Abstract
Study Design Retrospective case series.
Objective StaXx XD (Spine Wave, Inc., Shelton, CT, United States) is an expandable polyaryl-ether-ether-ketone (PEEK) wafer implant utilized in the treatment of lumbar degenerative disease. PEEK implants have been successfully used as interbody devices. Few studies have focused on expandable PEEK devices. The aim of the current study is to determine the radiographic and clinical outcome of expandable PEEK cages utilized for transforaminal lumbar interbody fusion in patients with lumbar degenerative diseases.
Methods Forty-nine patients who underwent lumbar interbody fusion with implantation of expandable PEEK cages and posterior instrumentation were included. The clinical outcome was evaluated using the visual analog scale (VAS) and the Oswestry Disability Index (ODI). Radiographic parameters including disk height, foraminal height, listhesis, local disk angle of the index level/levels, regional lumbar lordosis, and graft subsidence were measured preoperatively, postoperatively, and at latest follow-up.
Results At an average follow-up of 19.3 months, the minimum clinically important difference for the ODI and VAS back, buttock, and leg were achieved in 64, 52, 58, and 52% of the patients, respectively. There was statistically significant improvement in VAS back (6.42 versus 3.11, p < 0.001), VAS buttock (4.66 versus 1.97, p = 0.002), VAS leg (4.55 versus 1.96, p < 0.001), and ODI (21.7 versus 12.1, p < 0.001) scores. There was a significant increase in the average disk height (6.49 versus 8.18 mm, p = 0.037) and foraminal height (15.6 versus 18.53 mm, p = 0.0001), and a significant reduction in the listhesis (5.13 versus 3.15 mm, p = 0.005). The subsidence of 0.66 mm (7.4%) observed at the latest follow-up was not significant (p = 0.35).
Conclusions Midterm results indicate that expandable PEEK spacers can effectively and durably restore disk and foraminal height and improve the outcome without significant subsidence.
Keywords: lumbar spine, interbody fusion, expandable PEEK cage, disc height, graft subsidence, lumbar lordosis, Oswestry Disability Index, visual analog scale
Study Rationale and Context
Interbody cages are used in the surgical treatment of degenerative spinal disorders and allow for stabilization and indirect decompression of spinal structures.1 2 Polyaryl-ether-ether-ketone (PEEK) implants have been associated with successful outcome.3 4 The StaXx XD (Spine Wave, Inc., Shelton, CT, United States) expandable device is a PEEK implant with the ability to be expanded in situ after implantation.5
Objective
The aim of the current study is to determine the radiographic and clinical outcome of expandable PEEK cages utilized for transforaminal lumbar interbody fusion in patients with lumbar degenerative diseases.
Methods
Study Design
Retrospective case series. The study was approved by the institutional review board. All surgical devices used in this study are approved by the U.S. Food and Drug Administration.
Inclusion Criteria
Patients aged 18 years and older with lumbar spinal disease treated by lumbar interbody fusion using expandable PEEK cages and posterior instrumentation. There are various indications for performing an interbody fusion. The most important include mechanical low back pain and radicular pain associated with spondylolisthesis, recurrent disk herniations, severe discogenic low back pain caused by degenerative disk disease (DDD), postlaminectomy instability, malalignment, trauma, and treatment of pseudarthrosis.6
Exclusion Criteria
Tumor pathology.
Patient Population
According to the inclusion and exclusion criteria, all patients who underwent lumbar interbody fusion using expandable PEEK cages and posterior instrumentation between 2009 and 2011 were included. The total number of patients included in the study was equal to the total number of cases treated during the time interval for the same disease states, because no patients with tumor pathology (the exclusion criteria) were treated with expandable cages within the study period. All patients had radiographic and/or clinical outcome data.
Surgical Technique
After decompression of the spinal pathology, a diskectomy was performed using pituitary rongeurs and shavers. The end plates were carefully prepared and then the bone graft or substitute and an expandable StaXx XD PEEK cage were inserted. The implant has no graft chamber and the bone graft needs to be placed surrounding the cage. The PEEK cage is 7 mm in the collapsed state when inserted and can be expanded in situ (Fig. 1) to an appropriate height with distraction of the disk space, up to 15 mm. The final height was determined based on the adjacent disk levels as well as based on the manual resistance experienced during delivery of the individual wafers. Posterior instrumentation was then inserted using the standard techniques. Various bone graft substitutes or expanders were used for fusion. In the open procedures, a posterolateral fusion was performed in addition to the interbody fusion. In minimally invasive cases, only an interbody fusion was performed.
