Abstract
STUDY QUESTION
Does laboratory testing after syndromic screening for sexually transmitted infections (STIs) reduce the rate of intrauterine contraception (IUC) removal among women living with HIV/AIDS (WLHA)?
SUMMARY ANSWER
Additional laboratory testing after syndromic screening for STIs did not affect the likelihood that a woman would remove an IUC immediately or within 1 year of IUC use or the frequency of post-insertion unscheduled clinic visits. In low-risk WLHA, the incidence rate of IUC removal is low with or without laboratory testing.
WHAT IS KNOWN ALREADY
Fear of infectious morbidity remains an obstacle to uptake of IUC by WLHA. The value of laboratory testing after syndromic screening for STI before the insertion of IUC remains uncertain.
STUDY DESIGN, SIZE, DURATION
We enrolled WLHA from 2 September to 6 December 2013 and followed them up to 31 December 2014. After syndromic screening, 703 women free of STIs were randomized to either additional laboratory screening or no additional screening for STI before IUC insertion. The randomization sequence was generated by an independent statistician and randomization numbers placed in opaque sequentially numbered sealed envelopes. All women randomized had an IUC inserted and in all 672 participants completed the 1-year follow-up. The study staff who followed up the participants were blinded to the study allocation groups. Incidence rate ratios (IRRs) were used to compare the incidence rates of IUC removal, unscheduled clinic attendance and IUC continuation between the two groups.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women eligible to participate were 18–49 years old at study entry, in a relationship with a male partner, wanted to avoid pregnancy for at least 1 year and were undergoing HIV/AIDS care at Mulago Hospital, Uganda. Participants completed a baseline questionnaire and up to four follow-up questionnaires until discontinuation of IUC, loss to follow-up or end of study observation after 12 months.
MAIN RESULTS AND THE ROLE OF CHANCE
The rate of IUC removal was 8.8% (29/331) in the no additional screening group and 8.0% (27/341) in the additional laboratory screening group [IRR 1.1 (95% CI 0.63–1.93)]. Unscheduled clinic attendances were similar in the two groups at 1 year of IUC insertion: 13.6% (45/331) in the no additional screening group and 12.3% (42/241) in the additional laboratory screening group. During the 1-year follow-up, only five women, three from the no additional screening group and two from the additional laboratory screening group, developed pelvic inflammatory disease (PID), as defined by established diagnostic criteria.
LIMITATIONS, REASONS FOR CAUTION
We were not able to carry out STI risk assessment directly from the men thus women with high-risk partners could have been included in the study and this may be responsible for the lack of a demonstrable effect of additional laboratory screening on incidence rates of IUC removals and unscheduled clinic attendance. The diagnosis of PID was based on clinical signs and symptoms; therefore, subclinical PID could have been missed.
WIDER IMPLICATIONS OF THE FINDINGS
Among WLHA, the incidence rate of IUC removal is low and IUC continuation high. Syndromic screening for STIs could be sufficient in indentifying WLHA who are suitable for IUC use. However, our findings are only generalizable to women in HIV/AIDS care who have access to good follow-up.
STUDY FUNDING/COMPETING INTEREST(S)
The study was supported by Medical Education for Equitable Services to all Ugandans, a Medical Education Partnership Initiative grant number 5R24TW008886 from the office of Global AIDS Coordinator and the US Department of Health and Human Services, Health Resources and Services Administration and National Institutes of Health. Additional funding was from the Swedish International Development Agency, Swedish Research Council (SIDA/VR). The authors have no competing interests to declare.
TRIAL REGISTRATION NUMBER
This trial was registered at Pan African Clinical Trial, Registry. PACTR 201308000561212.
Keywords: acquired immunodeficiency syndrome, intrauterine contraceptive device, IUC removal, laboratory screening, sexually transmitted infections
Introduction
Intrauterine contraception (IUC), which includes the Levonorgestrel intrauterine system (LNG-IUS) and copper T 380A (Cu T 380A) intrauterine device, is a dependable and cost-effective method of contraception (Luukkainen et al., 1987; French et al., 2004; Trussell, 2004; Kulier et al., 2007; Trussell et al., 2009). It is also long acting and does not rely on daily user motivation for its continued effectiveness (Trussell, 2004; Mansour et al., 2010). Most women are eligible for IUC use (World Health Organisation, 2009) as it does not interfere with any form of medications and generally has minimal or no systemic side effects. These excellent characteristics have made IUC very popular. It is the commonest reversible method relied on by family planning acceptors in the world today (United Nations, 2013). It would be an excellent choice for women living with HIV/AIDS (WLHA) as it does not interact with antiretroviral drugs (Heikinheimo et al., 2006; Tseng and Hills-Nieminen, 2013).
