Abstract
Mechanical forces play critical roles in the development and remodeling processes of bone. As an alternative cell source for bone engineering, adipose-derived stem cells (ASCs) should be fully investigated for their responses to mechanical stress. Similarly, the osteogenic potential, stimulated by mechanical stress, should be compared with bone marrow stromal cells (BMSCs), which have been clinically used for bone tissue engineering. In this study, ASCs and BMSCs were osteogenic-induced for 48 hours, and then subjected to uniaxial mechanical stretching for 2 or 6 hours. Cell orientation, osteogenic regulatory genes, osteogenic genes and ALP activities were measured and compared between ASCs and BMSCs. ASCs could align in a perpendicular way to the direction of stretching stress, while BMSCs did not present a specific alignment. Both 2 and 6 hours mechanical stretching could enhance the mRNA expression of Osx and Runx2 in BMSCs and ASCs, while OCN mRNA only increased in ASCs after 6 hours mechanical loading. Mechanical stretching enhanced the BMP-2 mRNA expression in ASCs, while only after 6 hours of mechanical loading significantly increased the BMP-2 gene expression in BMSCs. Significant differences only exist between ASCs and BMSCs loaded at 2 hours of mechanical stretching. It is concluded that ASCs are more rapid responders to mechanical stress, and have greater potential than BMSCs in osteogenesis when stimulated by mechanical stretching, indicating their usefulness for bone study in a rat model.
Keywords: mechanotransduction, stem cells, osteoblasts, adipocytes
Introduction
Bone mesenchymal stem cells (BMSCs) and adipose-derived stem cells (ASCs) have both been used experimentally for gene therapy and tissue engineering applications (1,2). Currently, BMSCs are obtained by aspiration of 10−40 mL of bone marrow from the iliac crest, or at the time of bone marrow biopsy, and isolated by their adherence properties (3). ASCs can be obtained from subcutaneous adipose tissue by liposuction surgeries, and these procedures yield anywhere from 100 mL to 3L of lipoaspirate tissue (4). As a result, ASCs can be found in abundant quantities and harvested by minimally, invasive procedures. In recent studies, ASCs could differentiate along multiple cell lineage pathways in a regulatable and reproducible manner (5). Thus, ASCs are multipotent and hold promise for a range of therapeutic applications (6).
A number of studies have compared the differences between BMSCs and ASCs, in terms of multi-lineage potentials, surface markers, as well as origins, with contradictory results being generated. Cai et al. reported that BMSCs and ASCs were both smooth muscle actin (SMA) positive pericytes (7,8), which agrees with Yang et al.'s results that ASCs were CD34 negative and SMA positive, and belonged to a subset of pericytes (9). On the other hand, Lin et al. claimed ASCs were CD34 positive and SMA negative and thus, were not pericytes (10). De Ugartea et al. found that ASCs and BMSCs, obtained from the same patient, did not have any significant differences for multi-lineage differentiation capacity (4). Noëla et al. suggested that in vitro, as well as in vivo, ADSC displayed the same ability as MSC, to differentiate into chondrocytes or osteoblasts (11). However, opposite results have also indicated that the ASCs might have an inferior potential for both osteogenesis and chondrogenesis, compared with the BMSCs (12,13).
Mechanical force is one of the fundamental biological factors that affect fracture healing and bone remodeling. Most studies report that mechanical stress promotes osteogenic differentiation of BMSCs or osteoblasts (14–21). Mechanical stress might also up-regulate the expression of osteogenic genes of ASC (22–25). Results from our previous study have shown that cyclic tensile mechanical loading of long duration could promote the expression of BMP-2 and Runx2 and thus, osteogenic differentiation of ASCs in osteogenic medium (26) or adipogenic medium (27). However, limited studies were found concerning a comparison of osteogenic potential, stimulated by mechanical stress between ASCs and BMSCs. Regarding the whole bone regeneration process being affected by mechanical stress, we determined that a comparison of osteogenic differentiation by mechanical stretching between BMSCs and ASCs was necessary.
The present study was proposed to compare the effects of mechanical stretching on osteogenic differentiation of BMSCs and ASCs. ASCs and BMSCs from the same rat and the same passage were subjected to flow cytometry for surface markers and loaded by mechanical stretch, and the cell orientation, osteogenic regulatory genes and osteogenic genes and ALP activities were measured and compared.