Fig. 1.
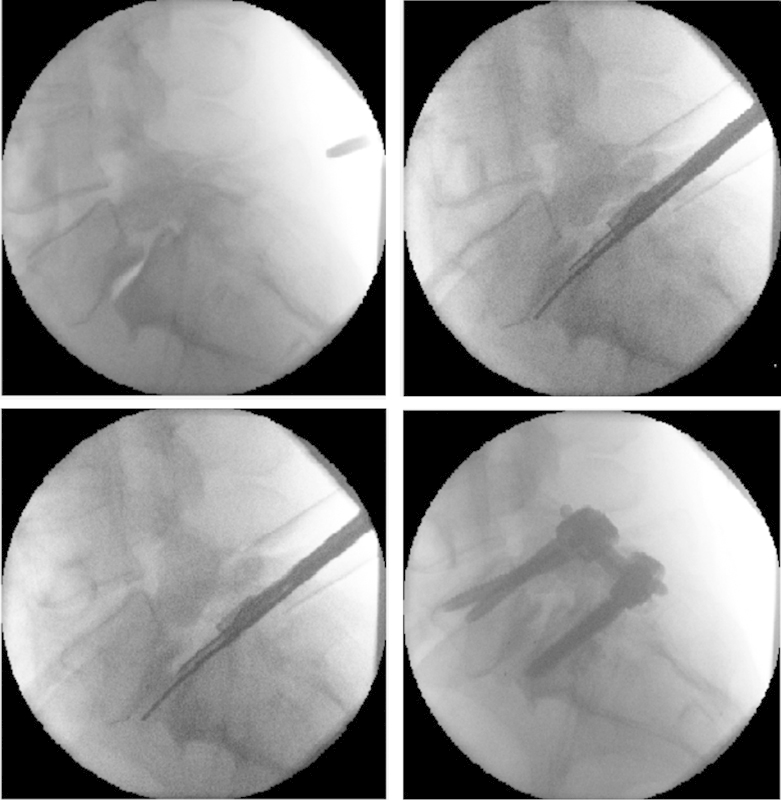
Example distraction of L5–S1 disk space resulting from in situ expansion of an interbody cage. Top left: L5–S1 disk space prior to surgery. Top right: Implantation of the expandable polyaryl-ether-ether-ketone (PEEK) spacer prior to expansion. Bottom left: In situ expansion of the PEEK spacer. Bottom right: The end of the operation.
Outcome
The demographic and perioperative data was recorded. The radiographic parameters including the disk height, left/right foraminal height, listhesis, local disk angle at the index level/levels, and regional lumbar lordosis were measured preoperatively, immediately postoperatively, and at the latest follow-up. Disk height and local disk angle were defined as the distance and the angle between the end plates immediately above and below the referenced levels of index on the lateral plane. Both anterior and posterior disk heights were measured and an average was then made. Foraminal height was defined as the distance between the inferior border of the immediate rostral pedicle and the superior border of the immediate caudal pedicle. Regional lumbar lordosis was defined as the angle between the superior end plate of L1 and the superior end plate of S1 on the lateral plane. Subsidence was defined as the difference between the average disk height on the postoperative versus the latest follow-up imaging, and listhesis as the extent of displacement of a vertebra in relation to the vertebrae below (Fig. 2). Radiographic fusion was defined by the presence of bony bridging across the intervertebral space on computed tomography (CT) imaging or as less than 4 degrees of motion on dynamic radiographs. CT scans were the preferred imaging module for our fusion evaluation; however, in the cases where CT scans were not available, alternatively dynamic flexion-extension radiographs were assessed. Fusion was evaluated by a board certified neuroradiologist. Clinical outcomes were evaluated preoperatively and at latest follow-up using the visual analog scale (VAS) for back, buttock, and leg pain, as well as the Oswestry Disability Index (ODI). The mean follow-up was 19.3 ± 6.37 months.
Fig. 2.
