Abstract
Survival of children with chronic medical illnesses is leading to an increase in secondary osteoporosis due to impaired peak bone mass (PBM). Insulin-like growth factor type 1 (IGF-1) levels correlate with the pattern of bone mass accrual and many chronic illnesses are associated with low IGF-1 levels. Reduced serum levels of IGF-1 minimally affect the integrity of the skeleton, whereas recent studies suggest that skeletal IGF-I regulates PBM. To determine the role of IGF-1 in postnatal bone mass accrual regardless of source, we established an inducible type 1 Igf receptor Cre/lox knockout mouse model, in which the type 1 Igf receptor was deleted inducibely in the mesenchymal stem cells (MSCs) from 3–7 weeks of age. The size of the mouse was not affected as knockout and wild type mice had similar body weights and nasoanal and femoral lengths. However, bone volume and trabecular bone thickness were decreased in the secondary spongiosa of female knockout mice relative to wild type controls, indicating that IGF-1 is critical for bone mass. IGF-1 signaling in MSCs in vitro has been implicated to be involved in both migration to the bone surface and differentiation into bone forming osteoblasts. To clarify the exact role of IGF-1 in bone, we found by immunohistochemical analysis that a similar number of Osterix–positive osteoprogenitors were on the bone perimeter, indicating migration of MSCs was not affected. Most importantly, 56% fewer osteocalcin-positive mature osteoblasts were present on the bone perimeter in the secondary spongiosa in knockout mice versus wild type littermates. These in vivo data demonstrate that the primary role of skeletal IGF-1 is for the terminal differentiation of osteoprogenitors, but refute the role of IGF-1 in MSC migration in vivo. Additionally, these findings confirm that impaired IGF-1 signaling in bone MSCs is sufficient to impair bone mass acquisition.
Keywords: osteoblasts, knockout mice, cell migration, IGF receptor, nestin
Introduction
Bone mass normally peaks in mid to late adolescence, plateaus for several years and then declines over time (1–5). Acquisition of a higher peak bone mass (PBM) in adolescence is associated with reduced subsequent fracture risk, whereas impaired acquisition of PBM or loss of bone in childhood is associated with greater fracture risk. Chronic illness in childhood impairs PBM acquisition. Survival of many chronic illnesses in childhood are leading to a growing incidence of secondary osteoporosis (6). The prevention of secondary osteoporosis in children with chronic illnesses depends on protecting the bones of this at-risk population. However, before treatment can be developed, the regulation of cellular signaling mechanisms involved in the acquisition of bone mass needs to be further elucidated.
The only cells that form new bone are osteoblasts, which are non-replicative mononuclear cells derived from mesenchymal stem cells (MSC) (7,8). Thus, bone formation is dependent on MSCs and signals that determine MSC recruitment, replication, differentiation, and death are critical determinants for bone mass. In humans, insulin-like growth factor type 1 (IGF-1) and several of its binding proteins are positively associated with acquisition of PBM and the maintenance of bone mineral density (BMD) (9–11) and low IGF-1 levels have been found to be a risk factor for osteoporosis and fracture risk (3,12–19). IGF-1 cell signaling in cells of the osteoblast lineage have helped define the precise relationship between IGF-1 and bone mass acquisition. For example, we recently found that Igf-1 in mice stimulates osteoblast differentiation by activation of mammalian target of rapamycin and is essential to maintain proper bone microarchitecture and mass (20). Others have found that IGF-1 signaling in mature osteo-blasts enhances the function of the mature osteoblast (21). Data has also implicated IGF-1 as a mitogenic factor for MSCs in culture (22–25). However, as multiple other cytokines and growth factors stimulate similar cell signaling pathways, the contribution of IGF-1 signaling in vivo to MSC cell migration remains unknown.
