Abstract
About 70% of patients with primary membranous nephropathy (MN) have circulating anti-phospholipase A2 receptor (PLA2R) antibodies that correlate with disease activity, but their predictive value in post-transplant (Tx) recurrent MN is uncertain. We evaluated 26 patients, 18 with recurrent MN and 8 without recurrence, with serial post-Tx serum samples and renal biopsies to determine if patients with pre-Tx anti-PLA2R are at increased risk of recurrence as compared to seronegative patients and to determine if post-Tx changes in anti-PLA2R correspond to the clinical course. In the recurrent group, 10/17 patients had anti-PLA2R at the time of Tx vs. 2/7 patients in the non-recurrent group. The positive predictive value of pre-Tx anti-PLA2R for recurrence was 83%, while the negative predictive value was 42%. Persistence or reappearance of post-Tx anti-PLA2R was associated with increasing proteinuria and resistant disease in many cases; little or no proteinuria occurred in cases with pre-Tx anti-PLA2R and biopsy evidence of recurrence in which the antibodies resolved with standard immunosuppression. Some cases with positive pre-Tx anti-PLA2R were seronegative at the time of recurrence. In conclusion, patients with positive pre-Tx anti-PLA2R should be monitored closely for recurrent MN. Persistence or reappearance of antibody post-Tx may indicate a more resistant disease.
Keywords: Membranous nephropathy, recurrent disease, kidney transplant, M-type phospholipase A2 receptor
Introduction
Primary (idiopathic) membranous nephropathy (MN) is an organ-specific autoimmune disease caused by circulating autoantibodies that target glomerular podocyte antigens. The predominant target antigen is the M-type phospholipase A2 receptor (PLA2R) and anti-PLA2R antibodies are found in the serum of 70% patients with active primary MN(1–6). Moreover, PLA2R is typically detected in the glomerular immune deposits of patients with primary MN by immunohistology where it can serve as a footprint of PLA2R-associated MN even after patients have entered an immunological remission and serum anti-PLA2R is no longer detectable(1, 7–9).
About a third of patients with primary MN may develop end-stage renal disease (ESRD)(10, 11) and many are suitable candidates for kidney transplantation. Unfortunately, recurrence can occur in approximately 40% of patients, usually within the first year(12). Recurrent MN varies in severity from a subclinical finding apparent only on protocol kidney biopsy to a severe disease manifesting by heavy proteinuria and a high risk of allograft failure (13, 14). Case reports and small case series have shown that early identification of clinical recurrence and treatment with rituximab (RTX) can induce remission and avoid progressive kidney failure (14, 15). However there has been no way to predict which patients with end-stage MN will develop recurrent MN or to detect its early occurrence other than by protocol biopsies.
The primary purpose of this study was to determine if knowledge of a patient’s anti-PLA2R status at the time of transplantation has utility in forecasting their risk of recurrent MN. If, as suggested by previous case studies, anti-PLA2R antibodies are able to transfer the disease to the transplanted kidney(4, 16, 17), one would predict that those patients with persistently circulating antibodies at and after the time of transplantation would be at the greatest risk of recurrence. Given this theoretical risk, we set out to determine if MN recurs more frequently in patients that are seropositive for anti-PLA2R than in those that are seronegative at the time of transplantation. To do this we took advantage of a case series in which patients with end-stage primary MN were followed with serial post-Tx protocol biopsies and serum collections before and after Tx. The longitudinal nature of the study enabled us to follow the clinical and serological outcome of the cases with histological and clinical recurrence.
Materials and Methods
Patient Population
We identified 37 patients with ESRD who underwent kidney Tx at the Mayo Clinic due to primary MN between the years 2000–2010. We included all patients with available protocol biopsies, as well as at least one available stored serum sample in the Mayo Clinic Transplant Center Serum/Tissue Bank. Protocol biopsies were initiated at our institution in 1998 and occur at time of transplantation, 4, 12, 24 and 60 months post-Tx. We additionally attempted to retrieve native kidney biopsies from both Mayo Clinic and outside institutions. This study was conducted with the approval of the Mayo Clinic Institutional Review Board (IRB # 09-004518).
Biopsies
Recurrent MN was defined by the presence of positive IgG staining in a capillary loop pattern by immunofluorescence (IF) and subepithelial deposits on electron microscopy (EM)(18).
Tx Immunosuppression
The majority of patients with both recurrent and non-recurrent MN received thymoglobulin for induction (14/18 vs. 6/8, respectively). Two patients received thymoglobulin and RTX for induction, one for a positive cross-match [4] and one for an ABO-incompatible Tx [24]. The remaining patients received either basiliximab [7, 10, 18] or daclizumab [25] for induction. The majority of patients (80%) received tacrolimus, mycophenolate mofetil and steroids for maintenance immunosuppression.
Anti-PLA2R Western blotting and ELISA
All available pre- and post-Tx serum samples were assayed both by Western blotting (WB) and with a commercial enzyme-linked immunosorbent assay (ELISA; Euroimmun US, Morris Plains, NJ).
For WB, extracts of human glomeruli (containing native PLA2R) and recombinant human PLA2R (rPLA2R) were electrophoresed and immunoblotted with patients’ sera diluted at 1:25, as previously described(1). The anti-PLA2R level was semi-quantitated using a scale from 0 to 3 according to band intensity as compared to a well-characterized positive control. All weakly positive WB signals were repeated and confirmed as positive signals.
In cases where all samples from an individual patient were found to be negative for the IgG4 subclass of anti-PLA2R, the pre-Tx sample was re-assayed for total IgG anti-PLA2R to detect the potential presence of other IgG subclasses. In the patients with positive tissue staining for PLA2R and negative serum anti-PLA2R at the time of histologic recurrence with a previously positive serum anti-PLA2R (see below), the samples were repeated at dilution of 1:10 to increase sensitivity.
ELISA for anti-PLA2R was carried out according to the manufacturer’s instructions. In brief, sera diluted to 1:100 were incubated 30 min with PLA2R coated microplates (Euroimmun US) and detected by incubation with anti-human-IgG HRP conjugate that recognizes all human IgG subclasses. A highly positive index patient serum provided in the ELISA kit was used to generate a standard curve consisting of five calibrators (2, 20, 100, 500, and 1500 relative units [RU]/ml). In addition, we included our well-characterized positive control with each ELISA plate.