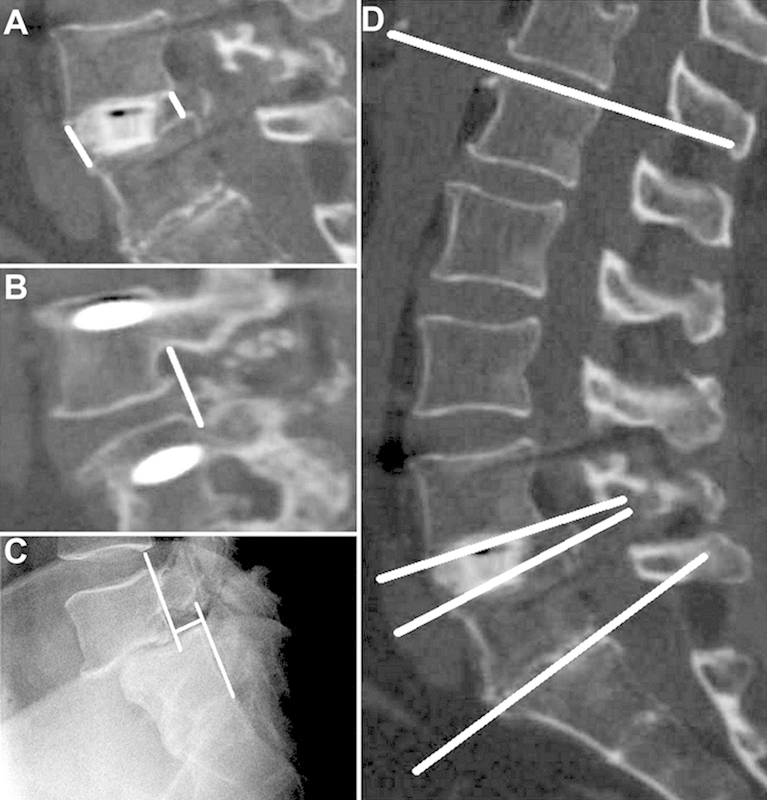
Examples of radiologic outcome measurements. (A) Anterior and posterior disk heights. (B) Foraminal height. (C) Listhesis. (D) Regional lumbar lordosis (the top and the bottom most lines) and the local disk angle at the index level/levels (the pair of lines located in the middle).
Analysis
Both the clinical and radiographic outcomes were analyzed using Student paired sample t test. Descriptive statistics were used for the patient demographics and data. Statistical analyses were conducted in SPSS (PASW) Version 18.0 (SPSS Inc., Chicago, Illinois, United States).
Results
Based on the inclusion and exclusion criteria, all 49 patients who underwent lumbar interbody fusion using expandable StaXx XD PEEK spacer and bilateral pedicle screw instrumentation between 2009 and 2011 were eligible for inclusion. All met the pathology criteria, and there were no cases with tumor. Of the patients, 72% had minimally invasive transforaminal lumbar interbody fusion procedures (MIS TLIF) and 28% had open revision surgeries for outside referrals and other indications. The demographics and surgical details are summarized in Tables 1 and 2, respectively. Clinical and radiographic outcome are reported in Tables 3 4 to 5 and Figs. 3 4 to 5.
Table 1. Patient demographics (n = 49).
| Variable | n (%) or average ± SD |
|---|---|
| Age | 59.6 ± 15.46 |
| Gender (M/F) | 21/28 |
| BMI | 28.7 ± 7.25 |
| Current smoker | 12 (24.4%) |
| Diabetic | 6 (12.2%) |
| Current steroid medication user | 2 (4.1%) |
| Previous surgery at index level | 14 (28.6%) |
| At an outside center | 7 (14.3%) |
| At our own center | 5 (10.2%) |
| At both outside and our own center | 2 (4.1%) |
| Preoperative diagnosis | |
| Spondylolisthesis | 29 (59.1%) |
| Degenerative disk disease | 14 (28.6%) |
| Previous nonunion/pseudarthrosis | 5 (10.2%) |
| Post laminectomy syndrome | 4 (8.1%) |
| Instrumentation/mechanical failure | 6 (12.2%) |
| Scoliosis | 1 (2%) |
| Trauma | 1 (2%) |
Abbreviations: BMI, body mass index; SD, standard deviation.
Table 2. Operative data.
| Variable | n (%) |
|---|---|
| Total number of operated levels | 62 |
| Fusion level | |
| T12–L1 | 2 (3.3%) |
| L1–L2 | 3 (4.8%) |
| L2–L3 | 3 (4.8%) |
| L3–L4 | 8 (12.9%) |
| L4–L5 | 26 (41.9%) |
| L5–S1 | 20 (32.3%) |
| PEEK cage as part of single level MIS TLIF | 36 (73.5%) |
| PEEK cage(s) as part of multilevel open surgery | 13 (26.5%) |
| Unilateral cage | 83.7% |
| Bilateral cages | 16.3% |
| Final cage height (mm)a | 10.9 ± 0.99 |
| Graft substance used for fusion | |
| Si-CaP only | 61.7% |
| Si-CaP and bone morphogenic protein | 23.6% |
| Si-CaP and autograft/demineralized bone matrix | 14.7% |
Abbreviations: MIS, minimally invasive; PEEK, polyaryl-ether-ether-ketone; Si-CaP, silicate calcium phosphate; TLIF, transforaminal lumbar interbody fusion.
