 5, A and B). Therefore we investigated the half-life of NHE3 initially on the plasma membrane by surface biotinylation, as described previously (Cavet et al., 2001). We determined the total surface biotinylated NHE3 protein remaining with cells at 0–24 h after initial biotinylation of surface protein in control and SNX27-KD SK-CO15 cells. The amounts of biotinylated NHE3 protein left at different time periods were normalized to surface NHE3 at time 0 (cells always at 4°C). These results (Figure 7, A and B) demonstrated that plasma membrane NHE3 in control and SNX27-KD cells has different degradation rates. Whereas control cells had NHE3 plasma membrane half-life of ∼12.5 h, SNX27-KD cells had a much shorter plasma membrane half-life of ∼7.5 h (Figure 7B).
FIGURE 7:
Half-life of plasma membrane but not total NHE3 depends on SNX27. (A) Plasma membrane NHE3 protein in control and SNX27 shRNA–expressing cells was biotinylated at 4°C using NHS-SS-biotin. Cells were incubated at 37°C and harvested at various time points. After solubilization, biotinylated protein was recovered with streptavidin-agarose beads and analyzed by Western blotting as described. A single representative blot of three independent experiments with similar results. (B) Densitometric analyses of Western blots. The amount of biotinylated NHE3 protein left at different time periods was normalized to surface NHE3 at time 0 (cells always at 4°C). Error bars represent the SE of four experiments. *p < 0.05 values are comparison between control and SNX27-depleted cells. (C) Total NHE3 levels in control and SNX27- depleted cells treated with cycloheximide (100 μM) over the indicated periods. (D) Densitometric analyses of Western blots were performed to calculate percentage of NHE3 remaining over indicated time periods. A representative blot for four independent experiments is shown. Error bars represent the SE of four experiments.
Although we did not notice any change in total NHE3 expression between control and SNX27-KD cells, to further confirm this observation, we performed degradation (half-life of total NHE3) assays from 0 to 24 h in SK-CO15 control and SNX27-KD cells treated with cycloheximide (100 μM) to block synthesis of new proteins. Cell lysates from different time points were separated by SDS–PAGE and transferred to nitrocellulose membranes. Figure 7, C and D, showed that even though the surface half-life was much shorter in SNX27-KD cells than in control, the half-lives of total NHE3 were not significantly different. This is not a cell type–specific but instead is a substrate-specific effect, as the other known cargoes of SNX27 in these cell types showed higher degradation of total protein expression in response to SNX27-KD (Figure 2F). These results show that SNX27 regulates the retention or stability of NHE3 on the plasma membrane.
DISCUSSION
Epithelial cells contain multiple PDZ proteins, which are localized to specific subcellular domains, including the brush border (BB), basolateral membranes, and tight junctions, as well as in the Golgi (CAL) and endosomes (sorting nexin 27; Cheng et al., 2002, 2004; Brone and Eggermont 2005). The major identified class of PDZ domain–containing proteins in the BB is the NHERF family, although the unrelated Shank2 is also present. These proteins regulate different aspects of transport protein function. Use of NHE3 as an example shows that the contributions of PDZ domain–containing proteins depend on their different binding partners. For instance, the NHERF1 and NHERF2 C-terminal ERM protein-binding domains mediate a network of protein connectivity linking integral membrane proteins to actin (Bretscher et al., 2000). None of the NHERF proteins localizes to endosomes at steady state. Acute regulation of NHE3 involves changes in the rate of endocytosis/exocytosis between plasma membrane and recycling endosomes. Therefore this study was undertaken to define the role endosomal PDZ domain proteins might play in endosome-to–plasma membrane trafficking of NHE3. Phylogenetic analysis showed that the NHERF family members are most highly related to each other, with the next-most-related protein being SNX27 (M. Donowitz, A. Sharma, and C. Brett, unpublished results; Joubert et al., 2004; Thelin et al., 2005). SNX27 (also called Mrt1) is a unique member of the PDZ domain–containing protein family, which is widely expressed and contains a PX domain that binds specifically to phosphatidylinositol 3-phosphate, which is enriched on the cytoplasmic surface of early/sorting endosomes (Lunn et al., 2007; Rincon et al., 2007). Here we demonstrate that NHE3 directly interacts with SNX27, which is mediated by the PDZ domain of SNX27. In addition, the majority of the SNX27–PDZ domain interaction is mediated by the PDZ motif at the C-terminus of NHE3 (-E-S-T-H-M).
Recently SNX27 has emerged as an important sorting protein that maintains surface levels of multiple proteins. Recent studies using an unbiased global analysis of SNX27-mediated sorting found that it affected >100 cell surface proteins, which include the glucose transporter GLUT1, the Menkes disease copper transporter ATP7A, zinc and amino acid transporters, and multiple signaling receptors (β2AR, TGFβ-receptor1, etc.). All of these proteins require the SNX27–retromer complex to prevent lysosomal degradation and maintain surface levels (Temkin et al., 2011; Steinberg et al., 2013). These reports, including our present results, are different from the previously proposed roles of SNX27 in promoting endocytosis or lysosomal delivery of PDZ motif–bearing cargo (Joubert et al., 2004; Lunn et al., 2007). The possible explanation is that the previous studies relied entirely on SNX27 overexpression, which is known to produce additional effects consistent with expression-dependent differences in the functional effects of other sorting nexins (Carlton et al., 2005). To avoid the additional effects caused by the overexpression system, we chose a model that contains endogenous NHE3 and SNX27 proteins. Here we demonstrated with shRNA-mediated knockdown and subsequent rescue experiments that loss of SNX27 in parallel decreased the apical localization and basal activity of NHE3 in intestinal epithelial cells. In addition, the change in activity and surface expression depends on the PDZ domain of SNX27. This conclusion was reached because the reduced NHE3 activity and surface expression that resulted from knockdown of SNX27 was reversed by knocking-in wild-type SNX27-rat, whereas the PDZ-domain mutant of the same construct was unable to duplicate the rescue. Our results are in agreement with a previous detailed study showing that SNX27 is a critical sorting protein for PDZ motif–directed endosome-to–plasma membrane traffic of the β2ARs (Lauffer et al., 2010; Loo et al., 2014).
