Abstract
Severe burn injury triggers the body's nonspecific adaptive responses to acute insult, including the systemic inflammatory and stress responses, as well as the sympathetic response to immobilization. These responses trigger inflammatory bone resorption followed by glucocorticoid-induced apoptosis of osteoblasts and probably osteocytes. Because these patients are catabolic, they suffer concomitant muscle wasting and negative nitrogen balance. The use of anabolic agents such as recombinant human growth hormone and oxandrolone results in improved bone mineral content and muscle strength after approximately 1 year. Use of bisphosphonates within the first 10 days of a severe burn completely blocks the resorptive bone loss and has the added advantage of appearing to preserve muscle protein from excessive breakdown. The mechanism for the protective effect on muscle is not currently known. However, if the effect of bisphosphonates on muscle can be confirmed, it raises the possibility that bone communicates with muscle.
Introduction: burn injury as a model for bone and muscle loss
Burn injury is a relatively uncommon condition. Severe burn injury is more uncommon still. Furthermore, burn care is highly specialized and care for these victims is provided by a small group of highly trained clinicians. Moreover, burn injury involves multiple organ system dysfunction and, inevitably, sepsis. This involvement creates a highly complex set of metabolic interactions. Therefore, it is important to explain why burn injury is a good model for bone loss and muscle catabolism.
To begin with, the relative rarity of burn injury is a reason to believe that man did not evolve any responses to deal specifically with burns. Therefore, the mechanism or mechanisms by which humans adapt to burn injury are nonspecific adaptive responses which might be brought to bear in other conditions as well. These nonspecific adaptive responses result in consequent bone and muscle loss following severe burn injury. In particular, we are referring to the inflammatory response and the stress response, as well as in part to the response to immobilization. And, despite the complex metabolic abnormalities seen with severe burns, the features of bone and muscle dysfunction in children and adults are remarkably similar.1,2
The nonspecific adaptive responses: inflammation
The first adaptive response to be discussed is the inflammatory response. Destruction of the skin barrier by burn injury permits entrance of pathogenic microorganisms directly into the bloodstream. The resultant systemic inflammatory response, which occurs immediately following the injury, gives rise to production of pro-inflammatory cytokines, most notably interleukin (IL)-1β and IL-6, by peripheral blood mononuclear cells.2 The IL-6 can then shift bone marrow production from erythropoietic to predominantly myelopoietic cells,3 helping to sustain the inflammatory response.
In addition, IL-1β and IL-6 stimulate osteoblasts and, likely, osteocytes, to produce the ligand of the receptor activator of the nuclear transcription factor NF-κB, otherwise known as RANK ligand, or simply RANKL. RANKL in turn stimulates marrow stem cells to differentiate into osteoclasts with a consequent increase in osteoclastogenesis and bone resorption such that severely burned children lose over 7% of their lumbar spine bone mineral density in the first 3–6 weeks following the burn and about 3% of their total body bone mineral content in the first 6 months following the injury.4 These findings suggest that trabecular bone may be more affected than cortical bone since lumbar spine is mostly trabecular bone and the total body consists of approximately 80% cortical bone. In a sheep model of burn injury, histological scalloping of the bones is detectable at 5 days post-burn, while the urinary C telopeptide of type I collagen, CTx, is elevated within the first 24 h.5
At the same time, these cytokines can upregulate the parathyroid gland calcium-sensing receptor, leading to hypercalciuric hypoparathyroidism6 and urinary calcium wasting,7 thus making repair of the bone loss difficult.
Immobilization
Immobilization will also contribute to resorptive bone loss at this stage.5 The mechanism for this is not entirely clear but appears to involve increased sympathetic tone,8 which may be part of the stress response.2 Endogenous glucocorticoids and catecholamines are released as part of this response. Osteoblasts contain β2 adrenergic receptors,8 which, when activated, will stimulate RANKL production. This set of responses also occurs early following the burn injury. However, when osteoblasts become apoptotic, which will be dealt with in the next section, it is unclear how immobilization would continue to mediate bone loss.