Average ± standard deviation.
Table 3. Clinical outcome.
| Outcome variable | Preoperative | Latest follow-up | p Value |
|---|---|---|---|
| VAS backa | 6.4 ± 0.6 | 3.1 ± 0.6 | <0.001b |
| VAS buttocka | 4.6 ± 0.5 | 1.9 ± 0.5 | 0.002b |
| VAS lega | 4.5 ± 0.5 | 1.9 ± 0.5 | <0.001b |
| Oswestry Disability Indexa | 44.0 ± 3.2 | 24.2 ± 3.8 | <0.001b |
Results are mean ± standard error.
p values <0.05 are considered to be statistically significant.
Table 4. Radiographic outcome.
| Variable | Preoperative | Latest follow-up | p Value |
|---|---|---|---|
| Disk heighta | 6.5 ± 0.62 | 8.2 ± 0.61 | 0.037b |
| Foraminal heighta | 15.6 ± 0.67 | 18.5 ± 0.57 | <0.001b |
| Listhesisa | 5.1 ± 0.77 | 3.1 ± 0.36 | 0.005b |
| Local disk anglea | 6.5 ± 0.85 | 8.1 ± 0.93 | 0.122 |
| Lumbar lordodsisa | 45.8 ± 2.73 | 48.0 ± 2.14 | 0.248 |
Results are mean ± standard error.
p values <0.05 are considered to be statistically significant.
Table 5. Subsidence.
| Immediate postoperative | Latest follow-up | p Value | |
|---|---|---|---|
| Disk heighta | 8.8 ± 0.42 | 8.2 ± 0.61 | 0.351 |
Results are mean ± standard error.
Fig. 3.
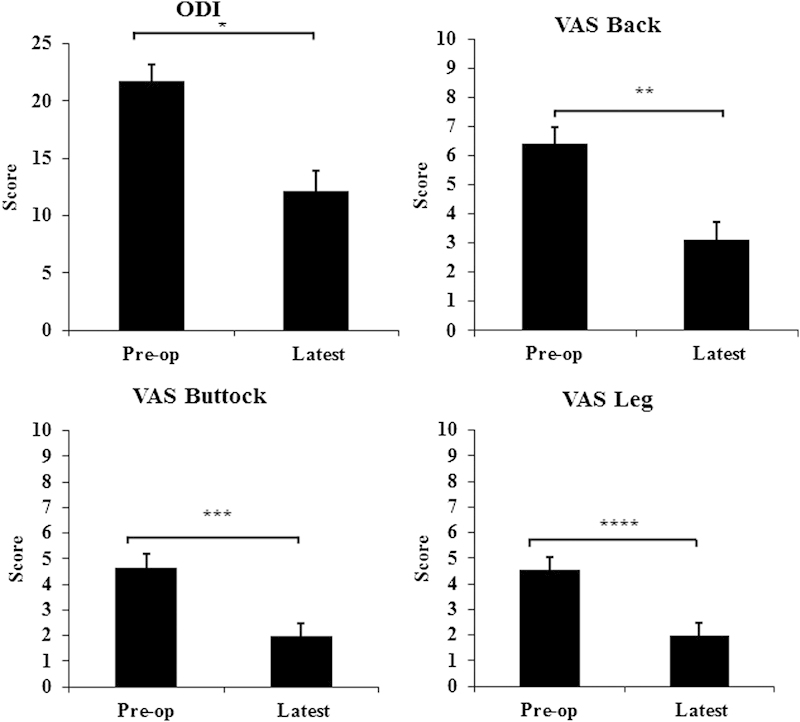
Clinical outcomes of 40/49 patients at the latest follow-up on average 19.3 months after the surgery; Oswestry Disability Index (ODI), visual analog scale (VAS) back, VAS buttock, and VAS leg were all significantly improved. *p < 0.001; **p < 0.001; ***p = 0.002; ****p < 0.001.
Fig. 4.