IUC is under utilized in Africa, where only 2% of family planning users opt for it (Clifton et al., 2008; Buhling et al., 2014). In Uganda, only 0.5% of family planning acceptors choose IUC for fertility regulation (Uganda Bureau of Statistics, 2012). This low uptake of IUC is due to the fear of pelvic inflammatory disease (PID) that may follow the insertion of IUC in women at high risk of sexually transmitted infections (STIs). WLHA are a high-risk group for STIs (Asavapiriyanont et al., 2013) with increased risk of acquiring cervical Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections (Venkatesh et al., 2011); yet, many women with STIs remain asymptomatic.
There is concern that among women with current STI, the process of inserting an IUC may facilitate the ascendance of microorganisms from the lower to the upper genital tract leading to the development of PID (Mohllajee et al., 2005). Evidence suggests that the risk of PID among IUC users is significantly increased immediately after insertion (Farley et al., 1992). Therefore, PID associated with IUC occurs in the first 20 days of its initiation (Farley et al., 1992; Grimes, 2000; Mohllajee et al., 2005). It is also related to background STI prevalence in a given setting (Grimes et al., 1999; Mohllajee et al., 2006). When compared with women in low STI settings, women in settings with high STI prevalence are at an increased risk of PID following IUC insertion (Steen and Shapiro, 2004).
In high income settings, high-risk women undergo laboratory testing for STIs before insertion of IUC (Centre for Disease Control and Prevention, 2013). However, in low to middle income countries like Uganda such services are not readily available. The only form of STI screening available is syndromic screening, which relies on client demographic history and clinical examination to exclude STIs.
The aim of this study was to determine if laboratory testing versus no additional testing for STI after syndromic screening prior to IUC insertion would reduce the rate of IUC removal among WLHA at Mulago Hospital, Uganda.
Methods
Trial design
This was a randomized controlled trial conducted from September 2013 to December 2014, in Mulago National Referral Hospital, Uganda. Participants included in this study were women aged 18–49 years, lived 20 km or less from the hospital, were married or had a stable sexual relationship, wanted to avoid pregnancy for at least 1 year and were undergoing HIV/AIDs care at Mulago Hospital. Women were excluded if they had menstrual irregularities, uterine abnormalities, severe dysmenorrhoea, had AIDS but were not on antiretroviral therapy and if they had STIs currently or in the 3 months preceding enrolment. Women who reported their sexual partners to have multiple extramarital partners or a recent history of STI were also excluded.
Setting
This study was carried out in the Makerere University Joint Aids Program (MJAP) and the family planning clinics located in Mulago Hospital, Department of Obstetrics and Gynaecology. Mulago Hospital is the national referral hospital for Uganda and teaching hospital for Makerere University College of Health Sciences. The MJAP is an AIDS care facility with an annual attendance of ∼20 000 clients, 60% of whom are women of reproductive age. It offers services such as voluntary counselling and testing for HIV, management of opportunistic infections, antiretroviral therapy, contraceptive counselling, prevention of mother to child transmission of HIV and condoms for the prevention of STI/HIV transmission and unwanted pregnancy. Response to HIV/AIDS care is monitored using quarterly CD4 cell counts. Patients whose CD4 cell count reduce or fail to increase get HIV viral load estimated. Annually, 30 000 clients are seen at the associated family planning clinic and ∼3000 women opt for IUC. Eight midwives with over 10 years' experience of contraceptive service provision including insertion and removal of IUC run the clinic.
Interventions
Eligible women were randomly allocated after syndromic screening to additional laboratory screening or no additional screening for STIs prior to insertion of IUC.