Tracking the in vivo fate of MSCs has not previously been performed due to the lack of an endogenous marker for bone marrow MSCs. Nestin is an intermediate filament protein, expressed in embryonic and developing myogenic and neurogenic tissues and has classically been used to trace neural stem cells (26). Recently, Nestin expression has been identified in bone marrow MSCs (27), which may be genetically marked to trace their fate. During the lineage progression of osteoblast differentiation, it is well accepted that bone marrow MSCs first commit to osteoprogenitor cells that express Osterix. Osteoprogenitors further differentiate into mature osteoblasts, which produce matrix proteins such as osteocalcin. Using an inducible Nestin-CreER mouse, we first confirmed that these cells differentiate into the osteoblast lineage in vivo. We then deleted IGF1R in mice in Nestin expressing cells during the pubertal growth spurt. We found that impairment of IGF1R signaling resulted in lower bone volume and trabecular thickness in the knockout mice compared to the wild type littermates without having an effect on the overall growth of the mouse. Importantly, immunostaining for Osterix and Osteocalcin revealed a similar number of osteoprogenitors on the bone surface, but decreased numbers of mature osteoblasts on the bone surface. These findings confirm our previous results in the importance of IGF-1 signaling for terminal osteoblast differentiation. As the number of osteoprogenitor cells able to migrate to the cell surface was similar to wild type controls, these findings suggest that IGF-1 signaling in vivo is not essential for MSC migration.
Methods
Mice
We obtained mice with Nestin-Cre recombinase fused to a mutated estrogen receptor (Nestin-CreER) in a C57BL/6 background, ROSA26-EYFP in C57BL/6 background, and Igf1r with loxP sites flanking exon 3 (Igf1rfl/fl) mice in a mixed C57Bl/6 × 129 background from the Jackson Laboratory. To confirm that Nestin expressing cells differentiate into the osteoblast lineage, we crossed the Nestin-CreER transgenic mice with ROSA26-EYFP mice to obtain the reporter mice, Nestin-CreER::ROSA26-EYFP+/−. In these mice, administration of tamoxifen allows nuclear transport of the Cre-recombinase resulting in deletion of the loxP-flanked stop sequence and permanent expression of YFP (Figure 1). To generate mice with inducible deletion of IGF1R in MSCs, we crossed Nestin-CreER transgenic mice with Igf1rfl/fl mice. We then intercrossed the F1 offspring to generate the following F2 offspring: Nestin-CreER::Igf1rfl/fl (homozygous IGF1R inducible knockout mice, hereafter referred to as MSC-Igf1r−/−), Nestin-CreER::Igf1rfl/+ (heterozygous IGF1R inducible knockout mice), and mice without either the Nestin-CreER or a floxed Igf1r allele (hereafter referred to as wild type) (Figure 2). DNA was isolated from tail biopsies using phenol/chloroform extraction. We performed polymerase chain reaction using primers and conditions as described by Jackson Laboratory for each genetic modification. At the time of weaning, 21 days, all experimental mice were injected with tamoxifen 0.1 mg·kg−1 body weight every 3 days for 4 weeks. Body weight was recorded every three days and nasoanal length was measured weekly using a caliper with an accuracy of 0.1 mm. Animals were sacrificed at 7 weeks. All animals were maintained in the Animal Facility of the Johns Hopkins University School of Medicine. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University, Baltimore, MD, USA.
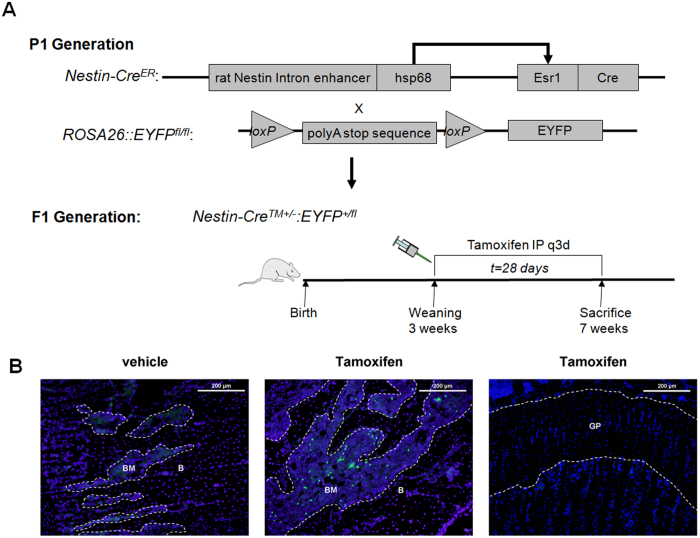
Figure 1.