Materials and methods
Cell Culture
ASCs were isolated as described by Zuk et al. (28) with minor modifications. Eighteen four-week-old Sprague-Dawley rats were prepared with standard sterile technique to excise the inguinal fat pads, following protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Sichuan University. The obtained tissue was washed twice with phosphate-buffered saline supplemented with streptomycin sulfate and penicillin (PBS), incubated in α-modified Eagle's medium (α-MEM) and finely minced into small pieces of 0.5 cm3. The fine tissue was digested by 0.05% type 1 collagenase with vigorous shaking for 40 min at 37 °C. Floating populations were removed by centrifugation at 1 200 r·min-1 for 8 min and cells were pelleted. A single-cell suspension was obtained and re-suspended in culture medium composed of α-MEM supplemented with antibiotics (penicillin-streptomycin solution), sodium bicarbonate and 10% fetal bovine serum (Gibco, UK), and cells were finally seeded on the plastic flask for the final isolation step selected for the plastic adherent populations.
BMSCs were isolated from the same rat after the ASCs. The inguinal fat pads were excised using published standard protocols. Femur and tibia bones from Sprague-Dawley rats were sawn and gelatinous marrow were extracted under sterile conditions. Briefly, gelatinous bone marrow were suspended in PBS and dispersed mechanically by passing through syringes with a needle of 20-gauge. The cells were resuspended in PBS and layered on top of a percoll gradient (d =1.073 g·mL−1). The percoll gradient consisted of an equal volume of suspending and percoll. The cell pellet was centrifuged immediately over the percoll gradient for 20 min at 900g. The intermediate zone was enriched in BMSCs, which were collected by a Pasteur pipette. The enriched BMSCs suspended in percoll were diluted with an equal volume of PBS and centrifuged at 900g for 10 min. The resulting pellet was suspended in culture medium composed of α-MEM supplemented with antibiotics (penicillin-streptomycin solution), sodium bicarbonate and 10% fetal bovine serum (Gibco, UK), which is the same as that in which the ASCs were kept.
ASCs and BMSCs were cultured at 37 °C in a 90% humidified atmosphere and 5% CO2. The third passage was used for the following test.
Flow Cytometry
To investigate the similarity of source of BMSC and ASCs, the phenotypic profile of BMSCs and ASCs were investigated by flow cytometry for CD34, CD44 and CD146. Flow cytometry was performed on a FAC scan argon laser cytometer. Cells were harvested in 0.25% trypsin/EDTA and fixed for 30 min in ice-cold 4% formaldehyde. Following fixation, cells were washed in flow cytometry buffer (FCB; PBS, 2% FBS, 0.2% Tween-20). Cell aliquots (1×106 cells) were incubated in FCB containing monoclonal antibodies to CD34, CD44, CD90 and CD146 (Biolegend). Control cells were incubated with the same concentration of isotype-matched control antibodies of irrelevant specificity. After washing twice with washing buffer, CD34 and CD146 expression was assessed with a FACScan (BD Biosciences Immunocytometry Systems, San Jose, CA). To analyze CD44 expression, cells were incubated with fluorescein isothiocyanate (FITC, 0.1 mg·mL−1)-conjugated secondary antibody (Alex Fluo, Invitrogen) and washed twice with washing buffer.
Induction of osteogenic differentiation
When cells were totally attached to the plates, the culture medium was replaced with osteogenic medium containing α-MEM supplemented with 10% FBS, 0.1 μmol·L−1 dexamethasone (Sigma), 10 mmol·L−1 glycerol phosphate (Sigma), and 50 μmol·L−1 ascorbic acid-2-phosphate (Sigma). The cell density at initial induction period was 1×105·mL−1.
Application of cyclic tensile stretch
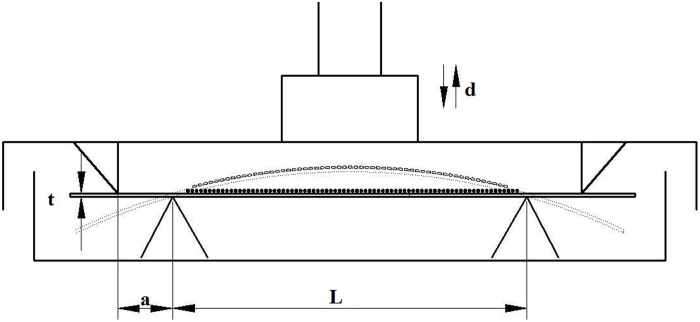
Cyclic tensile stretching of the cells was conducted on a four-point bending mechanical loading device, as shown in figure 1, the loading box has two metal wedges on the lid and two on the bottom. The loading plate with cells attached on the upper surface of the loading box. When the lid is pushed downwards by a stepper motor, the loading plate will bend in between the wedges, thus the cells on the upper surface were subjected to tensile stretch. The magnitude of the strain was calculated according to the following formula: ϵ=td·[a(L−1.33a)]−1, where ϵ refers to the strain applied on the cells, t refers to the thickness of the plate, d refers to the vertical displacement of the lid, L refers to the length of the plate and a refers to the distance between the wedges on the lid and the bottom.