Study procedures
Eligible women were identified from the MJAP clinic by research assistants who were trained nurses-midwives. The women were educated about family planning and the study introduced to them as they awaited care at the MJAP clinic. Women who were interested in long acting reversible contraception were referred to the family planning clinic were method-specific counselling was done. Women, who opted for IUC, were conducted through an informed consent procedure and gave written informed consent. The women underwent syndromic screening for STI, which involved history taking and a physical examination using a syndromic logarithm used to treat STIs. Women were interviewed about their socio-demographic characteristics, reproductive and sexual history including, history of suffering or being treated for STI in the past 3 months, number of sexual partners in past 12 months, menstrual bleeding pattern, contraceptive use including condom, use of cotrimoxazole and antiretroviral therapy. A thorough physical, abdominal and pelvic examination was done. Women who did not have abdominal and cervical motion or adnexal tenderness, purulent vaginal or cervical discharge and inflammation of the cervix or vagina were deemed free of STI and fit for IUC insertion. Women, who were free of STI, were randomized to additional laboratory screening or no additional screening prior IUC insertion. The women who were randomized to no additional screening had the IUC inserted right away after syndromic screening, whereas those randomized to additional laboratory screening had an endo-cervical swab taken. A sterile cotton swab was inserted in the cervix, rotated clockwise through 360° and kept in a transport medium at room temperature and sent to the laboratory for analysis. PCR was carried out to detect the presence of NG or CT and the results were sent to the clinic on the same day. Women who were free of STIs had IUC placed and those with STIs had IUC inserted after completion of antibiotic treatment as per the hospital protocol.
Follow-up
All the women were followed up for 12 months. The participants were reviewed at 1, 3, 6 and 12 months. If a woman missed a scheduled visit she was contacted by telephone and reminded of the visit. During the scheduled visits, participants were asked about the condition of their health, if the IUC had been removed, if she experienced any complications and if they had visited other health facilities and the medications they had taken. In addition, the women were asked about the presence of lower abdominal pain, purulent vaginal discharge, fever, number of sexual partners, if the intrauterine contraceptive had been expelled and ART records reviewed to determine the CD4 cell count. They underwent a physical examination and noted the presence of abdominal tenderness. Pelvic examination was conducted to check for the presence of IUC strings, cervical or adnexal tenderness, adnexal masses, purulent vaginal discharge, inflammation of vagina or cervix and findings recorded on standard clinical record forms. Women clinically diagnosed with PID were managed according to the hospital protocol. We also reviewed latest available medical records of participants who were lost to follow-up.
Outcomes
The primary outcome measure was IUC removal for any reason other than partial spontaneous expulsion within 1 year of IUC insertion. We assigned the date the IUC was removed as the removal date. For the secondary outcome measure, use of medical services after IUC insertion, our definition included unscheduled clinic visits as well as scheduled study visits at which the participant reported a gynaecological complaint for which she would have sought attention. We also collected information to calculate the rates of complete or partial IUC expulsion and treatment for suspected PID within the 1 year of IUC insertion. We defined heavy bleeding as prolonged menstrual flow of more than 7 days or passage of blood clots. PID was defined as described by the United States centre for disease control and prevention (Centre for Disease Control and Prevention, 2013). Under this classification, PID was defined as lower abdominal pain in the presence of cervical motion or adnexal tenderness, body temperature >38°C, adnexal mass and the presence of purulent vaginal discharge. All the women where IUC strings were not seen at pelvic examination had a pelvic ultrasound scan to confirm if the IUC had been expelled. Expulsion was complete if the IUC was fully extruded through the cervical canal into the vagina and partial if the IUC was extruded from the uterine cavity into the cervical canal.
Sample size
We assumed that laboratory testing after syndromic screening would lower the risk of infectious morbidity by 55% (relative) when compared with syndromic screening alone prior to IUC Insertion. We estimated the risk of infectious morbidity to be 10.7% in the syndromic arm based on the literature (Morrison et al., 2001). Given a power of 80% and a loss to follow of 9% we estimated the sample size of 703 participants.
Interim analyses and stopping guidelines
We performed one interim analysis when the accumulating follow-up data had accrued approximately half the estimated sample size according to O'Brien-Fleming boundaries (DeMets error-spending function) at a level α = 0.05 (two sided); the significance level for the final analysis was α = 0.0459. A standardized test statistic was calculated for the rate of IUC removal and all adverse effects based on accrued data. The Data Safety and Monitoring Board recommended continuation of the study.
Randomization: sequence generation
The individual patient was the unit of randomization. An independent statistician in the School of Public Health, Makerere University, created the randomization sequence using a computer generated randomization list. The randomization sequence was created using STATA 12 software package. Computer generated randomization codes were used to generate the randomization list, which was sent to the family planning clinic. Permutated block size of 6 and 8 were used and these were varied at random.
Randomization: allocation concealment
We used sequentially numbered, identical, opaque, sealed envelopes to conceal allocation from clinicians, research personnel and participants. After syndromic screening, women who were eligible for IUC insertion were randomized to additional laboratory screening or no additional screening prior to IUC insertion.
