Abstract
Addition of N-acetylgalactosamine to threonine and serine is the first step in the synthesis of O-glycosidically linked oligosaccharides. A UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase (EC 2.4.1.41) from porcine submaxillary glands was recently purified to electrophoretic homogeneity, and polyclonal antibodies against the purified transferase were raised. Immunoblots of porcine, bovine, and ovine submaxillary gland extracts with the anti-transferase antibodies gave a single band and the antibodies reacted equally well with the purified glycosylated and N-glycanase-treated transferase. Immunoelectron microscopic localization of the transferase was achieved in Lowicryl K4M thin sections and frozen-thawed thin sections of porcine and bovine submaxillary gland by using the protein A-gold technique. Specific gold particle labeling was observed in the cis Golgi apparatus and smooth-membraned vesicular structures in close topological relation with it. Labeling was undetectable in the rough endoplasmic reticulum, its transitional elements, and smooth-membraned structures close to them, the trans Golgi apparatus, mucin droplets, and the plasma membrane. The onset of labeling for peptide-bound GalNAc as detected with Vicia villosa isolectin G4 mirrored the transferase immunolocalization as directly shown by double labeling and extended into the trans Golgi apparatus and mucous droplets. Apomucin immunolabeling was found throughout the endoplasmic reticulum and the intermediate compartment and partially overlapped the region of transferase labeling in the Golgi apparatus as demonstrated by double immunolabeling. Thus, the initial step of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase-mediated O-glycosylation in porcine and bovine submaxillary gland cells occurs in the cis Golgi apparatus. The possible involvement of the intermediate compartment remains to be clarified.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Hirschberg C. B. Subcellular site of synthesis of the N-acetylgalactosamine (alpha 1-0) serine (or threonine) linkage in rat liver. J Biol Chem. 1987 Mar 25;262(9):4153–4159. [PubMed] [Google Scholar]
- Brada D., Kerjaschki D., Roth J. Cell type-specific post-Golgi apparatus localization of a "resident" endoplasmic reticulum glycoprotein, glucosidase II. J Cell Biol. 1990 Feb;110(2):309–318. doi: 10.1083/jcb.110.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
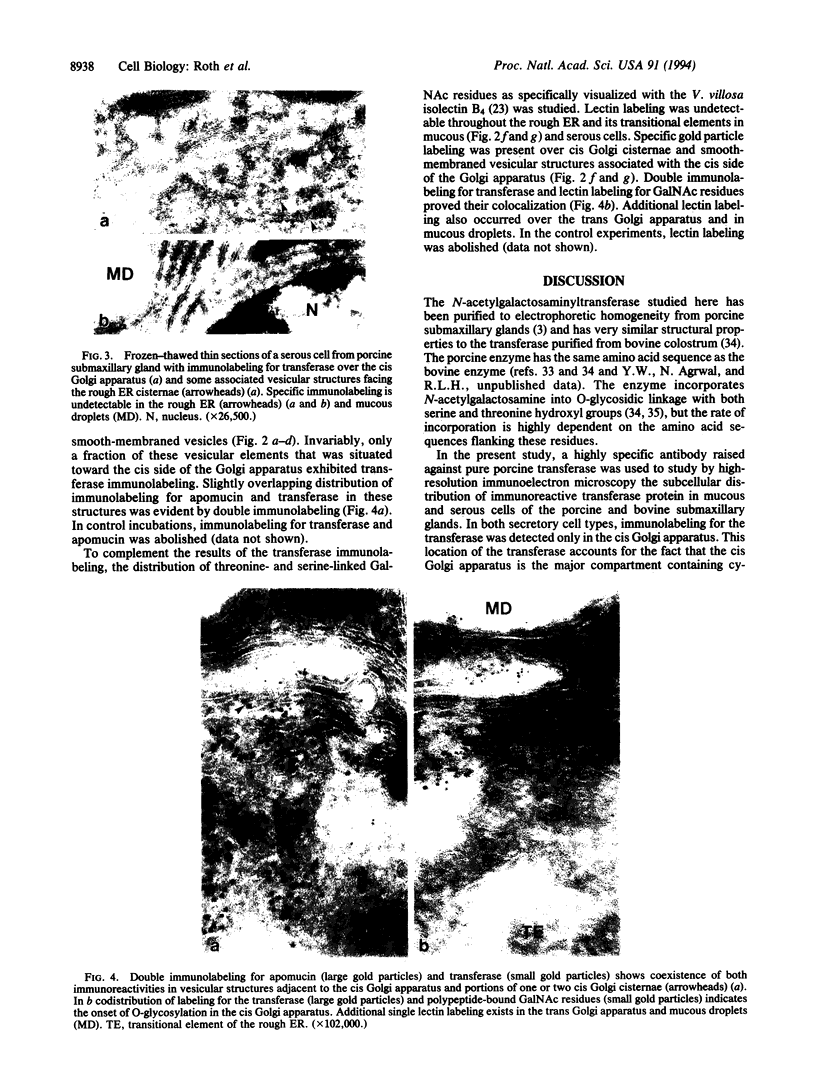
- Deschuyteneer M., Eckhardt A. E., Roth J., Hill R. L. The subcellular localization of apomucin and nonreducing terminal N-acetylgalactosamine in porcine submaxillary glands. J Biol Chem. 1988 Feb 15;263(5):2452–2459. [PubMed] [Google Scholar]
- Eckhardt A. E., Timpte C. S., Abernethy J. L., Toumadje A., Johnson W. C., Jr, Hill R. L. Structural properties of porcine submaxillary gland apomucin. J Biol Chem. 1987 Aug 15;262(23):11339–11344. [PubMed] [Google Scholar]
- Elhammer A., Kornfeld S. Two enzymes involved in the synthesis of O-linked oligosaccharides are localized on membranes of different densities in mouse lymphoma BW5147 cells. J Cell Biol. 1984 Jul;99(1 Pt 1):327–331. doi: 10.1083/jcb.99.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger A., Pavelka M. Subdomains of the rough endoplasmic reticulum in colon goblet cells of the rat: lectin-cytochemical characterization. J Histochem Cytochem. 1992 Jul;40(7):919–930. doi: 10.1177/40.7.1607641. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K., Warren G., Tokuyasu K. T. Immunoelectron microscopy using thin, frozen sections: application to studies of the intracellular transport of Semliki Forest virus spike glycoproteins. Methods Enzymol. 1983;96:466–485. doi: 10.1016/s0076-6879(83)96041-x. [DOI] [PubMed] [Google Scholar]
