Abstract
Objective
To evaluate the efficacy and safety of CyberKnife® treatment for locally-advanced pancreatic cancer (LAPC).
Methods
The efficacy of CyberKnife® treatment was analyzed in 59 LAPC patients treated between October 2006 and September 2014. The median tumor volume was 27.1 mL (13.0–125.145 mL). The median prescribed dose was 45 Gy (35–50 Gy), delivered in 5 fractions (3–8 fractions). The overall survival (OS) rates and freedom from local progression (FFLP) rates were estimated using the Kaplan–Meier survival curve.
Results
The median follow-up for all patients was 10.9 months (3.2–48.7 months) and 15.6 months (3.9–37.6 months) among surviving patients. The median OS was 12.5 months, and the 1-year and 2-year survival rates were 53.9% and 35.1%, respectively. The 1-year FFLP rate was 90.8% based on the computed tomography (CT) evaluation. Grade 1–2 acute and late-stage gastrointestinal (GI) reactions were observed in 61% of the patients. One patient experienced grade 3 toxicity.
Conclusion
Excellent clinical efficacy was obtained after treatment of LAPC using CyberKnife®, with minimal toxicity.
Keywords: pancreatic cancer, stereotactic body radiotherapy, CyberKnife®, local control, toxicity
Introduction
Pancreatic cancer is recognized as a malignant tumor with poor prognosis. At present, morbidity remains close to the mortality rate, and the 5-year survival rates for patients at all stages are still approximately 5%.1 Only 20% of patients are suitable for surgical treatment.2 The main reasons for inoperability include locally advanced lesions, distant metastasis, or other concurrent disease. Patients with unresectable pancreatic cancer have a poor prognosis, and effective nonsurgical treatment methods are very limited. Currently, 50.4 Gy of radiotherapy concurrent with chemotherapy based on 5-fluorouracil (5-FU)/gemcitabine has become the standard treatment. Unfortunately, the 5-year survival rate is very low, and local progression is very common. The disappointing results provided by traditional pancreatic cancer treatment methods have driven research attention to new systemic cytotoxic drugs and more aggressive radiotherapy measures.
Stereotactic body radiotherapy (SBRT) is a highly accurate radiotherapy technique. It can deliver a radiotherapy dose precisely to the tumor site over 1–5 fractions, while the surrounding normal tissues are maximally protected.3 However, due to the motion of the abdominal organs during the respiratory cycle, its clinical application is greatly limited. CyberKnife® (Accuracy, Sunnyvale, CA, USA) is a device that can track the motion of tumors and execute real-time location adjustments. It includes three key components: 1) an advanced and deft linear accelerator; 2) a robotic arm that allows manipulation of the accelerator at different directions and angles; and 3) a tumor tracking system. This system uses two connected structures, gold fiducial markers placed in the tumor or the surrounding tissue and an external camera system, to establish a movement model for the chest wall, track tumor movement in the abdomen, and synchronously adjust the movement of the accelerator. Therefore, the CyberKnife® system can achieve submillimeter grade accuracy through real-time tracking of gold fiducial markers in the body combined with a real-time respiratory model and continuous monitoring to correct for tumor movement during treatment.4 From October 2006 to September 2014, a total of 59 patients with unresectable pancreatic cancer were treated with CyberKnife®, and the preliminary results are reported.
Material and methods
Patient population
The data for 59 patients with locally advanced pancreatic cancer (LAPC) who received CyberKnife® treatment between October 2006 and September 2014 were retrospectively analyzed. The inclusion criteria included the following: 1) pancreatic cancer confirmed by biopsy; 2) unresectable lesion confirmed by a multidisciplinary team (MDT); and 3) a life expectancy of at least 12 weeks. Five patients underwent biliary drainage before treatment, and 90% of patients received chemotherapy before or after treatment.