Stress
Following acute bone resorption, by approximately two weeks post-burn, osteoblasts disappear from the bone surface9 and markers of bone marrow stem cell differentiation into osteoblasts are significantly reduced.9 At approximately the same time, urinary deoxypyridinoline excretion, a biomarker specific for bone resorption, is reduced,2 indicating that bone has become hypodynamic with both decreased formation and decreased resorption. These hypodynamic changes occur despite the continued high circulating concentrations of IL-1β and IL-62 indicating that the osteoblasts, and, likely, the osteocytes, are apoptotic. While there is not as yet direct evidence supporting osteocyte apoptosis, there is certainly failure to generate quantities of RANKL sufficient to maintain normal bone resorption. The most likely explanation for this particular set of changes is the increased production of endogenous glucocorticoids, with 24-h urine-free cortisol excretion ranging from 3–8 times the upper limit of normal.2,9
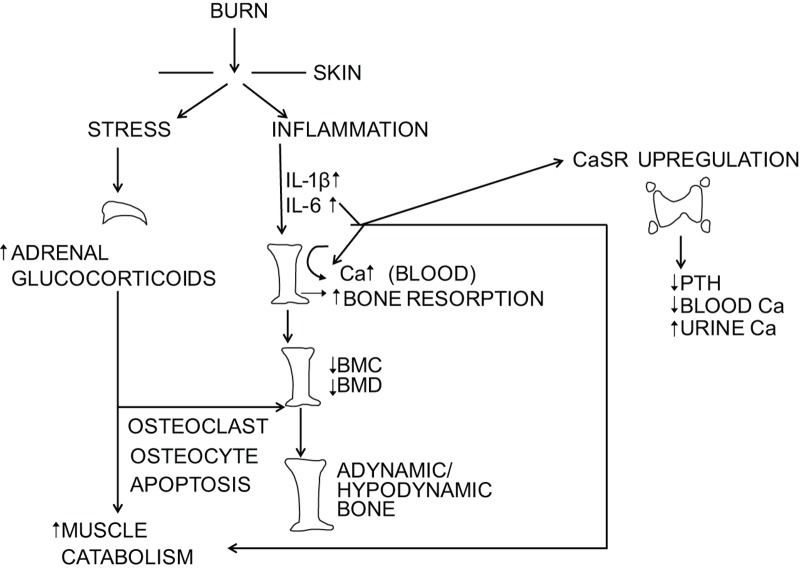
Concomitant with the abnormal bone dynamics is muscle wasting resulting from increased muscle protein breakdown and nitrogen-wasting.10 Using a standard protocol for stable isotope study of muscle protein kinetics,11 an infusion of labeled phenylalanine is given intravenously over 8 h. Phenylalanine is neither synthesized nor oxidized by muscle; therefore, once a steady state has been established, the rate of labeled phenylalanine disappearance from arterial blood is considered an index of muscle protein synthesis and the rate of labeled phenylalanine appearance in venous blood is considered a measure of muscle protein breakdown. In a typical study, labeled phenylalanine is infused intravenously over 8 h. Over the last 3 h, unlabeled amino acids are also infused. This provides additional substrate for muscle protein synthesis; normally, what is seen is a spike in synthetic rate. However, inevitably, muscle protein breakdown exceeds muscle protein synthesis.10 Both endogenous glucocorticoids and pro-inflammatory cytokines have been implicated in the breakdown of skeletal muscle12 (Figure 1).
Figure 1.
Schematic diagram illustrating the effect of burn injury on the inflammatory and stress responses and their effect on bone. BMC, bone mineral content; BMD, bone mineral density; CaSR, extracellular calcium sensing receptor; IL, interleukin; PTH, parathyroid hormone.
Thus, both the systemic inflammatory response and the stress response to severe burn injury may contribute to the loss of both bone and muscle. This possibility raises the issue of whether loss of muscle contributes to the loss of bone and vice versa.