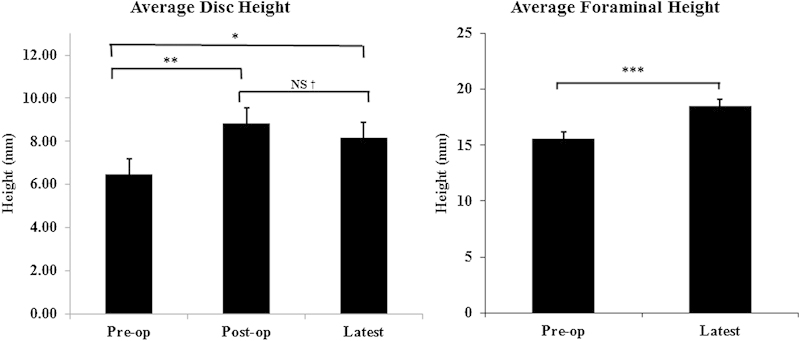
Radiologic outcomes of 45/49 patients at the latest follow-up on average 19.3 months. Average disk height and average foraminal height were significantly improved. NS, not significant. *p = 0.026; **p = 0.0001; † p = 0.35; ***p = 0.0001.
Fig. 5.
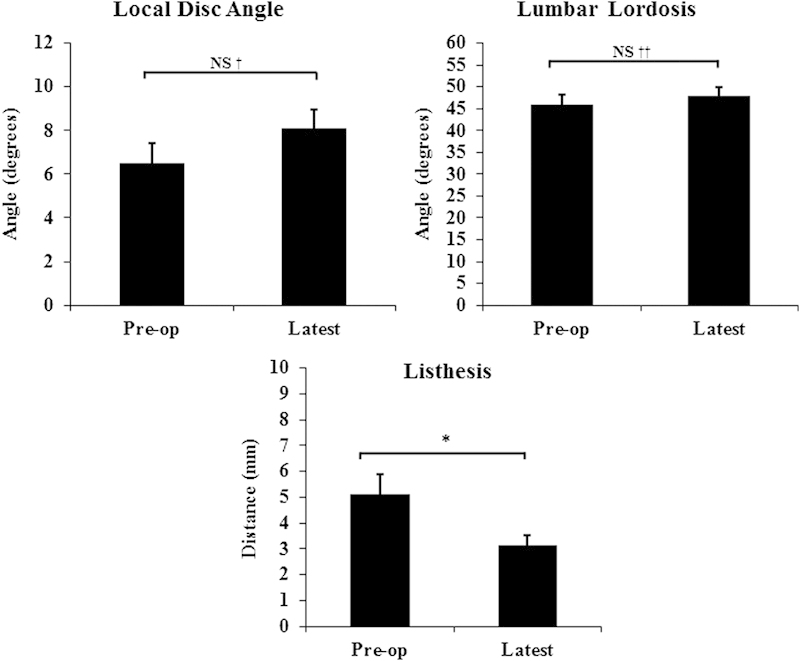
Radiologic outcomes of 45/49 patients (58/62 levels) at the latest follow-up on average 19.3 months. Listhesis was significantly improved. However, the local disk angle and regional lumbar lordosis were not significantly changed. NS, not significant. † p = 0.12; †† p = 0.24; *p = 0.005.
Clinical outcome was available in 92% of the cases. At an average follow-up of 19.3 ± 6.37 months, there was significant improvement in all clinical outcome parameters. Minimum clinically important difference (MCID) was defined as a change of 12 points in the ODI and change of 3 points for the VAS.7 8 9 10 11 MCID for the ODI and VAS back, buttock, and leg were achieved in 64, 52, 58, and 52% of the patients, respectively. Radiographic outcome was available in 82% of the patients. The subsidence from postoperative values to the latest of 0.66 mm (7.4%) was not statistically significant. The fusion rate and the complications are presented in Tables 6 and 7, respectively. Fusion was evaluated using CT scans in 85% of the patients with radiographic outcome, and it was evaluated by dynamic flexion-extension radiographs in the remaining 15%. Compared with silicate calcium phosphate (Si-CaP) as a graft material, combined use of Si-CaP and bone morphogenic protein resulted in a statistically higher fusion rate. Similarly, statistically higher fusion rates were observed with a combination of Si-CaP and autograft and/or demineralized bone matrix. No patients were completely lost to follow-up. The majority of patients had both clinical and radiographic results available. The few patients who did not have clinical results available had radiographic results, and similarly those few who lacked radiographic results had clinical results.
Table 6. Fusion rate.
| Group of patients | No. of cases fused (%) |
|---|---|
| All patients (evaluated in 34 patients with available latest follow-up CT scans) | 17/34 (50%) |
| Patients with Si-CaP and bone morphogenic protein | 5/8 (62.5%) |
| Patients with Si-CaP and autograft and/or demineralized bone matrix | 5/5 (100%) |
Abbreviations: CT, computed tomography; Si-CaP, silicate calcium phosphate.