Tissue staining for PLA2R
We examined all available serial sections from formalin fixed paraffin blocks (3 micron). Sections were deparaffinized with xylene and treated with proteinase K (20 mg/ml, 15 min at 37°C; Invitrogen) for antigen retrieval. Sections were incubated with rabbit polyclonal anti-PLA2R1 (1:100; Sigma/Atlas) for 30 min. As we anticipated weak PLA2R staining in patients with early stages of recurrence or in patients with resolving MN, amplification was used in all cases to optimize staining. The amplification process included sequential blocking with streptavidin and biotin (15 min; Streptavidin/Biotin blocking kit, Vector Laboratories) followed by biotinylated goat anti-rabbit IgG (1:200 for 45 min; Vector Laboratories) and DyLight 488 Streptavidin (1:100 for 45 min; Vector Laboratories).
All staining was done on coded sections without knowledge of the histological or clinical diagnosis and included a known positive control and known negative controls (lupus nephritis or normal human kidney). All glomeruli were photographed at 200x using an Olympus DP72 camera attached to a Nikon epifluorescence microscope. The coded images were scored as positive, negative, or equivocal by five observers, with high inter-observer agreement. Any images on which there was disagreement were reviewed together by all five observers to reach a consensus.
HLA typing
HLA typing of donors and recipients were reviewed and this data is provided in the supplement (Supplementary Table S1).
IgG subtyping
We performed IgG subtyping (IgG subclass 1-4) on a single post-Tx biopsy specimen in patients with recurrent disease at the time point closest to the time of initial recurrence, when available. Results shown in Supplementary Table S2.
Results
Patient characteristics
The data and samples for this study were from patients with primary MN that received a kidney Tx at the Mayo Clinic between 2000 and 2010. Of the 37 patients with idiopathic MN as their native renal disease, 21 had histologic recurrence post-Tx on protocol biopsy (57%) and 16 had no evidence of recurrence. Eleven patients were excluded due to lack of consent (n=10) or availability of serum (n=1), and therefore our analysis is based on a total of 26 patients: 18 with recurrent MN and 8 without recurrence (Table 1).
Table 1.
Patient Characteristics
| Characteristics | Non- recurrent n=8 | Recurrent n = 18 | P-value* |
|---|---|---|---|
|
| |||
| Age at initial diagnosis | 41.5 ± 11.3 | 41.7 ± 13.6 | 0.87 |
|
| |||
| Age at transplant | 52.5 ± 10.5 | 51.3 ± 11.1 | 0.80 |
|
| |||
| Sex (no, % male) | 6(75%) | 11(61%) | 0.67 |
|
| |||
| Race (no, % Caucasian) | 8(100%) | 15(83%) | 0.53 |
|
| |||
| Donor type (no, %) | |||
| Living Related Donor | 3(37.5%) | 10(56%) | 0.135 |
| Living Unrelated Donor | 1(12.5%) | 6(33%) | |
| Cadaveric Donor | 4(50%) | 2(11%) | |
|
| |||
| Biopsy-proven MN in native kidney (no, %) | 8(100%) | 16(89%) | 1.0 |
|
| |||
| Prior transplants (no, %) | 0 | 4(22%) | 0.28 |
|
| |||
| Standard triple immunosuppressant therapy (no, %) | 6(75%) | 15(83%) | 0.63 |
|
| |||
| Induction with Thymoglobulin (no, %) | 6(75%) | 14(77%) | 1.0 |
• Mean ± standard deviation unless otherwise indicated.
Comparisons in baseline characteristics were performed by unpaired t-test, two tailed U test (Mann–Whitney) or Wilcoxon signed-rank test for continuous variables and Fisher’s exact test for binary variables.
The median time to recurrence, defined as a histologic recurrence regardless of level of proteinuria, was 4.1 months (IQR 2.6–38.5 months). The median proteinuria and mean serum creatinine at the time of recurrence was 533 mg/day (IQR 253-1679 mg/day) and 1.80 ± 1.05 mg/dl, respectively.
Comparison of anti-PLA2R Western blotting and ELISA
Using the manufacturer’s definition for anti-PLA2R negativity as < 14 RU/ml, there was 92% (160/174) concordance between WB and ELISA (Supplementary Table S3). Some have suggested that any titer above 2 RU/ml by the commercial ELISA may be considered positive (19). Using this definition, 10 of the 14 samples would be re-classified as seropositive. Due to the increased sensitivity of WB over ELISA, we have reported anti-PLA2R seropositivity based on the WB signal rather than ELISA (see Supplement).
Predictive value of pre-Tx anti-PLA2R
Pre-Tx serum was available from 24 patients at a median of 2 days (range 1–7) prior to Tx. Seropositivity for anti-PLA2R is shown on a per-patient basis in Tables 2 and 3, along with tissue staining for PLA2R in the allograft, clinical features at time of recurrence and clinical course of the recurrent (1–18; Table 2) and non-recurrent (19–26; Table 3) patients. Median follow-up was 98 months (IQR 74-135) in non-recurrent patients and 88 months (IQR 64-122) in recurrent patients.
Table 2.
Clinical features at time of recurrence and clinical course for recurrent (1–18) patients.
| Recurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Recurrence | Pre-Tx Ab / level by WB | Time (mos) | Urine Protein (mg/day) | Ab | Tissue staining for PLA2R** | Treatment with RTX | Change in Ab levels post-Tx and/or RTX | Clinical condition at last follow-up including response to RTX, rejections, last biopsy findings, most recent proteinuria, ESRD and/or death |
| 1 | Y | NEG | 0.5 | 2312 | NEG | NEG | YES | NEG post-Tx | CR, stage IV deposits EM |
| 2 | Y | NEG | 68 | 512 | NEG | NEG | YES | NEG post-Tx | CR, neg IF/stage I-II deposits EM |
| 3 | Y | NEG | 4 | 205 | NEG | NEG | YES | NEG post-Tx | PR, relapsedtwice more, active disease (proteinuria 2600 mg/24hrs) with additional RTX planned |
| 4 | Y | NEG | 33 | 1278 | NEG | NEG | YES | NEG post-Tx | Positive cross-match Tx, NR to RTX, progressed to ESRD, death 7 years post-Tx |
| 5 | Y | POS/3 | 0.3 | 217 | POS/0.5* | POS | YES | POS post-Tx, NEG after 2nd RTX | See Figure 4. |
| 6 | Y | POS/2 | NA¥ | 2099¥ | NEG¥ | NA | YES | NEG post-Tx | PR, proteinuria 700 mg/day, no follow-up biopsies |
| 7 | Y | POS/2 | 5 | 930 | POS/2 | NA | YES | POS post-Tx, NEG after RTX | CR, stage IV deposits EMwith negative PLA2R tissue staining on later biopsy. |
| 8 | Y | POS/3 | 3 | 148 | POS/3 | POS | YES | POS post-Tx, NEG after 1st RTX dose then POS again. NEG post 2nd RTX dose | See Figure 5. |
| 9 | Y | NA | 5 | 2296 | POS/2 | POS | YES | POS post-Tx, NEG after RTX | CR, stage IV deposits EM |
| 10 | Y | POS/2 | 0.5 | 265 | POS/0.5 | POS | YES | POS post-Tx, NEG after RTX, then POS again | CR initially, now with active disease on biopsy with proteinuria 300 mg/day |
| 11 | Y | POS/3 | 3 | 554 | NEG | POS | YES | NEG post-Tx | See Figure 2. |
| 12 | Y | POS/3 | 4 | 1544 | NA€ | POS | NO | NEG post-Tx | Increased MMF dose -> CR, no deposits, neg PLA2R on subsequent biopsy |
| 13 | Y | POS/2 | 24 | 2082 | NEG | POS | NO | NEG post-Tx | Developed ACR, allograft loss, then 2nd txp withundetectableanti-PLA2R. 2y later without clinical or histology recurrence. Neg biopsy staining for PLA2R. |
| 14 | Y | POS/2 | 54 | 508 | NEG | POS | NO | POS post-Tx, then NEG | Stage III deposits/pos PLA2R on recent biopsy. |
| 15 | Y | POS/2 | 2.5 | 345 | NEG | NA | NO | NEG post-Tx | See Figure 3. |
| 16 | Y | NEG | 3 | 1075 | NEG | NEG | NO | NEG post-Tx | Proteinuria 460 mg/day, ill-defined deposits on EM |
| 17 | Y | NEG | 2.5 | 99 | NEG | NEG | NO | NEG post-Tx | Proteinuria 490 mg/day. |
| 18 | Y | NEG | 4 | 338 | NEG | POS | NO | NEG post-Tx | Historical POS Ab prior toprevious Tx. In this 2nd Tx Persistent undetectable Ab with positive PLA2R staining. proteinuria 100 mg/day |
As tissue was not available on all patients from the biopsy with first recurrence, if PLA2R staining was ever positive in setting of other features of recurrent MN post-Tx, it is listed as (+).
PR is defined as proteinuria ≤ 3.5 g/24h; CR equals proteinuria ≤ 0.3g/24h.
No antibody at time of recurrence, received RTX for AMR and Ab immediately after RTX treatment positive,
No protocol biopsies available, only biopsy is at time of clinical MN recurrence at 120 months, values provided for 120 month visit.
No serum available at time of recurrence, all subsequent Abs negative.
WB = western blotting, NEG = negative, POS = positive/semiquantitative Ab by Western blot, NA = not available, mos = months, Ab = anti-PLA2R antibody, RTX = rituximab, CR = complete response, PR = partial response, NR = no response, EM = electron microscopy, AMR = acute antibody-mediated rejection, ACR = Acute cellular rejection, MMF = mycophenolate mofetil.
Table 3.
Clinical features and clinical course for non-recurrent (19-26) patients.
| Patient | Recurrence | Pre-Tx Ab | Change in Ab levels post-Tx and/or RTX | Clinical condition at last follow-up includingresponse to RTX, rejections, last biopsy findings, most recent proteinuria, ESRD and/or death |
|---|---|---|---|---|
| 19 | N | NEG | Neg PLA2R on biopsy, negative Abs post-Tx | |
| 20 | N | POS/3 | NEG post-Tx | Equivocal PLA2R on biopsy, negative Abs post-Tx |
| 21 | N | NEG | Neg PLA2R on biopsy, negative Abs post-Tx* | |
| 22 | N | NEG | Neg PLA2R on biopsy, negative Abs post-Tx, 2 episodes ACR, progressed to ESRD¥ | |
| 23 | N | POS/3 | NEG post-Tx | Equivocal PLA2R on biopsy, negative Abs post-Tx, proteinuria ~ 1000 mg/24hrs, on sirolimus |
| 24 | N | NA | ABO-incompatible Tx, Neg PLA2R on biopsy, negative Abs post-Tx, death 6 years post-Tx | |
| 25 | N | NEG | Liver-kidney Tx, Neg PLA2R on biopsy, negative Ab by Western blot | |
| 26 | N | NEG | Neg PLA2R on biopsy, negative Abs post-Tx |
Native biopsy was negative for PLA2R antigen.
Native biopsy was positive for PLA2R antigen. NEG = negative, POS = positive/semiquantitative Ab by Western blot, NA = not available. ACR = Acute cellular rejection.
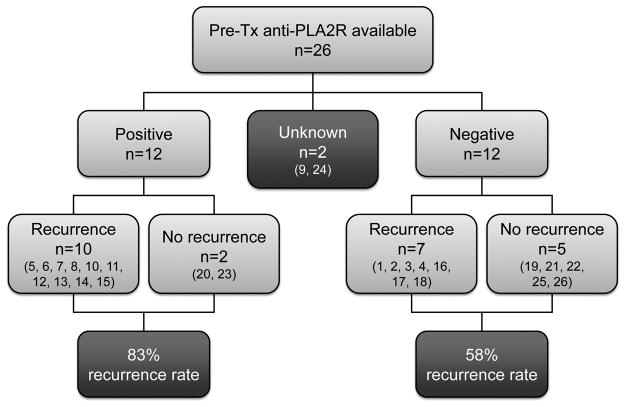
In those with recurrent MN, 17/18 had available pre-Tx samples of which 10/17 (59%) were seropositive for anti-PLA2R by Western blot. In the group of MN patients without recurrence, 7/8 had available pre-Tx serum samples, of which 2/7 (29%) were seropositive for anti-PLA2R. The association of pre-Tx anti-PLA2R reactivity with MN recurrence is illustrated in Figure 1. The positive predictive value (PPV) of pre-Tx anti-PLA2R for recurrence was 83%. Patient 9 did not have a pre-Tx sample, but was seropositive for anti-PLA2R two months after Tx with positive glomerular staining for PLA2R. Of the 12 patients who were seronegative for anti-PLA2R pre-Tx, 5 have not recurred and 7 have recurred, which yields a negative predictive value (NPV) of 42%.