In confluent polarized monolayers, NHE3 is localized at the apical and subapical region, where it is present in multiple pools (Li et al., 2001; Cha et al., 2004; Alexander et al., 2005). These pools are rapidly activated or inhibited by changes in trafficking, which probably mimics renal and intestinal physiology (Cha et al., 2004; Alexander et al., 2005). Given that SNX27 is known to localize to early endosomes (Lauffer et al., 2010), we hypothesized that NHE3 might transiently interact with SNX27 during intracellular trafficking. Using tagged NHE3 protein as well as antibody staining of native proteins following endocytosis, we showed overlap of the two proteins on the EEA1-containing early endosome. NHE3 traffics in multimeric complexes that are regulated by PDZ-domain protein interactions involving NHERF proteins (Cha et al., 2004; Cha et al., 2010). Thus it is likely that there are other proteins that interact with the SNX27-NHE3 complex to regulate endosomal trafficking. Analysis of all proteins in the SNX27-NHE3 complex and an examination of multiple endosomal pools of NHE3 proteins will be required to fully understand the role of SNX27 in regulating NHE3 activity.
Under basal conditions, NHE3 cycles between the plasma membrane and the endosomal recycling compartment (D'souza et al., 1998; Kurashima et al., 1998; Janecki et al., 2000a, b; Hu et al., 2001; Akhter et al., 2002). As part of digestive physiology, NHE3 is acutely stimulated and inhibited by changes in trafficking, and we previously identified the role of some PDZ-domain proteins under different physiological conditions (Zachos et al., 2009; Cha et al., 2010; Yang et al., 2013). However, our previous studies focused on the role of PDZ proteins in cytoskeleton association of NHE3. In contrast, in the present study, we explored the role of the PDZ-domain protein SNX27 in NHE3 trafficking in detail. Biochemical internalization and recycling assays revealed that although NHE3 was internalized at similar relative rates in SNX27-depleted cells, the return to the cell surface was severely inhibited by knocking down SNX27. This is in accordance with other reports showing PDZ domain–mediated protein interactions in determining the endomembrane trafficking for several membrane proteins (Lin and Huganir 2007; Cushing et al., 2008; Lauffer et al., 2010). Surprisingly, depletion of SNX27 decreased the stability of NHE3 on the plasma membrane but not the stability of total NHE3, as confirmed by degradation assays in cells treated with cycloheximide to block protein synthesis. Unlike other proteins regulated by SNX27 (e.g., Glut-1 and β2AR), this finding is unique to NHE3. There could be two possible explanations for this finding: 1) other FERM-like domain–containing proteins in early endosomes mediate retrieval of NHE3 from the lysosomal pathway in absence of SNX27, and/or 2) the SNX27-dependent exocytic pool, which has the same half-life as the total NHE3 pool, is stabilized by another SNX27-dependent protein, perhaps one the exocytosis of which also requires SNX27. In the absence of this additional protein, surface NHE3 stability/surface half-life is reduced. This pool of NHE3 is exocytosed rapidly in response to serum, since by 60 min after serum stimulation, the NHE3 activity is no longer dependent on the presence of SNX27. However, further studies are needed to understand this process in detail.
Several apical-domain pools of NHE3 in epithelial cells have been defined previously. These include NHE3 in lipid rafts and outside lipid rafts and NHE3 bound to megalin (with heavier density and less activity) or not bound to megalin (lighter density and more activity). Intracellular NHE3 also exists in two pools, one of which is rapidly exocytosed, whereas the other moves to the apical membrane with much slower kinetics (Alexander et al., 2005, 2007; Alexander and Grinstein 2006). Serum-stimulated exocytosis of NHE3 had two phases, with loss of stimulation with SNX27-KD only at shorter time points (<60 min). On the basis of our results and previous reports, we believe that SNX27 plays a major role in trafficking of NHE3 from an intracellular pool that responds to stimuli to increase NHE3 by rapid exocytosis rather than the pool that moves more slowly and that both pools are involved in stimulation of NHE3 activity by serum.
Vesicle membrane trafficking and recycling is important to maintain proper transporter function in polarized epithelial cells. Our data established the role of a PDZ-domain protein (SNX27) that is directly involved in cellular trafficking and hence in maintaining normal physiology in epithelial cell. Collectively the results of the present study provide several lines of evidence indicating that in epithelial cells, SNX27 has a necessary role in PDZ motif–directed exocytosis of membrane proteins and that it mediates this sorting function directly from the early endosome. This finding sheds light on the concept of motif-directed molecular sorting in the recycling pathway and supports its physiological significance.
MATERIALS AND METHODS
Materials
Glutathione Sepharose 4B resin was from GE Healthcare Life Science (Pittsburgh, PA). Amylose resin and rabbit anti-MBP were from New England Biolabs (Ipswich, MA). BCECF-AM, nigericin, and Hoechst 33342 were from Life Technologies (Grand Island, NY). Mouse anti–glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and mouse anti-actin were from Sigma-Aldrich (St. Louis, MO). Rabbit anti-GFP, Alexa Fluor 488– and 568–conjugated goat anti-mouse and anti-rabbit secondary antibodies, and Alexa Fluor 568–conjugated phalloidin were from Life Technologies. Mouse anti-HA was from Covance (Princeton, NJ). Mouse anti-GST was from Cell Signaling Technology (Danvers, MA). IRdye-700– and IRdye-800–conjugated goat anti-mouse and goat anti-rabbit secondary antibodies were from Rockland Immunochemicals (Gilbertsville, PA) and were used with the Odyssey system (LI-COR, Lincoln, NE) for Western blot analysis. Mouse anti-GFP, mouse anti-SNX27, rabbit anti-β2AR, and rabbit anti-Glut-1 were from Abcam (Cambridge, MA)
Plasmid pcDNA3.1-HA-NHE3 was constructed previously (Murtazina et al., 2006). To study the role of the C-terminal-end putative class I PDZ binding motif (829-STHM) of NHE3, we made HA-NHE3∆C4 in pcDNA3.1/Hygro+vector with HindIII/XbaI cloning site. HA-NHE3∆C4 (HA-NHE3 with deleted last four amino acids 829-STHM) was made by PCR using sense primer ATAAGCTTGATGTCAGGGCGCGGGGG and antisense primer GCTCTAGATCACTCGGGGTGTTCAGCGCC. Anti-NHE3 polyclonal antibodies were raised previously against a synthetic peptide corresponding to amino acids 809–831 (NH2-DSFLQADGHEEQLQPAAPESTHM-COOH) of the COOH terminus of rabbit NHE3 (Kim et al., 1999; Murtazina et al., 2006).
