Abstract
Tissue engineering is promising to meet the increasing need for bone regeneration. Nanostructured calcium phosphate (CaP) biomaterials/scaffolds are of special interest as they share chemical/crystallographic similarities to inorganic components of bone. Three applications of nano-CaP are discussed in this review: nanostructured calcium phosphate cement (CPC); nano-CaP composites; and nano-CaP coatings. The interactions between stem cells and nano-CaP are highlighted, including cell attachment, orientation/morphology, differentiation and in vivo bone regeneration. Several trends can be seen: (i) nano-CaP biomaterials support stem cell attachment/proliferation and induce osteogenic differentiation, in some cases even without osteogenic supplements; (ii) the influence of nano-CaP surface patterns on cell alignment is not prominent due to non-uniform distribution of nano-crystals; (iii) nano-CaP can achieve better bone regeneration than conventional CaP biomaterials; (iv) combining stem cells with nano-CaP accelerates bone regeneration, the effect of which can be further enhanced by growth factors; and (v) cell microencapsulation in nano-CaP scaffolds is promising for bone tissue engineering. These understandings would help researchers to further uncover the underlying mechanisms and interactions in nano-CaP stem cell constructs in vitro and in vivo, tailor nano-CaP composite construct design and stem cell type selection to enhance cell function and bone regeneration, and translate laboratory findings to clinical treatments.
Introduction
Bone fracture is a substantial public health issue, and the need for bone regeneration is increasing dramatically as the world population ages.1–3 Bone defects are one of the leading causes of morbidity and disability in elderly patients, leading to decreases in overall health and quality of life.1 In the United States, an estimated two million people suffer from osteoporosis-related bone fractures annually, which costs nearly $20 billion per year.2,3 There is an urgent need for bone reconstruction, not only for osteoporosis-related fractures, but also for trauma, congenital bone malformations, skeletal diseases and tumor resections. Approximately 600 000 bone graft procedures are performed each year in the United States, and about 2.2 million of such procedures are performed worldwide annually.4 In the majority of these cases, either autografts or allografts are currently used.4 However, although autografting is regarded as the gold standard, it is accompanied with risks of donor site morbidity and limited availability.5 On the other hand, allografting is limited by potential infection and a high nonunion rate with host tissues.6 Therefore, bone tissue engineering is being explored as a promising alternative, and an important approach involves the use of nanostructured biomaterials and stem cells.7–10
The use of nanostructured biomaterials in bone regeneration is inspired by the native bone architecture. Bone possesses a complex organic–inorganic nanocomposite structure. The organic phase is mainly composed of type I collagen, which is arranged into nanofibers ranging from 50 to 500 nm in diameter.11 The inorganic phase consists of non-stoichiometric hydroxyapatite (HA) crystals with lengths of about 100 nm, widths of 20–30 nm and thicknesses of 3–6 nm, which are embedded between the collagen fibers.12,13 In 2011, the European Commission adopted the following definition of a nanomaterial: ‘A natural, incidental or manufactured material containing particles, in an unbound state or as an aggregate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range of 1 nm–100 nm’.14 Some literatures more broadly use the prefix ‘nano-’ to also include structures slightly exceeding 100 nm to a few hundred nanometers. It is of great interest to develop biomimetic nanostructured scaffolds to mimic native bone. Calcium phosphate (CaP) biomaterials are of special interest because they mimic the major inorganic component of bone, are bioactive and can form intimate and functional interfaces with neighboring bone. Various forms of CaP are widely studied for bone regeneration research. Commonly used CaPs include monocalcium phosphate monohydrate, monocalcium phosphate anhydrous, dicalcium phosphate dihydrate, dicalcium phosphate anhydrous, octacalcium phosphate, α- and β-tricalcium phosphate (TCP), amorphous CaP (ACP), calcium-deficient hydroxyapatite and HA.15 Using different synthetic methods, nano-CaP crystals with diverse structures have been fabricated including particles, spheres, rods/needles/wires/fibers/whiskers, sheets/disks/flakes/platelets/strips and various 3D architectures.16 Importantly, several reports showed that nano-CaP biomaterials exhibited physicochemical and biological characteristics better than conventional-sized CaPs, due to nano-CaPs being more similar to bone nanocrystals.17,18 Thus, nano-CaPs have great potential in bone repair and augmentation.
A number of recent reviews on nanomaterials covered topics on materials selection (metals, ceramics, polymers and composites)19 and synthesis and processing techniques (e.g., blasting, etching and anodization for metals; electrospinning, phase separation, self-assembly and precipitation for ceramics/polymers/composites; and lithography, laser ablation and nanoimprinting for nanotopography).20,21Other reviews covered nanotopography22 and its influence on cells.23 Others reviewed nanopolymers (natural and synthetic polymers)24 and nanocomposites (e.g., biopolymers, polymer/ceramic, metal/ceramic).25 Still other reviews focused on nanofibers,26 nanoparticles27 and multiple applications in regenerative medicine (e.g., tissue engineering, cell therapy, diagnosis, and drug and gene delivery).28 To avoid repetition, the present review focuses on nanostructured CaP biomaterials, highlighting their interactions with stem cells including cell attachment, orientation and morphology, osteogenic differentiation, as well as cell encapsulation and delivery for in vivo bone regeneration.
Nanostructured calcium phosphate biomaterials
Nanostructured calcium phosphate cement (CPC)
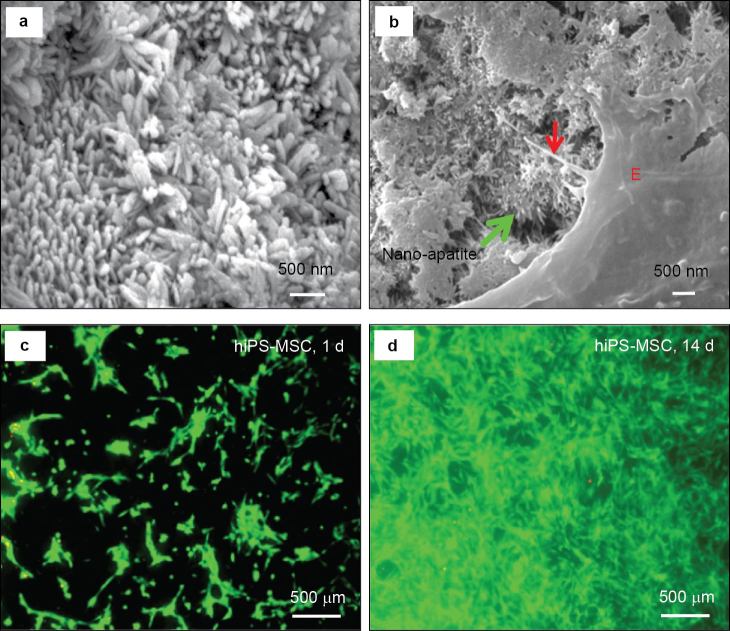
CPCs are self-setting synthetic bone graft materials.29–34 The first CPC consisted of a mixture of tetracalcium phosphate (TTCP: Ca4(PO4)2O) and dicalcium phosphate anhydrous (DCPA: CaHPO4) and was developed in 1986 (referred to as CPC).35 CPC was approved in 1996 by the Food and Drug Administration for repairing craniofacial defects.36 When mixed with an aqueous solution to form a paste, CPC can self-harden to form HA in situ. CPC has good biocompatibility, in situ hardening and molding capabilities and injectability, enabling minimally invasive applications.29–37 Recent studies enhanced the mechanical, physical and biological properties of CPC through the introduction of absorbable fibers,38 chitosan,39 mannitol porogen,40 gas-foaming agents,41 alginate microbeads42 and biofunctionalization.43,44 These approaches improved mechanical strength, setting time, degradability, macroporosity, cell attachment and delivery of cells and growth factors. Scanning electron microscopy revealed the formation of nano-sized elongated HA crystals in CPC (figure 1a). These nanocrystals had a diameter of about 100 nm.45,46 Osteoblasts, human bone marrow mesenchymal stem cells (hBMSCs), human umbilical cord MSCs (hUCMSCs) (Figure 1b), human embryonic stem cell-derived MSCs (hESC-MSCs) and human induced pluripotent stem cell-derived MSCs (hiPSC-MSCs) (Figure 1c and 1d) all responded favorably when attaching to the nano-apatite structure of CPC. The interactions between various stem cells and nanostructured CPC are addressed in another section of this review.