Validation of inducible Nestin-Cre lineage fate. A: Nestin-CreER mice were crossed with ROSA26-EYFP mice to generate the reporter strain, Nestin-CreER::ROSA26-EYFP+/−. Mice were treated with either vehicle or tamoxifen, beginning at the time of weaning (3 weeks) and continued every 3 days for a total of 4 weeks. Mice were sacrificed at 7 weeks. Hsp68=heat shock protein 68. Esr1=mutated estrogen receptor. B: Representative distal femoral sections stained for YFP (green). Mice treated with vehicle did not show any leakiness of YFP expression (left panel). Mice treated with tamoxifen showed that YFP expression was limited to cells residing in the bone marrow or on the bone surface (middle panel), as no YFP expression was noted in growth plate chondroctyes (right panel). DAPI stains nuclei blue. B=bone. BM=bone marrow. GP=growth plate. White dotted lines outline the bone surface. Scale bars=200 μm.
Figure 2.
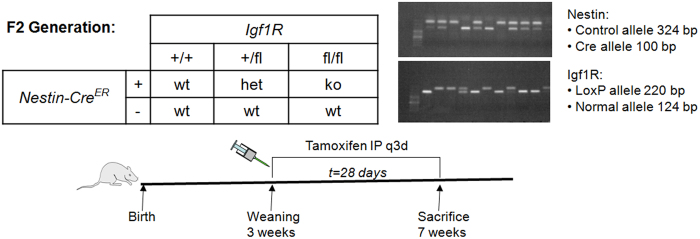
Experimental design for deletion of IGF1R in Nestin+ MSCs. Nestin-CreER mice were crossed with IGF1Rfl/fl mice to generate the F1 generation, Nestin-CreER::IGF1R+/fl. The F1 generation was intercrossed to generate the desired genotypes in the F2 generation. Genotypes were confirmed by PCR amplification of the endogenous control, Cre, and loxP alleles. Tamoxifen was administered beginning at 3 weeks and continued every 3 days for a total of 4 weeks. MSC-Igf1r−/− (ko) mice (ko) expressed the Nestin-Cre allele and were homozygous for the Igf1r floxed allele, whereas as wild type (wt) mice were littermates that were either negative for the Nestin-CreER or Igf1r floxed allele.
Microcomputed tomography (μCT) and histomorphometric analysis
Femura and tibiae obtained from mice were dissected free of soft tissue, fixed overnight in 10% neutral buffered formalin for 24 h, dehydrated in 70% ethanol for 3 days, and analyzed by a high resolution μCT (SkyScan 1076 in-vivo CT, SKYSCAN) installed with softwares NRecon v1.6, CTan v1.9, and CTVol v2.0. The scanner was set at a voltage of 89 kVp, a current of 112 μA and a resolution of 8.67 μm per pixel. Coronal, sagittal, and transverse images of the distal femora were used to perform three-dimensional resconstruction and histomorphometric analysis of trabecular bone. The secondary spongiosa area was selected for analysis, defined as femoral metaphyseal trabecular bone originating 1 mm from the epiphyseal growth plate and extending caudally 1.5 mm.
Histology and immunohistochemistry
Femura and tibiae were isolated and fixed in 10% neutral buffered formalin for 1–2 days, decalcified in 10% EDTA (pH 7.4) for 2–4 weeks, and embedded in paraffin. Four μm-thick longitudinally-oriented sections including metaphysis were deparaffinized and rehydrated, and then processed for hematoxylin and eosin (H&E) (Thermo Scientific), tartrate resistant acid phosphatase (TRAP) staining (Sigma-Aldrich) and immunohistochemical staining using primary antibodies against Osterix (Abcam, 1:200 dilution) or Osteocalcin (Takara, 1:200 dilution). For immunohistochemical staining, we incubated the sections with primary antibodies overnight at 4 °C and used a horseradish peroxidase-streptavidin detection system (Dako) to detect immunoactivity followed by counter-staining with hematoxylin (Sigma-Aldrich). We performed histomorphometric measurements of two-dimensional parameters of the trabecular bone in 2 mm squares in the distal femoral secondary spongiosa, defined as >1 mm distal to the lowest point of growth plate with Osteo-MeasureXP Software (OsteoMetrics, Inc.). We selected all histomorphometric parameters in five random visual fields per specimen, and measured four sections per animal in each group.