Figure 1.
Distribution of pre-Tx anti-PLA2R in those with and without MN recurrence (numbers according to Table 2/3).
Tissue staining for PLA2R
A total of 59 follow-up biopsies on all recurrent and non-recurrent MN patients were available (2-3 follow-up biopsies per patient) for PLA2R staining (Table 4). Nine sections had no glomeruli or no tissue, and hence were not informative, leaving one recurrent patient [6] without tissue for PLA2R staining.
Table 4.
Summary of post-Tx biopsy findings in recurrent patients (1–18).
| Pt # | Pre- Tx Ab | Biopsy findings at recurrence | Follow up biopsy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgM | C1q | C3 | C4d | MN stage | LM (glom) | PLA2R* | Other | IgG/MN stage | Other findings | ||
| 1 | 0 | + | N | + | N | +++ | −− | III-IV | Thick BM | N | ATN | N/IV | Mild AS |
| 2 | 0 | +++ | Tr | +++ | ++ | +++ | G+++/P TC- | −− | Spikes/pi nholes | N | N/II | Severe IF/TA | |
| 3 | 0 | +++ | N | N | N | N | PTC− | 0-I | Normal | N | ++/II | Mild IF/TA | |
| 4 | 0 | ++ | N | + | ++ | + | G+++/P TC++ | I | Normal | N | TG | N/A | |
| 5 | 3 | +++ | N | Tr | N | Tr | Gtr/PTC +++ | −− | Normal | + | AMR | ++/II-III | ACR |
| 6 | 2 | ++ | N | + | N | Tr | G+++/P TC- | II-III | Thick BM | N/A | N/A | ||
| 7 | 2 | +++ | N | N | N | + | −− | −− | Deposits | N/A | N/IV | Secondary FSGS | |
| 8 | 3 | +++ | N | Tr | N | Tr | −− | I | Normal | + | +++/no EM | Mild IF/TA | |
| 9 | −− | +++ | N | N | N | N | G −/PTC − | I | Thick BM | + | N/IV | Early DGS | |
| 10 | 2 | ++ | N | N | N | N | G++/PT C− | 0-I | Normal | + | +++/I | Mild IF/TA | |
| 11 | 3 | +++ | N | N | N | N | PTC− | I | Deposits | + | Tr/III | Mild IF/TA | |
| 12 | 3 | +++ | N | N | N | +++ | −− | I | Irregular BM | + | N/no deposits | Early BM dup | |
| 13 | 2 | + | N | N | + | ++ | −− | 0 | Irregular BM | + | N/no EM | ||
| 14 | 2 | +++ | N | N | N | + | G+++/P TC− | I | Thick BM/spikes | + | ++/III | Early diabetic changes. | |
| 15 | 2 | + | N | N | N | Tr | G+/PTC− | I | Normal | N/A | ATN | N/no deposits | Ischemia/ATN, sclerosis |
| 16 | 0 | ++ | N | N | N | N | G+++/P TC− | −− | Pinholes | N | Tr/ill-defined deposits | Mild IF/TA | |
| 17 | 0 | + | N | Tr | N | N | G+++/P TC− | I | Normal | N | ATN | ++/no deposits | Mod AS |
| 18 | 0 | +++ | N | Tr | Tr | ++ | G+++/P TC− | I | Deposits | + | ++/II | Mild IF/TA | |
As tissue was not available on all patients from the biopsy with first recurrence, if PLA2R staining was ever positive in setting of other features of recurrent MN post-Tx, it is listed as (+). Pre-Tx Ab measured by Western blot, semi-quantitative levels. Mos = months. +−+++, trace (Tr) = intensity of staining on immunofluorescence in glomerular capillaries. N=negative. G = glomerular, PTC = peritubular capillaries. LM = light microscopy. BM = glomerular basement membrane. N/A = not available. AS = arteriosclerosis. IF/TA = interstitial fibrosis/tubular atrophy. AMR = antibody-medicated rejection, ACR = acute cellular rejection. ATN = acute tubular necrosis
In the recurrent MN group, none of the patients consistently seronegative for anti-PLA2R pre- and post-Tx had positive tissue staining. Hence, the tissue staining did not reveal any additional cases of PLA2R-associated MN. In cases of known PLA2R-associated MN, all available sections at the time of recurrence stained positive for PLA2R. Four patients with PLA2R-associated MN had positive tissue staining at the time of recurrence but were seronegative for anti-PLA2R [11, 13, 14, 18].
Only one patient [7] with known PLA2R-associated recurrent MN and a biopsy showing 2+ IgG and trace C3 on IF exhibited negative PLA2R staining. In this case, EM showed stage 4 MN, at which time the antigen may have been resorbed from the deposits.
In the non-recurrent group, none of the tissue sections were positive for PLA2R.
We located13 native biopsies from both Mayo Clinic and outside institutions; however, only 7 had glomeruli for review. Five of these patients [5, 8, 9, 18, 22] were positive for PLA2R and 2 were negative [3, 21] (Supplementary Table S2 and Table 3).
Change in post-Tx anti-PLA2R
The post-Tx course of patients who were seropositive for anti-PLA2R pre-Tx was variable. We will discuss selected cases with and without recurrent MN in the following sections and further case descriptions are in the supplement. Complete remission (CR) was defined as proteinuria ≤ 0.3 g/day and partial remission (PR) was defined as proteinuria 3.5 g/day and a > 50% reduction from pretreatment levels of proteinuria. No response (NR) included a < 50% reduction, no change, or an increase in proteinuria.
Positive pre-Tx anti-PLA2R with recurrent MN
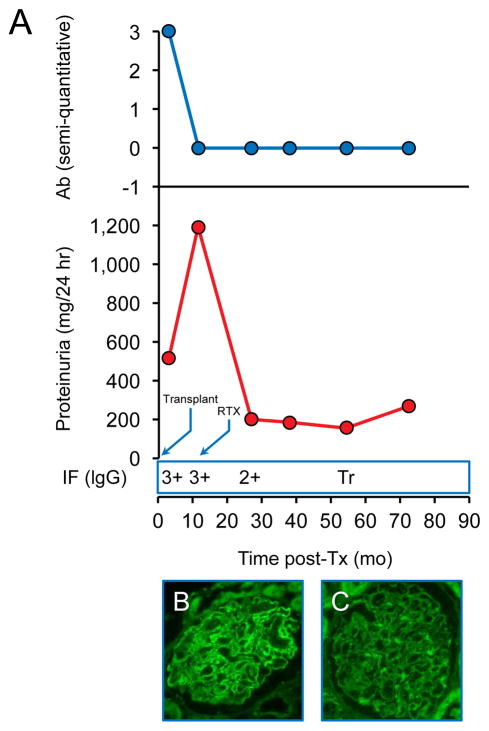
Patient 11 was diagnosed with recurrent MN by biopsy with positive PLA2R staining 3 months post-Tx, at which point his anti-PLA2R antibody was already negative. His proteinuria increased from 0.5g/day at the time of recurrence to >1g/day almost a year later while his anti-PLA2R antibody remained undetectable and a decision was made to treat with RTX (Figure 2). He responded well and eventually achieved a CR with improving biopsy features of MN, including decreased staining for tissue PLA2R.