Table 7. Complications (n = 49).
| Variable | n (%) |
|---|---|
| Perioperative inpatient complications | |
| Dural tear | 4 (8.2%) |
| Wound infection | 2 (4.1%) |
| Reoperations at index level | 4 (8.1%) |
| Nonunion | 3 (6.1%) |
| Adjacent level disease | 1 (2.0%) |
We compared single-level cases with multilevel cases (radiographic and clinical outcome). In clinical parameters, interestingly last follow-up ODI, VAS buttock, and VAS leg were lower in multilevel cases (ODI 5.0 versus 13.3, p = 0.02; VAS buttock 0.0 versus 1.9, p = 0.002; VAS leg 0.16 versus 1.9, p = 0.006). VAS back and fusion were similar in the two groups. The observed difference could be attributed partially to the small size of the study group, requiring caution when interpreting the results. At a minimum, the comparisons showed that the outcome was not worse in multilevel cases. The only radiographic parameter that was found to be different between the two groups was the last follow-up was foraminal height, which was higher in single-level cases (18.7 ± 2.86 versus 13.3 ± 4.2, p = 0.006). Other radiographic parameters were similar.
There were no statistically significant differences between the primary and secondary surgeries in the radiographic outcome and/or fusion rate. Neither were there significant differences between MIS TLIF and other open procedures in the same parameters. Bilateral cage implantation in open revision cases resulted in a significantly greater improvement in the foraminal height at the latest follow-up (p = 0.028) and also showed a tendency toward better improvement in regional lumbar lordosis (p = 0.058).
Different expandable cages used for TLIF and their specific characteristics are summarized in Table 8.
Table 8. Different expandable cages used for TLIF.
| Device | Cage | Substance | Types | Height size | Footplates |
|---|---|---|---|---|---|
| StaXx XD Expandable Device (Spine Wave, Inc., Shelton, CT, United States) |

|
PEEK | Convex/lordotic | Starts at 7 mm; expands in situ in 1-mm increments, up to 15 mm | Width 9 and 11 mm; length 22, 25, and 29 mm |
| CALIBER expandable lumbar fusion device (Globus Medica, Inc., Audubon, PA, United States) |

|
PEEK and titanium | Parallel and lordotic options | Starts at 8 mm; expansion options from 8 to 17 mm | Width 10 and 12 mm; length 22, 26, and 30 mm |
| AccuLIF TL (CoAlign Innovations, Allendale, NJ, United States) |

|
Titanium | Lordotic | Starts at 6 mm; expansion options from 6 to 16 mm | Width 11 mm; length 30 mm |
| SmArtCage-L Expandable and self-positioning interbody fusion cage dedicated to TLIF (SmartSpine SAS, Saint Victoret, France) |

|
Peek-Optima (with the addition of BaSo4) | Biconvex | Starts at 7 mm; four different heights: 7, 9, 11, and 13 mm | Width 15 mm; length 30 mm |
Note: The SmArtCage-L device is currently not utilized in the U.S. practice for TLIF surgery. The device requires FDA approval. The other three devices are FDA approved.
Abbreviations: FDA, U.S. Food and Drug Administration; PEEK, polyaryl-ether-ether-ketone; TLIF, transforaminal lumbar interbody fusion.
Discussion
Subsidence is a concern with utilization of expandable cages; it is partially attributable to the small footplate and overdistraction of the device.12 13 14 Other factors implied to have an impact on the subsidence include position of the cage, spinal region, bone mineral density, and the underlying diagnosis.13 14 15 16 Subsidence can potentially result in a loss of decompression of the spinal elements and loss of lordosis. Supplementation with pedicle screws minimizes subsidence and results in a better outcome.13 14 On the other hand, the study of Yao et al suggested that the development of subsidence is not necessarily associated with a worse outcome.17 We did not see significant subsidence over time, and no correlation was found between cage expansion and the degree of subsidence. Our other radiographic findings showed statistically significant improvement in the disk height and foraminal height, as well as the extent of listhesis but a nonsignificant change in the local disk angle and/or the regional lumbar lordosis.
The fusion rate in our study was lower compared with previously published studies, in part probably due to utilization of different graft materials; nonetheless, no relationships were found between the fusion rates and the clinical outcome. Despite the low fusion rate, the patients were doing clinically well, which suggests that the nonsatisfactory radiographic fusion outcomes may not necessarily lead to unfavorable clinical outcomes. There were four cases of reoperation at the index level (8.1%), three of which were due to nonunion. The indication for the other reoperation was adjacent-level disease.
