Abstract
A decade ago, only two hormones, parathyroid hormone and 1,25(OH)2D, were widely recognized to directly affect phosphate homeostasis. Since the discovery of fibroblast growth factor 23 (FGF23) in 2000 (1), our understanding of the mechanisms of phosphate homeostasis and of bone mineralization has grown exponentially. FGF23 is the link between intestine, bone, and kidney together in phosphate regulation. However, we still do not know the complex mechanism of phosphate homeostasis and bone mineralization. The physiological role of FGF23 is to regulate serum phosphate. Secreted mainly by osteocytes and osteoblasts in the skeleton (2,3), it modulates kidney handling of phosphate reabsorption and calcitriol production. Genetic and acquired abnormalities in FGF23 structure and metabolism cause conditions of either hyper-FGF23 or hypo-FGF23. Hyper-FGF23 is related to hypophosphatemia, while hypo-FGF23 is related to hyperphosphatemia. Both hyper-FGF23 and hypo-FGF23 are detrimental to humans. In this review, we will discuss the pathophysiology of FGF23 and hyper-FGF23 related renal phosphate wasting disorders (4).
Keywords: FGF23, Klotho, hypophosphatemic rickets, XLH, ADHR, ARHR, ENS, OGD, NF, McCune Albright syndrome, DMP-1, PHEX
Introduction
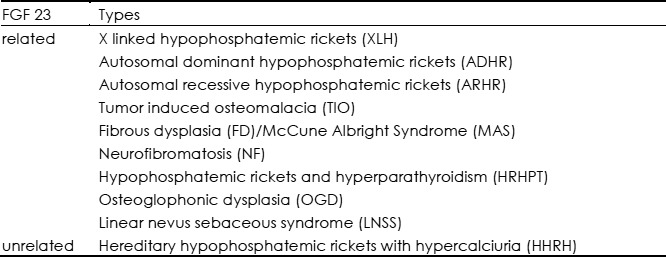
According to pathogenesis, ricketsis classified into calicopenic rickets, phosphopenicrickets, and a miscellaneous group associated with direct inhibitors of mineralization (5). In general, most instances of nutritional rickets are calicopenic, whereas heritable causes are usually phosphopenic. In this review we focus on hypophosphatemic rickets or osteomalacia. The causes of hypophosphatemia are various, of whichthe most prominent one is decreased reabsorption of phosphate in the proximal tubule.Although renal tubular disease can result in excessive renal phosphate wasting, in most hypophosphatemic disordersno abnormalities are found in the proximal tubule (6,7). It is speculated that an unknown factor is responsible for this phenomenon. The discovery of fibroblast growth factor 23 (FGF23), a member of the FGF family, which mediates renal tubular defects in phosphate reabsorption, has given new light to understanding hypophosphatemic disorders. FGF23 was identified by positional cloning in 2000 as the gene responsible for autosomal dominant hypophosphatemic rickets (ADHR) (1). Subsequent analyses indicate that several kinds of hypophosphatemic rickets are associated with high circulatory levels of FGF23. Thus, hypophosphatemic rickets can now be divided into types that are FGF23-mediated and those that are not (4). Table 1 lists FGF23 related and unrelated hypophophatemic rickets/osteomalacia.
Table 1. Types of hypophosphatemic rickets/osteomalcia.
Phosphate homeostasis
Phosphate comprises about 1% of total body weight. About 85% of total body phosphate resides in the bone, 14% in the cells, and only 1% in the serum and extracellular fluids. Maintenance of serum phosphate within its normal range allows for optimal mineralization of bone without deposition in vascular and other soft tissues. Serum phosphate concentration is determined by the balance among intestinal absorption of phosphate from the diet, its storage in bone, and its excretion in the urine. The proximal tubule is responsible for the reabsorption of phosphate filtered at the glomerulus and is the primary regulator of phosphate balance in the body. Transportation in the proximal tubule is driven primarily by sodium—potassium ATPase, which is located in the baso-lateral membrane of the cell (8–11). Under normal conditions, about 85% of the filtered phosphate is reabsorbed via the sodium—phosphate co-transporter (NaPi2a and NaPi2c) in the proximal tubule (9,11). Parathyroid hormone (PTH) is one of the most potent hormonal regulators of phosphate transport and promotes renal excretion of phosphate. It has now become clear that the mechanism of action of PTH is to stimulate endocytosis of the NaPi2a co-transporters from the apical membrane of the proximal tubule cells (9,12). FGF23 is also an important factor resulting in renal phosphate wasting. FGF23 acts in conjunction with PTH to decrease phosphate reabsorption by down-regulating NaPi2a and NaPi2c expression in the brush border of the proximal tubule (13–16). This, in turn, results in hyperphosphaturia and hypophosphatemia. FGF23 is a counter-regulatory hormone for 1,25(OH)2D in the bone– kidney feedback loop. 1,25(OH)2D stimulates FGF23 production, resulting in increased circulating FGF23, which in turn suppresses 1, 25(OH)2D concentrations. The mechanism will be discussed later. Thus, conditions associated with FGF23 excess characteristically have suppressed or inappropriately normal circulating 1,25(OH)2D concentrations in the face of hypophosphatemia.
Long term hypophosphatemia can result in rickets in children, while it can result in osteomalacia in adults. The clinical signs of hypophosphatemic rickets include squared skull and bowing of the legs, while adults typically present with bone pain and pathologic fracture. In the growth plate, hypophosphatemia causes arrest of apoptosis in the hypertrophic chondrocytes leading to rickets, while in the osteoblasts, hypophosphatemia inhibits maturation and mineralization, leading to osteomalacia (17).
FGF23 and phosphate regulation
The discovery of FGF23
Prader was the first to propose the idea of a circulating factor that could cause phosphate wasting (18). The first evidence that a circulating factor was responsible for the hypophosphatemia of phosphaturic disorders was demonstrated by Meyer et al and Nesbitt et al (19,20). The first to support this concept in humans were the findings from Miyauchi et al (21). This phosphaturic substance was termed ‘phosphatonin’ by Econs and Drezner (22) because of its ability to lower blood phosphorus levels. The first identification of FGF23 as the putative phosphatonin was when mutations in FGF23 were identified as the cause of autosomal dominant hypophosphatemic rickets (ADHR) (1). Since then, FGF23 has been found to be related to numerous hypophosphatemic disorders.
The structure of FGF23
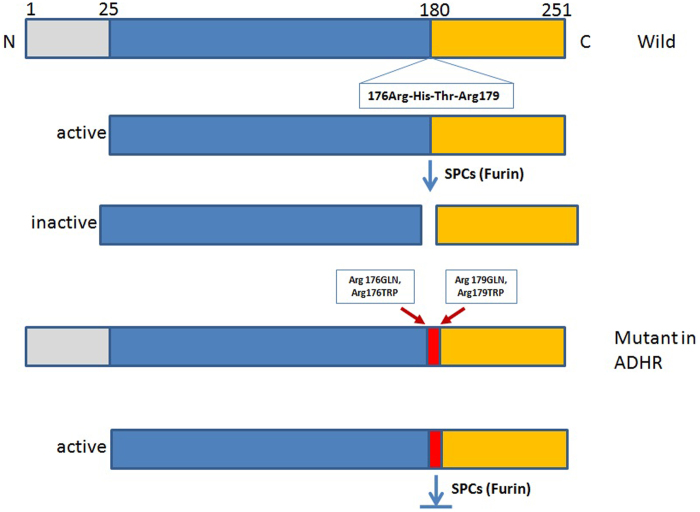
FGF23 is a glycoprotein with 251 amino acids. There is a signal peptide of 24 amino acids in the N-terminal portion of the FGF23 protein. Next to the signal peptide is the FGF homology region, which binds to FGF receptors (FGFR) in the tissue. Its C-terminal peptide binds to its co-receptor Klotho which is also a transmembrane protein. Both the N and C terminals are participants in the hormone's activity. The intact FGF23 is cleaved prior to secretion between Arg179 and Ser180 by furin recognizing Arg176-X-X-Arg179 motif. Both C-terminal FGF23 and N-terminal FGF23 are inactive. Figure 1 is the structure and function of FGF23. Mutations near this site in the RXXR furin-like cleavage domain of FGF23 (R176Q and R179W) impair proteolytic inactivation of FGF23, resulting in high FGF23 levels and leading to autosomal dominant hypophosphatemic rickets (ADHR) (23).