Figure 1.
Nanostructured CaP and cell interactions. (a) Nano-sized HA crystals in CPC; (b) cytoplasmic extensions of hUCMSCs (red arrow) anchored to the apatite nano-crystals (green arrow); (c, d) proliferation of hiPSC-MSCs on nano-apatite CPC as indicated by live/dead staining (adapted from Refs. 45, 71 and 110, with permission).
Another strategy to obtain a nanostructured CPC is to reduce the starting particle size of CPC to the nanoscale level. Brunner et al. used a flame-spray synthesis method to prepare amorphous TCP nanoparticles.47 Due to the higher surface area, amorphous TCP nanoparticles significantly accelerated the setting time and the conversion to apatite during the self-hardening of CPC. The addition of nanoparticulate amorphous TCP favored the nucleation of smaller crystals and promoted the formation of nano-apatite crystals (100–200 nm) in CPC.15,47
Nanostructured CaP composites
Composite approaches can be used to improve the mechanical properties of nanostructured CaP in order to satisfy clinical needs in load-bearing areas. Combining natural or synthetic polymers with nanostructured CaP is a promising strategy, since bone tissue itself is a nanocomposite of HA and collagen. Many degradable polymers have been explored for this purpose, such as collagen fibers,48 silk fibrion,49 gelatin,50 chitosan,51 poly-L-lactide,52 poly-DL-lactide-co-glycolide (PLGA)53 and poly(vinylalcohol).54 The compositions and properties of several recently-developed nanostructured CaP composites are briefly reviewed in Table 1. Each type of polymer has its own characteristics to contribute to the property improvement of the composite. Collagen is the most abundant polymer in bone tissue. By incorporating collagen into the composite, it provides more cell recognition sites and accelerates biomaterial’s degradation rate, thus allowing fast replacement by new bone.48,55,56 However, the use of collagen is limited as it is costly, and its potential of antigenicity and pathogen transmission.48,55,56 Gelatin is a denatured form of collagen, which is free of immunogenic concerns. Gelatin contains integrin binding sites which are important for cell adhesion.50 Other natural polymers such as chitosan and silk are especially known for their excellent mechanical properties.49,51 Synthetic polymers represent another category, with the main advantage of avoiding immunogenicity and disease transmission, and possessing flexibility in property controls.52–54 In general, the composite approach can yield novel materials with improved mechanical properties and better bioactivity which promotes cell adhesion ions and enhances new bone formation. However, a main potential downside of including polymers into nano-CaP biomaterials is the excessive aggregation of nanoparticles.57 A major challenge in developing polymer/CaP nanocomposites is how to homogeneously disperse nanoparticles in the polymer matrix.57 Several approaches have been proposed to overcome this problem, including hydroxyapatite nanoparticle modification and biomimetic process in synthesis.57
Table 1. Nanostructured CaP composites for bone repairs.
| Materials | Fabrication technique | Dimension features | Properties | References |
|---|---|---|---|---|
| Gelatin nanospheres/CaP nanocrystals colloidal composite gels | CaP nanocrystals: wet-chemical precipitation | Needle-shaped apatitic crystals with an average length of 173±52 nm and a width of 30±8 nm | Nano-CaP enhanced gel elasticity, shear-thinning, self-healing behavior and gel stability; reduced the degradation rate; fine-tuned the release of growth factors; supported attachment, spreading and proliferation of rat BMSCs | [50] |
| nHA/polyelectrolyte (chitosan/hyaluronic acid) complex | In situ crystallization of HA precursors | Nanoparticles: from 54 to 147 nm | The scaffold was excellent for hBMSC penetration, growth and proliferation | [58] |
| PLLA/chitosan/nano-CaP | Freeze casting | Average crystallite size: 16.5 nm | The addition of nano-CP and chitosan decreased porosity, swelling ability and degradation of the scaffold, increased the mechanical strength | [52] |
| Nanobiphasic CaP/PVA scaffold | Emulsion foam freeze–drying | Nano-CaP particles: an average width of 50 nm and length of 100 nm | Good cytocompatibility, no negative effects on hBMSC cell growth and proliferation | [54] |
| Silk/nano-CaP | In situ synthesis and salt leaching | Nano-CaP particles <200 nm | Nano-CaP improved mechanical performance and induced higher amount of new bone formation | [49] |
Abbreviations: PLLA, poly-L-lactide; PVA, poly(vinylalcohol).
Nanostructured CaP coatings
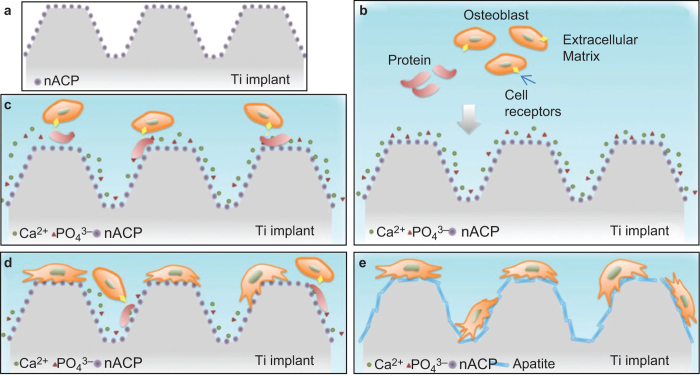
Another important application of nanostructured CaP is coating on metallic or other implants to enhance the bioactivity and osteoconductivity of bioinert materials. CaP can facilitate osteointegration with natural bone through the formation of an apatite layer.21 In addition, nanoscale modification of implant surfaces has been suggested to further increase its biomimicry and bioactivity.59 Nanostructured CaP coatings can be prepared by a biomimetic coating method,60 sputter deposition,61 ectrochemical deposition62 and plasma-spraying methods.63 CaP coatings with different compositions (e.g., HA, brushite or apatite),64,65 crystallinity (amorphous or crystalline)66 and surface features of the substrate (e.g., polished or roughened)60 can all affect osseointegration in vivo. Figure 2 shows schematically the biological response of a nanostructured ACP (nACP)-coated titanium implant.66 In an aqueous medium, nACP can readily release Ca2+ and PO43− ions. They can bind with serum proteins that facilitate cell attachment and subsequent formation of apatite on the implant surfaces.66 To decrease the unfavorable effects of rapid dissolution of the nACP coating, more stable CaP phases such as poorly-crystalline nano-apatite or highly-crystalline nano-HA (nHA) can be used.66 Poorly-crystalline nano-apatite showed a favorable effect on the osteogenic differentiation of osteoblasts.66
Figure 2.
Schematic of biological response of a nACP-coated titanium implant (Ti implant). (a) Ti implant coated with ACP nanoparticles; (b) release of Ca2+ and PO43− ions from ACP hydrolysis after implantation; (c) cell binding on the implant surface with the help of serum proteins and integrin receptors; (d) cell proliferation on the implant surface; (e) formation of apatite on the implant surface (adapted from Ref. 66, with permission).