- Hagen F. K., Van Wuyckhuyse B., Tabak L. A. Purification, cloning, and expression of a bovine UDP-GalNAc: polypeptide N-acetyl-galactosaminyltransferase. J Biol Chem. 1993 Sep 5;268(25):18960–18965. [PubMed] [Google Scholar]
- Hanover J. A., Elting J., Mintz G. R., Lennarz W. J. Temporal aspects of the N- and O-glycosylation of human chorionic gonadotropin. J Biol Chem. 1982 Sep 10;257(17):10172–10177. [PubMed] [Google Scholar]
- Hauri H. P., Schweizer A. The endoplasmic reticulum-Golgi intermediate compartment. Curr Opin Cell Biol. 1992 Aug;4(4):600–608. doi: 10.1016/0955-0674(92)90078-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Hollander T., Lehman D. J., Thomsen D. R., Elhammer A. P. Isolation and expression of a cDNA clone encoding a bovine UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase. J Biol Chem. 1993 Jun 15;268(17):12609–12616. [PubMed] [Google Scholar]
- Hsu V. W., Yuan L. C., Nuchtern J. G., Lippincott-Schwartz J., Hammerling G. J., Klausner R. D. A recycling pathway between the endoplasmic reticulum and the Golgi apparatus for retention of unassembled MHC class I molecules. Nature. 1991 Aug 1;352(6334):441–444. doi: 10.1038/352441a0. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. Bidirectional membrane traffic between the endoplasmic reticulum and Golgi apparatus. Trends Cell Biol. 1993 Mar;3(3):81–88. doi: 10.1016/0962-8924(93)90078-f. [DOI] [PubMed] [Google Scholar]
- Niemann H., Boschek B., Evans D., Rosing M., Tamura T., Klenk H. D. Post-translational glycosylation of coronavirus glycoprotein E1: inhibition by monensin. EMBO J. 1982;1(12):1499–1504. doi: 10.1002/j.1460-2075.1982.tb01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale M. C., Erra M. C., Malagolini N., Serafini-Cessi F., Leone A., Bonatti S. Post-translational processing of an O-glycosylated protein, the human CD8 glycoprotein, during the intracellular transport to the plasma membrane. J Biol Chem. 1992 Dec 15;267(35):25196–25201. [PubMed] [Google Scholar]
- Pathak R. K., Merkle R. K., Cummings R. D., Goldstein J. L., Brown M. S., Anderson R. G. Immunocytochemical localization of mutant low density lipoprotein receptors that fail to reach the Golgi complex. J Cell Biol. 1988 Jun;106(6):1831–1841. doi: 10.1083/jcb.106.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzelt C., Weber B. Early O-glycosidic glycosylation of proglucagon in pancreatic islets: an unusual type of prohormonal modification. EMBO J. 1986 Sep;5(9):2103–2108. doi: 10.1002/j.1460-2075.1986.tb04472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Recycling of proteins between the endoplasmic reticulum and Golgi complex. Curr Opin Cell Biol. 1991 Aug;3(4):585–591. doi: 10.1016/0955-0674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Perez-Vilar J., Hidalgo J., Velasco A. Presence of terminal N-acetylgalactosamine residues in subregions of the endoplasmic reticulum is influenced by cell differentiation in culture. J Biol Chem. 1991 Dec 15;266(35):23967–23976. [PubMed] [Google Scholar]