Cell culture and construction of SNX27-knockdown cells
Rat SNX27b (SNX27b) cDNA was kindly provided by Jae Cheng (Johns Hopkins University, Baltimore, MD). The SNX27b PDZ (GYGF → GAGA) point mutation was generated using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) following the manufacturer's instructions using primers 5′-AAGTCCGAATCCGGCGCCGGCGCCAACGTGCGGGGCCAA-3′ and 5′-TTGGCCCCGCACGTTGGCGCCGGCGCCGGATTCGGACTT -3′.
cDNAs of human GFP-Rab4, Rab5 and Rab11 were kindly provided by James Goldenring (Vanderbilt University School of Medicine, Nashville, TN). Human embryonic kidney 293 cells and human intestinal polarized epithelial cell lines Caco-2BBe and SK-CO15 (kindly provided by Asma Nusrat, Emory University, School of Medicine, Atlanta, GA) were used for these studies. HEK293T cells were used to generate HA-NHE3 stably expressing cells by transient transfection of pcDNA3.1-HA-NHE3 with G418 selection. Stable HEK293-HA-NHE3 cells were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS), penicillin (50 mU/ml), streptomycin (50 μg/ml), and G418 (1000 μg/ml) at 37°C with 5% CO2 and 95% humidity. SK-CO15 cells were grown on membranes (Transwells or filterslips; Corning, Corning, NY; Zachos et al., 2009) and maintained in DMEM supplemented with 10% FBS, penicillin (50 mU/ml), streptomycin (50 μg/ml), 1 mM sodium pyruvate, 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 1× nonessential amino acids. Stable SNX27 knockdown (SNX27-KD) cells (SK-CO15 and HEK-293) were generated using lenti-shRNA constructs. In brief, gene sequence–specific shRNA clones were constructed within the lentivirus plasmid vector pLKO.1-puromycin (Sigma-Aldrich). Three constructs were tested to generate lentiviral transduction particles:
- A:
CCGGGTGTGTTCAATACGAGTAATTCTCGAGAATTACTCGTATTGAACACACTTTTT
- B:
CCGGCACGCCATATTTCAATTACATCTCGAGATGTAATTGAAATATGGCGTGTTTTT
- C:
CCGGCAACGGTTACAGTCAGGGTTACTCGAGTAACCCTGACTGTAACCGTTGTTTTT
The production of lentiviral particle and lentiviral transduction was done as described previously (Zachos et al., 2009; Sarker et al., 2011; Yang et al., 2014). Stable cell lines of SK-CO15 and HEK293 with expression of SNX27 knocked down were generated by infecting cells with the lenti-shRNA particles, and selection was achieved by inclusion of 2 and 1 μg/ml puromycin for SK-CO15 and HEK-293-HA-NHE3 cells, respectively, in the culture medium. Knockdown of protein expression was verified by Western blot analysis. SNX27 shRNA construct C was ineffective in reducing endogenous SNX27 expression in SK-CO15 cells, whereas constructs A and B knocked down endogenous SNX27 expression in SK-CO15 cells by ∼80% and ∼50%, respectively. Construct A was used to knock down SNX27 in SK-CO15 and HEK-293-HA-NHE3 cells. As a transduction control, cells were transduced with a lentivirus plasmid vector containing shRNA that does not match any known human gene (Sigma-Aldrich). Infected cells were maintained under selection pressure of puromycin.
Expression of recombinant proteins in bacteria and purification
cDNAs encoding rat SNX27b were generated using the following primer pairs:
- SNX27-FL (aa 1–539):
5′-CGCAAGGGATCCATGGCGGACGAGGACGGG-3′ and 5′-GAAAGGGAATTCCTAGGTGGCCACATCCCT-3′
- PDZ (aa 1–156):
-
5′-CGCAAGGGATCCATGGCGGACGAGGACGGG-3′ and
5′-CTTTTCGAATTCCTATGTGTAATCATAAAA-3′
- FERM (aa 272–539):
-
5′-GTGTCTGGATCCGATGTGGAGCTGAGA-3′ and
5′-GAAAGGGAATTCCTAGGTGGCCACATCCCT-3′
- PDZ+PX (aa 1–266):
5′-CGCAAGGGATCCATGGCGGACGAGGACGGG-3′ and
- /PDZ-GAGA+PX:
5′-ATTGTAGAATTCCTAATTCTCATGAGATTCTGA-3′
- PX+FERM (aa 158–539):
-
5′-TACACAGGATTCGAAAAGCAAGCAAGT-3′ and
5′-GAAAGGGAATTCCTAGGTGGCCACATCCCT-3′
These constructs were cloned into the BamHI-EcoRI site of pGEX-4T-1vector.
NHE3 recombinant protein expression and purification
cDNA fragments encoding rabbit NHE3 carboxyl-terminus fragment 642C (amino acids P642–M832) were amplified by PCR and inserted into the previously constructed vector pMBP (Yang et al., 2014) between BamHI and XhoI.
Expression constructs were transformed into BL21(DE3) strain (EMD Millipore, Billerica, MA). When the bacterial culture reached an OD of ∼0.8, protein expression was induced with 0.3 mM isopropyl-β-d-thiogalactoside at 16°C overnight. MBP-tagged proteins were purified with a column packed with amylose resin by gravity flow following the New England Biolabs manuals of the pMAL system. GST-tagged protein was purified in a gravity-flow column following the instructions from GE Healthcare. Purified proteins were concentrated by Amicon Ultra-15 Centrifugal filter units (EMD Millipore), supplemented with 10% glycerol and 10 mM dithiothreitol, and stored at −80oC.