Stem cell interactions with nano-cap biomaterials
An ideal orthopedic repair material is more than just fillers for bone defects. It also serves as a scaffold to provide chemical, mechanical and topographical cues to regulate cell behavior. Many studies revealed that nanostructured biomaterials promoted the process of bone regeneration by supporting cell adhesion, spreading, proliferation and differentiation.23,67 Understanding the interactions between stem cells and nanostructured materials is of utmost importance in designing and fabricating novel biomaterials that can guide cell behaviors in a desirable way. The present review article focuses on recent studies on the interactions between stem cells and nanostructured CaP scaffolds for bone tissue engineering both in vitro and in vivo. For biomaterial interactions with other cell types (e.g., fibroblastic cells, endothelial cells, epithelial cells and macrophage cells) and nanomaterials other than CaP-based biomaterials, several review articles already exist.23,67
Previous studies investigated the behavior of various types of stem cells attaching to nanostructured CPCs with different compositions. All the tested stem cells, including rat68 and hBMSCs,62 hUCMSCs,63,69 hESC-MSCs64 and hiPSC-MSCs,65 attached well to CPC scaffolds containing apatite nanocrystals. Scanning electron microscopy examination showed that the cells anchored to nano-apatite crystals via cytoplasmic processes and exhibited a healthy spreading polygonal morphology (Figure 1b). The cytoplasmic extensions are crucial for cell adhesion, migration and formation of cell–cell junctions. The nano-apatite surfaces favorably supported proliferation of these MSCs. In another study, a nanofiberous scaffold with gradients in amorphous calcium phosphate nanoparticles (nACP) was fabricated by a two-spinnerette electrospining.72 The adhesion and proliferation of MC3T3-E1 murine pre-osteoblasts was enhanced in the gradient regions, indicating that higher nACP content yielded a better cell response.72 Therefore, stem cells can have strong interactions with nanostructured CaP biomaterials, and nanostructures can be tailored to guide and enhance cell function.
Another key aspect is cell orientation and morphology. Nano-sized surface structures provide important cues to regulate cell orientation and morphology.23,67 Among the various two-dimensional topographical nanoscale features (e.g., grooves, pits, wells, steps, pillars, pores, ridges, etc.), nanogrooves appeared to provide the most powerful and clear cues in regulating cell orientation and morphology.23,67 Regardless of different substrates and cell types, in most cases, cells aligned their shape and elongation in the long axis of the grooves, with the organization of actin and other cytoskeletal proteins to be parallel to the grooves.23 However, since nano-CaP is less maneuverable in fabrication than polymers or metals, it is more challenging to produce highly patterned nano-CaP surface features. Irregular surface geometry due to the non-uniform distribution of nHA may not promote cell orientation in a polarized direction. Hence, there are few studies reporting the particular alignment patterns of cells on nano-CaP implants. However, the addition of fibers can help induce directionality for the cells. For example, Jose et al.73 produced an aligned nanofibrous PLGA/collagen/nHA scaffold with fiber diameters of 100–350 nm. hMSCs assumed an aligned morphology along the direction of the fiber orientation.73 Electrospinning is a versatile technique to produce aligned nanofibrous scaffolds. By controlling the spinning conditions, parameters, and compositions of the solution, oriented fibers with different compositions and diameters can be obtained. The aligned nanostructures of the biomaterial can effectively induce an aligned morphology for most types of seeded cells.74
Nanostructured materials also have significant effects on cell differentiation. There is growing evidence indicating that nanofeatures are a compelling determinant of stem cell lineage commitment.75–78 With regard to the effects of nano-CaP on cell differentiation, a summary of recent literature is presented in Table 2. In nearly all instances, the incorporation of nano-CaP promoted or directed osteogenic differentiation of cells, manifested by the upregulation of osteogenic gene expression, synthesis of minerals and enhanced alkaline phosphatase (ALP) activity. Several studies reported that nanotopography can stimulate MSC differentiation in the absence of osteogenic supplements.79–81 Nanocomposites comprising of type I collagen and nHA were especially efficient at inducing rapid mineralization by cells, even in the absence of osteogenic supplements in culture medium.79 nHA and nHA–PLGA composites successfully induced osteogenic differentiation for hMSCs without the addition of osteogenic factors, achieving osteogenic differentiation comparable to that of directly adding BMP-7-derived short peptide (DIF-7c) into the culture medium.80 This was consistent with another study showing that the presence of TCP or HA nanocrystals on a poly-caprolactone (PCL) nanofiber surface positively affected hBMSC differentiation toward an osteogenic commitment, achieving a high level of osteogenic differentiation, similar to that via osteogenic supplements.81 Currently, the underlying mechanism of how nano-CaP materials dictate the osteogenic differentiation of MSCs is not fully understood. However, parameters such as the biomaterial’s chemical composition, surface roughness, nanomaterial size and stiffness/elastic modulus have been proposed to impact stem cells' fate. The following four factors of nano-CaP materials appear to influence the osteogenic differentiation of MSCs. First, nano-CaP materials serve as a calcium and phosphate ion source.82 Ca2+, PO43− and HPO42− ions, released into the surrounding tissue, are able to regulate osteoblast functions83 and create a local supersaturation of ions which cause the reprecipitation of biological carbonated apatite on the scaffold.82,84 Second, the large surface area and nanostructure of the material can provide more binding sites for cell receptors, and aid the adsorption and retention of circulating osteogenic factors such as BMPs which contribute to osteogenesis.85–87 Third, properties such as nanoscale roughness, nanotopography and nanocrystallinity can induce changes in cell membrane receptors and activate specific mechanotransduction pathways that direct cell fate in favor of osteogenesis.88,89 Fourth, the presence of CaP nanoparticles in composites can enhance the mechanical properties of the scaffold. Cells can sense the increased matrix stiffness and differentiate towards osteogenic lineage.90–92 These factors and their combined effects on the stem cells are highly complex, and require further investigation.
Table 2. Summary on the effects of nano-CaP biomaterials on cell differentiation.