Treatment with anabolic agents
Because burned children are hypermetabolic, with resting energy expenditure ranging from 1.2 to 1.8 times normal, anabolic agents initially appeared to be the treatment of choice. The only anabolic agents that were available for study in pediatric populations, were recombinant human growth hormone and oxandrolone, a non-aromatizable androgen that had previously had limited use in pediatrics. Of interest is that children were shown to have transient growth hormone deficiency following severe burns,13 the explanation for which was not readily apparent. This transient deficiency was associated with low circulating concentrations of insulin-like growth factor 1 (IGF-1), generally considered to be anabolic to bone. Treatment with recombinant human growth hormone at a dose of 0.05 mg·kg−1 per day subcutaneously quickly raised IGF-1 concentrations in the blood to normal levels. However, while serum concentrations of osteocalcin were also initially low, raising circulating IGF-1 concentrations in the blood failed to raise osteocalcin concentration in the blood to normal levels.13 The failure of an anabolic agent to produce a rise in serum osteocalcin concentration in the presence of normal circulating levels of IGF binding protein 3 raises the question of the effect of inflammatory cytokines and endogenous glucocorticoids and catecholamines on the IGF-1 receptor. This has yet to be addressed.
What was learned from the studies involving recombinant human growth hormone and oxandrolone14–17 is that improvement in lean body mass by measurement with dual energy X-ray absorptiometry, usually indicating muscle mass, precedes improvement in bone mass by about 3–6 months, the entire process taking about 12 months after initiating therapy. This finding suggests that skeletal loading following increased muscle mass as a result of treatment with anabolic agents contributes to increased bone mass and that muscle may be the primary factor in improving bone mineral content. Inasmuch as recombinant human growth hormone is a glucocorticoid antagonist and elevated endogenous glucocorticoid production persists for approximately 1 year following burn injury,18 it is possible that the reason that it takes 12 months to begin to see a positive effect on bone mass is due to persistence of glucocorticoid interference with the anabolic agents.
Vitamin D deficiency
Also of note is that vitamin D has an effect on muscle function via the genomic and non-genomic expression of the vitamin D receptor in muscle fibers; vitamin D receptor signaling may play a role in myoblast differentiation and proliferation.19 So, what are the effects of the post-burn vitamin D status on muscle and, therefore, on bone? This is very difficult to determine. Inasmuch as vitamin D Binding Protein is low in acutely burned adults,1 it is difficult to know whether serum 25-hydroxyvitamin D concentrations are truly low or an artifact resulting from the low serum concentrations of vitamin D Binding Protein and albumin. This question will be partially addressed below.
Anti-resorptives
Bisphosphonates have had limited use in children in part due to concern regarding the length of time this class of drugs remains in bone. Their only application following clinical trials has been with osteogenesis imperfecta. Given their apparent safety and efficacy, a randomized controlled double-blind study was carried out in burned children and the drug pamidronate was shown to completely prevent the resorptive bone loss4 with the effect of one or two doses given acutely post-burn lasting for up to 2 years.20 The continued bone mineral accretion observed in these children is presumably due to continued modeling. This result was understandable given the pathophysiology of bone loss described above. What happened next, however, was surprising and is still not fully understood.
Because several of the subjects who participated in the randomized controlled study of pamidronate also participated in the stable isotope infusion studies of muscle protein kinetics, the results from these latter studies that were performed in subjects receiving pamidronate or placebo controls were reviewed.
The muscle protein kinetic studies were carried out according to the protocol previously described.11 Data were available only in a small number of subjects.12 However, what was found was really quite surprising. Firstly, the muscle protein synthetic rate, which invariably rises when unlabeled amino acids are infused, did not increase in the group receiving pamidronate. Furthermore, muscle protein breakdown was significantly lower in the pamidronate group than in the placebo group despite a large standard deviation seen in both groups.12 The net muscle protein balance, synthesis minus breakdown, was, as expected, negative in the subjects receiving placebo, but positive in those who received pamidronate.12
To check that these differences were not an artifact of the stable isotope protocol, residual muscle biopsy specimens for fiber diameter, an indirect measure of muscle strength, were determined. These studies demonstrated that the subjects receiving pamidronate had significantly greater muscle fiber diameter of their vastus lateralis biopsies than did their placebo controls.12 Additionally, we looked at lower limb muscle strength by measuring peak torque at 9 months post-burn in some of the subjects who participated in the randomized controlled study of pamidronate. Although the number of participants was small, analysis of the data showed that those who had received pamidronate had lower limb peak torque values equivalent to physically fit age-matched controls while those who received placebo tended to have less lower limb strength by ANOVA.12 Thus, pamidronate administration appeared to do something to improve muscle protein kinetics, but what?