Figure 1.
Schematic structure of fibroblast growth factor (FGF) 23. The FGF23 structure is schematically illustrated. FGF23 has a disulfide bond in the FGF-like sequence and internal cleavage site immediately after the R176X177X178R179 consensus sequence for convertase and cleaved into two peptides.
Regulation of FGF23
FGF23 is almost exclusively produced by osteocytes and osteoblasts in response to high serum phosphate levels and 1,25(OH)2D (17,24), although aberrant production may occur in mesenchymal tumors associated with hypophosphatemic osteomalacia (25) and in the tissue of fibrous dysplasia, as in McCune-Albright syndrome with rickets (26). However, it is unclear how FGF23 secretion by bone cells is regulated. Serum phosphate and active vitamin D are positive regulators of FGF23. When serum phosphate or vitamin D levels are high, FGF23 level is elevated to increase renal phosphate wasting and to decrease active vitamin D levels. In addition to being regulated by phosphate and vitamin D, some clinical evidence suggests that FGF23 production is regulated by PHEX, DMP-1, and ENPP1 genes, which encode distinct protein products, but the molecular mechanisms whereby FGF23 is regulated by these factors are unknown (27–31). PHEX, DMP-1, matrix extra cellular phosphoglycoprotein (MEPE), and acidic serine aspartate-rich MEPE associated motif (ASARM) peptides have been proposed to dynamically regulate FGF23 expression in bone (31,32). Normally, the PHEX-DMP-1 binding initiates a signaling pathway that reduces FGF23 expression, but in XLHR and ARHR, mutations in PHEX or DMP-1, respectively, result in hypophosphatemia through increased FGF23 expression and stability which causes phosphate wasting (25,33–35).
FGF23 mode of action as a phosphatonin
The physiologic effect of FGF23 is on phosphate metabolism. Although receptors to FGF23 are present in many tissues, only the kidney and parathyroid gland respond to the hormone. The reason is that the phosphaturic actions of FGF23 require FGF receptors and essential cofactor Klotho to form a heterotrimer complex (36–38). Previous studies have found that the N-terminal portion of FGF23 interacts with FGFR 1c, while the C-terminal binds to Klotho and both interactions appear to be important for bioactivity of FGF23 (29).
Klotho, a single-pass transmembrane protein, is predominantly expressed in distal convoluted tubules in the kidney and the epithelium of the choroid plexus in the brain (39) and to a lesser extent, in the parathyroid glands (40). It serves as an obligate co-receptor, enabling FGF23 to interact with its receptor. Thus, Klotho is the modifier dictating which tissues will respond to FGF23.
As mentioned above, FGF23 acts on the kidney to promote phosphate excretion (13,41–43). The site of FGF23 action in the kidney is controversial. A “cross-talk” between the distal and proximal tubules is postulated for FGF23-induced phosphaturia based on the original notion that Klotho is exclusively expressed in the distal convoluted tubule, and phosphate reabsorption and regulation solely resides in the proximal tubule (44). There may be some proximal tubule expression, but it is clear that the majority of renal Klotho expression is in the DCT (45), While a distal-proximal cross-talk is still possible, FGF23 likely also has direct action on the proximal tubule (46). Another important function of FGF23 is to regulate serum vitamin D levels. The active form of vitamin D (1,25-dihydroxyvitamin D3) is synthesized in the kidney from its inactive precursor (25-hydroxyvitamin D3) with 1-α-hydroxylase encoded by the Cyp2 7B1 gene and is inactivated with 24-hydroxylase encoded by the Cyp24 gene. FGF23 suppresses Cyp27B1 gene expression and increases Cyp24 gene expression, so that 1-α-hydroxylase (CYP27B1) activity is decreased and 24-hydroxylase (CYP24) activity is increased, leading to reduced 1,25-(OH)2D concentrations (47).
Elevated concentrations of FGF23 are responsible for impaired bone mineralization. Induced hypophosphatemia is largely responsible for the features of rickets and osteomalacia, since serum phosphorus concentration plays an important role in the process of growth plate mineralization. What is less clear is whether or not hypophosphatemia is solely responsible for the osteomalacia. Recent studies have found that FGF23 (and soluble Klotho) may directly impact bone in diseases with elevated FGF23 levels (48,49).
High FGF23 and disorders with abnormal phosphate metabolism
Since its discovery in 2000, FGF23 has been found to be related to a number of hereditable and acquired phosphate wasting disorders. Genetic disorders include X-linked dominant hypophosphatemic rickets, autosomal recessive hypophosphatemic rickets (35,50,51), autosomal dominant hypophosphatemic rickets (1), hypophosphatemic rickets associated with McCune—Albright syndrome (52–54) and Linear sebaceous nevus syndrome (55–57). Acquired disorders include tumor induced osteomalacia. Table 2 lists a number of FGF23-mediated hypophosphatemic disorders. Table 3 lists the biochemical findings of the various forms of hypophosphatemic rickets.
Table 2. FGF23 mediated hypophosphatemic disorders.
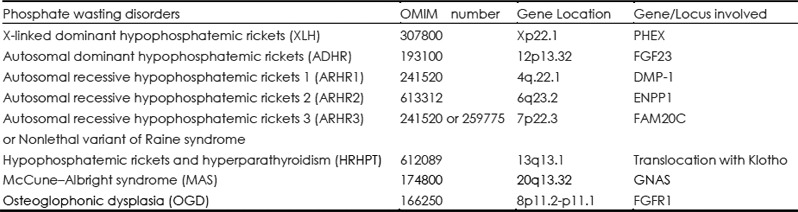
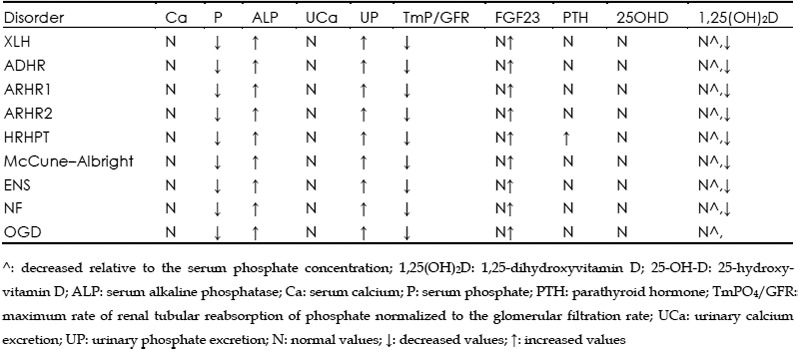
Table 3. Biochemical findings of the various forms of hypophosphatemic rickets due to genetic mutation/translocation.