| Materials | Dimension features | Stem cell types | Effects on cell differentiation | References |
|---|---|---|---|---|
| CPC–chitosan–RGD | Nano-apatite crystals | hESC-MSCs | hESC-MSCs expressed high levels of osteogenic markers (ALP, RUNX2, COLI and OCN), and synthesized bone mineral in vitro | [70] |
| CPC | Nano-apatite crystals | hiPSC-MSCs | hiPSC-MSCs differentiated into the osteogenic lineage and synthesized bone minerals in vitro | [71] |
| A non-rigid CPC with microbeads and fiber | Nano-apatite crystals | hUCMSCs | ALP, OCN and COL1 gene expressions of hUCMSCs were greatly increased, and the cells synthesized bone minerals in vitro | [94] |
| CaP coating on different topographic surfaces of metal discs | Nano-sized crystals: <100 nm | Ovine BMSCs | Nano-CaP crystals and flatter topographies enhanced MSC proliferation while rougher, microscale topographies enhanced osteogenic differentiation of MSCs | [60] |
| Mineralized nanofibers: collagen fibrils containing CaP | Collagen fibril diameters of 760±240 nm, 270±120 nm and 120±30 nm, respectively | hADMSCs | Inclusion of CaP enhanced hADMSC proliferation. Only the CaP containing groups exhibited a statistical increase in ALP activity | [48] |
| Nanocrystalline CaP/chitosan composite | Nanocrystalline CaP: 100 nm | hBMSCs | The proliferation of hBMSCs on nanocrystalline CaP/chitosan film was higher than that on nano-amorphous and microparticle CaP/chitosan films, whereas osteogenic differentiation was highest on the scattered microparticle CaP/chitosan film | [93] |
| nHA/PLGA | Nanoparticles: an average particle size of 36 nm | hMSCs | nHA and nHA–PLGA composites promote osteogenic differentiation of human MSCs, comparable with direct injection of BMP-7-derived short peptide (DIF-7c) into culture media | [80] |
| nHA/PCL nanofibrous scaffoldnTCP/PCL nanofibrous scaffold | HA nanocrystals average size: 20–70 nmbeta-TCP nanocrystals average size: 100 nm | hMSCs | The incorporation of nano-sized HA or TCP into the PCL nanofibers increased the activity of ALP and mRNA expression levels of osteoblast-related genes in total absence of osteogenic supplements | [81] |
| nHA-coated genipin-chitosan conjugation scaffold (HGCCS) | 3D interconnected nHA network with 150 nm pore diameter and 20 nm wall thickness | Rat BMSCs | nHA induced the highest mRNA expression of osteogenic differentiation makers and mineralized ECM | [95] |
Notably, hMSCs behaved differently in response to different scales of CaP crystals and topography. In one study, three types of chitosan/CaP films with different surface features were fabricated by a solvent-casting method.93 The proliferation of hMSCs cultured on the nanocrystalline CaP/chitosan film was higher than that on nano-amorphous or microparticle CaP/chitosan films. Osteogenic differentiation achieved the highest level on the scattered microparticle CaP/chitosan film.93 Similar observations were reported by Garcia-Gareta et al.,60 showing that nano-sized CaP crystals and relatively flat topographies enhanced MSCs proliferation, while rougher, microscale topographies enhanced osteogenic differentiation of MSCs. Both of these reports indicated that nano-sized CaP features enhanced cell proliferation, while micro-sized CaP composites enhanced the osteogenic differentiation of the cells.60,93
In vivo bone regeneration
Several in vivo studies have shown favorable bone regeneration via nanostructured CaP biomaterials. The effect of a composite’s particle size on alveolar bone reconstruction was studied in a rat osteoporotic alveolar bone model.53 Micro- and nanoparticulate CaP/PLGA composites were applied to restore bone defects (1.6 mm in diameter and 1.8 mm deep). The new bone amount generated by the nanoparticulate CaP/PLGA group was more pronounced than the micro-particulate CaP/PLGA group. Adding autologous plasma further promoted the bone regeneration, with the best bone regeneration via the nano-particulate CaP/PLGA/plasma composite.53 Fricain et al., compared the bone regeneration potential of scaffolds composing of natural hydrophilic polysaccharides pullulan and dextran, supplemented with or without nHA in five animal models.87 This study included two heterotopic implantations (subcutaneously in mice and intramuscularly in goat), and three orthotopic models of critical-size defects in three different bony sites (femoral condyle of rat, a transversal mandibular defect and a tibial osteotomy in goats). The results indicated that only the nHA-incorporated scaffold induced a biological apatite layer and favored the formation of a dense mineralized tissue subcutaneously in mice, as well as osteoid tissues after intramuscular implantation in goats. The addition of nHA induced early bone regeneration in all three orthotopic models regardless of the site of implantation.87 These phenomena clearly demonstrated that new bone formation via nano-sized CaP composites is superior to conventional-sized counterparts in vivo.
Beneficial in vivo effects can be further obtained by seeding stem cells to nanostructured CaP materials. When a nHA/collagen I nanocomposite was enriched with hMSCs for subcutaneous implantation, the time it took for the development of a bone matrix was shortened from 2 weeks to 1 week.79 In another study on ectopic implantation, nHA/CS/PLGA scaffolds combined with pre-osteogenic hUCMSCs achieved the most bone regeneration.96 Chai et al. seeded human periosteum-derived cells onto 3D-functionalized porous nCaP–Ti6Al4V hybrids.97 These hybrids induced ectopic bone formation, which was highly dependent on the physicochemical properties of the CaP coating in a cell density-dependent manner. Only a small amount of new bone spicules was found around the hybrids when one million cells were seeded, while substantial bone formation was observed throughout the whole hybrids when three million cells were seeded.97 Besides seeding stem cells, the co-application of growth factors in nano-CaP scaffolds can further enhance bone regeneration. For example, in a rabbit calvaria bone defect model, the triple application of MSC/nHA/platelet-rich growth factor yielded 29.45% and 44.55% of new bone area at 6 and 12 weeks, respectively.98 In contrast, bone formation in nHA, nHA/platelet-rich growth factor and nHA/MSC at 6 and 12 weeks were 11.35% and 32.53%, 29.10% and 39.74%, and 25.82% and 39.11%, respectively.98
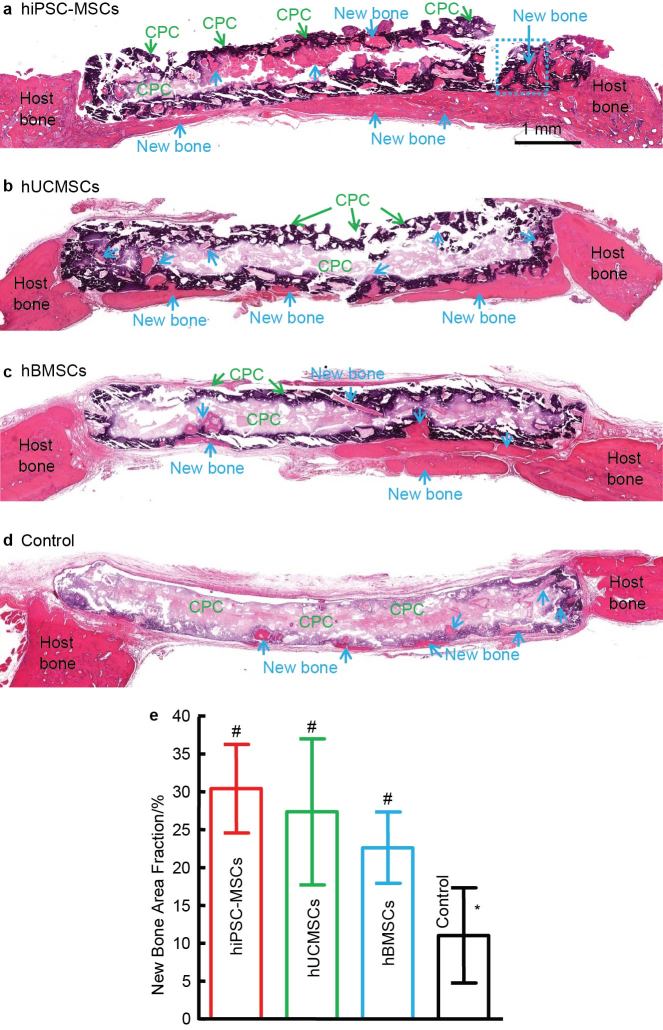
Therefore, combining stem cells with nano-CaP scaffolds can greatly enhance bone regeneration. Regarding the types of stem cells, hBMSCs are the gold standard in stem cell-based bone regeneration and have been successfully used in clinics.99 However, the use of hBMSCs is hampered by an invasive procedure to harvest, limited availability, donor site morbidity, and loss of potency of stem cells obtained from seniors and patients with diseases and disorders.100 Therefore, other stem cell sources are critically needed for bone regeneration. Currently, there are three broad types of stem cells: adult stem cells, ESCs and iPSCs. Adult stem cells can be found in stem cell niches such as bone marrow, adipose tissue, umbilical cord, placenta, etc. They exhibit a lineage-restricted differentiation potential and exhibit multipotency.101 In contrast, ESCs and iPSCs can give rise to cell types of all three germ layers, which is known as pluripotency. This feature makes these stem cells an extremely valuable and potent cell source. ESCs are obtained from the inner cell mass of blastocysts, while iPSCs are derived from reprogramming of somatic differentiated cells.102 hESCs involve complex cell culture procedures, risks of tumorigenicity and ethical concerns. Patient-specific hiPSCs with similar proliferation and differentiation ability to hESCs has the potential to overcome some of these hurdles. Thus, iPSCs are potentially the new frontier for cell-based regenerative medicine research. Several studies focused on the comparison of various types of stem cells in combination with nano-CaP biomaterials to repair bone defects. Chen et al.103 compared the in vivo bone regeneration efficiency of hUCMSCs and hBMSCs on a nano-crystalline CPC–chitosan–RGD scaffold in a nude rat cranial bone defect model. It was found that the bone-forming ability of hUCMSC–CPC constructs matched that of hBMSC–CPC constructs. 103 Our recent study revealed that, when seeded on macroporous CPC scaffolds, hiPSC-MSCs had good osteogenic capability comparable to hUCMSCs and the gold-standard hBMSCs (Figure 3). After implanting stem cell–CPC constructs in critical-sized cranial defects in rats, the new bone area fraction at 12 weeks for hiPSC-MSC–CPC constructs was (30.4±5.8)%, which was 2.8-fold the (11.0±6.3)% of CPC control without cell seeding (P<0.05). No significant differences in new bone area fraction were detected among hiPSC-MSCs, hUCMSCs and hBMSCs groups (P>0.1). The new bone exhibited an organized morphology which is typical of mature bone, manifested by the appearance of bone matrix with osteocytes and blood vessels, and with newly formed bone being lined by osteoblasts (Figure 4). Thus, hiPSC-MSCs and hUCMSCs may represent viable alternatives to hBMSCs and hESCs for bone regeneration. Reddy et al.104 compared MSCs from four different sources (human placenta, umbilical cord, fetal bone marrow and adipose tissue), cultured in the presence of nanosized biphasic ceramics. Placental MSCs demonstrated the best osteogenic potential based on expression of osteogenic markers and complete regeneration of bone defect in the femur of rats when seeded with nanoceramic with a Ca/P ratio of 1.58. Therefore, constructs using stem cells and nano-CaP scaffolds are highly promising for bone regeneration in vivo.