Figure 2.
Patient 11. Recurrence diagosed at 3 months post-Tx. A - Changes in proteinuria, semi-quantitative Ab levels and IgG staining by immunofluorescence (IF) over time. (Tr=trace). B–Positive PLA2R staining at 3 months post-Tx. C- Weakly positive PLA2R staining 40 months after treatment with RTX.
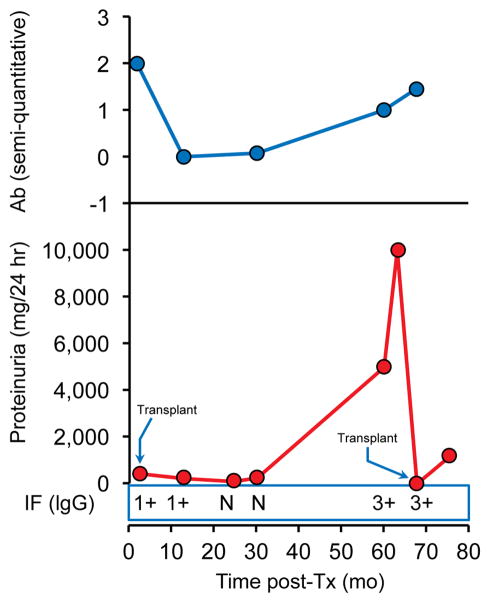
Patient 15 exhibited histologic changes indicating very early recurrent MN at 2.5 months post-Tx. The next available serum sample at 13 months post-Tx showed disappearance of the antibodies without additional treatment other than the Tx immunosuppression. He had minimal proteinuria as well as a histologic resolution of recurrent MN by protocol biopsy in the setting of documented anti-PLA2R seronegativity four years post-Tx. However, his antibodies reappeared and in the fifth year post-Tx, he recurred with significant proteinuria and lost his graft within months and so was re-transplanted a few months later without receiving RTX. The antibodies were still detected at time of second Tx and he developed early histologic recurrence with minimal proteinuria (Figure 3).
Figure 3.
Patient 15. Recurrence diagnosed 2.5 months post-first Tx and 5 months post-second Tx. Changes in proteinuria, semi-quantitative Ab levels and IgG staining by immunofluorescence (IF) over time. (N=negative). Tissue for PLA2R staining was not available.
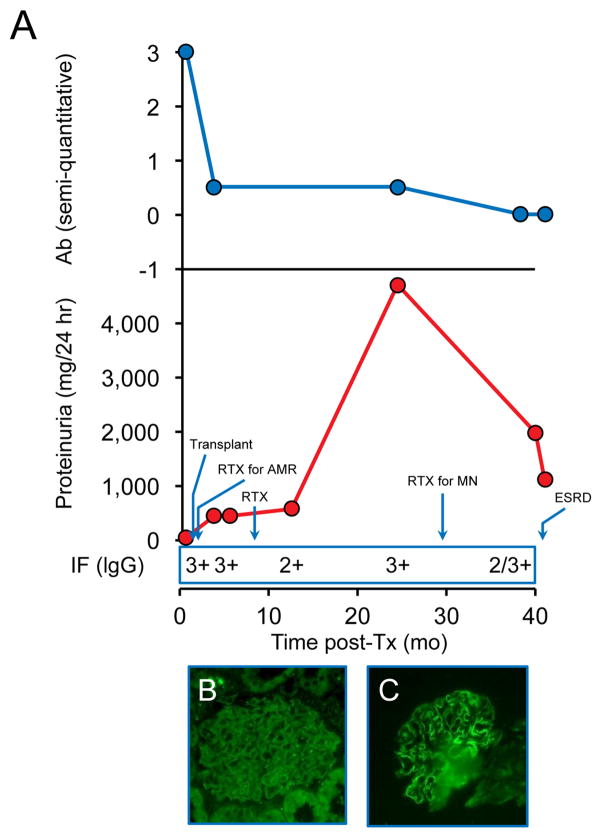
Patient 5 was treated early with RTX for the simultaneous presence of recurrent MN and antibody-mediated rejection. The anti-PLA2R levels decreased but remained persistent at a low level, and ongoing MN was detected on subsequent biopsies with positive PLA2R staining. She ultimately reached ESRD, due to a combination of recurrent disease and chronic rejection (Figure 4).
Figure 4.
Patient 5. Recurrence diagnosed at 0.3 months post-Tx. A- Changes in proteinuria, semi-quantitative Ab levels and IgG staining by immunofluorescence (IF) over time. B- Weakly-positive PLA2R staining at 0.3 months post-Tx in setting of antibody-mediated rejection. C- Positive PLA2R staining at 18 months post-Tx.