XLH
Clinical features
XLH is the most commonly inherited form of the renal phosphate wasting disorder with a prevalence of 1/20 000 (58). The defective gene is on the X-chromosome, and female carriers are affected (i.e., an X-linked dominant disorder). Clinical manifestations vary in severity. It frequently manifests during late infancy when the child begins walking. The patient develops skeletal deformities that primarily include bowing of the long bones and widening of the metaphyseal region. The latter is most common at costochondral junctions (rachitic rosary) (59,60). These deformities are associated with diminished growth velocity, often resulting in short stature. Later in life, patients develop osteomalacia, enthesopathy (calcified ligaments and teno-osseous junctions), degenerative joint disease, and continued dental disease in particular tooth decay and dental abscesses. With medical therapy these abnormalities can be improved, but cannot be completely resolved.
Biochemical findings
Hypophosphatemia and low-normal circulating 1,25-dihydroxyvitamin D [1,25(OH)2D] levels are typical biochemical findings. Serum alkaline phosphatase (ALP) activity and 24-hour urine phosphate are elevated in children, while serum calcium is normal, as is circulating 25-OHD. Tubular reabsorption of phosphate (TRP) is decreased.
Genetics
Genetic linkage analysis has revealed inactivating mutations in the phosphate regulating gene with homology to endopeptidases on the X chromosome (PHEX), a gene located on Xp22 (61,62). PHEX protein is expressed in various tissues, including the kidney, but is most abundant in mature osteoblasts and odontoblasts. PHEX is a member of the M13 family of neutral endopeptidases. It is an integral membrane glycoprotein, which activates or degrades peptides. PHEX is secreted largely by osteocytes, but also by osteoblasts, and is important for normal matrix mineralization. Its role in the mineralization process is unclear at present. Serum intact FGF23 level is elevated in most XLH patients (63). FGF23 was initially referred to as a substrate of PHEX (64), but subsequent research found that FGF23 is not a direct substrate of PHEX (65). The mechanism by which PHEX regulates FGF23 remains unclear. It is now thought that it might control mineralization by binding proteins such as DMP-1 and matrix extracellular phosphoglycoprotein, which are both members of the SIBLING proteins and contain ASARM peptides, preventing their proteolysis and the release of ASARM peptides which inhibit mineralization (66).
ADHR
Clinical features
ADHR is a rare disorder that was first described by Bianchine et al in 1971 (58). Clinical and biochemical findings of ADHR patients are similar to those of XLHR patients. But ADHR, unlike XLHR, shows variable and incomplete penetrance with variable symptomatology and biochemical findings depending on the age at presentation. Patients who manifest the disease in their childhood develop short stature, rickets, bone pain, lower extremity deformities, and dental abscess. Some of the children have spontaneous resolution of symptoms during adulthood. On the other hand, patients with ADHR who manifest the disease in adulthood have symptoms similar to patients with TIO. Adults have bone pain, weakness, osteomalacia, and fractures/pseudofractures, but do not have short stature nor lower extremity deformities. Interestingly, the majority of patients who develop the disease in adulthood are women, and pregnancy triggers the onset of symptoms (67). Studies in ADHR humans have suggested that iron deficiency may be a trigger for dysregulation of FGF23, thus inducing active disease (68). Therefore, the onset of the disease is the product of gene-environment interactions. Imel et al also showed that serum iron was negatively correlated to both C-terminal FGF23 and intact FGF23 in ADHR patients (67). These studies suggested that iron status may regulate FGF23 metabolic pathways, and that low iron status results in increased FGF23 mRNA.
ADHR is caused by heterozygous mutations in the gene encoding FGF23 which is on 12p13 (1,69). Mutations identified in ADHR are missense mutations, and in each case, the mutation alters an arginine residue at either position 176 or 179. The mutations, which involve the proprotein convertase (furin) cleavage site, prevent the proteolytic processing of FGF23 to its inactive Nand C-terminal peptides. Mutant FGF23 proteins exhibit increased stability, are more active than wild-type FGF23, in vivo (70,71), and are likely present at elevated concentrations in ADHR patients (72). Thus, in ADHR patients, high circulating levels of FGF23 are due to decreased FGF23 degradation.
ARHR
Autosomal-recessive hypophosphatemic rickets (ARHR), is a rare disorder that is recently recognized (3,35). Clinical and biochemical findings of the affected individuals are similar to ADHR and XLH. Clinical features include rickets, skeletal deformities, dental defects, and affected individuals develop sclerotic bone lesions and enthesopathies. The clinical presentation of ARHR is not found at birth. Affected individuals present signs of rickets/osteomalacia later during childhood and even in adulthood (73,74). ARHR type 1 is caused by inactivating mutations in dentin matrix protein 1 (DMP-1), a member of the small integrin-binding ligand N-linked glycoprotein family of extracellular matrix proteins that augment mineralization (75,76). DMP-1 is widely expressed, but particularly abundant in bone, where it is synthesized by osteoblasts. It is involved in the regulation of transcription in undifferentiated osteoblasts. DMP-1 belongs to the SIB-LING protein family, which includes osteopontin, MEPE, bone sialoprotein II, and dentin sialoprotein, and whose genes are clustered on chromosome 4q21. DMP-1 undergoes phosphorylation during the early phase of an osteoblast's maturation and is subsequently exported into the extracellular matrix where it regulates the nucleation of hydroxyapatite. FGF23 levels are elevated or inappropriately normal for the low serum phosphate levels. Loss of function of DMP-1results in increased transcription of FGF23 by osteocytes, but the mechanism is not clear (35).
Recently, Levi-Litan et al, Lorenz-Depiereux et al and Saito et al identified an inactivating mutation in the ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) gene that cause ARHR type 2 (50,51,77). The gene product ectonucleotide pyrophosphatase/phosphodiesterase 1 is a cell surface enzyme responsible for generating inorganic pyrophosphate, which inhibits mineralization (51). Typically, loss-of-function mutations in ENPP1 cause generalized arterial calcification of infancy (GACI). However, hypophosphatemic rickets has also been found in patients with ENPP1 mutation. It remains unclear how the loss-of-function mutation in ENPP1 causes hypophosphatemic rickets (and not GACI), but it has been speculated that it might be through increased secretion of FGF23 (24). A recent study demonstrated that the FGF23 level is elevated in patients with mutations in ENPP1 (50). However, the mechanism has yet to be defined.
Another type ARHR with hypophosphatemia, hyperphosphaturia, dental anomalies, intracerebral calcifications and osteosclerosis of the long bones in the absence of rickets, was called nonlethal variant of Raine syndrome or ARHR type 3. Using whole exome sequencing, Rafaelsen et al identified compound heterozygous mutations in family with sequence similarity 20, member C (FAM20C) in patients with ARHR type 3. DMP1 is phosphorylated by FAM20C, with partial loss of Fam20c function, DMP1 is not properly phosphorylated, seems to be a mechanism that is involved in the pathways that generate FGF23-dependent hypophosphatemia (78).
Current and future treatments in hypophosphatemic rickets
Basic therapy
Renal phosphate wasting is the principal pathophysiological abnormality that leads to all these disorders. Current treatment for patients with FGF23-dependent hypophosphatemic rickets is based on the association of activated vitamin D metabolites (calcitriol or alfacalcidol) and oral inorganic phosphate salts. Treatment during growth partially corrects leg deformities, decreases the number of surgeries, and may improve adult height. Early initiation of treatment appears to optimize height outcomes (79,80). However, the improvement is often partial (81).
Although an impaired growth hormone (GH)-insulin like growth factor-I axis is not the primary cause of short stature in XLHR patients, GH is useful to improve growth in poorly growing XLHR patients (82). However, the results are not conclusive, as some patients do not have a recovery of growth during GH treatment. Orthopedic surgery is indicated when healing of skeletal deformities of the lower limbs by medical treatment is unsatisfactory.