Coimmunoprecipitation
Coimmunoprecipitation experiments were performed using lysates from Caco-2BBe cells infected with HA-NHE3 adenovirus (Sarker et al., 2011). Cell lysates were prepared in lysis buffer (60 mM HEPES, pH 7.4, 150 mM NaCl, 3 mM KCl, 5 mM EDTA trisodium, 3 mM lysis buffer ethylene glycol tetraacetic acid, 1 mM Na3VO4, and 1% Triton X-100 with protease inhibitor cocktail; Sigma-Aldrich). Anti-HA affinity matrix was washed with this buffer three times. Aliquots (2 mg of protein) of lysate were incubated with 15 μl of monoclonal anti-HA affinity matrix at 4°C for 4 h on a rotating shaker. Beads were washed five times with the same buffer and eluted with 2.5× Laemmli buffer. The input and eluted samples were separated by SDS–PAGE and transferred to nitrocellulose membranes. The immunoblots were probed with polyclonal anti-HA or monoclonal anti-SNX27 primary antibodies, followed by fluorescently labeled secondary antibody (IRDye 800 or Alexa Fluor 680) according to the manufacturer's protocol, and bands were visualized by the Odyssey system.
GSH-resin pull down
For interaction studies, 1 nmol of recombinant GST-tagged protein was used as bait for pull downs. As prey, 1 nmol of purified MBP-tagged recombinant protein or 1 mg of cell lysate was used as indicated. The volume of the final mixture was adjusted to 500 μl with the lysis buffer (25 mM HEPES, pH 7.4, 150 mM NaCl, 50 mM NaF, 1 mM Na3VO4, 0.5% Triton X-100, and protease inhibitors). GSH-resin (Glutathione Sepharose 4B resin) was washed with lysis buffer three times. Each bait–prey mixture was mixed with 10 μl of resin and incubated at 4°C for 3 h on a rotating shaker. Resin was washed with the same lysis buffer four times and then eluted with lysis buffer supplemented with 10 mM glutathione. The input and elution samples were analyzed by SDS–PAGE and Western blotting.
Immunofluorescence
SK-CO15 cells were grown on Transwell filters (Corning) until 6–7 d postconfluence. Triple-HA-tagged rabbit NHE3 in replication-deficient adenovirus (Sarker et al., 2011) was transiently infected into SK-CO15 cells for endocytosis-based colocalization analysis. Transiently transfected SK-CO15 cells were incubated with 5 μg/ml HA or GFP antibody with 10% FBS for 1 h at 4°C. After binding, cells were incubated for 1 h at 37°C. The cells were then washed in ice-cold phosphate-buffered saline (PBS), and surface-bound antibody was stripped from the cells with cold PBS, pH 2.5, for 2 × 1 min. After the acid rinse, cells were washed once in PBS and fixed in cold 4% paraformaldehyde for 20 min, blocked with 1% bovine serum albumin (BSA) in PBS for 10 min, and incubated with anti-mouse Alexa Fluor 594– and anti-rabbit Alexa Fluor 488–conjugated secondary antibodies (1:100) in 0.1% saponin (Sigma-Aldrich) in PBS with Hoechst dye for 1 h at room temperature. Cells were washed three times with PBS and mounted with Gel Mount (Sigma-Aldrich) and then examined with a Zeiss LSM510 confocal fluorescence microscope. Results were from six to eight individual experiments.
HEK-293-HA-NHE3 control or SNX27-KD cells were grown on tissue culture coverslips to ∼60% confluence. These cells were then transfected with GFP-Rab4, Rab5, Rab11, SNX27wt, or SNX27-GAGA (PDZ mutant) using Lipofectamine 2000 (Life Technologies). At 1 d posttransfection, cells were fixed with 3% paraformaldehyde/PBS for 20 min at 4°C, and the residual formaldehyde was neutralized with 20 mM glycine in PBS for 10 min. Cells were then permeabilized for 30 min in 0.1% saponin/PBS before being blocked for 30 min in 1% BSA/PBS supplemented with 10% FBS. Cells were incubated with respective primary antibody in 1% BSA/PBS for 60 min at room temperature. After three 10-min washes in 0.1% saponin/PBS, goat secondary antibodies (Alexa conjugates; Invitrogen) were added at 1:100 dilutions in 1% BSA/PBS, incubated for 30 min, and again washed three times for 10 min in 0.1% saponin/PBS. Cells were then mounted with Gel Mount and viewed on a Zeiss LSM510 confocal fluorescence microscope.
Measurement of Na+/H+ exchange activity
Cellular Na+/H+ exchange activity in HEK293-HA-NHE3 or SNX27-KD cells grown to ∼70% confluence on glass coverslips and postconfluent SK-CO15 cells grown on Transwell filters was determined fluorometrically using the intracellular pH-sensitive dye 2′,7′-bis(carboxyethyl)5-6-carboxyfluorescein-acetoxymethyl ester (BCECF-AM, 5 μM) as described previously (Levine et al., 1995). HEK-293 cells were exposed to 40 mM NH4Cl for 15-min dye loading, and SK-CO15 cells were exposed to 50 mM NH4Cl for the final 10 min of the 50-min dye loading period. Endogenous NHE1 in HEK-293 cells and NHE1 and NHE2 activity in SK-CO15 cells were inhibited by 10 or 50 μM HOE694, respectively, present in all solutions. At the end of each experiment, the fluorescence ratio was converted to pHi using the high–potassium/nigericin method (Levine et al., 1993). The initial rate (approximately the first minute) of NHE3 activity was quantitated and expressed as change in pHi/minute (Watson et al., 1991; Janecki et al., 1998) Mean ± SE was determined from four separate experiments.
Cell surface biotinylation and immunoblotting
Postconfluent (6–7 d) control and SNX27-KD SK-CO15 cells were grown on 10-cm-diameter filters (Corning). The cells were then serum starved for ∼3 h. All subsequent manipulations were performed at 4°C. For surface labeling of NHE3, cells were incubated with 1.5 mg/ml NHS-SS-biotin (biotinylation solution; Pierce Chemical, Rockford, IL) for 20 min and repeated once, solubilized with lysis buffer, and then incubated for 4 h with streptavidin-agarose beads. Western analysis and the quantification of the surface fraction were performed as described previously (Akhter et al., 2002; Singh et al., 2014a).