Figure 1.
Illustrations of the four-point bending mechanical loading device. L: distance between two outer pressure points; a: distance between the outer and inner pressure points; t: thickness of the loading plate; d: distance of the pressure point movement; the strain (ϵ) of cells attached to the upper side of the loading plate could be calculated as the following formula: ϵ=td·[a(L-1.33a)]−1.
Force-loading plates were made out of the bottom of the BD Falcon™ 75 cm2 cell culture flask (BD Bioscience, CA, USA), 7.8×3.8 cm in size and 1.2 mm thick. ASCs and BMSCs from the same rat in passage 3 were seeded on the loading plates at a density of 2×105 cells per plate, and the plates were divided into 2 hours loading groups and 6 hours loading groups with 3 samples for each group. Every plate was kept in glass culture dishes of 9 cm in diameter. When cells were totally attached to the plates 6 hours later, the culture medium was replaced with osteogenic medium. After osteo-induced for 48 hours, cells were subjected to uniaxial cyclic tensile stretch by four-point bending mechanical loading device with the following protocols: 2 000 uϵ, 1 HZ for 2 and 6 hours, respectively. All cells cultured on the plastic plate uniformly exposed to exactly the same tensile force. All measurements started 2 hours after the last strain cycle.
Analysis of mRNA by real-time PCR
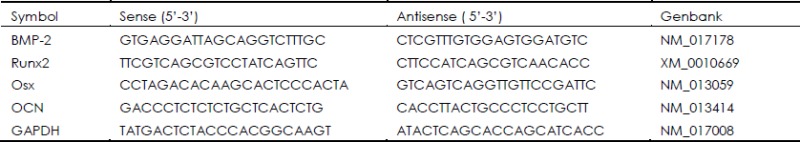
Total RNA of rat ASCs was extracted with Total Tissue/Cell RNA Extraction Kits (Watson, China) according to the manufacturer's protocol. Total RNA integrity was verified by 1.2% agarose gel electrophoresis and its yield and purity were confirmed by the ratio of A (260)/A (280) using the UV-spectroscopy. The cDNA synthesis was performed using transcriptor reverse transcriptase (Takara Biotechnology, Shiga, Japan). In order to establish the standard curve of a certain gene, cDNA samples were amplified with a RT-PCR kit (Tiangen Biotech, China) with specific designed primers displayed in Table 1. Real-time PCR was run in the ABI PRISM 7300 Sequence Detection system (Applied Biosystems, USA) using the hot-start DNA Master SYBR Green I Kit (Takara Biotechnology, Co., Ltd. Japan) with the following program: 95 °C for 10 min; 40 cycles of 95 °C for 15 s and 60 °C for 1 min, followed by melting curve analysis. The specificities of PCR products were verified by melting curve analyses between 60 °C and 95 °C. For each reaction, a melting curve was generated to test the primer dimer formation and false priming. Values of relative gene expression were normalized by the house-keeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Table 1. Primer sequences for real-time PCR assay.
Cellular ALP specific activity assay
After dynamic loading, BMSCs and ASCs were incubated in osteogenic medium for 2 hours. Cell extract was prepared with 0.1% Triton X-100. Cellular ALP activity was assayed at the end of the incubation with 10 mmol·L−1 p-nitrophenyl phosphate in 0.15 mol·L−1 sodium carbonate buffer (pH=10.3) and 1 mmol·L−1 MgCl2 and was normalized against cellular protein determined by the bicinchoninic acid assay (Pierce, Rockford, IL).
Statistical analysis
We performed three or more independent sets of experiments. Data were presented as means±SD. and was analyzed by t-test and one-way ANOVA. P<0.05 was considered to be statistically significant.