Figure 3.
Bone regeneration via CPC scaffolds containing hiPSC-MSCs, hUCMSCs and hBMSCs, and CPC without cells. (a–d) H&E; staining of stem cell-seeded CPC implanted in critical-sized cranial defects in nude rats. (e) Histomorphometry analysis of new bone area fraction. Bars with dissimilar marks (# and *) indicate values that are significantly different from each other (P<0.05). Each value is mean±s.d. (n=6). Scaffolds containing hiPSC-MSCs, hUCMSCs and hBMSCs exhibited better bone regeneration than CPC control without cells. There was no significant difference between hUCMSC and hBMSC groups (P>0.1).
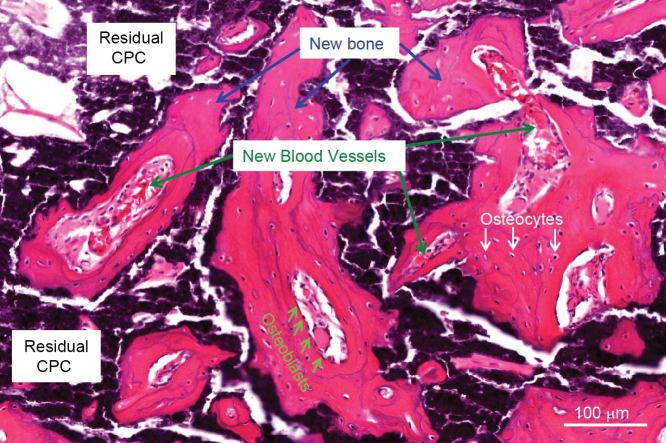
Figure 4.
High magnification of hiPSC-MSC-seeded nano-apatite CPC scaffold implanted in critical-sized cranial defect in nude rats. Osteoblasts were found around new bone. Osteocytes were found inside new bone. New blood vessels were found both within and around the new bone area. New bone areas were stained in pink red and marked with arrows. The white area was due to slight detachment of the tissue or decalcification of CPC. The black, dark purple and light purple areas were residual CPC material.
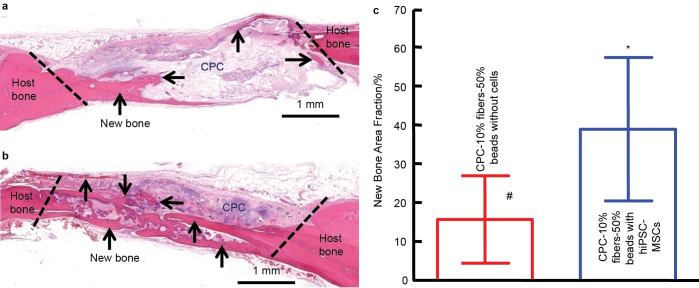
The above-mentioned studies only seeded cells onto the surfaces of preformed CaP scaffolds. This type of static cell seeding method has limitations of low seeding efficiency and minimal cell penetration into scaffold, leading to non-uniform distribution of cells.105 To address these problems, cell micro-encapsulation has been proposed as another approach to deliver cells. Alginate is commonly used for cell delivery owing to its gentle gelling ability, and free of detrimental effects to encapsulated cells. However, the degradation of alginate is undesirably slow and uncontrollable.106 Therefore, fast degradable alginate hydrogels were developed via partial oxidation, and the addition of fibrin further accelerated the degradation to as early as 4 days.107 Our recent study encapsulated hiPSC-MSCs in the fast degradable alginate-fibrin microbeads, mixed with CPC paste (which was reinforced by 10% absorbable fibers), and then transplanted into nude rats using a double cranial bone defect model. The CPC paste contained 50% of alginate-fibrin microbeads which encapsulated hiPSC-MSCs. The control group had the same CPC compositions with the same alginate-fibrin microbeads but without encapsulating cells. As shown in Figure 5, the cell-encapsulated group generated significantly more new bone than the counterpart without cell encapsulation at 3 months. The average new bone area fraction in the ‘CPC–10% fibers–50% beads with hiPSC-MSCs’ group was (38.9±18.4)%, more than twofold the (15.6±11.2)% of the control group (P<0.05) (Figure 5). In other studies, alginate microspheres and chitosan-coated alginate microspheres were used to encapsulate cells.108,109 Then the microspheres were dispersed in CPC paste for injection, one for subcutaneous implantation in nude mice,108 and the other for rabbit femoral condylar defects.109 In vivo implantation showed that cell-encapsulated groups degraded faster than the cell-free groups and exhibited higher mineralization and bone forming ability.108,109 The encapsulated cells were still detectable in the graft areas 8 weeks postoperatively.109 These in vivo results indicated that the encapsulated cells can be released from the microspheres and survived in vivo, while concomitantly creating pores in the scaffolds. These studies demonstrated that cell microencapsulation in combination with nano-CaP scaffolds is a promising tool for enhancing the bone tissue engineering efficacy.
Figure 5.
hiPSC-MSC encapsulation in alginate-fibrin microbeads in CPC paste implanted in critical-sized cranial defects in nude rats. (a) H&E; staining of constructs of CPC paste containing 10% fibers and 50% alginate-fibrin microbeads (CPC–10% fibers–50% beads) without cells. (b) CPC–10% fibers–50% beads encapsulating hiPSC-MSCs. New bone formation was indicated by the arrows. There was more new bone in cell-seeded CPC scaffolds than cell-free CPC scaffolds. The residual CPC was stained in purple or pink. Histomorphometry analysis of new bone area fraction (c). Bars with dissimilar marks (# and *) indicate values that are significantly different from each other (P<0.05). Each value is mean±s.d. (n=5).