Endocytosis internalization and exocytic insertion assays
Endocytosis was measured by a protocol slightly modified from the reduced glutathione (GSH)–resistant endocytosis assay described previously by us (Lin et al., 2011b). Briefly, 6 d postconfluence, polarized SK-CO15 control and SNX27-knockdown cells were serum starved for 3 h and labeled with 1.5 mg/ml sulfo-NHS-SS biotin for 30 min at 4°C (this was repeated once), and then nonbound biotin was quenched as described. Cells were rinsed with PBS at 37°C and then incubated in serum-free medium at 37°C for 0, 5, 10, 15, or 20 min. Cells were rinsed with ice-cold PBS twice. Surface biotin was cleaved with GSH-containing buffer (150 mM GSH, 150 mM NaCl, and 50 mM Tris-HCl, pH 8.8) for 5 min. The biotinylated NHE3 that was endocytosed was protected from cleavage by GSH. Cells were then solubilized in lysis buffer, biotinylated proteins were retrieved with streptavidin beads, and internalized NHE3 was assayed as described via Western analysis and normalized to the surface NHE3 initially present.
To measure exocytic insertion of NHE3 in basal conditions, confluent serum-starved SK-CO15 control and SNX27-knockdown cells were rinsed with PBS-Ca-Mg two times at 4°C. Surface proteins accessible to NHS-SS-biotin were masked by reaction with membrane-impermeant NHS-acetate (1.5 mg/ml) at 4°C for a total time of 2 h, adding fresh NHS-acetate every 40 min. Then cells were incubated with quenching buffer (100 mM glycine in PBS-Ca-Mg) twice with gentle shaking for a total time of 20 min at 4°C. After quenching, cells were incubated with prewarmed serum-free medium for 5, 10, 20, and 30 min at 37°C. One set of cells treated with sulfo-NHS-acetate at 4°C but never warmed to 37°C served as the zero time point. Cells were then labeled with sulfo-NHS-SS-biotin (1.5 mg/ml) and treated with lysis buffer as described. The biotinylated fraction was precipitated with avidin-agarose beads. The resultant precipitate was subjected to SDS–PAGE, and biotinylated NHE3 was detected by quantitative Western blot analysis as described. Biotinylated protein was normalized to the total amount of NHE3 present in the lysate at each time point and expressed as percentage exocytosed.
Measurement of half-life of plasma membrane NHE3 using cell surface biotinylation
To determine the half-life of plasma membrane NHE3 in wild-type and SNX27-KD cells, a cell surface biotinylation method was used, as described previously (Cavet et al., 2001). The purpose of this experiment was to determine the stability of NHE3 on the cell surface in the presence and absence of SNX27.
Total NHE3 degradation assay
Postconfluent control and SNX27-KD SK-CO15 cells were grown on Transwell filters and treated with 100 μM cycloheximide for the indicated time points. Cells were lysed in PBS with 1% (vol/vol) Triton X-100, and NHE3 levels were determined by quantitative Western blotting. β-Actin fluorescence intensity was used to normalize the detected levels of NHE3. The untreated controls were set to 100%, and the level of detected NHE3 was calculated as the percentage of untreated control for each time point.
Statistical analysis
Experiments were repeated at least four times. Results are presented as means ± SE. Comparisons were performed by unpaired Student's t tests or analysis of variance for multiple comparisons.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01DK26523, R01DK61765, and P01DK072084 and National Institute of Diabetes and Digestive and Kidney Diseases Grant P30DK089502 to the Conte Hopkins Digestive Diseases Basic and Translational Research Core Center.
Glossary
Abbreviations used:
- NHE3
Na+/H+ exchanger 3
- NHERF
Na+/H+ exchange regulatory cofactor
- PDZ
postsynaptic density protein 95/discs large/zona occludens 1
- SNX27
sorting nexin 27.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-12-1597) on April 7, 2015.
REFERENCES
- Akhter S, Kovbasnjuk O, Li X, Cavet M, Noel J, Arpin M, Hubbard AL, Donowitz M. Na(+)/H(+) exchanger 3 is in large complexes in the center of the apical surface of proximal tubule-derived OK cells. Am J Physiol Cell Physiol. 2002;283:C927–940. doi: 10.1152/ajpcell.00613.2001. [DOI] [PubMed] [Google Scholar]
- Alexander RT, Furuya W, Szaszi K, Orlowski J, Grinstein S. Rho GTPases dictate the mobility of the Na/H exchanger NHE3 in epithelia: role in apical retention and targeting. Proc Natl Acad Sci USA. 2005;102:12253–12258. doi: 10.1073/pnas.0409197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RT, Grinstein S. Na+/H+ exchangers and the regulation of volume. Acta Physiol (Oxf) 2006;187:159–167. doi: 10.1111/j.1748-1716.2006.01558.x. [DOI] [PubMed] [Google Scholar]
- Alexander RT, Malevanets A, Durkan AM, Kocinsky HS, Aronson PS, Orlowski J, Grinstein S. Membrane curvature alters the activation kinetics of the epithelial Na+/H +exchanger, NHE3. J Biol Chem. 2007;282:7376–7384. doi: 10.1074/jbc.M608557200. [DOI] [PubMed] [Google Scholar]
- Bobulescu IA, Dwarakanath V, Zou L, Zhang J, Baum M, Moe OW. Glucocorticoids acutely increase cell surface Na+/H +exchanger-3 (NHE3) by activation of NHE3 exocytosis. Am J Physiol Renal Physiol. 2005;289:F685–691. doi: 10.1152/ajprenal.00447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Brone B, Eggermont J. PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes. Am J Physiol Cell Physiol. 2005;288:C20–C29. doi: 10.1152/ajpcell.00368.2004. [DOI] [PubMed] [Google Scholar]