Results
Phenotypic characterization of ASCs and BMSCs
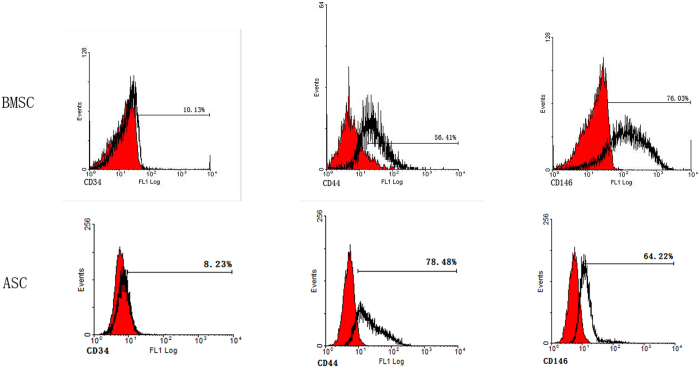
After isolation and culture for three days, both ASCs and BMSCs were negative for known haematopoietic marker CD34, and positive for mesenchymal markers CD44 and CD146 (Figure 2).
Figure 2.
Flow cytometry showing the expression of surface markers CD34, CD44, and CD146 on ASCs and BMSCs.
Primary culture and influence of cyclic tension stretch on cell orientation
The cell mass of ASCs grew slowly and single colonies could be identified after 2–3 days of culture, finally reaching 80% confluence after 5 days in primary culture. ASCs grew at a rapid rate in the second passage, and displayed a cell doubling time of 2 days and presented a homogeneous elongated spindle and polygonal fibroblastic shape. In contrast, BMSCs underwent a prolonged doubling time for 7 days in primary culture, and 3–4 days doubling time for secondary culture.
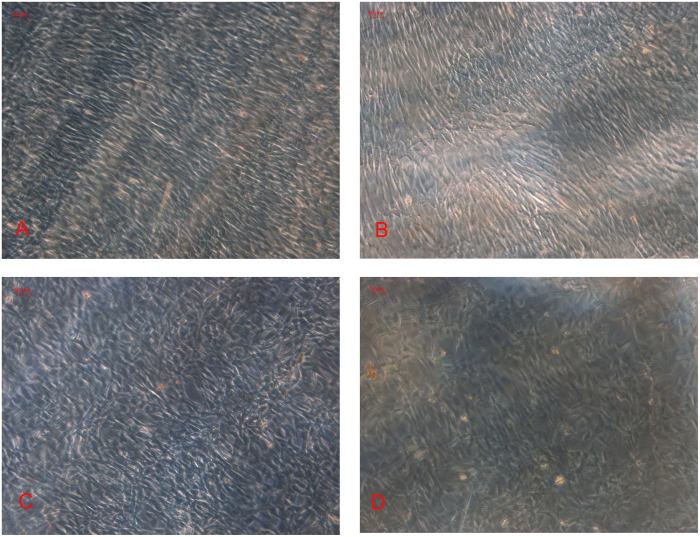
When subjected to cyclic stretching for 6 hours, ASCs presented a particular orientation away from the direction of the long axis of the plate (Figure 3A). In contrast, cells in the control group showed no particular orientation (Figure 3B). However, BMSCs did not present a specific orientation, neither in 6 mechanical loading groups, nor in control groups (Figure 3C and 3D).
Figure 3.
Effects of cyclic tensile stretch on the orientation of ASCs and BMSCs. ASCs and BMSCs of passage 3 were seeded at 2×105 per plate and cultured in osteogenic medium. A: After subjected to cyclic tensile stretch for 6 hours (1 Hz, 2 000 μϵ), ASCs presented a 90% confluence and an orientation perpendicular to the strain axis. B: ASCs in static controls and 2-hour duration group oriented randomly (magnification, ×100). BMSCs in mechanical loading groups (C) and control groups (D) did not present a specific orientation.