Statistical analysis
Statistical differences between two groups of data were analyzed with Student's t-test. Data are presented as means ± SD.
Results
Nestin expressing cells preferentially differentiate into osteoblast lineage cells postnatally
Cells expressing Nestin have previously been reported to represent a subset of bone marrow mesenchymal stem cells as they differentiate in vivo into multiple lineages, including osteoblasts, chondrocytes, and adipocytes (27). To verify the cell fate of Nestin+ cells in our tamoxifen inducible model, we crossed the Nestin-CreER mice with a reporter ROSA26-EYFP mouse. In the reporter mouse model (Nestin-CreER::ROSA26-EYFP+/−), tamoxifen treatment excises a loxP-polyA stop sequence, allowing YFP to be permanently expressed for the remainder of the cells life and its daughter cells (Figure 1A). We first validated and optimized the system for tracing bone marrow MSCs by screening different reporter lines. We traced the lineage fate of Nestin expression cells in mice injected with vehicle or tamoxifen every 3 days, beginning at 3 weeks of age and continuing for 4 weeks. We identified a lineage of Nestin-CreER::ROSA26-EYFP+/− mice that did not display any leaky YFP expression in the entire femur with vehicle injection (n=5) (Figure 1B, left panel). In the tamoxifen treated group, we found that the majority of YFP+ cells were located either in the bone marrow or on the bone surface (Figure 1B, middle panel). However, we did not detect any YFP+ cells in the growth plate chondrocytes (Figure 1B, right panel). In the inducible Nestin-CreER mouse model, during the pubertal growth spurt (3-7 weeks of age), Nestin expressing cells differentiate into the osteoblast lineage.
Deletion of IGF1R in MSCs results in decreased bone mass but does not affect growth
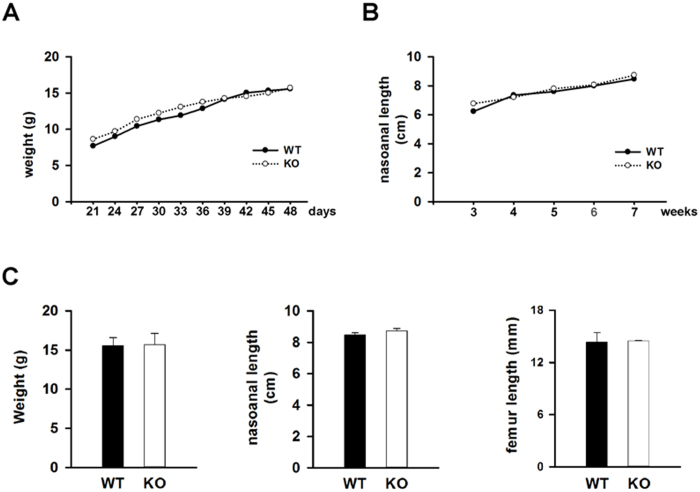
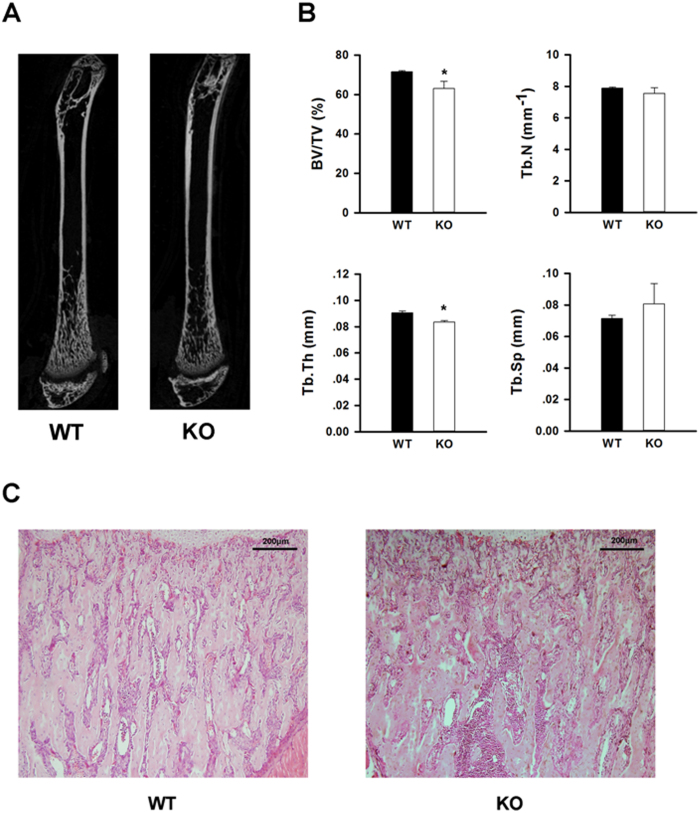