With the recognition of increased FGF23 levels in the pathogenesis of these renal phosphate wasting disorders, new therapeutic strategies are being developed. One study found that subcutaneous injection of salmon calcitonin in XLHR patients causes a significant and sustained drop in circulating levels of FGF23, with an increase in serum phosphate levels (83). Studies in Hyp mice have demonstrated that the inhibition of FGF23 overproduction by anti-FGF23 neutralizing antibodies can improve phosphate levels, renal tubular phosphate reabsorption, and bone mineralization (84,85). These results indicate that inhibition of FGF23 activity is a promising therapy for FGF23-dependent hypophosphatemia. However, further studies are needed to determine whether these findings in mice can be applied to humans.
Tumor-induced osteomalacia (TIO)
Tumor-induced osteomalacia (TIO), or oncogenic osteomalacia, is a rare paraneoplastic syndrome of abnormal phosphate and vitamin D metabolism caused by typically small endocrine tumors. Clinical symptoms include chronic bone pain, which is usually the first presentation, weakness, and fatigue in association with a high risk of fragility fractures. Due to under-recognition of the disease, the diagnosis is commonly delayed for years. At our hospital, patients frequently present with multiple fractures, height loss, and a generalized debilitated status. Biochemical hallmarks of the disorder are similar to hypophosphatemic rickets. The diagnosis is confirmed by the dramatic improvement of symptoms and correction of metabolic abnormalities following complete excision of the responsible tumor.
The tumors are usually very small in size and their locations are often obscure. They can arise in bone or soft tissue, in any part of the body, and they grow slowly.
Most histologic diagnoses have been classified as phosphaturic mesenchymal tumors (PMT). A characteristic histologic feature of such tumors is a background of spindle cells that tend to have low mitotic activity, prominent vascularity, osteoclast-like giant cells, or the presence of bony tissue. Although most of these tumors are thought to have a benign histologic appearance, malignant presentation and metastases can occur (86–90). While metastases are rare, infiltration of surrounding connective tissue is typically present, which has significant implications for surgical management and emphasizes the importance for wide surgical margins to avoid persistence or recurrence.
Numerous reports show elevation of FGF23 in some, but not all, patients with TIO (91,92). Removal of the tumor is associated with reduction in serum FGF23 concentrations, and there is a temporal association between the reduction in FGF23 concentration and the elevation in serum phosphate, decrease in renal phosphate wasting, and increase in 1,25(OH)2D3 concentrations (93,94). The diagnosis of TIO can be challenging because the tumors are often small and difficult to find. Bone scanning, computerized tomography (CT) (95), magnetic resonance imaging (MRI), Indium-111 pentetreotide or octreotide scintigraphy, and positron emission tomography (PET) have all been employed in an effort to localize the tumor (96). A stepwise approach, first performing functional tests and then anatomical tests, is advocated. In our hospital, we have successfully used 99Tcm-OCT scintigraphy to locate tumors in most patients with TIO as we previously reported (94). Therefore, we prefer octreotide scintigraphy as the first approach. As for patients who are octreotide negative, when a tumor is highly suspected, we use FDG-PET/CT. Recently, 68Ga-DOTANOC PET/CT has been explored as a means of finding TIO tumors (97). Once suspicious lesions have been identified with functional imaging, one should proceed to anatomical imaging such as X-rays, CT, and/or MRI scans to confirm the location of the tumor.
The treatment of choice for TIO is resection of the tumor with a wide margin to ensure complete resection. Resection with a wide surgical margin is very important, as recurrences of these tumors have been reported (89,90,98). Therefore, intermittent monitoring of patients after tumor resection should be performed. Tumor resection is almost always curative, and following complete resection of the tumor, there is relatively rapid improvement. FGF23 disappears rapidly from the circulation (99) and serum phosphate returns to normal within five days post operation (94). Most patients feel better within days to weeks of tumor removal. Bone healing starts immediately, but depending on the severity of the disease, it may take a year or more for significant clinical improvement.
When the tumor cannot be localized or is not surgically resectable, medical therapy with phosphate supplementation and calcitriol or alfacalcidiol is used. The treatment regimen that follows is essentially the same as that used in non-TIO hypophosphatemia. When initiating treatment, it is prudent to check weekly labs to guide titration of medications until treatment targets are reached. Future treatment will likely be guided by a better understanding of the biology of FGF23 and the nature of these tumors.
Fibrous dysplasia (FD)/McCune Albright Syndrome
McCune—Albright syndrome (MAS) is characterized by café-au-lait spots, polyostotic fibrous bone dysplasia, and multiple endocrine hyperfunctions, such as precocious puberty, hyperthyroidism, autonomous adrenal hyperplasia, and growth hormone secreting pituitary adenoma (100). Fibrous dysplasia (FD) is a focal and benign fibrous bone lesion that is caused by the activating mutation of the Gsa protein (101,102). Hypophosphatemic rickets is sometimes observed as a complication of MAS (103).
FD and MAS are caused by somatic activating mutations of the guanine nucleotide binding protein, alpha stimulating gene (GNAS1), the gene encoding the alpha-subunit of the stimulatory G-protein.
Renal phosphate wasting occurs in approximately 50% of patients with MAS and FD of bone. However, the cause of hypophosphatemia is unclear. Recently, Riminucci et al (26) reported the important role of FGF23 as a cause of hypophosphatemia in MAS. However, hypophosphatemia is not always associated with MAS patients (104). Hypophosphatemia appears as the age of the MAS patients increases, which is usually accompanied by advanced bone fibrous dysplasia lesions (105). It is plausible that overproduction of FGF23 is dependent on the severity of FD bone lesions, which may be associated with increased serum FGF23 levels, and which could explain the presence or absence of hypophosphatemia in MAS patients. In situ hybridization analysis of FGF23 mRNA expression identified “fibrous” cells, osteogenic cells, and cells associated with microvascular walls as specific cellular sources of FGF23 in FD. Production of FGF23 by FD tissue may play an important role in the renal phosphate wasting syndrome associated with FD/MAS (26).
Treatment with bisphosphonates has been shown to reduce serum FGF23 levels, which result in a reduction of renal phosphate wasting. The mechanisms underlying the reduction of FGF23 by bisphosphonates are unclear.
Neurofibromatosis (NF)
Skeletal lesions are not uncommon in neurofibromatosis. Most of them are considered to be dysplastic in nature. Association of osteomalacia or rickets with NF has only rarely been documented (106,107). Osteomalacia occurring in NF is quite distinct from the more common skeletal affection seen in the disease and its pathogenesis is still unknown. Osteomalacia associated with NF1 is characterized by later onset in adulthood, renal phosphate loss with hypophosphataemia, multiple and pseudofractures in typical cases. Treatment with oral phosphate and vitamin D is effective (108,109). It is hypothesized that melatonin deficiency in cases of NF might play a role in the pathogenesis of hyperphosphaturea (110). We have a few patients who are diagnosed with neurofibromatosis and osteomalacia, while FGF23 is positive in the neurofibroma bundle of a few of the patients (data not published yet).