- Cai L, Loo LS, Atlashkin V, Hanson BJ, Hong W. Deficiency of sorting nexin 27 (SNX27) leads to growth retardation and elevated levels of N-methyl-D-aspartate receptor 2C (NR2C) Mol Cell Biol. 2011;31:1734–1747. doi: 10.1128/MCB.01044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Rutherford A, Cullen P. Sorting nexins—unifying trends and new perspectives. Traffic. 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- Carlton JG, Cullen PJ. Sorting nexins. Curr Biol. 2005;15:R819–R820. doi: 10.1016/j.cub.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Cavet ME, Akhter S, Murtazina R, Sanchez de Medina F, Tse CM, Donowitz M. Half-lives of plasma membrane Na(+)/H(+) exchangers NHE1–3: plasma membrane NHE2 has a rapid rate of degradation. Am J Physiol Cell Physiol. 2001;281:C2039–C2048. doi: 10.1152/ajpcell.2001.281.6.C2039. [DOI] [PubMed] [Google Scholar]
- Cha B, Kenworthy A, Murtazina R, Donowitz M. The lateral mobility of NHE3 on the apical membrane of renal epithelial OK cells is limited by the PDZ domain proteins NHERF1/2, but is dependent on an intact actin cytoskeleton as determined by FRAP. J Cell Sci. 2004;117:3353–3365. doi: 10.1242/jcs.01180. [DOI] [PubMed] [Google Scholar]
- Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, et al. cGMP inhibition of Na+/H +antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem. 2005;280:16642–16650. doi: 10.1074/jbc.M500505200. [DOI] [PubMed] [Google Scholar]
- Cha B, Tse M, Yun C, Kovbasnjuk O, Mohan S, Hubbard A, Arpin M, Donowitz M. The NHE3 juxtamembrane cytoplasmic domain directly binds ezrin: dual role in NHE3 trafficking and mobility in the brush border. Mol Biol Cell. 2006;17:2661–2673. doi: 10.1091/mbc.E05-09-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha B, Zhu XC, Chen W, Jones M, Ryoo S, Zachos NC, Chen TE, Lin R, Sarker R, Kenworthy AK, et al. NHE3 mobility in brush borders increases upon NHERF2-dependent stimulation by lyophosphatidic acid. J Cell Sci. 2010;123:2434–2443. doi: 10.1242/jcs.056713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Moyer BD, Milewski M, Loffing J, Ikeda M, Mickle JE, Cutting GR, Li M, Stanton BA, Guggino WB. A Golgi-associated PDZ domain protein modulates cystic fibrosis transmembrane regulator plasma membrane expression. J Biol Chem. 2002;277:3520–3529. doi: 10.1074/jbc.M110177200. [DOI] [PubMed] [Google Scholar]
- Cheng J, Wang H, Guggino WB. Modulation of mature cystic fibrosis transmembrane regulator protein by the PDZ domain protein CAL. J Biol Chem. 2004;279:1892–1898. doi: 10.1074/jbc.M308640200. [DOI] [PubMed] [Google Scholar]
- Cinar A, Chen M, Riederer B, Bachmann O, Wiemann M, Manns M, Kocher O, Seidler U. NHE3 inhibition by cAMP and Ca2+ is abolished in PDZ-domain protein PDZK1-deficient murine enterocytes. J Physiol. 2007;581:1235–1246. doi: 10.1113/jphysiol.2007.131722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- Cushing PR, Fellows A, Villone D, Boisguerin P, Madden DR. The relative binding affinities of PDZ partners for CFTR: a biochemical basis for efficient endocytic recycling. Biochemistry. 2008;47:10084–10098. doi: 10.1021/bi8003928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'souza S, Garcia-Cabado A, Yu F, Teter K, Lukacs G, Skorecki K, Moore HP, Orlowski J, Grinstein S. The epithelial sodium-hydrogen antiporter Na+/H +exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem. 1998;273:2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. NHERF family and NHE3 regulation. J Physiol. 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M, Mohan S, Zhu CX, Chen TE, Lin R, Cha B, Zachos NC, Murtazina R, Sarker R, Li X. NHE3 regulatory complexes. J Exp Biol. 2009;212:1638–1646. doi: 10.1242/jeb.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Kim KH, Jo MJ, Lee JH, Yang J, Doctor RB, Moe OW, Lee J, Kim E, Lee MG. Shank2 associates with and regulates Na+/H +exchanger 3. J Biol Chem. 2006;281:1461–1469. doi: 10.1074/jbc.M509786200. [DOI] [PubMed] [Google Scholar]
- Hu MC, Fan L, Crowder LA, Karim-Jimenez Z, Murer H, Moe OW. Dopamine acutely stimulates Na+/H +exchanger (NHE3) endocytosis via clathrin-coated vesicles: dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem. 2001;276:26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- Hussain NK, Diering GH, Sole J, Anggono V, Huganir RL. Sorting Nexin 27 regulates basal and activity-dependent trafficking of AMPARs. Proc Natl Acad Sci USA. 2014;111:11840–11845. doi: 10.1073/pnas.1412415111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecki AJ, Janecki M, Akhter S, Donowitz M. Basic fibroblast growth factor stimulates surface expression and activity of Na(+)/H(+) exchanger NHE3 via mechanism involving phosphatidylinositol 3-kinase. J Biol Chem. 2000a;275:8133–8142. doi: 10.1074/jbc.275.11.8133. [DOI] [PubMed] [Google Scholar]
- Janecki AJ, Janecki M, Akhter S, Donowitz M. Quantitation of plasma membrane expression of a fusion protein of Na/H exchanger NHE3 and green fluorescence protein (GFP) in living PS120 fibroblasts. J Histochem Cytochem. 2000b;48:1479–1492. doi: 10.1177/002215540004801105. [DOI] [PubMed] [Google Scholar]
- Janecki AJ, Montrose MH, Zimniak P, Zweibaum A, Tse CM, Khurana S, Donowitz M. Subcellular redistribution is involved in acute regulation of the brush border Na+/H +exchanger isoform 3 in human colon adenocarcinoma cell line Caco-2. Protein kinase C-mediated inhibition of the exchanger. J Biol Chem. 1998;273:8790–8798. doi: 10.1074/jbc.273.15.8790. [DOI] [PubMed] [Google Scholar]
- Jemth P, Gianni S. PDZ domains: folding and binding. Biochemistry. 2007;46:8701–8708. doi: 10.1021/bi7008618. [DOI] [PubMed] [Google Scholar]