To explore the role of IGF-1 signaling in MSC fate and bone formation postnatally, we crossed the Nestin-CreER mouse with Igf1rfl/fl mice to generate the desired genotypes (Figure 2). Tamoxifen was administered to all experimental animals beginning at the time of weaning (3 weeks) and continued every 3 days for a total of 4 weeks, including the time period of the pubertal growth spurt. MSC-Igf1r−/− mice were defined as mice that expressed the Nestin-Cre allele and homozygous for the Igf1r floxed allele, whereas the wild type (wt) mice were littermates that were either negative for the Nestin-cre or Igf1r floxed allele. No difference in weight or nasoanal length was noted between MSC-Igf1r−/− and wt female mice (n=5) (Figure 3A and 3B). Analysis of the long bones at 7 weeks of age showed no change in femoral length between groups (Figure 3C). However, CT analysis revealed a decrease in bone mass in the MSC-Igf1r−/− female mice compared to their wt female littermates (Figure 4). Specifically, MSC-Igf1r−/− mice had less trabecular bone as defined by a significant decrease in the percent of bone volume per tissue volume (BV/TV) (P=0.01) and trabecular thickness (Tb.Th) (P=0.002) relative to their wt littermates (Figure 4B). We also observed similar results by H&E-staining with decreased trabecular bone in mice tibiae (Figure 4C). Consistent with previous reports (20,28), our results demonstrate IGF-1 signaling postnatally is important for the acquisition of bone mass.
Figure 3.
Growth of mice with deletion of IGF1R in MSCs during pubertal growth spurt was not impaired. A: Weight during the course of tamoxifen injections; B: nasoanal length during the course of tamoxifen injections; C: Weight, nasoanal length, and femoral length at 7 weeks (n=5). Results presented at mean±SD. wt=wild type littermates; ko=MSC-Igf1r−/−.
Figure 4.
Deletion of IGF1R in MSCs resulted in decreased bone mass. A: Representative microcomputed tomography (μCT) image from wildtype (wt) and MSC-Igf1r−/− (ko) mice in 7-week-old female mice. B: Quantitative analysis of μCT parameters of trabecular bone in distal femoral metaphysis in wt and ko female mice: bone volume/tissue volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb. Th), and trabecular spacing (Tb. Sp). Data presented as mean±SD (n=5, ∗P<0.05). C: Representative H&E staining of tibiae from wt and ko mice. Scale bar=200 μm.