Linear nevus sebaceous syndrome (LNSS)
Linear nevus sebaceous syndrome (LNSS)/epidermal nevus syndrome (ENS) is a sporadic condition characterized by congenital epidermal nevi associated with anomalies in other organ systems, most commonly the central nervous system and skeleton (111). Abnormalities in the eyes, heart, or genitourinary system have also been reported (112). Hypophosphataemic rickets is rare (113–115) in ENS; the manifestation usually presents in the first years of life (114,116). It is generally accepted that it may represent a variant of tumor-induced rickets/osteomalacia (113,114,117,118) characterized by renal phosphate wasting and inappropriately low serum levels of 1, 25(OH)2D. The pathogenic mechanism involved in the onset of hypophosphataemic rickets in ENS is not fully clarified. It has been proposed that FGF23 is the putative phosphatonin, based on demonstration of its elevated blood levels in a patient with LNSS (56). Subsequent studies also found the same result (119,120). In typical tumor-induced osteomalacia, symptoms tend to be resolved after removal of the tumor (117). Excision of epidermal lesions with ENS may improve the hypophosphataemic rickets in some patients (47,56), while it has failed to heal rickets in most patients (115,116,118). Although it is possible that large amounts of FGF23 are autonomously secreted by LNS lesions, this factor was not found to be directly excreted from the LNS lesions (119). Further study is needed to understand the exact mechanism of how FGF23 is related to hypophosphatemic rickets in LNSS.
Osteoglophonic dysplasia (OGD)
Osteoglophonic dysplasia(OGD), also known as Fair-bank-Keats syndrome, is a very rare skeletal dysplasia with craniosynostosis, and multiple lucent metaphyseal defects. It is an autosomal dominant disorder characterized by short stature, although most cases are the result of de novo mutations (121). Recently, White et al identified several heterozygous missense mutations in fibroblast growth factor receptor 1 (FGFR1) (121). These mutations are in highly conserved residues comprising the extracellular (asparagine 330 to isoleucine, Asn330Ile) and transmembrane domains (tyrosine 374 to cysteine, Tyr374Cys; and cysteine 381 to arginine, Cys381Arg) of FGFR1, which seems to lead to constitutive receptor activation (122,123). Hypophosphatemia, secondary to renal phosphate wasting associated with inappropriately normal 1,25(OH)2D3 levels, is present in affected individuals (124,125). FGF23 levels are elevated in some OGD patients. The elevated levels of FGF23 result in renal phosphate wasting seen in this condition (121). It is thought that the skeletal lesions in OGD patients develop because the constitutive activation of the FGFR1 leads to an up-regulation of FGF23 secretion in the metaphyseal growth plate. However, the mechanism is not yet clear.
Hypophosphatemic rickets and hyperparathyroidism (HRHPT)
Hypophosphatemic rickets and hyperparathyroidism (HRHPT) is a syndrome featuring both hypophosphatemic rickets and hyperparathyroidism due to parathyroid hyperplasia as well as other skeletal abnormalities. Brownstein et al investigated a patient with hypophosphatemic rickets and hyperparathyroidism due to parathyroid hyperplasia (126). They found no mutation in PHEX, DMP-1, and FGF23. They found a de novo translocation with a breakpoint adjacent to alpha-Klotho, which encodes abeta-glucuronidase. Plasma alpha-Klotho levels, beta-glucuronidase activity, and circulating FGF23 levels were markedly elevated. Moreover, emerging evidence indicates that alpha-Klotho is critical for FGF23 signaling. Whether the elevated FGF23 level seen in the patient is the direct result of increased alpha-Klotho (for example, if degradation of FGF23 is prevented by interaction with alpha-Klotho), or is part of a negative-feedback loop responding to hyperparathyroidism is difficult to discern at present.
Conclusion
Rickets, due to inherited or acquired causes, remains a significant problem across the globe. Considerable advances have been made in identifying genes responsible for a number of the inherited causes of hypophosphatemic rickets and to clarify the pathways of regulation of phosphate metabolism. The discovery that FGF23 overproduction is a primary cause of hypophosphatemic rickets may suggest a new approach for the treatment of these disorders.
Dysregulation of FGF23 occurs in a number of acquired and inherited disorders of phosphate homeostasis. Further investigations are required to understand the regulation of FGF23 expression.
Acknowledgments
This paper was supported by the National Natural Science Foundation of China (No. 81070687 and 81170805); National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (Grant No. 2008ZX09312-016). Beijing Natural Science Foundation (No. 7121012).
References
- ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettifor JM, Thandrayen K. Hypophosphatemic rickets: unraveling the role of FGF23. Calcif Tissue Int. 2012;91:297–306. doi: 10.1007/s00223-012-9651-0. [DOI] [PubMed] [Google Scholar]
- Imel EA, Carpenter TO. Rickets: The skeletal disorders of impaired calcium or phosphate availability. Pediatr Endocrinol. 2013;2:357–378. [Google Scholar]
- Tenenhouse HS, Beck L. Renal Na(+)-phosphate cotransporter gene expression in X-linked Hyp and Gy mice. Kidney Int. 1996;49:1027–1032. doi: 10.1038/ki.1996.149. [DOI] [PubMed] [Google Scholar]
- Hruska KA, Rifas L, Cheng SL, Gupta A, Halstead L, Avioli L. X-linked hypophosphatemic rickets and the murine Hyp homologue. Am J Physiol. 1995;268:F357–F362. doi: 10.1152/ajprenal.1995.268.3.F357. [DOI] [PubMed] [Google Scholar]
- Rector FC., Jr. Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983;244:F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- Forster IC, Hernando N, Biber J, Murer H. Proximal tubular handling of phosphate: A molecular perspective. Kidney Int. 2006;70:1548–1559. doi: 10.1038/sj.ki.5001813. [DOI] [PubMed] [Google Scholar]
- Tenenhouse HS. Phosphate transport: molecular basis, regulation and pathophysiology. J Steroid Biochem Mol Biol. 2007;103:572–577. doi: 10.1016/j.jsbmb.2006.12.090. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol. 2007;27:503–515. doi: 10.1159/000107069. [DOI] [PubMed] [Google Scholar]
- Biber J, Hernando N, Traebert M, Volkl H, Murer H. Parathyroid hormone-mediated regulation of renal phosphate reabsorption. Nephrol Dial Transplant. 2000;15 Suppl 6:29–30. doi: 10.1093/ndt/15.suppl_6.29. [DOI] [PubMed] [Google Scholar]
- Quarles LD. Endocrine functions of bone in mineral metabolism regulation. J Clin Invest. 2008;118:3820–3828. doi: 10.1172/JCI36479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. 2003;285:E1–E9. doi: 10.1152/ajpendo.00016.2003. [DOI] [PubMed] [Google Scholar]
- Liu S, Tang W, Zhou J, Stubbs JR, Luo Q, Pi M, Quarles LD. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- Liu S, Gupta A, Quarles LD. Emerging role of fibroblast growth factor 23 in a bone-kidney axis regulating systemic phosphate homeostasis and extracellular matrix mineralization. Curr Opin Nephrol Hypertens. 2007;16:329–335. doi: 10.1097/MNH.0b013e3281ca6ffd. [DOI] [PubMed] [Google Scholar]