Influence of cyclic tension stretch on the expression of osteogenic genes
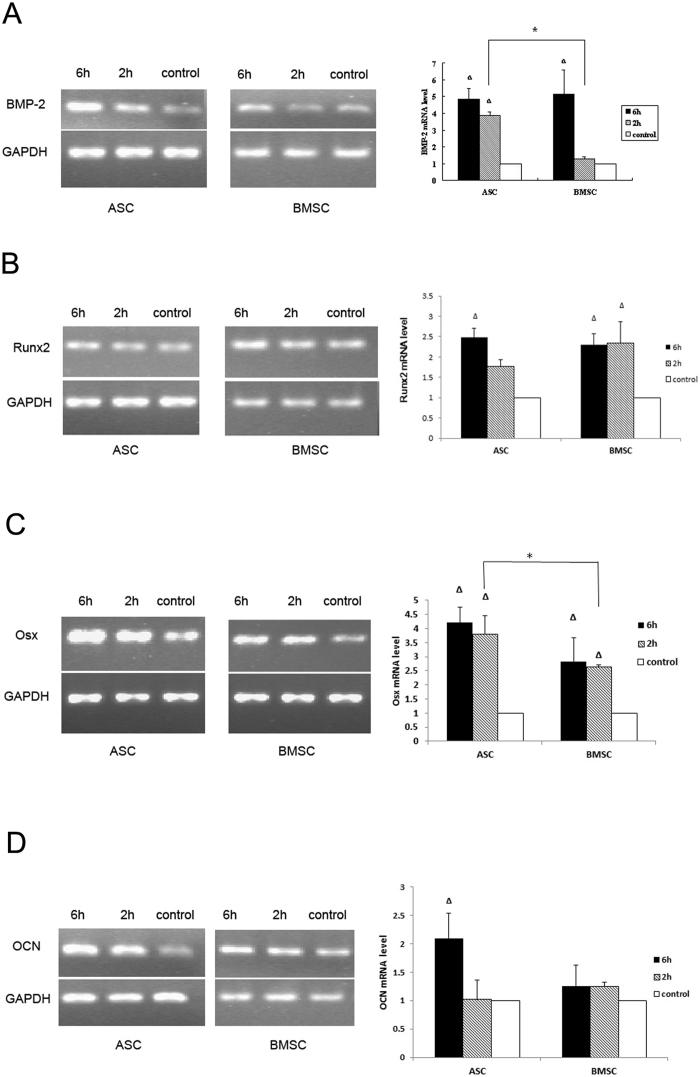
To investigate the regulatory genes during the osteogenic process treated by cyclic tension strain and osteogenic medium, we determined the mRNA level of BMP-2, a potential osteogenic differentiation factors in vitro, and Runx2, the molecular biomarker of osteogenic process and important transcription factor that regulate osteogenic differentiation.
No significant difference was observed between ASCs and BMSCs for BMP-2 relative expression when cells were loaded for 6 hours. However, when cells were mechanically loaded for 2 hours, mRNA of BMP-2 in ASCs significantly increased by 3-fold, while BMP-2 mRNA in BMSCs was not changed significantly (Figure 4). Runx2 mRNA, in both BMSCs and ASCs, were significantly increased when cells were loaded for 6 hours, while no significant difference was observed between ASCs and BMSCs for Runx2 mRNA, when cells were loaded for either 6 hours or 2 hours (Figure 4).
Figure 4.
The mRNA level of BMP-2 (A) in ASCs increased by 3-fold after two hours of mechanical stretch (P<0.01) and 4-fold after six hours of mechanical stretch (P<0.01) compared to the unloaded control; the mRNA level of BMP-2 in BMSCs increased by 4-fold after 6 hours (P<0.01). When cells were mechanically stretched for 2 hours, BMP-2 mRNA in ASCs increased more significantly (P<0.05) than BMSCs. The mRNA level of Runx2 (B) in ASCs increased by 1.5-fold after 6 hours of mechanical loading (P<0.05) compared to the unloaded control; the mRNA level of Runx2 in BMSCs increased by 2-fold after 2 and 6 hours of mechanical loading (P<0.05). No significant differences of Runx2 mRNA expression were observed between ASCs and BMSCs. The mRNA level of Osx (C) of ASCs and BMSCs were both significantly enhanced (P<0.01). When cells were mechanically stretched for 2 hours, Osx mRNA in ASCs increased more significantly than BMSCs (P<0.05). Mechanical stretch promoted the OCN mRNA (D) expression of ASCs rather than OCN mRNA in BMSCs. The mRNA level of OCN in ASCs increased by 1-fold when loaded for 6 hours (P<0.05) compared to the unloaded control. However, mRNA level of OCN in BMSCs did not changed significantly.