IGF-1 is necessary for MSC differentiation, not recruitment during bone remodeling
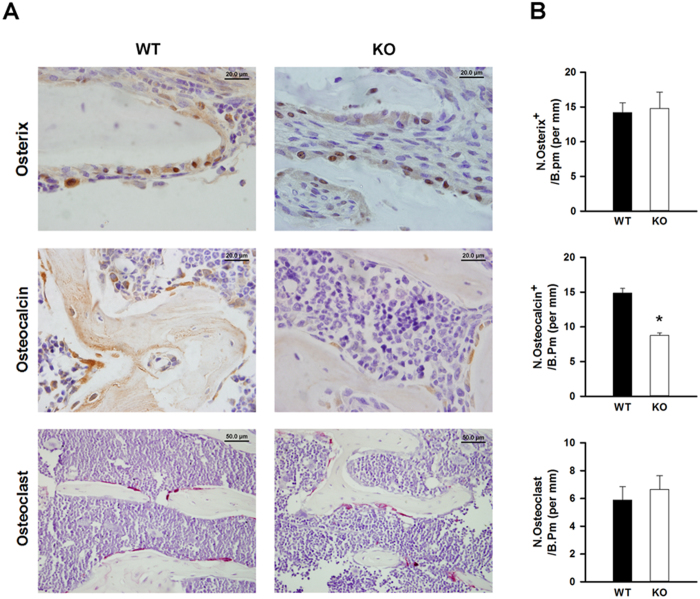
To examine the mechanism of the reduced bone mass in the MSC-Igf1r−/− mice, we performed immunohistochemical staining for Osterix and Osteocalcin to label osteoprogenitors and mature osteoblasts, respectively in mouse femora. IGF-1 signaling has been implicated to play a role in both MSC migration and differentiation (20,22–25). Therefore, to assess migration and differentiation of MSCs, we quantified the number of osteoprogenitors and mature osteoblasts by bone perimeter. Interestingly, staining for Osterix revealed a similar number of osteoprogenitor cells on the bone perimeter suggesting MSCs were able to migrate to the bone surface despite impaired IGF-1 signaling (Figure 5, top panel). Similar to our previous findings (20), the number of Osteocalcin+ cells was significantly less on the bone surface of MSC-Igf1r−/− mice compared to their wt littermates (P<0.001) (Figure 5, middle panel), confirming the role of IGF-1 signaling in terminal osteoblast differentiation. To ensure MSC specificity of Nestin expression, as osteoclasts are derived from the hematopoetic lineage, we performed TRAP staining. TRAP staining revealed a similar number of osteoclasts in MSC-Igf1r−/− mice compared to their wildtype littermates (Figure 5, bottom panel), further indicating that the decreased bone volume observed by μCT was due to decreased bone formation rather than enhanced bone resorption.
Figure 5.
Deletion of IGF1R in MSCs impairs osteoblast differentiation but not migration of MSCs. A: Representative immunohistochemical staining of Osterix for osteoprogenitor cells (brown, top panel) and Osteocalcin for mature osteoblasts (brown, middle panel) and tartrate resistant acid phosphatase staining for osteoclasts (pink, lower panel) of trabecular bone sections from distal femora of MSC-Igf1r−/− (ko) and wild type (wt) littermates. Scale bars for Osterix and Osteocalcin=20 μm; scale bar for osteoclasts=50 μm. B: Quantitative analysis of immunohistochemical staining for Osterix-positive cells (N. osterix+, top panel), Osteocalcin-positive cells (N. osteocalcin+, middle panel) and osteoclasts (lower panel) on the bone surface, measured as cells per millimeter of bone perimeter (/B.Pm). Date are presented as mean ± SD (n=4, ∗P<0.05).
Discussion
Bone modeling and remodeling are tightly regulated to ensure the integrity and strength of the skeleton. During the pubertal growth spurt, both occur with bone formation exceeding bone resorption and results in a rapid gain of bone mass. As the only cells that form bone are osteoblasts, the activity of osteoblasts is of utmost importance. An adequate supply of osteoblasts from their stem cells, bone marrow MSCs, is critical for bone formation as osteoblasts are non-replicative (7). Particularly, bone remodeling is temporally and spatially controlled and occurs continuously throughout the lifespan to maintain bone mass and architecture (5,7,20,27,29,30). During bone resorption by osteoclasts, multiple growth factors and cytokines are acvitated and released from the bone matrix to recruit MSCs and induce differentiation into mature osteoblasts in coupling bone formation with resorption (5,20,29,31,32). We have previously shown that TGFβ1 is activated during bone resorption and induces migration of MSCs from their quiescent phase and that IGF-1 released from bone matrix stimulates their osteoblast differentiation. However, several in vitro studies have suggested that IGF-1 may have a role in MSC migration as well (22–25). To clarify the specific role of IGF-1 on MSC function, in the current study, we further investigated the role of IGF-1 in MSCs during osteoblast differentiation using conditional knockout of IGF1R in the Nestin+ MSCs. Nestin+ MSCs are located in a perivascular distrubtion and have not yet migrated to the bone surface (27). We found that deletion of IGF1R in Nestin+ MSCs affected the differentiation, but not migration of MSCs during bone remodeling.