In our experience, the main advantage of expandable cages is that they allow insertion of a cage without retraction or with only minimal retraction of the thecal sac and nerve root, with little risk of nerve injury and facilitating MIS TLIF.
Finally, several limitations exist in the current study. It is a retrospective study of the clinical and radiographic outcome of PEEK expandable spacers. There is no control group in the study for comparison. Given that by the inclusion criteria, all patients should have been treated by expandable PEEK cages and posterior instrumentation, blinded assessment was not applicable to the current study. The lack of prospective randomization into intervention and control groups reduces the level of the evidence. The other main limitation of the study is the wide heterogeneity of the patient population, various diagnoses, and different procedures. The observed outcome in our patients may partially be attributed to and biased by confounding factors such as demographics, diagnoses, and/or procedures. In addition, given the heterogeneity of the study population and small number of cases, there is no sufficient statistical power in the current study to perform a full analysis of the data. In addition, unavailability of the latest follow-up CT scan studies for all patients and utilization of X-rays in several patients have made the fusion evaluation suboptimal, and the results should be interpreted with caution.
Prospective studies and longer follow-up are required to determine final outcome.
Conclusion
The expandable PEEK implant is an effective device for interbody distraction, with durable restoration of the disk height and the foraminal height at an average follow-up of over 1 year with significant improvements in both pain and functional scores. Subsidence was minimal.
Disclosures Marjan Alimi, Research grant: Baxter, AOSpine Benjamin Shin, none Michael Macielak, none Christoph P. Hofstetter, none Innocent Njoku, Jr., none Apostolos J. Tsiouris, none Eric Elowitz, none Roger Härtl, Consulting fees: DePuy-Synthes, Lanx, Brainlab, AOSpine
These authors contributed equally to the manuscript.
Editorial Perspective
The reviewers accepted this retrospective study by Alimi et al in recognition of the efforts of the authors to focus on clinical outcomes in addition to the more customary radiographic and surgical results reporting. The implant in question is a novel device that allows the surgeon to adapt the implant height to a desirable dimension within the disk space to facilitate vertebral segment reduction and improve alignment—all in the hope to decrease perioperative morbidity while improving patient outcomes. From a device standpoint, the main questions related to the introduction of a new interbody fusion technique mainly revolve around the question of intrinsic device durability, postimplantation stability, subsidence of the device into the end plates, and eventual fusion rates. Of course, the factors of perioperative complications and reoperation rates are relevant. Patient-reported outcomes include a pain scale, a functional outcomes tool, and some form of reporting of opiate and muscle relaxant use. Similarly, duration of follow-up and radiographic methods to rate fusion are contentious issues and are rightfully subject to criticism due to vagaries of analytic methods used—such as was the case in this article.
EBSJ has chosen to present this article as an example for reporting on early experiences with a new device. Ideally, authors presenting a new technology report on their experiences in a prospective fashion with a preplanned data set being collected and a denominator being monitored for comparison sake. Retrospective studies inherently hold a strong potential for observer bias and likely underreport complications, usually without intent.
From a methodological point of view, the reviewers point out inconsistent use of approach and bone-grafting techniques as an additional variable. The authors received praise from the EBSJ reviewers for reporting the MCID relative to their intervention. The question of what constitutes a meaningful improvement in patient well-being following interventions has become an increasingly requested measure of success. For instance, the MCID relative to the ODI is not a fixed number and is dependent upon the baseline scores. It is also affected by patient comorbidities and the types of interventions used.1 For instance, a hypothetical change from a patient with a high disability score of 90 to 80 might be more readily attainable than a jump from an ODI score of 10 to 0 with no disability reported. Beyond this kind of a ceiling or floor effect associated with many scales, the study of comparative responsiveness of subjects remains a fascinating insight into human nature and our attempts to gather scientifically meaningful insights from it.2 The discussion of how to calculate an MCID is ongoing and not easily resolved here, but the editors and reviewers of EBSJ invite readers to study this subject further by reading the reference articles listed below. The results reported by Alimi and colleagues are certainly encouraging and will hopefully prompt increased attention of clinical investigators to collect patient-reported outcomes and compare their results to better understand why we do what we do and how we can improve our care continuously.3