- Joubert L, Hanson B, Barthet G, Sebben M, Claeysen S, Hong W, Marin P, Dumuis A, Bockaert J. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci. 2004;117:5367–5379. doi: 10.1242/jcs.01379. [DOI] [PubMed] [Google Scholar]
- Karthikeyan S, Leung T, Ladias JA. Structural determinants of the Na+/H +exchanger regulatory factor interaction with the beta 2 adrenergic and platelet-derived growth factor receptors. J Biol Chem. 2002;277:18973–18978. doi: 10.1074/jbc.M201507200. [DOI] [PubMed] [Google Scholar]
- Kim GH, Ecelbarger C, Knepper MA, Packer RK. Regulation of thick ascending limb ion transporter abundance in response to altered acid/base intake. J Am Soc Nephrol. 1999;10:935–942. doi: 10.1681/ASN.V105935. [DOI] [PubMed] [Google Scholar]
- Kurashima K, Szabo EZ, Lukacs G, Orlowski J, Grinstein S. Endosomal recycling of the Na+/H +exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. J Biol Chem. 1998;273:20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- Lahuna O, Quellari M, Achard C, Nola S, Meduri G, Navarro C, Vitale N, Borg JP, Misrahi M. Thyrotropin receptor trafficking relies on the hScrib-betaPIX-GIT1-ARF6 pathway. EMBO J. 2005;24:1364–1374. doi: 10.1038/sj.emboj.7600616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer BE, Melero C, Temkin P, Lei C, Hong W, Kortemme T, von Zastrow M. SNX27 mediates PDZ-directed sorting from endosomes to the plasma membrane. J Cell Biol. 2010;190:565–574. doi: 10.1083/jcb.201004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Kwon W, Kim JH, Choi JW, Kawano K, Cha B, Dartt DA, Zoukhri D, Donowitz M. Ca2+-dependent inhibition of NHE3 requires PKC alpha which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol. 2003;285:C1527–1536. doi: 10.1152/ajpcell.00017.2003. [DOI] [PubMed] [Google Scholar]
- Levine SA, Montrose MH, Tse CM, Donowitz M. Kinetics and regulation of three cloned mammalian Na+/H +exchangers stably expressed in a fibroblast cell line. J Biol Chem. 1993;268:25527–25535. [PubMed] [Google Scholar]
- Levine SA, Nath SK, Yun CH, Yip JW, Montrose M, Donowitz M, Tse CM. Separate C-terminal domains of the epithelial specific brush border Na+/H +exchanger isoform NHE3 are involved in stimulation and inhibition by protein kinases/growth factors. J Biol Chem. 1995;270:13716–13725. doi: 10.1074/jbc.270.23.13716. [DOI] [PubMed] [Google Scholar]
- Li X, Galli T, Leu S, Wade JB, Weinman EJ, Leung G, Cheong A, Louvard D, Donowitz M. Na+-H +exchanger 3 (NHE3) is present in lipid rafts in the rabbit ileal brush border: a role for rafts in trafficking and rapid stimulation of NHE3. J Physiol. 2001;537:537–552. doi: 10.1111/j.1469-7793.2001.00537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Murtazina R, Cha B, Chakraborty M, Sarker R, Chen TE, Lin Z, Hogema BM, de Jonge HR, Seidler U, et al. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H +exchange regulatory factor 2-dependent process. Gastroenterology. 2011a;140:560–571. doi: 10.1053/j.gastro.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Jin S, Duan X, Wang T, Martini S, Hulamm P, Cha B, Hubbard A, Donowitz M, Guggino SE. Chloride channel (Clc)-5 is necessary for exocytic trafficking of Na+/H +exchanger 3 (NHE3) J Biol Chem. 2011b;286:22833–22845. doi: 10.1074/jbc.M111.224998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo LS, Tang N, Al-Haddawi M, Dawe GS, Hong W. A role for sorting nexin 27 in AMPA receptor trafficking. Nat Commun. 2014;5:3176. doi: 10.1038/ncomms4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ML, Nassirpour R, Arrabit C, Tan J, McLeod I, Arias CM, Sawchenko PE, Yates JR, 3rd, Slesinger PA. A unique sorting nexin regulates trafficking of potassium channels via a PDZ domain interaction. Nat Neurosci. 2007;10:1249–1259. doi: 10.1038/nn1953. [DOI] [PubMed] [Google Scholar]
- Mohrmann K, van der Sluijs P. Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol Membr Biol. 1999;16:81–87. doi: 10.1080/096876899294797. [DOI] [PubMed] [Google Scholar]
- Munoz MB, Slesinger PA. Sorting nexin 27 regulation of G protein-gated inwardly rectifying K(+) channels attenuates in vivo cocaine response. Neuron. 2014;82:659–669. doi: 10.1016/j.neuron.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtazina R, Kovbasnjuk O, Chen TE, Zachos NC, Chen Y, Kocinsky HS, Hogema BM, Seidler U, de Jonge HR, Donowitz M. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol. 2011;301:C126–C136. doi: 10.1152/ajpcell.00311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtazina R, Kovbasnjuk O, Donowitz M, Li X. Na+/H +exchanger NHE3 activity and trafficking are lipid Raft-dependent. J Biol Chem. 2006;281:17845–17855. doi: 10.1074/jbc.M601740200. [DOI] [PubMed] [Google Scholar]
- Murtazina R, Kovbasnjuk O, Zachos NC, Li X, Chen Y, Hubbard A, Hogema BM, Steplock D, Seidler U, Hoque KM, et al. Tissue-specific regulation of sodium/proton exchanger isoform 3 activity in Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) null mice. cAMP inhibition is differentially dependent on NHERF1 and exchange protein directly activated by cAMP in ileum versus proximal tubule. J Biol Chem. 2007;282:25141–25151. doi: 10.1074/jbc.M701910200. [DOI] [PubMed] [Google Scholar]
- Peng Y, Amemiya M, Yang X, Fan L, Moe OW, Yin H, Preisig PA, Yanagisawa M, Alpern RJ. ET(B) receptor activation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Renal Physiol. 2001;280:F34–F42. doi: 10.1152/ajprenal.2001.280.1.F34. [DOI] [PubMed] [Google Scholar]