CyberKnife® treatment
Pretreatment staging was conducted on all patients using positron emission tomography–computed tomography (PET–CT) to confirm LAPC without distant metastasis. A fiducial marker (gold marker, 0.8 mm in diameter) was implanted into the tumor guided by B-ultrasound, CT, or endoscopic ultrasonography or during tumor resection. One week later, a CT scan was performed. In the multiplan treatment planning system, the gross tumor volume (GTV) of the tumor was delineated and expanded by 3 mm to form the planning target volume (PTV). Preventive radiation was not administered to adjacent lymph nodes. The CyberKnife® treatment used the Synchrony respiratory motion-tracking system, and patients donned a special Synchrony vest. The average treatment time was approximately 40 minutes.
After treatment, patients received imaging evaluations every 2 months. The local remission rates were classified using the Response Evaluation Criteria In Solid Tumors (RECIST) standard to describe the changes in the treatment areas. The Common Terminology Criteria for Adverse Events (CTCAE) 3.0 were used to evaluate treatment-associated complications.
Statistical analysis
Results were analyzed using the Statistical Package for Social Science (SPSS) 20.0. A Kaplan–Meier curve was used to calculate the overall survival (OS) rates and the freedom from local progression (FFLP) rates. Local progression was defined as any disease progression in the treatment area. OS was calculated starting from the date of receipt of CyberKnife® treatment until the date of the final follow-up or death. A value of P<0.05 was considered statistically significant.
Results
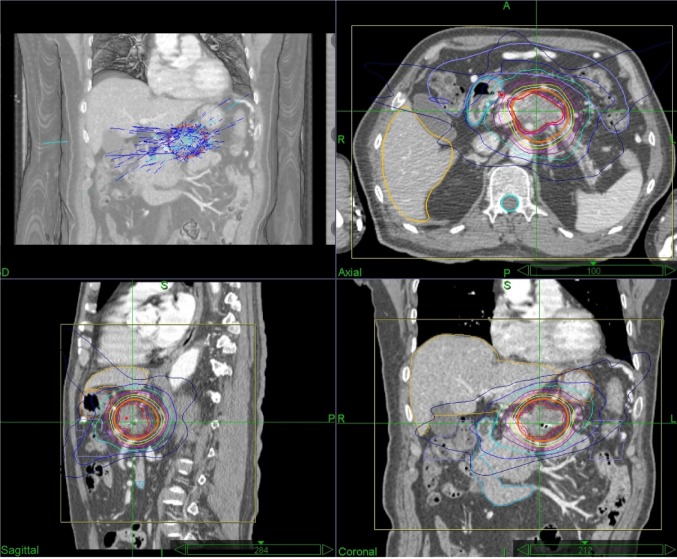
The characteristics of the patients are listed in Table 1. This group of patients included 27 males and 32 females. The median age was 62 years (28–86 years). The median follow-up duration for patients was 10.9 months (3.2–48.7 months) and 15.6 months (3.9–37.6 months) among surviving patients. The median tumor volume was 27.1 mL (13.0–125.1 mL). The median dose was 45 Gy (35–50 Gy), delivered in 5 fractions (3–8 fractions). The median isodose line was 75% (Figure 1).
Table 1.
Clinicopathologic characteristics of 59 patients with locally advanced pancreatic cancer
| Clinical characteristics | Number of cases (%) |
|---|---|
| Sex | |
| Male | 27 (45.8) |
| Female | 32 (54.2) |
| Median age (years) | 62 (28–86) |
| Lesion location | |
| Pancreatic head | 40 (67.8) |
| Pancreatic body and tail | 19 (32.2) |
| Pathological type | |
| Adenocarcinoma | 54 (91.5) |
| Others | 5 (8.5) |
| Fiducial implantation method | |
| Intraoperative | 5 (8.5) |
| B-ultrasound | 31 (52.5) |
| CT | 18 (30.5) |
| Endoscopic ultrasonography | 5 (8.5) |
Abbreviation: CT, computed tomography.
Figure 1.
Representative planning CT and isodose distributions with SBRT for patients with LAPC.
Notes: The representative patient had axial, sagittal, and coronal images taken, and red and purple lines indicate GTV and PTV (GTV +3 mm), respectively. SBRT was performed using five fractions of 45 Gy prescribed to the 75% isodose line.