References
- 1.Blumenthal S L Ohnmeiss D D; NASS. Intervertebral cages for degenerative spinal diseases Spine J 200334301–309. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham B W, Polly D W Jr. The use of interbody cage devices for spinal deformity: a biomechanical perspective. Clin Orthop Relat Res. 2002;(394):73–83. doi: 10.1097/00003086-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S M, Devine J N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845–4869. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toth J M, Wang M, Estes B T, Scifert J L, Seim H B III, Turner A S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials. 2006;27(3):324–334. doi: 10.1016/j.biomaterials.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Spine Wave Spine Wave Announces U.S. FDA's 510K Clearance of the StaXx® IB System, an Intervertebral Body Fusion Device Available at: http://www.marketwired.com/press-release/spine-wave-announces-us-fdas-510k-clearance-staxxr-ib-system-intervertebral-body-fusion-1783730.htm. Accessed May 28, 2015
- 6.Holly L T, Schwender J D, Rouben D P, Foley K T. Minimally invasive transforaminal lumbar interbody fusion: indications, technique, and complications. Neurosurg Focus. 2006;20(3):E6. doi: 10.3171/foc.2006.20.3.7. [DOI] [PubMed] [Google Scholar]
- 7.Beurskens A J, de Vet H C, Köke A J, van der Heijden G J, Knipschild P G. Measuring the functional status of patients with low back pain. Assessment of the quality of four disease-specific questionnaires. Spine (Phila Pa 1976) 1995;20(9):1017–1028. doi: 10.1097/00007632-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Farrar J T, Portenoy R K, Berlin J A, Kinman J L, Strom B L. Defining the clinically important difference in pain outcome measures. Pain. 2000;88(3):287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 9.Farrar J T, Young J P Jr, LaMoreaux L, Werth J L, Poole R M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 10.Fritz J M, Irrgang J J. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther. 2001;81(2):776–788. doi: 10.1093/ptj/81.2.776. [DOI] [PubMed] [Google Scholar]
- 11.Hägg O Fritzell P Nordwall A; Swedish Lumbar Spine Study Group. The clinical importance of changes in outcome scores after treatment for chronic low back pain Eur Spine J 200312112–20. [DOI] [PubMed] [Google Scholar]
- 12.Lau D Song Y Guan Z La Marca F Park P Radiological outcomes of static vs expandable titanium cages after corpectomy: a retrospective cohort analysis of subsidence Neurosurgery 2013724529–539., discussion 528–529 [DOI] [PubMed] [Google Scholar]
- 13.Bhatia N N, Lee K H, Bui C N, Luna M, Wahba G M, Lee T Q. Biomechanical evaluation of an expandable cage in single-segment posterior lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37(2):E79–E85. doi: 10.1097/BRS.0b013e3182226ba6. [DOI] [PubMed] [Google Scholar]
- 14.Karikari I O Grossi P M Nimjee S M et al. Minimally invasive lumbar interbody fusion in patients older than 70 years of age: analysis of peri- and postoperative complications Neurosurgery 2011684897–902., discussion 902 [DOI] [PubMed] [Google Scholar]
- 15.Kim M C, Chung H T, Cho J L, Kim D J, Chung N S. Subsidence of polyetheretherketone cage after minimally invasive transforaminal lumbar interbody fusion. J Spinal Disord Tech. 2013;26(2):87–92. doi: 10.1097/BSD.0b013e318237b9b1. [DOI] [PubMed] [Google Scholar]
- 16.Lam F C, Alkalay R, Groff M W. The effects of design and positioning of carbon fiber lumbar interbody cages and their subsidence in vertebral bodies. J Spinal Disord Tech. 2012;25(2):116–122. doi: 10.1097/BSD.0b013e31820ef778. [DOI] [PubMed] [Google Scholar]
- 17.Yao N, Wang W, Liu Y. Percutaneous endoscopic lumbar discectomy and interbody fusion with B-Twin expandable spinal spacer. Arch Orthop Trauma Surg. 2011;131(6):791–796. doi: 10.1007/s00402-010-1222-0. [DOI] [PubMed] [Google Scholar]
References
- 1.Copay A G, Subach B R, Glassman S D, Polly D W, Schuler T C. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Kim J K, Park E S. Comparative responsiveness and minimal clinically important differences for idiopathic ulnar impaction syndrome. Clin Orthop Relat Res. 2013;471(5):1406–1411. doi: 10.1007/s11999-013-2843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glassman S D, Copay A G, Berven S H, Polly D W, Subach B R, Carreon L Y. Defining substantial clinical benefit following lumbar spine arthrodesis. J Bone Joint Surg Am. 2008;90(9):1839–1847. doi: 10.2106/JBJS.G.01095. [DOI] [PubMed] [Google Scholar]