Randomization: implementation
Women who opted for IUC after counselling were escorted by the study coordinator to the examination room. The women underwent abdominal, pelvic and vaginal speculum examination to determine if they had signs of STI. The study coordinator allocated a study number to the participants if they were found free of STIs after syndromic screening. The next sealed opaque envelope was opened by the study coordinator and the participant randomized to receive either additional laboratory screening or no additional screening. The participants randomized to no additional screening had the IUC inserted right away at the time of randomization, whereas those randomized to additional laboratory screening had to wait in the clinic for 2–4 h to get the STI laboratory results before the IUC was inserted.
Blinding
The care providers involved in the follow-up and assessment of the outcomes were blinded to the study allocation groups. The study coordinator and nurses inserting the IUC were not involved in the participants' follow-up.
Statistical methods
The data were double entered using EPIDATA version 3.1 statistical package, cleaned, coded and exported to STATA version 12 for analysis. Analyses were done using the intention-to-treat principle and the results reported according to the CONSORT guidelines (Schulz et al., 2010). All participants were included in the group to which they were initially assigned. Incidence rates were calculated to determine the instantaneous risk of experiencing the primary and secondary outcomes. The results for the comparison of the two groups for the main and secondary outcomes are presented as incidence rate ratios (IRRs) with the corresponding 95% confidence intervals and P-values.
Ethical approval
This study was approved by the Makerere University College of Health Sciences Higher Degrees Research and Ethics Committee REC 2009-110 and the Uganda National Council for Science and Technology HS 1335. The women gave written informed consent. This trial was registered at Pan African Clinical Trial, Registry PACTR201308000561212.
Results
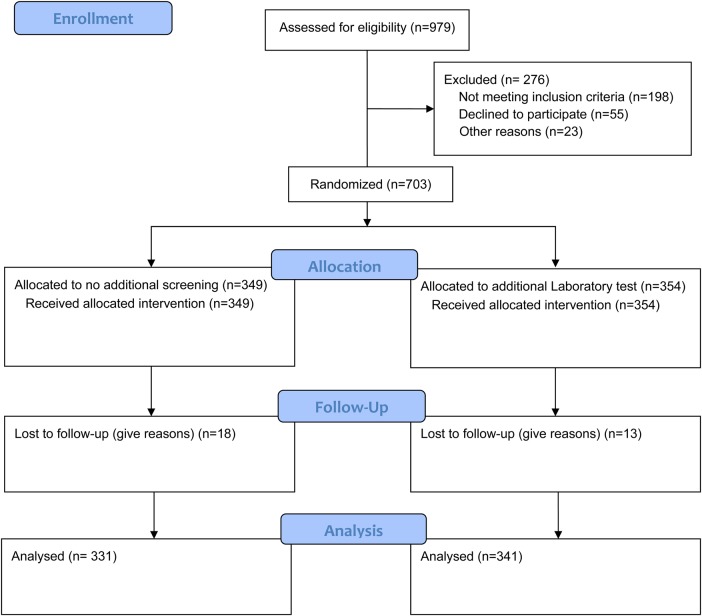
Between 2 September and 6 December 2013, 1500 women were approached to participate in the study (Fig. 1). Among them, 703 women were enrolled and randomly assigned to either additional laboratory screening or no additional screening for STI prior to IUC insertion. The participants were followed up to 31 December 2014. In case, an STI was detected during laboratory testing, the participant was treated with antibiotics according to the hospital protocol and IUC inserted after completion of treatment. After randomization, no participant changed her choice of the IUC. Thus, all the women who were randomized received an IUC. Of the 703 women, 349 had no additional screening and 354 had additional laboratory screening for STIs. At 1 year, the loss to follow-up was 31/703 (4.4%); 18/349 (5.2%) in the no additional screening group and 13/354 (3.7%) women in the additional laboratory screening group. The randomization produced treatment groups that were balanced in all important respects (Table I). The overall mean age was 29.9 ± 6.3 years; most (67%) had disclosed their HIV status to their spouses; on average, participants had known their HIV sero status for 4.6 ± 3.8 years. The mean age of sexual debut was 17 years. Most participants met the recommended patients' profile outlined in the prescribing information for the Copper T 380A. The mean number of living children was 3.0 ± 1.6, nearly all 606 (86.2%) reported single sexual partners over the past 1 year and 118 (16.8%) indicated having had symptoms of STI. The majority 518 (73.7%) had a CD4 count of more than 350 cells/mm3, most 618 (87.9%) were using antiretroviral drugs, majority 578 (82.2%) were using cotrimoxazole prophylaxis and 5.9% (21/354) in the additional laboratory screening group tested positive for NG or CT infections.