- Tiosano D, Hochberg Z. Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab. 2009;27:392–401. doi: 10.1007/s00774-009-0079-1. [DOI] [PubMed] [Google Scholar]
- Prader A, Illig R, Uehlinger E, Stalder G. Rickets following bone tumor. Helv Paediatr Acta. 1959;14:554–565. [PubMed] [Google Scholar]
- Meyer RA, Jr., Meyer MH, Gray RW. Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res. 1989;4:493–500. doi: 10.1002/jbmr.5650040407. [DOI] [PubMed] [Google Scholar]
- Nesbitt T, Coffman TM, Griffiths R, Drezner MK. Cross transplantation of kidneys in normal and Hyp mice. Evidence that the Hyp mouse phenotype is unrelated to an intrinsic renal defect. J Clin Invest. 1992;89:1453–1459. doi: 10.1172/JCI115735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi A, Fukase M, Tsutsumi M, Fujita T. Hemangio-pericytoma-induced osteomalacia: tumor transplantation in nude mice causes hypophosphatemia and tumor extracts inhibit renal 25-hydroxyvitamin D 1-hydroxylase activity. J Clin Endocrinol Metab. 1988;67:46–53. doi: 10.1210/jcem-67-1-46. [DOI] [PubMed] [Google Scholar]
- Econs MJ, Drezner MK. Tumor-induced osteomalacia—unveiling a new hormone. N Engl J Med. 1994;330:1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- Ubaidus S, Li M, Sultana S, De Freitas PH, Oda K, Maeda T, Takagi R, Amizuka N. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc (Tokyo) 2009;58:381–392. doi: 10.1093/jmicro/dfp032. [DOI] [PubMed] [Google Scholar]
- Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:31656–31663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Shimizu Y. Fibroblast growth factor 23 as a phosphotropic hormone and beyond. J Bone Miner Metab. 2011;29:507–514. doi: 10.1007/s00774-011-0298-0. [DOI] [PubMed] [Google Scholar]
- Alon US. Clinical practice. Fibroblast growth factor (FGF)23: a new hormone. Eur J Pediatr. 2011;170:545–554. doi: 10.1007/s00431-010-1382-5. [DOI] [PubMed] [Google Scholar]
- Gattineni J, Baum M. Genetic disorders of phosphate regulation. Pediatr Nephrol. 2012;27:1477–1487. doi: 10.1007/s00467-012-2103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter TO. The expanding family of hypophosphatemic syndromes. J Bone Miner Metab. 2012;30:1–9. doi: 10.1007/s00774-011-0340-2. [DOI] [PubMed] [Google Scholar]
- Baroncelli GI, Toschi B, Bertelloni S. Hypophosphatemic rickets. Curr Opin Endocrinol Diabetes Obes. 2012;19:460–467. doi: 10.1097/MED.0b013e328358be97. [DOI] [PubMed] [Google Scholar]
- Penido MG, Alon US. Phosphate homeostasis and its role in bone health. Pediatr Nephrol. 2012;27:2039–2048. doi: 10.1007/s00467-012-2175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, David V, Li H, Dai B, Feng JQ, Quarles LD. Over-expression of the DMP1 C-terminal fragment stimulates FGF23 and exacerbates the hypophosphatemic rickets phenotype in Hyp mice. Mol Endocrinol. 2012;26:1883–1895. doi: 10.1210/me.2012-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi T, Umemura S, Shintani S, Ooshima T. Phex mutation causes overexpression of FGF23 in teeth. Arch Oral Biol. 2008;53:99–104. doi: 10.1016/j.archoralbio.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Bastepe M, Benet-Pages A, Amyere M, Wagenstaller J, Muller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Juppner H, Strom TM. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens. 2006;15:437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa H, Kawakami E, Kaneko I, Kuwahata M, Ito M, Kusano K, Saito H, Fukushima N, Miyamoto K. Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflugers Arch. 2003;446:585–592. doi: 10.1007/s00424-003-1084-1. [DOI] [PubMed] [Google Scholar]
- Segawa H, Yamanaka S, Ohno Y, Onitsuka A, Shiozawa K, Aranami F, Furutani J, Tomoe Y, Ito M, Kuwahata M, Imura A, Nabeshima Y, Miyamoto K. Correlation between hyperphosphatemia and type II Na-Pi cotransporter activity in klotho mice. Am J Physiol Renal Physiol. 2007;292:F769–F779. doi: 10.1152/ajprenal.00248.2006. [DOI] [PubMed] [Google Scholar]
- Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- Farrow EG, Davis SI, Summers LJ, White KE. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009;20:955–960. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297:F282–F291. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, Richards WG. Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int. 2011;89:140–150. doi: 10.1007/s00223-011-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin JE, Maeda N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23:939–948. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Schnabel D, Tiosano D, Hausler G, Strom TM. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet. 2010;86:267–272. doi: 10.1016/j.ajhg.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet. 2010;86:273–278. doi: 10.1016/j.ajhg.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan WG, Nibbe AF, Schwartz TB, Ray RD. Fibrous dysplasia of bone with vitamin D resistant rickets: a case study. Metabolism. 1968;17:988–998. doi: 10.1016/0026-0495(68)90004-8. [DOI] [PubMed] [Google Scholar]
- Dent CE, Gertner JM. Hypophosphataemic osteomalacia in fibrous dysplasia. Q J Med. 1976;45:411–420. [PubMed] [Google Scholar]
- Dachille RD, Goldberg JS, Wexler ID, Shons AR. Fibrous dysplasia-induced hypocalcemia/rickets. J Oral Maxillofac Surg. 1990;48:1319–1322. doi: 10.1016/0278-2391(90)90491-j. [DOI] [PubMed] [Google Scholar]
- Zutt M, Strutz F, Happle R, Habenicht EM, Emmert S, Haenssle HA, Kretschmer L, Neumann C. Schimmelpenning-Feuerstein-Mims syndrome with hypophosphatemic rickets. Dermatology. 2003;207:72–76. doi: 10.1159/000070948. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Jueppner HW, Deyoung BR, O'Dorisio M S, Given KS. Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet A. 2005;134:233–236. doi: 10.1002/ajmg.a.30599. [DOI] [PubMed] [Google Scholar]
- Srinivas UM, Tourani KL. Epidermal nevus syndrome with hypophosphatemic renal rickets with hypercalciuria: a bone marrow diagnosis. Int J Hematol. 2008;88:125–126. doi: 10.1007/s12185-008-0127-y. [DOI] [PubMed] [Google Scholar]
- Bianchine JW, Stambler AA, Harrison HE. Familial hypophosphatemic rickets showing autosomal dominant inheritance. Birth Defects Orig Artic Ser. 1971;7:287–295. [PubMed] [Google Scholar]
- Pettifor JM. What's new in hypophosphataemic rickets? Eur J Pediatr. 2008;167:493–499. doi: 10.1007/s00431-007-0662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W, Meng X, Jiang Y, Li M, Xing X, Pang L, Wang O, Pei Y, Yu LY, Sun Y, Hu Y, Zhou X. Three novel mutations of the PHEX gene in three Chinese families with X-linked dominant hypophosphatemic rickets. Calcif Tissue Int. 2007;81:415–420. doi: 10.1007/s00223-007-9067-4. [DOI] [PubMed] [Google Scholar]