- Rincon E, Santos T, Avila-Flores A, Albar JP, Lalioti V, Lei C, Hong W, Merida I. Proteomics identification of sorting nexin 27 as a diacylglycerol kinase zeta-associated protein: new diacylglycerol kinase roles in endocytic recycling. Mol Cell Proteomics. 2007;6:1073–1087. doi: 10.1074/mcp.M700047-MCP200. [DOI] [PubMed] [Google Scholar]
- Sarker R, Valkhoff VE, Zachos NC, Lin R, Cha B, Chen TE, Guggino S, Zizak M, de Jonge H, Hogema B, Donowitz M. NHERF1 and NHERF2 are necessary for multiple but usually separate aspects of basal and acute regulation of NHE3 activity. Am J Physiol Cell Physiol. 2011;300:C771–C782. doi: 10.1152/ajpcell.00119.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seet LF, Hong W. The Phox (PX) domain proteins and membrane traffic. Biochim Biophys Acta. 2006;1761:878–896. doi: 10.1016/j.bbalip.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Singh V, Lin R, Yang J, Cha B, Sarker R, Tse CM, Donowitz M. AKT and GSK-3 are necessary for direct ezrin binding to NHE3 as part of a C-terminal stimulatory complex: role of a novel ser-rich nhe3 c-terminal motif in nhe3 activity and trafficking. J Biol Chem. 2014a;289:5449–5461. doi: 10.1074/jbc.M113.521336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Yang J, Chen TE, Zachos NC, Kovbasnjuk O, Verkman AS, Donowitz M. Translating molecular physiology of intestinal transport into pharmacologic treatment of diarrhea: stimulation of Na+ absorption. Clin Gastroenterol Hepatol. 2014b;12:27–31. doi: 10.1016/j.cgh.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavare JM, Cullen PJ. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol. 2013;15:461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, Milewski M, Cutting GR, Guggino WB, Langford G, Stanton BA. PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:40099–40105. doi: 10.1074/jbc.M206964200. [DOI] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jager S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin WR, Hodson CA, Milgram SL. Beyond the brush border: NHERF4 blazes new NHERF turf. J Physiol. 2005;567:13–19. doi: 10.1113/jphysiol.2005.091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes JL, Tang J, McDermott MI, Kuo JC, Zimmerman SP, Wincovitch SM, Waterman CM, Milgram SL, Playford MP. Sorting nexin 27 protein regulates trafficking of a p21-activated kinase (PAK) interacting exchange factor (beta-Pix)-G protein-coupled receptor kinase interacting protein (GIT) complex via a PDZ domain interaction. J Biol Chem. 2011;286:39403–39416. doi: 10.1074/jbc.M111.260802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weering JR, Verkade P, Cullen PJ. SNX-BAR proteins in phosphoinositide-mediated, tubular-based endosomal sorting. Semin Cell Dev Biol. 2010;21:371–380. doi: 10.1016/j.semcdb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao Y, Zhang X, Badie H, Zhou Y, Mu Y, Loo LS, Cai L, Thompson RC, Yang B, et al. Loss of sorting nexin 27 contributes to excitatory synaptic dysfunction by modulating glutamate receptor recycling in Down's syndrome. Nat Med. 2013;19:473–480. doi: 10.1038/nm.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AJ, Levine S, Donowitz M, Montrose MH. Kinetics and regulation of a polarized Na(+)-H+ exchanger from Caco-2 cells, a human intestinal cell line. Am J Physiol. 1991;261:G229–G238. doi: 10.1152/ajpgi.1991.261.2.G229. [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Wang Y, Wang F, Greer C, Steplock D, Shenolikar S. A C-terminal PDZ motif in NHE3 binds NHERF-1 and enhances cAMP inhibition of sodium-hydrogen exchange. Biochemistry. 2003;42:12662–12668. doi: 10.1021/bi035244l. [DOI] [PubMed] [Google Scholar]
- Wente W, Stroh T, Beaudet A, Richter D, Kreienkamp HJ. Interactions with PDZ domain proteins PIST/GOPC and PDZK1 regulate intracellular sorting of the somatostatin receptor subtype 5. J Biol Chem. 2005;280:32419–32425. doi: 10.1074/jbc.M507198200. [DOI] [PubMed] [Google Scholar]
- Yang J, Singh V, Cha B, Chen TE, Sarker R, Murtazina R, Jin S, Zachos NC, Patterson GH, Tse CM, et al. NHERF2 protein mobility rate is determined by a unique C-terminal domain that is also necessary for its regulation of NHE3 protein in OK cells. J Biol Chem. 2013;288:16960–16974. doi: 10.1074/jbc.M113.470799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Singh V, Chen TE, Sarker R, Xiong L, Cha B, Jin S, Li X, Tse CM, Zachos NC, Donowitz M. NHERF2/NHERF3 protein heterodimerization and macrocomplex formation are required for the inhibition of NHE3 activity by carbachol. J Biol Chem. 2014;289:20039–20053. doi: 10.1074/jbc.M114.562413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Amemiya M, Peng Y, Moe OW, Preisig PA, Alpern RJ. Acid incubation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Cell Physiol. 2000;279:C410–419. doi: 10.1152/ajpcell.2000.279.2.C410. [DOI] [PubMed] [Google Scholar]
- Yoo BK, Yanda MK, No YR, Yun CC. Human intestinal epithelial cell line SK-CO15 is a new model system to study Na(+)/H(+) exchanger 3. Am J Physiol Gastrointest Liver Physiol. 2012;303:G180–G188. doi: 10.1152/ajpgi.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos NC, Li X, Kovbasnjuk O, Hogema B, Sarker R, Lee LJ, Li M, de Jonge H, Donowitz M. NHERF3 (PDZK1) contributes to basal and calcium inhibition of NHE3 activity in Caco-2BBe cells. J Biol Chem. 2009;284:23708–23718. doi: 10.1074/jbc.M109.012641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H +exchange. Annu Rev Physiol. 2005;67:411–443. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]