To investigate the effect of mechanical strain on the osteogenic differentiation process of ASCs, the Osx and OCN mRNA expression was quantitatively analyzed. Osx mRNA, in both ASCs and BMSCs, were significantly increased by 2 and 6 hours of mechanical stretching. However, a significant difference only existed after 2 hours of loading between ASCs and BMSCs. Osx mRNA was relatively more increased in ASCs than in BMSCs (Figure 4). OCN mRNA was only significantly increased in ASCs, compared with control group when cells were loaded for 6 hours, and no significant difference was detected between ASCs and BMSCs when cells were loaded for either 2 or 6 hours (Figure 4).
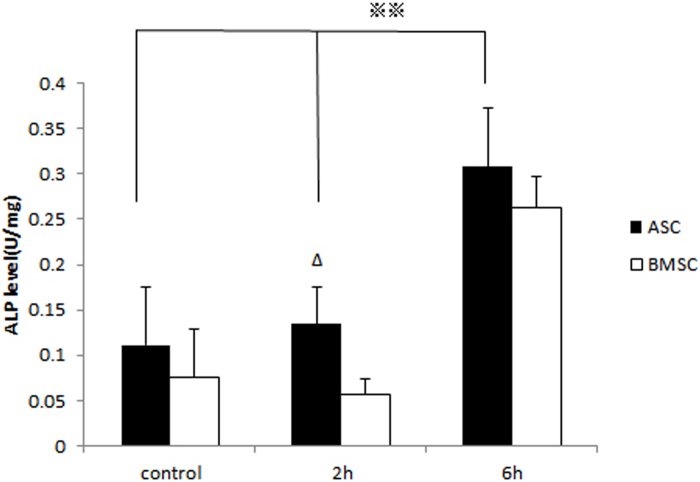
Influence of cyclic stretching on ALP activity
ALP is a marker enzyme of osteoblasts and is involved in the biomineralization process of bone matrix. ALP specific activity was assessed by enzyme histochemistry, recommended by the International Federation of Clinical Chemists (IFCC). ALP activity in ASCs was significantly enhanced by mechanical loading for 2 and 6 hours and in BMSCs, it was promoted only by 6-hour loading. In the 2-hour groups, significantly higher ALP activity was observed in the ASCs than BMSCs (Figure 5).
Figure 5.
Mechanical stretch promoted the ALP activity of ASCs rather than BMSCs. After being mechanically loaded for 2 hours, ALP activity in ASCs was significantly promoted (*P<0.05).
Discussion
In this study, primary culture of rat ASCs and BMSCs were obtained, and flow cytometry revealed similar phenotypic characteristics of them. Both types of cells were osteogenic-induced for 48 hours, and then subjected to uniaxial mechanical for 2 hours or 6 hours. The cell orientation, osteogenic regulatory genes and osteogenic genes and ALP activities were measured and compared between ASCs and BMSCs.
ASCs and BMSCs are similar in the expression of surface markers according to our study as well as previously published reports (7–9,29). Specifically, we found CD34, a surface marker of hematopoietic cells, negative or weakly positive, in both ASCs (8.23%) and BMSCs (10.13%), whereas CD146, a surface marker of mesenchymal stem cells, was positive in both (64.22% and 76.03%), implying a shared origin of ASCs and BMSCs.
ASCs aligned in a perpendicular way to the direction of stretching stress, while BMSCs did not present a specific alignment pattern. The change in cell alignment, as a response to mechanical loading, coincided with previously published reports, that cells responded to uniaxial cyclic stretching by reorganizing their long axes close to 100–110 degrees from the orientation of loading after a prolonged stimulation, though the cells they employed were BMSCs (30,31). The reason why BMSCs in our study did not show specific alignment pattern, was possibly because of the relatively short loading time (6 hours), compared to Koike's (48 hours) (31), and the comparatively high density of cells compared to Zhang's (30) as shown in the figures. Both 6 hours and 2 hours of mechanical stretching could enhance mRNA transcription of Osx and Runx2 in BMSCs and ASCs, while OCN mRNA transcription only increased in ASCs by 6 hours of mechanical loading. Mechanical stretching could enhance the BMP-2 mRNA expression in ASCs, while only 6 hours mechanical loading could significantly increase the BMP-2 gene expression in BMSCs.
Significant differences only exist between ASCs and BMSCs after being loaded by two hours of mechanical stretching, which might suggest that ASCs are faster mechano-responsive cells than BMSCs; the expressions of osteogenic genes and osteogenic regulatory genes after six hours of loading were not significantly different between ASCs and BMSCs, indicating ASCs have similar osteogenic capacity with BMSCs under mechanical strain.