- A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99:1200–1209. doi: 10.1172/JCI119276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia WB, Jiang Y, Li M, Xing XP, Wang O, Hu YY, Zhang HB, Liu HC, Meng XW, Zhou XY. Levels and dynamic changes of serum fibroblast growth factor 23 in hypophosphatemic rickets/osteomalacia. Chin Med J (Engl) 2010;123:1158–1162. [PubMed] [Google Scholar]
- Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- David V, Martin A, Hedge AM, Drezner MK, Rowe PS. ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate. Am J Physiol Renal Physiol. 2011;300:F783–F791. doi: 10.1152/ajprenal.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Econs MJ, McEnery PT. Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab. 1997;82:674–681. doi: 10.1210/jcem.82.2.3765. [DOI] [PubMed] [Google Scholar]
- Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96:3541–3549. doi: 10.1210/jc.2011-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse K, Woelfel D, Strom TM. Loss of renal phosphate wasting in a child with autosomal dominant hypophosphatemic rickets caused by a FGF23 mutation. Horm Res. 2001;55:305–308. doi: 10.1159/000050018. [DOI] [PubMed] [Google Scholar]
- White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang O, Xia W, Jiang Y, Li M, Xing X, Hu Y, Liu H, Meng X, Zhou X. FGF23 analysis of a Chinese family with autosomal dominant hypophosphatemic rickets. J Bone Miner Metab. 2012;30:78–84. doi: 10.1007/s00774-011-0285-5. [DOI] [PubMed] [Google Scholar]
- Imel EA, Econs MJ. Fibroblast growth factor 23: roles in health and disease. J Am Soc Nephrol. 2005;16:2565–2575. doi: 10.1681/ASN.2005050573. [DOI] [PubMed] [Google Scholar]
- Perry W, Stamp TC. Hereditary hypophosphataemic rickets with autosomal recessive inheritance and severe osteosclerosis. A report of two cases. J Bone Joint Surg Br. 1978;60-B:430–434. doi: 10.1302/0301-620X.60B3.681423. [DOI] [PubMed] [Google Scholar]
- Scriver CR, Reade T, Halal F, Costa T, Cole DE. Autosomal hypophosphataemic bone disease responds to 1,25-(OH)2D3. Arch Dis Child. 1981;56:203–207. doi: 10.1136/adc.56.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010;25:591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow EG, White KE. Recent advances in renal phosphate handling. Nat Rev Nephrol. 2010;6:207–217. doi: 10.1038/nrneph.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Shimizu Y, Hori M, Taguchi M, Igarashi T, Fukumoto S, Fujitab T. A patient with hypophosphatemic rickets and ossification of posterior longitudinal ligament caused by a novel homozygous mutation in ENPP1 gene. Bone. 2011;49:913–916. doi: 10.1016/j.bone.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Rafaelsen SH, Raeder H, Fagerheim AK, Knappskog P, Carpenter TO, Johansson S, Bjerknes R. Exome sequencing reveals FAM20c mutations associated with fibroblast growth factor 23-related hypophosphatemia, dental anomalies, and ectopic calcification. J Bone Miner Res. 2013;28:1378–1385. doi: 10.1002/jbmr.1850. [DOI] [PubMed] [Google Scholar]
- Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26:1381–1388. doi: 10.1002/jbmr.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan C, Guegan K, Offiah A, Neill RO, Hiorns MP, Ellard S, Bockenhauer D, Hoff WV, Waters AM. Growth in PHEX-associated X-linked hypophosphatemic rickets: the importance of early treatment. Pediatr Nephrol. 2012;27:581–588. doi: 10.1007/s00467-011-2046-z. [DOI] [PubMed] [Google Scholar]
- Makitie O. Early Treatment Improves Growth and Biochemical and Radiographic Outcome in X-Linked Hypophosphatemic Rickets. Journal of Clinical Endocrinology & Metabolism. 2003;88:3591–3597. doi: 10.1210/jc.2003-030036. [DOI] [PubMed] [Google Scholar]
- Zivicnjak M, Schnabel D, Staude H, Even G, Marx M, Beetz R, Holder M, Billing H, Fischer DC, Rabl W, Schumacher M, Hiort O, Haffner D, Hypophosphatemic Rickets Study Group of the Arbeitsgemeinschaft fur Padiatrische E, Gesellschaft fur Padiatrische N Three-year growth hormone treatment in short children with X-linked hypophosphatemic rickets: effects on linear growth and body disproportion. J Clin Endocrinol Metab. 2011;96:E2097–E2105. doi: 10.1210/jc.2011-0399. [DOI] [PubMed] [Google Scholar]
- Liu ES, Carpenter TO, Gundberg CM, Simpson CA, Insogna KL. Calcitonin administration in X-linked hypophosphatemia. N Engl J Med. 2011;364:1678–1680. doi: 10.1056/NEJMc1010928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aono Y, Hasegawa H, Yamazaki Y, Shimada T, Fujita T, Yamashita T, Fukumoto S. Anti-FGF-23 neutralizing antibodies ameliorate muscle weakness and decreased spontaneous movement of Hyp mice. J Bone Miner Res. 2011;26:803–810. doi: 10.1002/jbmr.275. [DOI] [PubMed] [Google Scholar]
- Shimada T, Fukumoto S. FGF23 as a novel therapeutic target. Adv Exp Med Biol. 2012;728:158–170. doi: 10.1007/978-1-4614-0887-1_10. [DOI] [PubMed] [Google Scholar]
- Wyman AL, Paradinas FJ, Daly JR. Hypophosphataemic osteomalacia associated with a malignant tumour of the tibia: report of a case. J Clin Pathol. 1977;30:328–335. doi: 10.1136/jcp.30.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico H, Fernandez-Miranda E, Sanz J, Gomez-Castresana F, Escriba A, Hernandez ER, Krsnik I. Oncogenous osteomalacia: a new case secondary to a malignant tumor. Bone. 1986;7:325–329. doi: 10.1016/8756-3282(86)90251-6. [DOI] [PubMed] [Google Scholar]
- Harvey JN, Gray C, Belchetz PE. Oncogenous osteomalacia and malignancy. Clin Endocrinol (Oxf) 1992;37:379–382. doi: 10.1111/j.1365-2265.1992.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Ogose A, Hotta T, Emura I, Hatano H, Inoue Y, Umezu H, Endo N. Recurrent malignant variant of phosphaturic mesenchymal tumor with oncogenic osteomalacia. Skeletal Radiol. 2001;30:99–103. doi: 10.1007/s002560000306. [DOI] [PubMed] [Google Scholar]
- Uramoto N, Furukawa M, Yoshizaki T. Malignant phosphaturic mesenchymal tumor, mixed connective tissue variant of the tongue. Auris Nasus Larynx. 2009;36:104–105. doi: 10.1016/j.anl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Cai Q, Hodgson SF, Kao PC, Lennon VA, Klee GG, Zinsmiester AR, Kumar R. Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med. 1994;330:1645–1649. doi: 10.1056/NEJM199406093302304. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Suzuki H, Ogura S, Imai R, Yamazaki Y, Yamashita T, Miyamoto Y, Okazaki H, Nakamura K, Nakahara K, Fukumoto S, Fujita T. Venous sampling for fibroblast growth factor-23 confirms preoperative diagnosis of tumor-induced osteomalacia. J Clin Endocrinol Metab. 2004;89:3979–3982. doi: 10.1210/jc.2004-0406. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Xia WB, Xing XP, Silva BC, Li M, Wang O, Zhang HB, Li F, Jing HL, Zhong DR, Jin J, Gao P, Zhou L, Qi F, Yu W, Bilezikian JP, Meng XW. Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: Report of 39 cases and review of the literature. J Bone Miner Res. 2012;27:1967–1975. doi: 10.1002/jbmr.1642. [DOI] [PubMed] [Google Scholar]