Figure 1.
Consort Flow diagram.
Table I.
Baseline characteristics of study participants.
| Characteristics | Syndromic screening (n = 349) |
Syndromic +Lab screening (n = 354) |
|---|---|---|
| Age (completed years) | 30.3 (6.2) | 29.5 (6.3) |
| Currently married | 258 (73.9) | 256 (72.3) |
| Years since HIV diagnosis | 4.9 (4.0) | 4.4 (3.7) |
| Reported disclosure of HIV status to partner | 224 (64.2) | 245 (69.2) |
| Reported use of Cotrimoxazole prophylaxis | 286 (82.0) | 292 (82.5) |
| Using ARV therapy | 310 (88.8) | 308 (87.0) |
| Cd4 count >350 cell/mm3 | 252 (72.2) | 266 (76.1) |
| Reproductive history | ||
| Age at first sexual encounter | 17.0 (2.3) | 17.1 (2.5) |
| Number of living children | 3.0 (1.6) | 2.9 (1.5) |
| Reported only one sexual partner in past year | 297 (85.1) | 309 (87.3) |
| Reported history of STI treatment | 59 (16.9) | 59 (16.7) |
Data are the mean (SD) or the number (%) of participants. ARV, antiretroviral therapy; STI, sexually transmitted infection.
The participants were followed up for a total of 330.9 years in the no additional screening group and 338.5 years in the additional laboratory screening group. The rate of IUC removal within 1 year of IUC insertion was 8.8% (29/331) in the no additional screening group and 8.0% (27/341) in the additional laboratory screening group [IRR 1.1 (95% CI 0.63–1.93)]. The most frequently reported reasons for IUC removal included heavy bleeding, cramping/abdominal pain and vaginal discharge (Table II). The rate of heavy bleeding was 1.8% (6/331) in the no additional screening group and 2.1% (7/341) in the additional laboratory screening group [IRR 0.9 (95% CI 0.24–3.05]. The rate of cramping or abdominal pain was 1.8% (6/331) in the no additional screening group and 1.5% (5/341) in the additional laboratory screening group [IRR 1.2 (95% CI 0.31–5.09)]. The rate of vaginal discharge was 2.1% (7/331) in the no additional screening group and 1.5% (5/341) in the additional laboratory screening group [IRR 1.4 (95% CI 0.39–5.72)]. All the removals were done at the request of the women except for one woman who did not respond after 48 h of antibiotic treatment for clinical PID. The rate of spontaneous IUC expulsion (complete and partial) was 5.1% (17/331) in the no additional screening group and 3.2% (11/341) in the additional laboratory screening group [IRR 1.1 (95% CI 0.63–1.93)]. Three participants in the no additional screening group and one in the additional laboratory screening group partially expelled the IUC. Study nurses discovered the partial expulsions during scheduled follow-up examinations but all complete expulsions were suspected by the woman. Within 1 year of insertion, only 0.9% (3/331) in the no additional screening group and 0.6% (2/341) in the additional laboratory screening group had PID that met accepted clinical diagnostic criteria as per US centre for disease control and prevention (Centre for Disease Control and Prevention, 2013) [IRR 1.06 (95% CI 0.91–1.23)]. They presented with lower abdominal pain and purulent discharge. On examination, they had lower abdominal tenderness, bilateral adnexal and cervical motion tenderness and axillary body temperature of 38°C. They were treated with oral antibiotics as per the hospital protocol and had IUC removed after 48 h of antibiotic treatment. They all fully recovered and switched to injection medroxyprogesterone acetate (DMPA Pharmacia & Upjohn Puurs, Belgium). The incidence of unscheduled visits within 1 year of IUC insertion was 13.6% (45/331) in the no additional screening and 12.3% (42/341) in the additional laboratory screening group (Table III). Lower abdominal pain 4.8% (16/331) in the no additional screening and 4.7% (17/341) in the additional laboratory screening group and prolonged or heavy bleeding 4.8% (16/331) among the no additional screening group and 4.6% (16/341) among the additional laboratory screening group were the commonest reasons for the unscheduled visits.
Table II.