Abbreviations: LAPC, locally advanced pancreatic cancer; CT, computer tomography; GTV, gross tumor volume; PTV, planning target volume; Gy, Gray; SBRT, stereotactic body radiation therapy.
The median OS was 12.5 months. The 1-year and 2-year survival rates were 53.9% and 35.1%, respectively (Figure 2). The 1-year FFLP was 90.8% based on the computed tomography (CT) evaluation (Figure 3). The median time to local progression was 13.85 months (5–36.5 months). Using the RECIST standard, 8 (13.6%) patients achieved complete remission, 31 (52.5%) patients had partial remission, and 12 (20.3%) patients had stable disease. Eight (13.6%) patients had disease progression. Twenty-eight patients had liver metastases, two patients had lung metastases, and one patient had adrenal metastasis. The median time to metastasis was 9.3 months.
Figure 2.
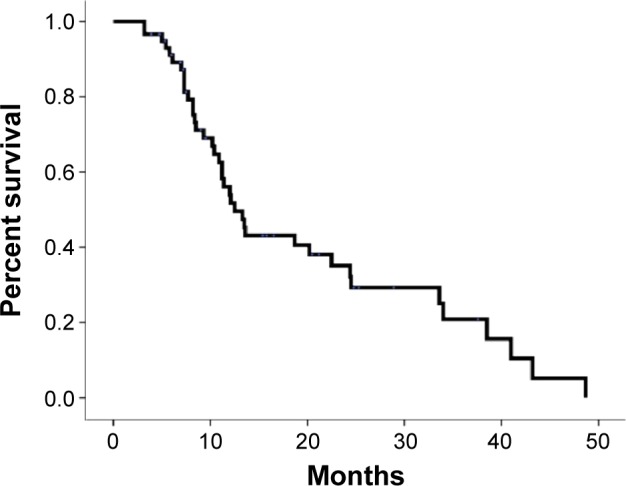
Actuarial OS of patients with LAPC.
Abbreviations: OS, overall survival; LAPC, locally advanced pancreatic carcinoma.
Figure 3.
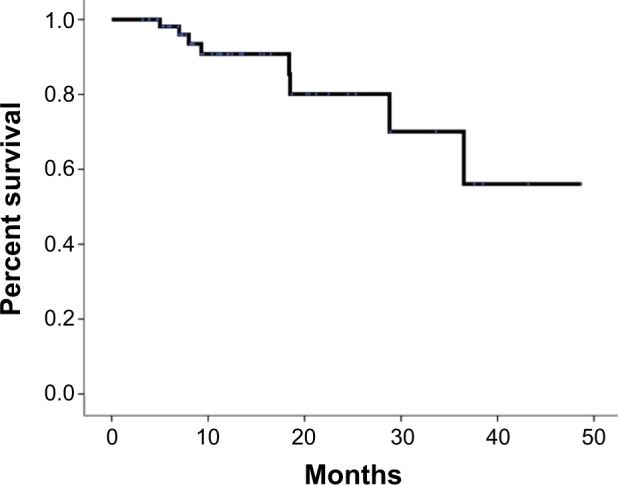
Actuarial FFLP of patients with LAPC.
Abbreviations: FFLP, freedom from local progression; LAPC, locally advanced pancreatic carcinoma.
During the process of placing the gold fiducial markers, no complications were observed. Grade 1–2 acute and late-stage gastrointestinal (GI) reactions occurred in 61% of patients, and the main manifestations were nausea, vomiting, abdominal pain, and diarrhea. One patient had a grade 3 late-stage GI reaction in the form of incomplete intestinal obstruction. There were cases of no grade 4 or above GI toxicity.