- White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Juppner H, Econs MJ. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab. 2001;86:497–500. doi: 10.1210/jcem.86.2.7408. [DOI] [PubMed] [Google Scholar]
- Farrow EG, White KE. Tumor-induced osteomalacia. Expert Rev Endocrinol Metab. 2009;4:435–442. doi: 10.1586/eem.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse E, Moessinger E, Rosenthal H, Laenger F, Brabant G, Petrich T, Gratz KF, Bastian L. Oncogenic osteomalacia: exact tumor localization by co-registration of positron emission and computed tomography. J Bone Miner Res. 2007;22:158–162. doi: 10.1359/jbmr.060909. [DOI] [PubMed] [Google Scholar]
- Clunie GP, Fox PE, Stamp TC. Four cases of acquired hypophosphataemic (‘oncogenic’) osteomalacia. Problems of diagnosis, treatment and long-term management. Rheumatology (Oxford) 2000;39:1415–1421. doi: 10.1093/rheumatology/39.12.1415. [DOI] [PubMed] [Google Scholar]
- Khosravi A, Cutler CM, Kelly MH, Chang R, Royal RE, Sherry RM, Wodajo FM, Fedarko NS, Collins MT. Determination of the elimination half-life of fibroblast growth factor-23. Journal of Clinical Endocrinology & Metabolism. 2007;92:2374–2377. doi: 10.1210/jc.2006-2865. [DOI] [PubMed] [Google Scholar]
- Spiegel AM, Shenker A, Weinstein LS. Receptor-effector coupling by G proteins: implications for normal and abnormal signal transduction. Endocr Rev. 1992;13:536–565. doi: 10.1210/edrv-13-3-536. [DOI] [PubMed] [Google Scholar]
- Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325:1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992;89:5152–5156. doi: 10.1073/pnas.89.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PA, van Dop C, Migeon CJ. McCune-Albright syndrome. Long-term follow-up. JAMA. 1986;256:2980–2984. [PubMed] [Google Scholar]
- Collins MT, Chebli C, Jones J, Kushner H, Consugar M, Rinaldo P, Wientroub S, Bianco P, Robey PG. Renal phosphate wasting in fibrous dysplasia of bone is part of a generalized renal tubular dysfunction similar to that seen in tumor-induced osteomalacia. J Bone Miner Res. 2001;16:806–813. doi: 10.1359/jbmr.2001.16.5.806. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Miyamoto KI, Ozono K, Taketani Y, Katai K, Miyauchi A, Shima M, Yoshikawa H, Yoh K, Takeda E, Okada S. Hypophosphatemic rickets accompanying McCune-Albright syndrome: evidence that a humoral factor causes hypophosphatemia. J Bone Miner Metab. 2001;19:287–295. doi: 10.1007/s007740170012. [DOI] [PubMed] [Google Scholar]
- Chadha M, Singh AP, Singh AP. Hypophosphataemic osteomalacia in neurofibromatosis. Acta Orthop Belg. 2009;75:847–850. [PubMed] [Google Scholar]
- Lambert J, Lips P. Adult hypophosphataemic osteomalacia with Fanconi syndrome presenting in a patient with neurofibromatosis. Neth J Med. 1989;35:309–316. [PubMed] [Google Scholar]
- Konishi K, Nakamura M, Yamakawa H, Suzuki H, Saruta T, Hanaoka H, Davatchi F. Hypophosphatemic osteomalacia in von Recklinghausen neurofibromatosis. Am J Med Sci. 1991;301:322–328. doi: 10.1097/00000441-199105000-00006. [DOI] [PubMed] [Google Scholar]
- Retnam VJ, Rangnekar DM, Bhandarkar SD. Neurofibromatosis with hypophosphatemic osteomalacia (Von Recklinghausen-Hernberg-Edgren-Swann syndrome) (a case report) J Assoc Physicians India. 1980;28:319–322. [PubMed] [Google Scholar]
- Abdel-Wanis M, Kawahara N. Hypophosphatemic osteomalacia in neurofibromatosis 1: hypotheses for pathogenesis and higher incidence of spinal deformity. Med Hypotheses. 2002;59:183–185. doi: 10.1016/s0306-9877(02)00254-2. [DOI] [PubMed] [Google Scholar]
- Solomon LM, Esterly NB. Epidermal and other congenital organoid nevi. Curr Probl Pediatr. 1975;6:1–56. doi: 10.1016/s0045-9380(75)80010-7. [DOI] [PubMed] [Google Scholar]
- Grebe TA, Rimsza ME, Richter SF, Hansen RC, Hoyme HE. Further delineation of the epidermal nevus syndrome: two cases with new findings and literature review. Am J Med Genet. 1993;47:24–30. doi: 10.1002/ajmg.1320470106. [DOI] [PubMed] [Google Scholar]
- Aschinberg LC, Solomon LM, Zeis PM, Justice P, Rosenthal IM. Vitamin D-resistant rickets associated with epidermal nevus syndrome: demonstration of a phosphaturic substance in the dermal lesions. J Pediatr. 1977;91:56–60. doi: 10.1016/s0022-3476(77)80444-7. [DOI] [PubMed] [Google Scholar]
- Carey DE, Drezner MK, Hamdan JA, Mange M, Ahmad MS, Mubarak S, Nyhan WL. Hypophosphatemic rickets/osteomalacia in linear sebaceous nevus syndrome: a variant of tumor-induced osteomalacia. J Pediatr. 1986;109:994–1000. doi: 10.1016/s0022-3476(86)80283-9. [DOI] [PubMed] [Google Scholar]
- Goldblum JR, Headington JT. Hypophosphatemic vitamin D-resistant rickets and multiple spindle and epithelioid nevi associated with linear nevus sebaceus syndrome. J Am Acad Dermatol. 1993;29:109–111. doi: 10.1016/s0190-9622(08)81813-0. [DOI] [PubMed] [Google Scholar]
- Skovby F, Svejgaard E, Moller J. Hypophosphatemic rickets in linear sebaceous nevus sequence. J Pediatr. 1987;111:855–857. doi: 10.1016/s0022-3476(87)80204-4. [DOI] [PubMed] [Google Scholar]
- Eyskens B, Proesmans W, van Damme B, Lateur L, Bouillon R, Hoogmartens M. Tumour-induced rickets: a case report and review of the literature. Eur J Pediatr. 1995;154:462–468. doi: 10.1007/BF02029356. [DOI] [PubMed] [Google Scholar]
- Oranje AP, Przyrembel H, Meradji M, Loonen MC, De Klerk JB. Solomon's epidermal nevus syndrome (type: linear nevus sebaceus) and hypophosphatemic vitamin D-resistant rickets. Arch Dermatol. 1994;130:1167–1171. [PubMed] [Google Scholar]
- Narazaki R, Ihara K, Namba N, Matsuzaki H, Ozono K, Hara T. Linear nevus sebaceous syndrome with hypophosphatemic rickets with elevated FGF-23. Pediatr Nephrol. 2012;27:861–863. doi: 10.1007/s00467-011-2086-4. [DOI] [PubMed] [Google Scholar]
- Sethi SK, Hari P, Bagga A. Elevated FGF-23 and parathormone in linear nevus sebaceous syndrome with resistant rickets. Pediatr Nephrol. 2010;25:1577–1578. doi: 10.1007/s00467-010-1485-2. [DOI] [PubMed] [Google Scholar]
- White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ. Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet. 2005;76:361–367. doi: 10.1086/427956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow EG, Davis SI, Mooney SD, Beighton P, Mascarenhas L, Gutierrez YR, Pitukcheewanont P, White KE. Extended mutational analyses of FGFR1 in osteoglophonic dysplasia. Am J Med Genet A. 2006;140:537–539. doi: 10.1002/ajmg.a.31106. [DOI] [PubMed] [Google Scholar]
- Roberts TS, Stephen L, Beighton P. Osteoglophonic dysplasia: dental and orthodontic implications. Orthod Craniofac Res. 2006;9:153–156. doi: 10.1111/j.1601-6343.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- Beighton P, Cremin BJ, Kozlowski K. Osteoglophonic dwarfism. Pediatr Radiol. 1980;10:46–50. doi: 10.1007/BF01644343. [DOI] [PubMed] [Google Scholar]
- Beighton P. Osteoglophonic dysplasia. J Med Genet. 1989;26:572–576. doi: 10.1136/jmg.26.9.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP. A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci U S A. 2008;105:3455–3460. doi: 10.1073/pnas.0712361105. [DOI] [PMC free article] [PubMed] [Google Scholar]