Before discussing possible explanations of the effect of pamidronate on muscle, it is necessary to return to the question of vitamin D and the effect of progressive vitamin D deficiency on muscle mass. D deficiency develops due to the failure of the skin to synthesize normal quantities of vitamin D3 from its 7-dehydrocholesterol precursor on ultraviolet radiation exposure.21 Because the administration of pamidronate appears to promote positive muscle protein balance in the presence of vitamin D deficiency, it is not likely that the D deficiency plays a major role in burn-induced sarcopenia, at least in the early stages. As the deficiency progresses, it is possible that it plays a role in continued muscle weakness during recuperation. However, this question has not been investigated.
Possible explanations for the apparent effects of pamidronate on muscle
At present there are no definitive answers to the question posed by the pamidronate study. Any answer other than the one that would suggest selection bias due to the small number of subjects enrolled in both the randomized clinical trial and the stable isotope infusion protocols and the retrospective nature of the examination12 would invoke a new paradigm. Possible explanations for the effect of bisphosphonates on muscle include: (i) extra-osseous effects of pamidronate; (ii) a paracrine effect of bone on muscle; or (iii) effects of bisphosphonates on the bone microenvironment.
Because the pharmacokinetics of bisphosphonates suggests that they can be released from bone and re-enter the blood,22 there is a possibility that they can be taken up by other tissues, including muscle. Another possibility, according to the work of Bellido and Plotkin,23 is that bisphosphonates prevent osteocyte apoptosis. If in fact the osteocytes are preserved, they may produce a factor or factors with a paracrine effect on muscles to help maintain muscle mass and function. Finally, preliminary data from Guise24 indicate that the bone microenvironment adversely affects muscle in breast cancer patients with bone metastases, resulting in a chain of events that cause calcium leakage from muscle and resulting in muscle weakness. These events appear to be blocked by administration of bisphosphonates. Could a similar mechanism be operative following burn injury?
All of these possibilities require investigation, but patients who suffer from osteoporosis and sarcopenia would be well served if treatment for bone loss also resulted in preservation of muscle mass.
Acknowledgments
The author notes that in August 2012, he served on the Bone Toxicity Advisory Board of Novartis Pharmaceutical Company. He has no other apparent conflicts of interest. This work was performed with partial support from P50 GM60338 from the National Institutes of Health and several grants from Shriners Hospitals for Children.
The author declares no conflict of interest.