- Piller V., Piller F., Fukuda M. Biosynthesis of truncated O-glycans in the T cell line Jurkat. Localization of O-glycan initiation. J Biol Chem. 1990 Jun 5;265(16):9264–9271. [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Roth J. Cytochemical localization of terminal N-acetyl-D-galactosamine residues in cellular compartments of intestinal goblet cells: implications for the topology of O-glycosylation. J Cell Biol. 1984 Feb;98(2):399–406. doi: 10.1083/jcb.98.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Greenwell P., Watkins W. M. Immunolocalization of blood group A gene specified alpha 1,3N-acetylgalactosaminyltransferase and blood group A substance in the trans-tubular network of the Golgi apparatus and mucus of intestinal goblet cells. Eur J Cell Biol. 1988 Apr;46(1):105–112. [PubMed] [Google Scholar]
- Roth J. Postembedding labeling on Lowicryl K4M tissue sections: detection and modification of cellular components. Methods Cell Biol. 1989;31:513–551. doi: 10.1016/s0091-679x(08)61625-8. [DOI] [PubMed] [Google Scholar]
- Roth J., Saremaslani P., Zuber C. Versatility of anti-horseradish peroxidase antibody-gold complexes for cytochemistry and in situ hybridization: preparation and application of soluble complexes with streptavidin-peroxidase conjugates and biotinylated antibodies. Histochemistry. 1992 Nov;98(4):229–236. doi: 10.1007/BF00271036. [DOI] [PubMed] [Google Scholar]
- Roth J. Subcellular organization of glycosylation in mammalian cells. Biochim Biophys Acta. 1987 Oct 5;906(3):405–436. doi: 10.1016/0304-4157(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Roth J., Taatjes D. J., Weinstein J., Paulson J. C., Greenwell P., Watkins W. M. Differential subcompartmentation of terminal glycosylation in the Golgi apparatus of intestinal absorptive and goblet cells. J Biol Chem. 1986 Oct 25;261(30):14307–14312. [PubMed] [Google Scholar]
- Saraste J., Kuismanen E. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin Cell Biol. 1992 Oct;3(5):343–355. doi: 10.1016/1043-4682(92)90020-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Clausen H., van Meer G., Hauri H. P. Localization of O-glycan initiation, sphingomyelin synthesis, and glucosylceramide synthesis in Vero cells with respect to the endoplasmic reticulum-Golgi intermediate compartment. J Biol Chem. 1994 Feb 11;269(6):4035–4041. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Strous G. J. Initial glycosylation of proteins with acetylgalactosaminylserine linkages. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2694–2698. doi: 10.1073/pnas.76.6.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989 Mar;21(3):163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- Tollefsen S. E., Kornfeld R. The B4 lectin from Vicia villosa seeds interacts with N-acetylgalactosamine residues alpha-linked to serine or threonine residues in cell surface glycoproteins. J Biol Chem. 1983 Apr 25;258(8):5172–5176. [PubMed] [Google Scholar]
- Tooze S. A., Tooze J., Warren G. Site of addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J Cell Biol. 1988 May;106(5):1475–1487. doi: 10.1083/jcb.106.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco A., Hendricks L., Moremen K. W., Tulsiani D. R., Touster O., Farquhar M. G. Cell type-dependent variations in the subcellular distribution of alpha-mannosidase I and II. J Cell Biol. 1993 Jul;122(1):39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Abernethy J. L., Eckhardt A. E., Hill R. L. Purification and characterization of a UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase specific for glycosylation of threonine residues. J Biol Chem. 1992 Jun 25;267(18):12709–12716. [PubMed] [Google Scholar]
- Wang Y., Agrwal N., Eckhardt A. E., Stevens R. D., Hill R. L. The acceptor substrate specificity of porcine submaxillary UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase is dependent on the amino acid sequences adjacent to serine and threonine residues. J Biol Chem. 1993 Nov 5;268(31):22979–22983. [PubMed] [Google Scholar]