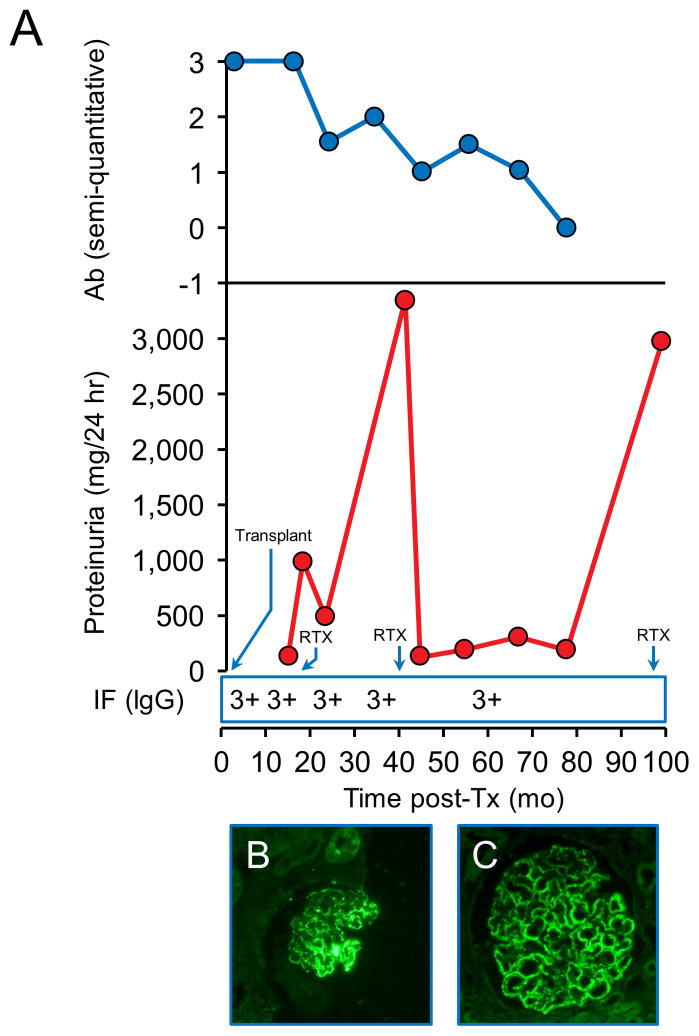
Patient 8 had a high pre-Tx anti-PLA2R level that remained high at the time of recurrence, 3 months post-Tx. Due to increasing proteinuria, RTX was given at 10 months post-Tx. The antibodies decreased after the first RTX course but then increased with worsening proteinuria up to > 3g/day and he was treated with a second course of RTX 3 years post-Tx, this time with achievement of a CR despite continued presence of the antibodies. Anti-PLA2R eventually resolved 3 years after the second RTX dose. His proteinuria worsened a third time to > 3g/day requiring a third RTX course. His protocol biopsies have shown features of ongoing MN on biopsy including positive PLA2R tissue staining (Figure 5).
Figure 5.
Patient 8. Recurrence diagnosed at 4 months post-Tx. A - Changes in proteinuria, semi-quantitative Ab levels and IgG staining by immunofluorescence (IF) over time. B–Positive PLA2R staining at 16 months post-Tx, after RTX. C- Positive PLA2R staining 5 years post-Tx, after two courses of RTX.
Positive pre-Tx anti-PLA2R without recurrent MN
Two patients [20, 23] had strongly positive pre-Tx anti-PLA2R but have not demonstrated evidence of recurrent MN on any protocol biopsies, starting at the 4-month point. However, in both cases, the first post-Tx serum sample was not available for anti-PLA2R testing until 2 and 5 years later, respectively, and both were seronegative.
Negative pre-Tx anti-PLA2R with and without recurrent MN
All seven patients with recurrent MN and 5 with no recurrence who were seronegative for anti-PLA2R pre-Tx remained seronegative during the post-Tx course.
Prior history of positive anti-PLA2R, negative pre-Tx with recurrent MN
Patient 18 is unique in that this was his second Tx, as his first was lost due to venous thrombosis. Prior to his first Tx, he was anti-PLA2R seropositive, but during the short period between transplants, the antibody disappeared such that he was seronegative at the time of his second Tx. His 4-month protocol biopsy showed recurrent MN with positive PLA2R tissue staining, although his serum remained negative for anti-PLA2R and he has not developed significant proteinuria.
Discussion
The primary outcome of this study to determine the predictive value of pre-Tx anti-PLA2R in recurrent MN is that recurrence occurs in a high proportion of patients with positive pre-Tx antibody (83%), but also that the risk of recurrence is as high as 58% in those that are seronegative pre-Tx (Figure 1). In our cohort, the PPV of positive pre-Tx anti-PLA2R for recurrent MN is 83% and the negative predictive value is 42%. Though the number of patients is small, further analysis of follow up data allows us to make broader statements that will be of value in the future. Although a positive test for anti-PLA2R pre-Tx predicts early recurrence of MN, in some cases this is a subclinical biopsy finding that may resolve with standard post-Tx immunosuppression. It has been previously established that symptomatic recurrence may lead to graft loss(12, 13, 21–23). In this study that was an infrequent outcome (3/18) because most cases of recurrence that were detected by protocol biopsy were effectively treated with RTX if they developed proteinuria (11/18). A particularly informative subset of patients was those that were seropositive pre-Tx and became seronegative without additional immunosuppression [14, 18, 20, 23]. In these cases, prospective serial measurements showing decline and disappearance of anti-PLA2R could be used clinically in place of biopsies to monitor for recurrent disease.
We propose that patients with primary MN may be separated into two distinct groups–those with PLA2R-associated MN and those with non-PLA2R-associated MN. We define those with PLA2R-associated MN in this study as patients with a positive serum anti-PLA2R either pre- or post-Tx and/or positive staining for PLA2R in kidney tissue in the native biopsy or at the time of recurrence. Patients with documented native or recurrent MN on biopsy with consistently negative serum and negative tissue staining are considered to have non-PLA2R-associated MN. Those patients who were seronegative pre-Tx and had no evidence of recurrence or no tissue available for staining at time of recurrence could potentially fall into either group, only prior information on anti-PLA2R or PLA2R staining of the native kidney biopsy at the time of original diagnosis could have distinguished them. Due to the lack of serum and limited availability of tissue samples from the time when patients were initially diagnosed with MN in their native kidneys, the recurrence rate amongst those with PLA2R associated and non-PLA2R associated MN cannot be determined in this cohort, although there was close concordance between the native kidney PLA2R positivity and pretransplant anti-PLA2R status in the few cases in which tissue was available. However, we were able to identify a single subject [22] whose native biopsy stained for PLA2R thus establishing the disease as PLA2R-associated MN. The finding of seronegativity at transplantation suggests a lack of immunologic activity, and is likely the reason why this patient had no evidence of recurrence.
Our study includes 18 patients with recurrence: 12 of them with PLA2R-associated MN (66%), 4 with non-PLA2R associated MN (22%), and 2 with unknown status (11%). This rate is compatible with the prevalence of anti-PLA2R in patients with primary MN. The overall recurrence rate in our cohort (57%) is higher than in previously published series (30-42%)(13, 24), but could be related to earlier histologic diagnosis with protocol biopsies, longer follow up of our cohort, and the high number of living related donor transplants in this cohort(25).