Outcome of IUC 1 year after insertion.
| Outcome type | Syndromic [n = 331; n (%)] | Syndromic+ lab [n = 341; n (%)] | IRR (CI) | P-value |
|---|---|---|---|---|
| Removed | ||||
| Heavy/prolonged bleeding | 6 (1.8) | 7 (2.1) | 0.9 (0.24–3.05) | 0.82 |
| Cramping/abdominal pain | 6 (1.8) | 5 (1.5) | 1.2 (0.31–5.09) | 0.75 |
| Vaginal discharge | 7 (2.1) | 5 (1.5) | 1.4 (0.39–5.72) | 0.55 |
| Pregnancy | 3 (0.9) | 2 (0.6) | 1.5 (0.17–18.38) | 0.67 |
| PID | 3 (0.9) | 2 (0.6) | 1.5 (0.17–18.38) | 0.67 |
| Husband objection | 4 (1.2) | 6 (1.8) | 0.7 (0.14–2.88) | 0.57 |
| All IUC removals | 29 (8.8) | 27 (8.0) | 1.1 (0.63–1.93) | 0.73 |
| Expelled | ||||
| Partly | 3 (0.9) | 1 (0.3) | 3.1 (0.25–161.16) | 0.36 |
| Fully | 14 (4.2) | 10 (3.0) | 1.4 (0.59–3.61) | 0.39 |
| All IUC expulsions | 17 (5.1) | 11 (3.2) | 1.6 (0.70–3.74) | 0.24 |
| IUC continuation | 302 (90.1) | 314 (90.3) | 1.0 (0.84–1.16) | 0.84 |
Lab, laboratory; IRR, incidence rate ratio; CI, confidence interval; PID, pelvic inflammatory disease; IUC, intrauterine contraception.
Table III.
Reasons participants attended unscheduled clinic within 1 year of IUC insertion.
| Outcome type | Syndromic [n = 331; n (%)] | Syndromic+ lab [n = 341; n (%)] |
|---|---|---|
| Cramping/abdominal pain | 16 (4.8) | 17 (5.0) |
| Prolonged/heavy bleeding | 16 (4.8) | 16 (4.7) |
| Pus discharge | 3 (0.9) | 2 (0.6) |
| Partial IUC expulsion | 3 (0.9) | 1 (0.3) |
| Complete IUC expulsions | 4 (1.2) | 6 (1.8) |
| Amenorrhea | 2 (0.6) | 2 (0.6) |
| Suspected pregnancy | 2 (0.6) | 2 (0.6) |
| Others | 10 (3.0) | 14 (4.1) |
| Overall unscheduled visits | 45 (13.6) | 42 (12.3) |
IUC, intrauterine contraception; Lab, laboratory test.
Discussion
Laboratory testing in addition to syndromic screening for STI had no overall effect on IUC removal rates at 1 year of IUC use. Similarly, a randomized clinical trial of prophylactic antibiotics prior to IUC insertion found no differences in IUC removals (Walsh et al., 1998). A secondary finding of public health significance was the rarity of PID following IUC insertion in this population. Our data show that after 1 year of follow-up, only five participants, three from the no additional screening group and two from the additional laboratory-screening group, met accepted criteria for the diagnosis of PID. Randomized trials aimed at interventions to reduce PID or infectious morbidity in IUC users have failed to demonstrate a significant difference between the intervention and placebo groups (Sinei et al., 1990; Walsh et al., 1998; Grimes et al., 1999). Our findings add to the body of growing evidence suggesting that PID among IUC users is low even in settings where STIs are prevalent (Sinei et al., 1998; Morrison et al., 2001; Shelton, 2001).
The secondary outcomes, including the rates of heavy bleeding, lower abdominal pain, IUC expulsions, purulent discharge and the frequency of unscheduled clinic attendance, were low and there were no statistical differences between the two groups. Our findings show that IUC was well tolerated and the continuation rate was high regardless of the screening group. The high continuation rate is in line with previous studies showing IUC continuation rates of more than 80% at 1 year of IUC use (Shaamash et al., 2005; Peipert et al., 2011). Although only 6.7% of the participants attended an unscheduled clinic visit, the research team identified clinically significant disorders in all these attendees and there were no differences between the two groups. This is contrary to studies that show that women in the placebo arm are more likely to attend unscheduled clinical visits (Sinei et al., 1990; Grimes et al., 1999).
In this controlled randomized trial, possible limitations include that we were not able to carry out STI risk assessment directly from the male partners. Thus, women with high-risk partners could have been included in the study and this may be responsible for the lack of a demonstrable effect of laboratory screening on IUC removal. The diagnosis of PID was based on clinical signs and symptoms and subclinical PID could have been missed (Jacobson and Westrom, 1969; Hadgu et al., 1998). Potential selection and confounding biases were minimized by concealment of the allocation sequence in opaque, sequentially numbered and sealed envelopes. Randomization was carried out only after the participant had undergone syndromic screening and found to be clinically free of infection. The study nurse and the principal investigators involved in the follow-up of the participants were masked to the intervention group. We also applied uniform diagnostic criteria to all suspected infections. Rates of loss to follow-up in the two groups were identical. Furthermore, the sample size was large and reflected a participant population representative of contemporary IUC acceptors.