Nestin is expressed in many other tissues, including multiple cells during embryonic development and neural stem cells (33). Studies in mice, however, have shown that Nestin expression in brain reaches a nadir around 18 days of life and that most cells that express Nestin postnatally are relatively inactive in adulthood unless an injury, such as hypoxia to the brain, occurs (33–35). Consistent with these reports, previous lineage tracing of Nestin+ MSCs in vivo revealed a relatively quiescent state in adult mice as minimal GFP positive cells could be identified one month after induction, whereas abundant GFP expression was detected 8 months after induction (27). We used an inducible model that with administration of tamoxifen allowed us to temporally delete the IGF1R after Nestin+ cells in the brain had reached the quiescent phase but at a time point in which the skeleton continues to undergo rapid growth. Our reporter mice, NestinER-YFP mice confirmed that the bone marrow Nestin+ cells actively differentiate into the osteoblast lineage during this time period, as indicated by abundant fluorescent staining. Contrary to the previous report, we found that Nestin+ MSCs are predominantly restricted to differentiate into the osteoblast lineage. This may be a reflection of the time period studied or a result of the non-endogenous enhancer used to drive Cre-expression. Deletion of the IGF1R in Nestin+ MSCs during this time period did not affect mortality nor impair weight gain or longitudinal growth, but did result in decreased bone formation.
The regulation of bone marrow MSCs in osteoblast differentiation in vivo is not completely clear, especially in regard to the temporal-spatial signaling control of specific actions of MSCs during bone modeling and remodeling. The development of a reproducible in vivo model to label bone marrow MSCs provides an opportunity to study differentiation of MSCs. Nestin is known to be expressed in bone marrow MSCS (7,27). In this study, we show that Nestin+ MSCs were able to differentiate into the osteoblast lineage and deletion of the IGF1R in Nestin+ MSCs impaired osteoblast differentiation and decreased trabecular bone formation. IGF-1 signaling is essential for terminal osteoblast differentiation of MSCs, whereas the recruitment of MSCs to the bone remodeling surface was not significantly affected.
Acknowledgments
This work was supported in part by the grants from the United States National Institute of Health NIDDK including T32DK07751 (JLC), the Diabetes Research and Training Center grant P60DK079637 (JSF), and DK057501 and DK08098 (XC).
References
- Rizzoli R, Bianchi ML, Garabedian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Schettler AE, Gustafson EM. Osteoporosis prevention starts in adolescence. J Am Acad Nurse Pract. 2004;16:274–282. doi: 10.1111/j.1745-7599.2004.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Agnusdei D, Gentilella R. GH and IGF-I as therapeutic agents for osteoporosis. J Endocrinol Invest. 2005;28:32–36. [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- Bianchi ML. How to manage osteoporosis in children. Best Pract Res Clin Rheumatol. 2005;19:991–1005. doi: 10.1016/j.berh.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E. The fate of circulating osteoblasts. N Engl J Med. 2005;352:2014–2016. doi: 10.1056/NEJMe058080. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- Amin S, Riggs BL, Melton LJ, III, Achenbach SJ, Atkinson EJ, Khosla S. High serum IGFBP-2 is predictive of increased bone turnover in aging men and women. J Bone Miner Res. 2007;22:799–807. doi: 10.1359/jbmr.070306. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kanatani M, Yamauchi M, Kaji H, Sugishita T, Baylink DJ, Mohan S, Chihara K, Sugimoto T. Serum levels of insulin-like growth factor (IGF); IGF-binding proteins-3, -4, and -5; and their relationships to bone mineral density and the risk of vertebral fractures in postmenopausal women. Calcif Tissue Int. 2006;78:18–24. doi: 10.1007/s00223-005-0163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Pennisi P, Wu Y, Zhao H, LeRoith D. Clinical relevance of systemic and local IGF-I. Endocr Dev. 2005;9:11–16. doi: 10.1159/000085718. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Mohan S, Sjogren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seck T, Scheppach B, Scharla S, Diel I, Blum WF, Bismar H, Schmid G, Krempien B, Ziegler R, Pfeilschifter J. Concentration of insulin-like growth factor (IGF)-I and -II in iliac crest bone matrix from pre- and postmenopausal women: relationship to age, menopause, bone turnover, bone volume, and circulating IGFs. J Clin Endocrinol Metab. 1998;83:2331–2337. doi: 10.1210/jcem.83.7.4967. [DOI] [PubMed] [Google Scholar]