Our biopsy protocol also provided a unique opportunity to verify whether the IgG1 subclass is dominant and predates IgG4 deposition in glomeruli as suggested by Huang et al. in native biopsies(20). Of the 7 patients (regardless of PLA2R status) that had biopsies available for IgG subtyping at the first time histologic recurrence was noted ([5, 6, 11, 14, 15, 16, 17], 6/7 had predominantly IgG4 staining in capillary walls (Supplementary Table S2). Similarly, IgG4 was the only or dominant subclass in most of the earliest biopsies of subjects with PLA2R-associated MN [5, 6, 9, 11, 14, 15]. A single case of known non-PLA2R-associated MN [16] had only IgG1 staining.
Recurrent MN
In patients with positive pre-Tx anti-PLA2R, the antibody can disappear within 13 months or less after Tx, which may be due to standard post-Tx immunosuppression, additional RTX treatment, or unknown factors related to antigen exposure in the allograft.
Early recurrence that resolved spontaneously could have been missed in some cases prior to the first protocol biopsy. In addition, a few patients were seronegative at time of recurrence and had positive tissue staining for PLA2R [11, 13, 14, 18]. In two of them [11, 14] one might argue that their high pre-Tx antibody levels induced an early recurrence but were no longer detectable in the serum by the time of the first protocol biopsy. Such reasoning cannot apply to patient 13 who recurred after 26 months and whose 4-month protocol biopsy did not have evidence of MN. Nor does it explain why patient 18 developed PLA2R-associated recurrent MN in his second allograft at a time when he was apparently seronegative and thus his kidney was never exposed to detectable anti-PLA2R. This finding of negative anti-PLA2R in the serum with positive tissue staining is consistent with prior studies (4) and may reflect very low levels of circulating antibodies that are consumed by the allograft and thus are undetectable by the most sensitive methods available. These low levels may be able to accumulate in the glomeruli over time and not only cause histological changes detectable by immunostaining but can also induce clinical recurrence and graft loss despite apparent seronegativity. These data also suggest that tissue staining for PLA2R may be a more sensitive modality for detecting early recurrence, though this method may be less useful in centers that do not perform protocol biopsies.
The persistence or reappearance of anti-PLA2R may herald a more aggressive disease. In six patients [5, 7, 8, 9, 10, 15], anti-PLA2R levels remained positive or re-appeared years after Tx and were accompanied by symptomatic disease with significant proteinuria.
Another insight from this study is that about half of those patients that were seronegative pre-Tx and post-Tx went on to develop symptomatic recurrent MN. As up to 20% of patients with idiopathic MN may have non-PLA2R-associated disease, we would expect that some of the patients in our cohort also have MN associated with one or more distinct, and as yet unidentified, podocyte antigens. However, assuming that at least some of these seronegative cases did indeed have PLA2R-associated MN in immunologic remission at the time of transplantation, this study provided an opportunity to determine if they reactivated when exposed to the new allograft. Notably, none of these cases became seropositive post-Tx, and none of those with available tissue had positive PLA2R immunostaining. These results suggest that post-Tx anti-PLA2R testing is of little value in cases that are seronegative pre-Tx unless they are known (from prior serological studies or PLA2R staining of their native kidney) to have PLA2R-associated MN.
Non-recurrent MN
There were two patients that were seropositive for anti-PLA2R pre-Tx that became seronegative post-Tx and had no evidence of recurrent MN on protocol biopsy, which suggests that the antibodies either failed to transfer the disease to the allograft or Tx immunosuppression rapidly induced suppression of anti-PLA2R production. In those patients seronegative for anti-PLA2R at the time of Tx, a proportion were likely cases of PLA2R-associated who were immunologically inactive, such as patient 22 who was positive for the PLA2R antigen on native biopsy but seronegative at the time of Tx. While in anti-glomerular basement membrane (GBM) disease, the recurrence rate is significantly reduced if circulating anti-GBM antibody levels have been undetectable for at least 12 months before Tx(26), the optimal duration of seronegativity in PLA2R-associated MN is unknown.
Our findings are consistent with the findings of Debiec et al, who have previously shown that the kinetics of anti-PLA2R post-Tx is quite variable and we found similar patterns of change anti-PLA2R post-Tx(4). Our study has the advantage of using protocol biopsies, allowing us to identify subclinical, histologic recurrence of MN, as well as more complete serologic follow up of anti-PLA2R, including both pre- and post-Tx samples.
Our study has several limitations. It is a small, retrospective study and so our analysis is limited by the availability of serum and tissue samples. The data is quite heterogeneous and our power to detect significant differences between groups is limited. In addition, we are unable to comment on the overall recurrence rate in PLA2R-associated vs. non-PLA2R-associated MN due to limited native kidney biopsy specimens.
In conclusion, pre-Tx anti-PLA2R testing may have clinical utility either in those with known PLA2R-associated disease or those who are seropositive at the time of transplant. Now that the assays are more widely available in the clinical setting, anti-PLA2R should be checked in all patients with primary MN being considered for transplantation and the native kidney biopsy should be stained for PLA2R in order to classify them as having PLA2R- or non-PLA2R-associated MN prior to transplantation. The presence of anti-PLA2R at the time of Tx very often leads to early recurrence of MN. Induction therapy and standard maintenance immunosuppression in some cases are sufficient to induce immunologic remission, but do not preclude the possibility of recurrence of PLA2R-associated MN. In those cases with persistence or reappearance of the antibody post-Tx the disease appears more resistant. The response to RTX is excellent and should be considered to prevent allograft loss.
Our study raises two practical issues. One is whether to postpone Tx until the circulating anti-PLA2R disappears and for how long to wait with an undetectable level before Tx? Second, considering the high recurrence rate documented in patients seropositive for anti-PLA2R at the time of transplantation, should RTX be given preventively to all patients with either pre- or post-Tx anti-PLA2R seropositivity? Future prospective studies are needed to establish the answers to these questions.
Supplementary Material
Acknowledgments
We would like to thank Dr. Joel Henderson from the Department of Pathology and Laboratory Medicine at Boston University Medical Center for guidance and advice with the tissue staining.
This work is supported by R01 DK090029 (DJS, LHB), R01 DK 097053 (LHB) from NIH/NIDDK and NEPTUNE, Nephrotic Syndrome Study Network, U-54-DK-083912 (RA).