Conclusion
In suitable candidates, IUC removal and PID are rare, with or without laboratory screening for STIs. In the absence of any statistical differences in IUC removals, bleeding, IUC expulsions, PID, purulent vaginal discharge and unscheduled clinic attendance, routine laboratory screening for WLHA at low risk of STI at the time of IUC insertion may be unwarranted. However, our findings are only generalizable to women in HIV/AIDS care who have access to good follow-up.
Authors’ roles
All authors contributed to the design of the study. O.K. wrote the drafts of the paper, and O.K. and N.T.M. performed the statistical analyses. All authors contributed to the interpretation of the study results, reviewed and approved the final manuscript.
Funding
This work was made possible by Medical Education for Equitable Services to All Ugandans a Medical Education Partnership Initiative grant number 5R24TW008886 from the Office of Global AIDS Coordinator and the U. S. Department of Health and Human Services, Health Resources and Services Administration and National Institutes of Health. Additional funding was from the Swedish International Development Agency, Swedish Research Council (SIDA/VR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the government.
Conflict of interest
None declared.
Acknowledgements
The authors would like to thank study participants and our research team for all their contributions to this study.
References
- Asavapiriyanont S, Lolekha R, Roongpisuthipong A, Wiratchai A, Kaoiean S, Suksripanich O, Chalermchockcharoenkit A, Ausavapipit J, Srifeungfung S, Pattanasin S. et al. Sexually transmitted infections among HIV-infected women in Thailand. BMC Public Health 2013;13:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhling KJ, Zite NB, Lotke P, Black K. Worldwide use of intrauterine contraception: a review. Contraception 2014;89:162–173. [DOI] [PubMed] [Google Scholar]
- Centre for Disease Control and Prevention. U.S. Selected Practice Recommendations for Contraceptive Use, 2013: adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2nd Edition. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6205a1.htm (24 November 2014, date last accessed). [PubMed]
- Clifton D, Kaneda T, Ashford L. Family Planning Worldwide, 2008 Data Sheet. Washington, DC, USA: Population Reference Bureau. [Google Scholar]
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet 1992;339:785–788. [DOI] [PubMed] [Google Scholar]
- French R, Sorhaindo AM, Van Vliet HAAM, Mansour DD, Robinson AA, Logan S, Helmerhorst FM, Guillebaud J, Cowan FM. Progestogen-releasing intrauterine systems versus other forms of reversible contraceptives for contraception. Cochrane Database Syst Rev 2004; doi:10.1002/14651858.CD001776.pub2. [DOI] [PMC free article] [PubMed]
- Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet 2000;356:1013–1019. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Lopez LM, Schulz KF. Antibiotic prophylaxis for intrauterine contraceptive device insertion. Cochrane Database Syst Rev 1999. doi:10.1002/14651858.CD001327. [DOI] [PMC free article] [PubMed]
- Hadgu A, Westrom L, Brooks CA, Reynolds GH, Thompson SE. Predicting acute pelvic inflammatory disease: a multivariate analysis. Am J Obstet Gynecol 1998;155:954–960. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O, Lehtovirta P, Suni J, Paavonen J. The levonorgestrel-releasing intrauterine system (LNG-IUS) in HIV-infected women--effects on bleeding patterns, ovarian function and genital shedding of HIV. Hum Reprod 2006;21:2857–2861. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Westrom L. Objectivized diagnosis of acute pelvic inflammatory disease. Diagnostic and prognostic value of routine laparoscopy. Am J Obstet Gynecol 1969;105:1088–1098. [DOI] [PubMed] [Google Scholar]
- Kulier R, O'Brien P, Helmerhorst FM, Usher-Patel M, d'Arcangues C. Copper containing, framed intrauterine devices for contraception. Cochrane Database Syst Rev 2007. doi:10.1002/14651858.CD005347.pub3. [DOI] [PubMed]