Conclusions
Nanostructured CaP biomaterials and scaffolds mimic natural bone, and have high surface-to-volume ratios, improved wettability and mechanical properties, and increased protein adsorption and other desirable properties, compared to conventional counterparts. Nano-CaP biomaterials have emerged as a promising class of biomimetic and bioactive scaffolds capable of directing cell behavior and cell fate and enhancing tissue formation in vivo. In general, nano-CaP scaffolds can support stem cell attachment and proliferation and induce osteogenic differentiation, in some cases without osteogenic supplements. The influence of nano-CaP on cell alignment is less prominent than polymers and metals, due to the non-uniform distribution of the nano-CaP crystals. Nano-CaP biomaterials can achieve significantly better bone regeneration in vivo than conventional CaP biomaterials. The combination of various types of stem cells with nano-CaP scaffolds can further accelerate bone regeneration, the effect of which can be even further promoted by growth factor incorporation. Cell microencapsulation in combination with nano-CaP scaffolds is a promising tool for bone tissue engineering applications to distribute cells throughout the interior of the scaffold. Further studies are needed to compare various types of nano-CaP compositions and nanostructures side-by-side in vivo, and compare the various types of stem cells in bone regeneration efficacy. Studies should also focus on understanding the bone tissue regeneration mechanisms via nano-CaP constructs, and tailoring the nano-CaP biomaterials and stem cells types to further enhance bone regeneration in vivo for various craniofacial and orthopedic applications.
Acknowledgments
We gratefully thank Dr Laurence C. Chow, Dr Carl G. Simon, Dr David J. Mooney, Dr Hongzhi Zhou and Dr Minghui Tang for fruitful discussions. This study was supported by NIH R01 DE14190 and R21 DE22625 (HX), National Science Foundation of China 31100695 and 31328008 (LZ), 81401794 (PW), Maryland Stem Cell Research Fund and University of Maryland School of Dentistry.
The authors declare no conflict of interest.
References
- Borgstrom F, Lekander I, Ivergard M et al. The International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS)—quality of life during the first 4 months after fracture. Osteoporos Int 2013; 24: 811–823. [DOI] [PubMed] [Google Scholar]
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007; 22: 465–475. [DOI] [PubMed] [Google Scholar]
- Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci 2013; 68: 1236–1242. [DOI] [PubMed] [Google Scholar]
- Marino JT, Ziran BH. Use of solid and cancellous autologous bone graft for fractures and nonunions. Orthop Clin North Am 2010; 41: 15–26. [DOI] [PubMed] [Google Scholar]
- Sen MK, Miclau T. Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury 2007; 38: S75–S80. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Smith WR, Mauffrey C et al. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: a retrospective cohort study in 182 patients. J Orthop Surg Res 2013; 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 2012; 40: 363–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe RJ, Mao J. Bone tissue engineering and regeneration: from discovery to the clinic—an overview. Tissue Eng Part B Rev 2011; 17: 389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducheyne P, Mauck RL, Smith DH. Biomaterials in the repair of sports injuries. Nat Mater 2012; 11: 652–654. [DOI] [PubMed] [Google Scholar]
- Steinert AF, Rackwitz L, Gilbert F, Noth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med 2012; 1: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler KE, Holmes DF, Trotter JA, Chapman JA. Collagen fibril formation. Biochem J 1996; 316: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis WJ, Silver FH. Mineral deposition in the extracellular matrices of vertebrate tissues: identification of possible apatite nucleation sites on type I collagen. Cells Tissues Organs 2009; 189: 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Sun K, Cui FZ, Landis WJ. Organization of apatite crystals in human woven bone. Bone 2003; 32: 150–162. [DOI] [PubMed] [Google Scholar]
- European Commission. Definition of a nanomaterial. Brussels: European Commission, 12/3/2013. Available at http://ec.europa.eu/environment/chemicals/nanotech/faq/definition_en.htm
- Dorozhkin SV. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater 2010; 6: 715–734. [DOI] [PubMed] [Google Scholar]
- Lin K, Wu C, Chang J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater 2014; 10: 4071–4102. [DOI] [PubMed] [Google Scholar]
- Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004; 25: 987–994. [DOI] [PubMed] [Google Scholar]
- Chan CK, Kumar TS, Liao S, Murugan R, Ngiam M, Ramakrishnan S. Biomimetic nanocomposites for bone graft applications. Nanomedicine (Lond) 2006; 1: 177–188. [DOI] [PubMed] [Google Scholar]
- Gardin C, Ferroni L, Favero L et al. Nanostructured biomaterials for tissue engineered bone tissue reconstruction. Int J Mol Sci 2012; 13: 737–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon RE, Wang L, Skoracki R, Mathur AB. Development of nanomaterials for bone repair and regeneration. J Biomed Mater Res B Appl Biomater 2013; 101: 387–397. [DOI] [PubMed] [Google Scholar]
- Alves Cardoso D, Jansen JA, Leeuwenburgh SC. Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration. J Biomed Mater Res B Appl Biomater 2012; 100: 2316–2326. [DOI] [PubMed] [Google Scholar]
- Kim HN, Jiao A, Hwang NS et al. Nanotopography-guided tissue engineering and regenerative medicine. Adv Drug Deliv Rev 2013; 65: 536–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E, Engel E, Planell JA, Samitier J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann Anat 2009; 191: 126–135. [DOI] [PubMed] [Google Scholar]
- Smith IO, Liu XH, Smith LA, Ma PX. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2009; 1: 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, John B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog Polym Sci 2013; 38: 1487–1503. [Google Scholar]
- Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011; 32: 9622–9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 2012; 55: 329–347. [DOI] [PubMed] [Google Scholar]
- Peran M, Garcia MA, Lopez-Ruiz E, Bustamante M, Jimenez G, Madeddu R, Marchal JA. Functionalized nanostructures with application in regenerative medicine. Int J Mol Sci 2012; 13: 3847–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LC. Next generation calcium phosphate-based biomaterials. Dent Mater J 2009; 28: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohner M. Design of ceramic-based cements and putties for bone graft substitution. Eur Cell Mater 2010; 20: 1–12. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Espanol M, Montufar EB, Perez RA, Mestres G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater 2010; 6: 2863–2873. [DOI] [PubMed] [Google Scholar]
- Sariibrahimoglu K, Wolke JG, Leeuwenburgh SC, Yubao L, Jansen JA. Injectable biphasic calcium phosphate cements as a potential bone substitute. J Biomed Mater Res B Appl Biomater 2014; 102: 415–422. [DOI] [PubMed] [Google Scholar]
- Grover LM, Wright AJ, Gbureck U et al. The effect of amorphous pyrophosphate on calcium phosphate cement resorption and bone generation. Biomaterials 2013; 34: 6631–6637. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu W, Schnitzler V, Tancret F, Bouler JM. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater 2014; 10: 1035–1049. [DOI] [PubMed] [Google Scholar]
- Brown WE, Chow LC. A new calcium phosphate water setting cement. In: Brown PW (ed.), Cements research progress. Westerville, OH: American Ceramics Society, 1986: 352–379. [Google Scholar]