Discussion
Although the distant metastasis rate of pancreatic cancer is high, local progression is also an important prognostic factor for pancreatic cancer. Local control is also very important for improving quality of life (such as controlling pain and avoiding GI obstruction). Considering the toxicity to the surrounding normal tissues (such as the intestines, kidney, and liver), traditional conventional fractionated radiotherapy can only administer an approximately 50 Gy radiation dose to primary tumors. Obviously, this dose is not sufficient, and the local failure rate is very high.5 Therefore, more powerful local treatment measures are needed to increase the local control rate of pancreatic cancer.
Koong et al6 first applied CyberKnife® to treat pancreatic cancer. In their Phase I study, the dose escalation to 25 Gy/1 f.6 In the follow-up of six evaluable patients, the local control rate was 100%. More importantly, most patients experienced benefits such as pain relief and weight gain. Schellenberg et al7 reported the results of their Phase II study. Sixteen patients received 25 Gy/1 f CyberKnife® treatment combined with standard-dose gemcitabine chemotherapy before and after treatment. In their study, only three patients had local progression (19%), and the median OS was 11.4 months.7 In our group of patients, the median survival period was 12.5 months, and the 1-year and 2-year survival rates were 53.9% and 35.1%, respectively, which were similar to the results of other studies. More importantly, only eight patients among all of these patients had local progression after treatment, which suggests that the majority of patients could benefit from CyberKnife® treatment. Compared with traditional radiotherapy, the advantages of this technology are: 1) Strengthens the treatment of primary tumors; 2) The accuracy of treatment is further increased, and the incidence of treatment-associated toxicity is decreased; and 3) The treatment time is shortened, thereby decreasing the interference of radiotherapy with systemic chemotherapy.
The major consequences of toxicity during the treatment are GI complications. Especially for pancreatic head carcinoma, the duodenum and the tumors are in close proximity, which makes it difficult to distinguish the tissues when defining the GTV. Hoyer et al8 reported that for their 22 patients using CyberKnife® a dose of 42 Gy/3 f was used. Acute reactions were most prominent on day 14 after treatment; five patients (22%) had severe mucositis, ulceration, and perforation as well as severe pain, nausea, and decline in physical fitness. Analysis of these patients showed that some intestinal tract tissue was included in the treatment area during the delineation of the GTV. In addition, the PTV was defined to expand 5 mm in the horizontal direction and 10 mm in the vertical direction, which might be the reason why there was a high incidence of toxicity leading to a decrease in the OS. We used fluorodeoxyglucose (FDG)-PET–CT fused images to delineate the GTV and applied the Synchrony respiratory motion-tracking system with the implantation of the fiducial marker; therefore, the degree of PTV expansion into normal tissues was minimized. In addition, we used a median dose of 45 Gy/5 f to decrease the radiation dose of each fraction, thus further decreasing the incidence of GI toxicity.
Conclusion
Treatment of LAPC using CyberKnife® is a new approach. Among all evaluable patients, it increases the local control rate of tumors and decreases the delay in systemic treatment. The benefits of local tumor control in pancreatic cancer are pain relief and the decreased risk of gastric or duodenal obstruction. Theoretically, increase in local control can inhibit or delay the occurrence of distant metastasis. Because all patients are at risk for distant metastasis, local treatment must be combined with more effective chemotherapy. With continued in-depth studies on SBRT combined with new types of chemotherapy drugs or targeted therapeutic drugs for pancreatic cancer, OS rates in LAPC patients will continue to improve. However, when CyberKnife® treatment is chosen, appropriate patients should be carefully selected. The treatment plan and the details of the treatment process should be carefully detailed, and the best GTV should be confirmed to the maximum extent necessary, while accuracy of the positioning should be ensured to reduce the incidence of complications.
Acknowledgments
The authors thank AJE for its linguistic assistance during the preparation of this manuscript.
Footnotes
Disclosure
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this paper. The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007;30:637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 4.Nuyttens JJ, van de Pol M. The CyberKnife radiosurgery system for lung cancer. Expert Rev Med Devices. 2012;9:465–475. doi: 10.1586/erd.12.35. [DOI] [PubMed] [Google Scholar]
- 5.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 6.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery inpatients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Schellenberg D, Kim J, Christman-Skieller C, et al. Single fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]