- Luukkainen T, Allonen H, Haukkamaa M, Holma P, Pyorala T, Terho J, Toivonen J, Batar I, Lampe L, Andersson K, et al. Effective contraception with the levonorgestrel -releasing intrauterine device: 12-month report of a European multicenter study. Contraception 1987;36:169–179. [DOI] [PubMed] [Google Scholar]
- Mansour D, Inki P, Gemzell-Danielsson K. Efficacy of contraceptive methods: A review of the literature. Eur J Contracept Reprod Health Care 2010;15:4–16. [DOI] [PubMed] [Google Scholar]
- Mohllajee AP, Curtis KM, Flanagan RG, Rinehart W, Gaffield ML, Peterson HB. Keeping up with evidence a new system for WHO's evidence-based family planning guidance. Am J Prev Med 2005;28:483–490. [DOI] [PubMed] [Google Scholar]
- Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception 2006;73:145–153. [DOI] [PubMed] [Google Scholar]
- Morrison CS, Sekadde-Kigondu C, Sinei SK, Weiner DH, Kwok C, Kokonya D. Is the intrauterine device appropriate contraception for HIV-1-infected women. BJOG 2001;108:784–790. [DOI] [PubMed] [Google Scholar]
- Peipert JF, Zhao Q, Allsworth JE, Petrosky E, Madden T, Eisenberg D, Secura G. Continuation and satisfaction of reversible contraception. Obstet Gynecol 2011;117:1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Br Med J 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaamash AH, Sayed GH, Hussien MM, Shaaban MM. A comparative study of the levonorgestrel-releasing intrauterine system Mirena versus the Copper T380A intrauterine device during lactation: breast-feeding performance, infant growth and infant development. Contraception 2005;72:346–351. [DOI] [PubMed] [Google Scholar]
- Shelton JD. Risk of clinical pelvic inflammatory disease attributable to an intrauterine device. Lancet 2001;357:443. [DOI] [PubMed] [Google Scholar]
- Sinei SK, Schulz KF, Lamptey PR, Grimes DA, Mati JK, Rosenthal SM, Rosenberg MJ, Riara G, Njage PN, Bhullar VB. Preventing IUCD-related pelvic infection: the efficacy of prophylactic doxycycline at insertion. Br J Obstet Gynaecol 1990;97:412–419. [DOI] [PubMed] [Google Scholar]
- Sinei SK, Morrison CS, Sekadde-Kigondu C, Allen M, Kokonya D. Complications of use of intrauterine devices among HIV-1-infected women. Lancet 1998;351:1238–1241. [DOI] [PubMed] [Google Scholar]
- Steen R, Shapiro K. Intrauterine contraceptive devices and risk of pelvic inflammatory disease: standard of care in high STI prevalence settings. Reprod Health Matters 2004;12:136–143. [DOI] [PubMed] [Google Scholar]
- Trussell J. Contraceptive failure in the United States. Contraception 2004;70:89–96. [DOI] [PubMed] [Google Scholar]
- Trussell J, Lalla AM, Doan QV, Reyes E, Pinto L, Gricar J. Cost effectiveness of contraceptives in the United States. Contraception 2009;79:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A, Hills-Nieminen C. Drug interactions between antiretrovirals and hormonal contraceptives. Expert Opin Drug Metab Toxicol 2013;9:559–572. [DOI] [PubMed] [Google Scholar]
- Uganda Bureau of Statistics (UBOS) and ICF International Inc. 2012. Uganda Demographic and Health Survey 2011. Kampala, Uganda: UBOS and Claverton, Maryland: ICF International Inc. http://www.ubos.org/onlinefiles/uploads/ubos/UDHS/UDHS2011.pdf (24 November 2014, date last accessed). [Google Scholar]
- United Nations, 2013. Trends in Contraceptive Methods Used Worldwide http://www.un.org/en/development/desa/population/publications/pdf/popfacts/PopFacts_2013–9_new.pdf (24 November 2014, date last accessed).
- Venkatesh KK, van der Straten A, Mayer KH, Blanchard K, Ramjee G, Lurie MN, Chipato T, Padian NS, de Bruyn G. African women recently infected with HIV-1 and HSV-2 have increased risk of acquiring Neisseria gonorrhoeae and Chlamydia trachomatis in the Methods for Improving Reproductive Health in Africa trial. Sex Transm Dis 2011;38:562–570. [DOI] [PubMed] [Google Scholar]
- Walsh T, Grimes D, Frezieres R, Nelson A, Bernstein L, Coulson A, Bernstein G. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. IUD Study Group. Lancet 1998;351:1005–1008. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. Medical Eligibility Criteria for Contraceptive Use, 4th edn Geneva: World Health Organization, 2009. http://whqlibdoc.who.int/publications/2010/9789241563888_eng.pdf (24 November 2014, date last accessed). [Google Scholar]