- Johansson AG, Lindh E, Blum WF, Kollerup G, Sorensen OH, Ljunghall S. Effects of growth hormone and insulin-like growth factor I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1996;81:44–48. doi: 10.1210/jcem.81.1.8550792. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Mellstrom D, Carlzon D, Orwoll E, Ljunggren O, Karlsson MK, Vandenput L. Older men with low serum IGF-1 have an increased risk of incident fractures: the MrOS Sweden study. J Bone Miner Res. 2011;26:865–872. doi: 10.1002/jbmr.281. [DOI] [PubMed] [Google Scholar]
- Seck T, Scheppach B, Scharla S, Diel I, Blum WF, Bismar H, Schmid G, Krempien B, Ziegler R, Pfeilschifter J. Concentration of insulin-like growth factor (IGF)-I and -II in iliac crest bone matrix from pre- and postmenopausal women: relationship to age, menopause, bone turnover, bone volume, and circulating IGFs. J Clin Endocrinol Metab. 1998;83:2331–2337. doi: 10.1210/jcem.83.7.4967. [DOI] [PubMed] [Google Scholar]
- Liu JM, Zhao HY, Ning G, Chen Y, Zhang LZ, Sun LH, Zhao YJ, Xu MY, Chen JL. IGF-1 as an early marker for low bone mass or osteoporosis in premenopausal and postmenopausal women. J Bone Miner Metab. 2008;26:159–164. doi: 10.1007/s00774-007-0799-z. [DOI] [PubMed] [Google Scholar]
- Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, Crane J, Frassica F, Zhang L, Rodriguez JP, Xiaofeng J, Shoshana Y, Shouhong X, Argiris E, Mei W, Xu C. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Monier-Faugere MC, Langub MC, Geng Z, Nakayama T, Pike JW, Chernausek SD, Rosen CJ, Donahue LR, Malluche HH, Fagin JA, Clemens TL. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology. 2000;141:2674–2682. doi: 10.1210/endo.141.7.7585. [DOI] [PubMed] [Google Scholar]
- Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780–784. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Nakasaki M, Yoshioka K, Miyamoto Y, Sasaki T, Yoshikawa H, Itoh K. IGF-I secreted by osteoblasts acts as a potent chemotactic factor for osteoblasts. Bone. 2008;43:869–879. doi: 10.1016/j.bone.2008.07.241. [DOI] [PubMed] [Google Scholar]
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. in vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von SD, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van HW, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Pang L, Lei W, Lu W, Li J, Li Z, Frassica FJ, Chen X, Wan M, Cao X. Inhibition of Sca-1-positive skeletal stem cell recruitment by alendronate blunts the anabolic effects of parathyroid hormone on bone remodeling. Cell Stem Cell. 2010;7:571–580. doi: 10.1016/j.stem.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill PA. Bone remodelling. Br J Orthod. 1998;25:101–107. doi: 10.1093/ortho/25.2.101. [DOI] [PubMed] [Google Scholar]
- Martin T, Gooi JH, Sims NA. Molecular mechanisms in coupling of bone formation to resorption. Crit Rev Eukaryot Gene Expr. 2009;19:73–88. doi: 10.1615/critreveukargeneexpr.v19.i1.40. [DOI] [PubMed] [Google Scholar]
- Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression—a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim J, Kim Y, Yang M, Jang H, Kang S, Kim JC, Kim SH, Shin T, Moon C. Differential patterns of nestin and glial fibrillary acidic protein expression in mouse hippocampus during postnatal development. J Vet Sci. 2011;12:1–6. doi: 10.4142/jvs.2011.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, D'Ercole JA, Ye P. Blunting type 1 insulin-like growth factor receptor expression exacerbates neuronal apoptosis following hypoxic/ischemic injury. BMC Neurosci. 2011;12:64. doi: 10.1186/1471-2202-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]