- Friedman CD, Costantino PD, Takagi S, Chow LC. BoneSource hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res 1998; 43: 428–432. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Espanol M, Montufar EB, Perez RA, Mestres G. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater 2010; 6: 2863–2873. [DOI] [PubMed] [Google Scholar]
- Zhou H, Weir MD, Xu HH. Effect of cell seeding density on proliferation and osteodifferentiation of umbilical cord stem cells on calcium phosphate cement–fiber scaffold. Tissue Eng Part A 2011; 17: 2603–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir MD, Xu HH. Osteoblastic induction on calcium phosphate cement-chitosan constructs for bone tissue engineering. J Biomed Mater Res A 2010; 94: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Weir MD, Xu HH. Mannitol-containing macroporous calcium phosphate cement encapsulating human umbilical cord stem cells. J Tissue Eng Regen Med 2012; 6: 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhou H, Tang M, Weir MD, Bao C, Xu HH. Gas-foaming calcium phosphate cement scaffold encapsulating human umbilical cord stem cells. Tissue Eng Part A 2012; 18: 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir MD, Xu HH. Human bone marrow stem cell-encapsulating calcium phosphate scaffolds for bone repair. Acta Biomater 2010; 6: 4118–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein-Han W, Liu J, Xu HH. Calcium phosphate cement with biofunctional agents and stem cell seeding for dental and craniofacial bone repair. Dent Mater 2012; 28: 1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein-Han W, LiuJ, Tang M, Chen W, Cheng L, Xu HH. Induced pluripotent stem cell-derived mesenchymal stem cell seeding on biofunctionalized calcium phosphate cements. Bone Res 2013; 4: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Weir MD, Simon CG. Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dent Mater 2008; 24: 1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LE, Xu HH, Simon CG Jr, Takagi S, Chow LC. Premixed rapid-setting calcium phosphate composites for bone repair. Biomaterials 2005; 26: 5002–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner TJ, Bohner M, Dora C, Gerber C, Stark WJ. Comparison of amorphous TCP nanoparticles to micron-sized alpha-TCP as starting materials for calcium phosphate cements. J Biomed Mater Res B Appl Biomater 2007; 83: 400–407. [DOI] [PubMed] [Google Scholar]
- Maas M, Guo P, Keeney M et al. Preparation of mineralized nanofibers: collagen fibrils containing calcium phosphate. Nano Lett 2011; 11: 1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LP, Silva-Correia J, Correia C et al. Bioactive macro/micro porous silk fibroin/nano-sized calcium phosphate scaffolds with potential for bone-tissue-engineering applications. Nanomedicine (Lond) 2013; 8: 359–378. [DOI] [PubMed] [Google Scholar]
- Wang H, Bongio M, Farbod K et al. Development of injectable organic/inorganic colloidal composite gels made of self-assembling gelatin nanospheres and calcium phosphate nanocrystals. Acta Biomater 2014; 10: 508–519. [DOI] [PubMed] [Google Scholar]
- Chesnutt BM, Viano AM, Yuan Y et al. Design and characterization of a novel chitosan/nanocrystalline calcium phosphate composite scaffold for bone regeneration. J Biomed Mater Res A 2009; 88: 491–502. [DOI] [PubMed] [Google Scholar]
- Jafarkhani M, Fazlali A, Moztarzadeh F, Moztarzadeh Z, Mozafari M. Fabrication and characterization of PLLA/chitosan/nano calcium phosphate scaffolds by freeze-casting technique. Ind Eng Chem Res 2012; 51: 9241–9249. [Google Scholar]
- Ignjatovic NL, Ajdukovic ZR, Savic VP, Uskokovic DP. Size effect of calcium phosphate coated with poly-DL-lactide-co-glycolide on healing processes in bone reconstruction. J Biomed Mater Res B Appl Biomater 2010; 94: 108–117. [DOI] [PubMed] [Google Scholar]
- Nie L, Chen D, Suo J et al. Physicochemical characterization and biocompatibility in vitro of biphasic calcium phosphate/polyvinyl alcohol scaffolds prepared by freeze–drying method for bone tissue engineering applications. Colloids Surf B Biointerfaces 2012; 100: 169–176. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Weir MD, Xu HH. Self-setting collagen-calcium phosphate bone cement: mechanical and cellular properties. J Biomed Mater Res A 2009; 91: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M, Itoh S, Ichinose S, Shinomiya K, Tanaka J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001; 22: 1705–1711. [DOI] [PubMed] [Google Scholar]
- Supova M. Problem of hydroxyapatite dispersion in polymer matrices: a review. J Mater Sci Mater Med 2009; 20: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Chen J, Yu Q, Zhang G, Yang S, Wu J, Zhang Q. Preparation and biocompatibility of nanohybrid scaffolds by in situ homogeneous formation of nano hydroxyapatite from biopolymer polyelectrolyte complex for bone repair applications. Colloids Surf B Biointerfaces 2012; 93: 100–107. [DOI] [PubMed] [Google Scholar]
- Tomsia AP, Lee JS, Wegst UG, Saiz E. Nanotechnology for dental implants. Int J Oral Maxillofac Implants 2013; 28: e535–e546. [DOI] [PubMed] [Google Scholar]
- Garcia-Gareta E, Hua J, Knowles JC, Blunn GW. Comparison of mesenchymal stem cell proliferation and differentiation between biomimetic and electrochemical coatings on different topographic surfaces. J Mater Sci Mater Med 2013; 24: 199–210. [DOI] [PubMed] [Google Scholar]
- McCafferty MM, Burke GA, Meenan BJ. Mesenchymal stem cell response to conformal sputter deposited calcium phosphate thin films on nanostructured titanium surfaces. J Biomed Mater Res A 2014; 102: 3585–3597. [DOI] [PubMed] [Google Scholar]
- Hernandez-Montelongo J, Gallach D, Naveas N et al. Calcium phosphate/porous silicon biocomposites prepared by cyclic deposition methods: spin coating vs electrochemical activation. Mater Sci Eng C Mater Biol Appl 2014; 34: 245–251. [DOI] [PubMed] [Google Scholar]
- Surmenev RA. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf Coat Technol 2012; 206: 2035–2056. [Google Scholar]
- Jimbo R, Coelho PG, Bryington M et al. Nano hydroxyapatite-coated implants improve bone nanomechanical properties. J Dent Res 2012; 91: 1172–1177. [DOI] [PubMed] [Google Scholar]
- Niu J, Yuan G, Liao Y et al. Enhanced biocorrosion resistance and biocompatibility of degradable Mg–Nd–Zn–Zr alloy by brushite coating. Mater Sci Eng C Mater Biol Appl 2013; 33: 4833–4841. [DOI] [PubMed] [Google Scholar]
- He W, Andersson M, de Souza PP et al. Osteogenesis-inducing calcium phosphate nanoparticle precursors applied to titanium surfaces. Biomed Mater 2013; 8: 035007. [DOI] [PubMed] [Google Scholar]
- Lord M, Foss M, Besenbacher F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010; 5: 66–78. [Google Scholar]
- Moreau JL, Xu HH. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate–chitosan composite scaffold. Biomaterials 2009; 30: 2675–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Burguera EF, Xu HH, Amin N, Ryou H, Arola DD. Fatigue and human umbilical cord stem cell seeding characteristics of calcium phosphate–chitosan–biodegradable fiber scaffolds. Biomaterials 2010; 31: 840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhou H, Weir MD, Tang M, Bao C, Xu HH. Human embryonic stem cell-derived mesenchymal stem cell seeding on calcium phosphate cement–chitosan–RGD scaffold for bone repair. Tissue Eng Part A 2013; 19: 915–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Chen W, Liu J, Weir MD, Cheng L, Xu HH. Human induced pluripotent stem cell-derived mesenchymal stem cell seeding on calcium phosphate scaffold for bone regeneration. Tissue Eng Part A 2014; 20: 1295–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam M, Young MF, Thomas V et al. Nanofiber scaffold gradients for interfacial tissue engineering. J Biomater Appl 2013; 27: 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose MV, Thomas V, Xu Y, Bellis S, Nyairo E, Dean D. Aligned bioactive multi-component nanofibrous nanocomposite scaffolds for bone tissue engineering. Macromol Biosci 2010; 10: 433–444. [DOI] [PubMed] [Google Scholar]