Abbreviations
- MN
Membranous nephropathy
- PLA2R
M-type phospholipase A2 receptor
- ESRD
End-stage renal disease
- RTX
Rituximab
- Tx
Transplant
- CR
Complete remission
- PR
Partial remission
- NR
No response
- SNP
Single nucleotide polymorphism
- PPV
Positive predictive value
- NPV
Negative predictive value
Footnotes
Financial interests
LHB and DJS are co-inventors on the US patent, Diagnostics for Membranous Nephropathy.
References
- 1.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009 Jul 2;361(1):11–21. doi: 10.1056/NEJMoa0810457. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A(2) receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011 Jun;6(6):1286–91. doi: 10.2215/CJN.07210810. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, et al. An immunofluorescence test for phospholipase-A(2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011 Aug;26(8):2526–32. doi: 10.1093/ndt/gfr247. Comparative Study Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 4.Debiec H, Martin L, Jouanneau C, Dautin G, Mesnard L, Rondeau E, et al. Autoantibodies specific for the phospholipase A2 receptor in recurrent and De Novo membranous nephropathy. Am J Transplant. 2011 Oct;11(10):2144–52. doi: 10.1111/j.1600-6143.2011.03643.x. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 5.Qin W, Beck LH, Jr, Zeng C, Chen Z, Li S, Zuo K, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011 Jun;22(6):1137–43. doi: 10.1681/ASN.2010090967. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh YJ, Yang SH, Kim DK, Kang SW, Kim YS. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One. 2013;8(4):e62151. doi: 10.1371/journal.pone.0062151. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2013 Jul;28(7):1839–44. doi: 10.1093/ndt/gfs439. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 8.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011 Feb 17;364(7):689–90. doi: 10.1056/NEJMc1011678. Letter Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 9.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013 May;26(5):709–15. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 10.Donadio JV, Jr, Torres VE, Velosa JA, Wagoner RD, Holley KE, Okamura M, et al. Idiopathic membranous nephropathy: the natural history of untreated patients. Kidney Int. 1988 Mar;33(3):708–15. doi: 10.1038/ki.1988.56. [DOI] [PubMed] [Google Scholar]
- 11.Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993 Jul 8;329(2):85–9. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 12.Dabade TS, Grande JP, Norby SM, Fervenza FC, Cosio FG. Recurrent idiopathic membranous nephropathy after kidney transplantation: a surveillance biopsy study. Am J Transplant. 2008 Jun;8(6):1318–22. doi: 10.1111/j.1600-6143.2008.02237.x. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 13.Poduval RD, Josephson MA, Javaid B. Treatment of de novo and recurrent membranous nephropathy in renal transplant patients. Semin Nephrol. 2003 Jul;23(4):392–9. doi: 10.1016/s0270-9295(03)00057-3. Review. [DOI] [PubMed] [Google Scholar]
- 14.El-Zoghby ZM, Grande JP, Fraile MG, Norby SM, Fervenza FC, Cosio FG. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant. 2009 Dec;9(12):2800–7. doi: 10.1111/j.1600-6143.2009.02851.x. [DOI] [PubMed] [Google Scholar]
- 15.Sprangers B, Lefkowitz GI, Cohen SD, Stokes MB, Valeri A, Appel GB, et al. Beneficial effect of rituximab in the treatment of recurrent idiopathic membranous nephropathy after kidney transplantation. Clin J Am Soc Nephrol. 2010 May;5(5):790–7. doi: 10.2215/CJN.04120609. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl R, Hoxha E, Fechner K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N Engl J Med. 2010 Jul 29;363(5):496–8. doi: 10.1056/NEJMc1003066. Case Reports Letter Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 17.Seitz-Polski B, Payre C, Ambrosetti D, Albano L, Cassuto-Viguier E, Berguignat M, et al. Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: a case series of 15 patients. Nephrol Dial Transplant. 2014 Jul 25; doi: 10.1093/ndt/gfu252. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez EF, Cosio FG, Nasr SH, Sethi S, Fidler ME, Stegall MD, et al. The pathology and clinical features of early recurrent membranous glomerulonephritis. Am J Transplant. 2012 Apr;12(4):1029–38. doi: 10.1111/j.1600-6143.2011.03903.x. [DOI] [PubMed] [Google Scholar]
- 19.Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, Ayalon R, Beck LH, Jr, Schlumberger W, et al. Evaluation of Anti-PLA2R1 as Measured by a Novel ELISA in Patients With Idiopathic Membranous Nephropathy: A Cohort Study. American Journal of Clinical Pathology. 2014 Jul;142(1):29–34. doi: 10.1309/AJCP8QMOY5GLRSFP. [DOI] [PubMed] [Google Scholar]
- 20.Huang CC, Lehman A, Albawardi A, Satoskar A, Brodsky S, Nadasdy G, et al. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol. 2013 Jun;26(6):799–805. doi: 10.1038/modpathol.2012.237. [DOI] [PubMed] [Google Scholar]
- 21.Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002 Jul 11;347(2):103–9. doi: 10.1056/NEJMoa013036. Comparative Study. [DOI] [PubMed] [Google Scholar]
- 22.Cosyns JP, Couchoud C, Pouteil-Noble C, Squifflet JP, Pirson Y. Recurrence of membranous nephropathy after renal transplantation: probability, outcome and risk factors. Clin Nephrol. 1998 Sep;50(3):144–53. Case Reports Review. [PubMed] [Google Scholar]
- 23.Monga G, Mazzucco G, Basolo B, Quaranta S, Motta M, Segoloni G, et al. Membranous glomerulonephritis (MGN) in transplanted kidneys: morphologic investigation on 256 renal allografts. Mod Pathol. 1993 May;6(3):249–58. Comparative Study Research Support, Non-U.S. Gov't. [PubMed] [Google Scholar]
- 24.El-Zoghby ZM, Stegall MD, Lager DJ, Kremers WK, Amer H, Gloor JM, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009 Mar;9(3):527–35. doi: 10.1111/j.1600-6143.2008.02519.x. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- 25.Andresdottir MB, Wetzels JF. Increased risk of recurrence of membranous nephropathy after related donor kidney transplantation. Am J Transplant. 2012 Jan;12(1):265–6. doi: 10.1111/j.1600-6143.2011.03818.x. Letter. [DOI] [PubMed] [Google Scholar]
- 26.Netzer KO, Merkel F, Weber M. Goodpasture syndrome and end-stage renal failure--to transplant or not to transplant? Nephrol Dial Transplant. 1998 Jun;13(6):1346–8. doi: 10.1093/oxfordjournals.ndt.a027889. Editorial Review. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.