References
- Klein GL, Herndon DN, Rutan TC et al. Bone disease in burn patients. J Bone Miner Res 1993; 8: 337–345. [DOI] [PubMed] [Google Scholar]
- Klein GL, Herndon DN, Goodman WG et al. Histomorphometric and biochemical characterization of bone following acute severe burns in children. Bone 1995; 17: 455–460. [DOI] [PubMed] [Google Scholar]
- Chou DB, Sworder B, Bouladoux N et al. Stromal derived IL6 alters the balance of myeloerythroid progenitors during toxoplasma gondi infection. J Leukoc Biol 2012; 92: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein GL, Wimalawansa SJ, Kulkarni G, Sherrard DJ, Sanford AP, Herndon DN. The efficacy of acute administration of pamidronate on the conservation of bone mass following severe burn injury in children: a double-blind, randomized, controlled study. Osteoporos Int 2005; 16: 631–635. [DOI] [PubMed] [Google Scholar]
- Klein GL, Xie Y, Qin YX et al. Preliminary evidence of early bone resorption in a sheep model of acute burn injury: an observational study. J Bone Miner Metab 2014; 32: 136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey ED, Chattopadhyay N, Bai M et al. Up-regulation of the parathyroid calcium-sensing receptor after burn injury in sheep: a potential contributory factor to post-burn hypocalcemia. Crit Care Med 2000; 28: 3885–3890. [DOI] [PubMed] [Google Scholar]
- Klein GL, Nicolai M, Langman CB, Cuneo BF, Sailer DE, Herndon DN. Dysregulation of calcium homeostasis after severe burn injury in children: possible role of magnesium depletion. J Pediatr 1997; 131: 246–251. [DOI] [PubMed] [Google Scholar]
- Kondo H, Nifuji A, Takeda S et al. Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J Biol Chem 2005; 280: 30192–30200. [DOI] [PubMed] [Google Scholar]
- Klein GL, Bi LX, Sherrard DJ et al. Evidence supporting a role of glucocorticoids in short-term bone loss in burned children. Osteoporos Int 2004; 15: 468–474. [DOI] [PubMed] [Google Scholar]
- Gore DC, Chinkes DL, Wolf SE, Sanford AP, Herndon DN, Wolfe RR. Quantification of protein metabolism in vivo for skin, wound and muscle in severe burn patients. J Parenter Enteral Nutr 2006; 30: 331–338. [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research. New York: John Wiley and Sons, 2004: 51–76. [Google Scholar]
- Borsheim E, Herndon DN, Hawkins HK, Suman OE, Cotter M, Klein GL. Pamidronate attenuates muscle loss after pediatric burn injury. J Bone Miner Res 2014; 29: 1369–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein GL, Wolf SE, Langman CB et al. Effects of therapy with recombinant human growth hormone on insulin-like growth factor system components and serum levels of biochemical markers of bone formation in children after severe burn injury. J Clin Endocrinol Metab 1998; 83: 21–24. [DOI] [PubMed] [Google Scholar]
- Hart DW, Herndon DN, Klein G et al. Attenuation of post-traumatic muscle catabolism and osteopenia by long-term growth hormone therapy. Ann Surg 2001; 233: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branski LK, Herndon DN, Barrow RE et al. Randomized controlled trial to determine the efficacy of long-term growth hormone treatment in severely burned children. Ann Surg 2009; 250: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KD, Thomas S, Mlcak RP, Chinkes DL, Klein GL, Herndon DN. Effects of long-term oxandrolone administration in severely burned children. Surgery 2004; 136: 219–224. [DOI] [PubMed] [Google Scholar]
- Porro LJ, Herndon DN, Rodriguez NA et al. Five-year outcomes after oxandrolone administration in severely burned children: a randomized clinical trial of safety and efficacy. J Am Coll Surg 2012; 214: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreira CT, Jeschke MG, Herndon DN. Beta blockade in burns. Novartis Found Symp 2007; 280: 238–248. [DOI] [PubMed] [Google Scholar]
- Wagatsuma A, Sakuma K. Vitamin D signaling in myogenesis: potential for treatment of sarcopenia. Biomed Res Int 2014; 2014: 121254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przkora R, Herndon DN, Sherrard DJ, Chinkes DL, Klein GL. Pamidronate preserves bone mass for at least 2 years following pamidronate administration for pediatric burn injury. Bone 2007; 41: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein GL, Chen TC, Holick MF et al. Synthesis of vitamin D in skin after burns. Lancet 2004; 363: 291–292. [DOI] [PubMed] [Google Scholar]
- Weiss HM, Pfaar U, Schweitzer A, Wiegand H, Skerjanec A, Schran H. Biodistribution and plasma protein binding of zoledronic acid. Drug Metab Dispos 2008; 36: 2043–2049. [DOI] [PubMed] [Google Scholar]
- Bellido T, Plotkin LI. Novel actions of bisphosphonates in bone: preservation of osteoblast and osteocyte viability. Bone 2011; 49: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise TA. The bone microenvironment (abstract). In:Proceedings of the 7th International Conference on Osteoporosis and Bone Research; 16–19 October 2014; Xiamen, China. IBMS: Chicago, IL, USA, 2014, BoneKEy Reports, in press. [Google Scholar]