- Beachley V, Katsanevakis E, Zhang N, Wen X. Highly aligned polymer nanofiber structures: fabrication and applications in tissue engineering. Adv Polym Sci 2012; 246: 171–212. [Google Scholar]
- Dalby MJ, Gadegaard N, Tare R et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 2007; 6: 997–1003. [DOI] [PubMed] [Google Scholar]
- Oh S, Brammer KS, Li YS et al. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci USA 2009; 106: 2130–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Liu L, Wu Z, Zhang Y, Chu PK. Effects of micropitted/nanotubular titania topographies on bone mesenchymal stem cell osteogenic differentiation. Biomaterials 2012; 33: 2629–2641. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim HN, Lim KT et al. Synergistic effects of nanotopography and co-culture with endothelial cells on osteogenesis of mesenchymal stem cells. Biomaterials 2013; 34: 7257–7268. [DOI] [PubMed] [Google Scholar]
- Liao S, Nguyen LT, Ngiam M et al. Biomimetic nanocomposites to control osteogenic differentiation of human mesenchymal stem cells. Adv Healthc Mater 2014; 3: 737–751. [DOI] [PubMed] [Google Scholar]
- Lock J, Liu H. Nanomaterials enhance osteogenic differentiation of human mesenchymal stem cells similar to a short peptide of BMP-7. Int J Nanomedicine 2011; 6: 2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polini A, Pisignano D, Parodi M, Quarto R, Scaglione S. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PloS One 2011; 6: e26211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barradas AM, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater 2011; 21: 407–429. [DOI] [PubMed] [Google Scholar]
- Wu X, Itoh N, Taniguchi T, Nakanishi T, Tanaka K. Requirement of calcium and phosphate ions in expression of sodium-dependent vitamin C transporter 2 and osteopontin in MC3T3-E1 osteoblastic cells. Biochim Biophys Acta 2003; 1641: 65–70. [DOI] [PubMed] [Google Scholar]
- Habibovic P, Bassett DC, Doillon CJ, Gerard C, McKee MD, Barralet JE. Collagen biomineralization in vivo by sustained release of inorganic phosphate ions. Adv Mater 2010; 22: 1858–1862. [DOI] [PubMed] [Google Scholar]
- LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev 2008; 108: 4742–4753. [DOI] [PubMed] [Google Scholar]
- Koegler P, Clayton A, Thissen H, Santos GN, Kingshott P. The influence of nanostructured materials on biointerfacial interactions. Adv Drug Deliv Rev 2012. ; 64: 1820–1839. [DOI] [PubMed] [Google Scholar]
- Fricain JC, Schlaubitz S, Le Visage C et al. A nano-hydroxyapatite—pullulan/dextran polysaccharide composite macroporous material for bone tissue engineering. Biomaterials 2013; 34: 2947–2959. [DOI] [PubMed] [Google Scholar]
- Nikukar H, Reid S, Tsimbouri PM, Riehle MO, Curtis AS, Dalby MJ. Osteogenesis of mesenchymal stem cells by nanoscale mechanotransduction. ACS Nano 2013; 7: 2758–2767. [DOI] [PubMed] [Google Scholar]
- Mendonca G, Mendonca DB, Simoes LG et al. The effects of implant surface nanoscale features on osteoblast-specific gene expression. Biomaterials 2009; 30: 4053–4062. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126: 677–689. [DOI] [PubMed] [Google Scholar]
- Pek YS, Wan AC, Ying JY. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 2010; 31: 385–391. [DOI] [PubMed] [Google Scholar]
- Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am J Physiol Cell Physiol 2008; 295: C1037–C1044. [DOI] [PubMed] [Google Scholar]
- Lee YT, Yu BY, Shao HJ et al. Effects of the surface characteristics of nano-crystalline and micro-particle calcium phosphate/chitosan composite films on the behavior of human mesenchymal stem cells in vitro. J Biomater Sci Polym Ed 2011; 22: 2369–2388. [DOI] [PubMed] [Google Scholar]
- TheinHan W, Weir MD, Simon CG, Xu HH. Non-rigid calcium phosphate cement containing hydrogel microbeads and absorbable fibres seeded with umbilical cord stem cells for bone engineering. J Tissue Eng Regen Med 2013; 7: 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zheng L, Zhao H et al. In vitro assessment of the differentiation potential of bone marrow-derived mesenchymal stem cells on genipin–chitosan conjugation scaffold with surface hydroxyapatite nanostructure for bone tissue engineering. Tissue Eng Part A 2011; 17: 1341–1349. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang YC, Zhou H, Guo YC, Su XX. Evaluation of in vitro and in vivo osteogenic differentiation of nano-hydroxyapatite/chitosan/poly(lactide-co-glycolide) scaffolds with human umbilical cord mesenchymal stem cells. J Biomed Mater Res A 2014; 102: 760–768. [DOI] [PubMed] [Google Scholar]
- Chai YC, Kerckhofs G, Roberts SJ et al. Ectopic bone formation by 3D porous calcium phosphate–Ti6Al4V hybrids produced by perfusion electrodeposition. Biomaterials 2012; 33: 4044–4058. [DOI] [PubMed] [Google Scholar]
- Behnia H, Khojasteh A, Kiani MT et al. Bone regeneration with a combination of nanocrystalline hydroxyapatite silica gel, platelet-rich growth factor, and mesenchymal stem cells: a histologic study in rabbit calvaria. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115: e7–e15. [DOI] [PubMed] [Google Scholar]
- Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther 2010; 21: 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Saggio I, Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther 2010; 21: 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther 2009; 17: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingham E, Oreffo RO. Embryonic and induced pluripotent stem cells: understanding, creating, and exploiting the nano-niche for regenerative medicine. ACS Nano 2013; 7: 1867–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu J, Manuchehrabadi N, Weir MD, Zhu Z, Xu HH. Umbilical cord and bone marrow mesenchymal stem cell seeding on macroporous calcium phosphate for bone regeneration in rat cranial defects. Biomaterials 2013; 34: 9917–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Wasnik S, Guha A, Kumar JM, Sinha A, Singh S. Evaluation of nano-biphasic calcium phosphate ceramics for bone tissue engineering applications: in vitro and preliminary in vivo studies. J Biomater Appl 2013; 27: 565–575. [DOI] [PubMed] [Google Scholar]
- Villalona GA, Udelsman B, Duncan DR et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng Part B Rev 2010; 16: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar SN, Edgar KJ. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials 2012; 33: 3279–3305. [DOI] [PubMed] [Google Scholar]
- Zhou H, Xu HH. The fast release of stem cells from alginate-fibrin microbeads in injectable scaffolds for bone tissue engineering. Biomaterials 2011; 32: 7503–7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao P, Wang J, Xie Q, Li F, Dong L, Xu T. Injectable calcium phosphate-alginate-chitosan microencapsulated MC3T3-E1 cell paste for bone tissue engineering in vivo. Mater Sci Eng C Mater Biol Appl 2013; 33: 4633–4639. [DOI] [PubMed] [Google Scholar]
- Wang J, Qiao P, Dong L, Li F, Xu T, Xie Q. Microencapsulated rBMMSCs/calcium phosphate cement for bone formation in vivo. Biomed Mater Eng 2014; 24: 835–843. [DOI] [PubMed] [Google Scholar]
- Xu HH, Zhao L, Detamore MS, Takagi S, Chow LC. Umbilical cord stem cell seeding on fast-resorbable calcium phosphate bone cement. Tissue Eng Part A 2010; 16: 2743–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]