For bone tissue engineering and bone regeneration, the advantage of adipose tissue, as a source of multilineage cells, is its relative abundance and ease of procurement by local excision, or suction-assisted liposuction (4,32), making ASCs a potential alternative to BMSCs. Previous studies have compared the characteristics of yield of adherent stromal cells, growth kinetics, cell senescence, multi-lineage differentiation capacity and gene transduction efficiency between ASCs and BMSCs. De Ugarte and Morizono found no significant differences between multi-lineage differentiation, and suggested that ASCs have the potential to serve as substitutes for BMSCs in tissue-engineering applications involving fat, bone, cartilage and possibly nervous tissue (33). Yoshimura and Muneta isolated rat MSCs from bone marrow and adipose tissue, and then compared the multi-differentiation potentials, and found that adipose stem cells have higher adipogenic potentials and similar osteogenic- and chondrogenic-potentials (32).
Results of the present study suggest that mechanical stretching has a similar effect on OCN and Runx2 transcription of ASCs and BMSCs, as well as BMP-2, Osx mRNA expression and ALP activity from ASCs and BMSCs, loaded by 6 hours mechanical loading. Nevertheless, BMP-2 mRNA, Osx mRNA and ALP activities were significantly enhanced by 2 hours mechanical loading in ASCs than BMSCs. In summary, ASCs respond to mechanical stretching faster, and achieve similar osteogenetic effects as BMSCs, which makes them a potential alternative to BMSCs as seed cells for bone tissue engineering.
Acknowledgments
This work was funded by the Peabody Foundation, Inc., the Constance and Anthony A Franchi Fund for Pediatric Orthopaedics at the MassGeneral Hospital for Children, and National Natural Science Foundation of China (81071273, 31170929, 81200810).
References
- Niemeyer P, Vohrer J, Schmal H, Kasten P, Fellenberg J, Suedkamp NP, Mehlhorn AT. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008;10:784–795. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- Panetta NJ, Gupta DM, Longaker MT. Bone regeneration and repair. Curr Stem Cell Res Ther. 2010;5:122–128. doi: 10.2174/157488810791268618. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Alfonso Z, Zuk PA, Elbarbary A, Zhu M, Ashjian P, Benhaim P, Hedrick MH, Fraser JK. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Estes BT, Diekman BO, Moutos FT, Gimble JM. Nicolas Andry Award: Multipotent adult stem cells from adipose tissue for musculoskeletal tissue engineering. Clin Orthop Relat Res. 2010;468:2530–2540. doi: 10.1007/s11999-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Lin Y, Friedrich CC, Neville C, Pomerantseva I, Sundback CA, Zhang Z, Vacanti JP, Hauschka PV, Grottkau BE. Bone marrow derived pluripotent cells are pericytes which contribute to vascularization. Stem Cell Rev. 2009;5:437–445. doi: 10.1007/s12015-009-9097-6. [DOI] [PubMed] [Google Scholar]
- Cai X, Lin Y, Hauschka PV, Grottkau BE. Adipose stem cells originate from perivascular cells. Biol Cell. 2011;103:435–447. doi: 10.1042/BC20110033. [DOI] [PubMed] [Google Scholar]
- Yang YI, Kim HI, Choi MY, Son SH, Seo MJ, Seo JY, Jang WH, Youn YC, Choi KJ, Cheong SH, Shelby J. Ex vivo organ culture of adipose tissue for in situ mobilization of adipose-derived stem cells and defining the stem cell niche. J Cell Physiol. 2010;224:807–816. doi: 10.1002/jcp.22188. [DOI] [PubMed] [Google Scholar]
- Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- Noël D, Caton D, Roche S, Bony C, Lehmann S, Casteilla L, Jorgensen C, Cousin B. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314:1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis cartilage. 2005;13:845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehl-horn AT, Pearce S, Kasten P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31:3572–3579. doi: 10.1016/j.biomaterials.2010.01.085. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kahn CJ, Chen HQ, Tran N, Wang X. Effect of uniaxial stretching on rat bone mesenchymal stem cell: orientation and expressions of collagen types I and III and tenascin-C. Cell Biol Int. 2008;32:344–352. doi: 10.1016/j.cellbi.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Mauney JR, Sjostorm S, Blumberg J, Horan R, O'Leary JP, Vunjak-Novakovic G, Volloch V, Kaplan DL. Mechanical stimulation promotes osteogenic differentiation of human bone marrow stromal cells on 3-D partially demineralized bone scaffolds in vitro. Calcif Tissue Int. 2004;74:458–468. doi: 10.1007/s00223-003-0104-7. [DOI] [PubMed] [Google Scholar]
- Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36:1047–1055. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- van Eijk F, Saris DB, Creemers LB, Riesle J, Willems WJ, van Blitterswijk CA, Verbout AJ, Dhert WJ. The effect of timing of mechanical stimulation on proliferation and differentiation of goat bone marrow stem cells cultured on braided PLGA scaffolds. Tissue Eng Part A. 2008;14:1425–1433. doi: 10.1089/ten.tea.2007.0081. [DOI] [PubMed] [Google Scholar]
- Potier E, Noailly J, Ito K. Directing bone marrow-derived stromal cell function with mechanics. J Biomech. 2010;43:807–817. doi: 10.1016/j.jbiomech.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Matziolis D, Tuischer J, Matziolis G, Kasper G, Duda G, Perka C. Osteogenic predifferentiation of human bone marrow-derived stem cells by short-term mechanical stimulation. Open Orthop J. 2011;5:1–6. doi: 10.2174/1874325001105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wu Y, Jiang Z, Jiang L, Fang B. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling. Int J Mol Med. 2012;29:1083–1089. doi: 10.3892/ijmm.2012.934. [DOI] [PubMed] [Google Scholar]
- Prè D, Ceccarelli G, Visai L, Benedetti L, Imbriani M, Cusella De Angelis MG, Magenes G. High-Frequency Vibration Treatment of Human Bone Marrow Stromal Cells Increases Differentiation toward Bone Tissue. Bone Marrow Res. 2013;2013:803450. doi: 10.1155/2013/803450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wagner DR, Bekerman E, Chiou M, James AW, Carter D, Longaker MT. Connective tissue growth factor in regulation of RhoA mediated cytoskeletal tension associated osteogenesis of mouse adipose-derived stromal cells. PLoS One. 2010;5:e11279. doi: 10.1371/journal.pone.0011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauß S, Dudziak S, Hagemann R, Barcikowski S, Fliess M, Israelowitz M, Kracht D, Kuhbier JW, Radtke C, Reimers K, Vogt PM. Induction of osteogenic differentiation of adipose derived stem cells by microstructured nitinol actuator-mediated mechanical stress. PLoS One. 2012;7:e51264. doi: 10.1371/journal.pone.0051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cai X, Wang J, Tang H, Yuan Q, Gong P, Lin Y. Mechanical stretch inhibits adipogenesis and stimulates osteogenesis of adipose stem cells. Cell Prolif. 2012;45:158–166. doi: 10.1111/j.1365-2184.2011.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Gong P, Lin Y, Zhang L, Li X, Yuan Q, Tan Z, Wang Y, Man Y, Tang H. Cyclic Tensile Stretch Modulates osteogenic differentiation of Adipose-derived stem cells via BMP-2 pathway. Arch Med Sci. 2010;6:152–159. doi: 10.5114/aoms.2010.13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba K, Yang X, Wu L, Wei X, Fu N, Fu Y, Cai X, Yao Y, Ge Y, Lin Y. Jagged-1-mediated activation of notch signalling induces adipo-genesis of adipose-derived stem cells. Cell Prolif. 2012;45:538–544. doi: 10.1111/j.1365-2184.2012.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from Human adipose tissue:implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- Niemeyer P, Kornacker M, Mehlhorn A, Seckinger A, Vohrer J, Schmal H, Kasten P, Eckstein V, Südkamp NP, Krause U. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteo-genic differentiation in vitro. Tissue Eng. 2007;13:111–121. doi: 10.1089/ten.2006.0114. [DOI] [PubMed] [Google Scholar]
- Yue Y, Yang X, Wei X, Chen J, Fu N, Fu Y, Ba K, Li G, Yao Y, Liang C, Zhang J, Cai X, Wang M. Osteogenic differentiation of adipose-derived stem cells prompted by low-intensity pulsed ultrasound. Cell Prolif. 2013;46:320–327. doi: 10.1111/cpr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Shimokawa H, Kanno Z, Ohya K, Soma K. Effects of mechanical strain on proliferation and differentiation of bone marrow stromal cell line ST2. J Bone Miner Metab. 2005;23:219–225. doi: 10.1007/s00774-004-0587-y. [DOI] [PubMed] [Google Scholar]
- Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]