Abstract
Objectives
The objectives of this study were to summarize antiretroviral drug concentrations in breast milk (BM) and exposure of breast-fed infants.
Methods
This was a systematic review of pharmacokinetic studies of HIV-positive women taking antiretrovirals that measured drugs in BM. The quality of pharmacokinetic and laboratory methods was assessed using pre-defined criteria. Pooled ratios and 95% CIs were calculated using the generalized inverse variance method and heterogeneity was estimated by the I2 statistic. PubMed Central, SCOPUS and LactMed databases were searched. No date or language restrictions were applied. Searches were conducted up to 10 November 2014. Clinical relevance was estimated by comparing ingested dose with the recommended therapeutic dose for each drug.
Results
Twenty-four studies were included. There was substantial variability in the clinical and laboratory methods used and in reported results. Relative to maternal plasma (MP), NRTIs accumulate in BM, with BM : MP ratios (95% CI estimates) from 0.89 to 1.21 (14 studies, 1159 paired BM and MP samples). NNRTI estimates were from 0.71 to 0.94 (17 studies, 965 paired samples) and PI estimates were from 0.17 to 0.21 (8 studies, 477 paired samples). Relative to the recommended paediatric doses, a breast-fed infant may ingest 8.4% (95% CI 1.9–15.0), 12.5% (95% CI 2.6–22.3) and 1.1% (95% CI 0–3.6) of lamivudine, nevirapine and efavirenz, respectively, via BM.
Conclusions
Transfer to untreated infants appears quantitatively important for some NRTIs and NNRTIs. The pharmacokinetic methods varied widely and we propose standards for the design, analysis and reporting of future pharmacokinetic studies of drug transfer during breastfeeding.
Keywords: ARV, mother-to-child transmission, PK
Introduction
Approximately 1.5 million HIV-positive women become pregnant each year.1 The infection of some 400 000 infants annually led the WHO to encourage provision of efavirenz-based antiretroviral (ARV) therapy for pregnant HIV-positive women [prevention of mother-to-child transmission (PMTCT) options B and B+].2 Increasing numbers of women will receive ARVs throughout breastfeeding.
In contrast to well-resourced settings,3–5 the WHO recommends exclusive breastfeeding in the developing world (since formula feeding is associated with high infant mortality)6 despite breastfeeding accounting for a significant proportion of all mother-to-child transmissions of HIV.5 Infants of ARV recipients who acquire HIV via breast milk (BM) have high rates of drug resistance,7,8 limiting treatment options and shortening life. Pharmacokinetic (PK) knowledge of ARV transfer to BM and breast-fed infants is essential to understand the safety of prolonged exposure through breastfeeding and limit the development of drug resistance.
The objectives of this study were to summarize from existing studies of breastfeeding mothers taking ARVs: (i) ARV concentrations in BM; and (ii) ARV transfer to breast-fed infants.
Methods
We wrote the protocol for this review before starting the analysis (available as Supplementary data at JAC Online), following PRISMA guidelines.9
Ethics
Ethics approval was not necessary for this systematic review.
Inclusion criteria
We sought to identify and analyse all studies that reported drug concentrations of any ARV in the BM of HIV-positive women on ARVs.
Search strategy
PubMed Central, SCOPUS and LactMed databases were searched using the keywords ‘antiretroviral’ and ‘breast’ and ‘milk’ and subsequently by replacing the generic term ‘antiretroviral’ with the name of each individual agent. No date or language restrictions were applied. The proceedings of relevant conferences and citation lists from review articles and included papers were searched. Searches were conducted up to 10 November 2014.
Data extraction
Data were extracted onto an Excel® spreadsheet by C. J. W. and L. J. E., with differences resolved by discussion amongst all authors. For each study, the author, date, country, study design including inclusion and exclusion criteria, sample size, ARV regimen(s) administered to the mother and whether or not infant ARVs were administered were recorded.
Methodological rigor and reporting quality
For clinical sampling, we assessed: (i) the PK sampling strategy in terms of number of BM samples obtained from each mother, timing of sampling relative to birth and to maternal dosing, or whether this was unclear; (ii) if the methods used to obtain BM were described sufficiently (volume of milk, manual or pump-assisted expression and storage of milk) for another investigator to be able to replicate the process; and (iii) whether the samples of maternal plasma (MP), infant plasma (IP) and BM were obtained at the same time, a different time or if this was unclear.
We assessed the quality of the laboratory methods using seven criteria: (i) if the laboratory method was described in sufficient detail for another investigator to be able to repeat the procedure; (ii) whether the authors validated or referred to assay validation for the BM matrix (yes/no); (iii) if drug was measured in whole BM, if the lipid fraction was skimmed off or if both of these methods were used; (iv) the method of drug extraction; (v) the choice of detection method used; (vi) if the assay sensitivity was reported (yes/no); and (vii) if detail regarding the internal standard was provided (yes/no).
Data analysis
Outcomes
Medians and IQRs for ARV concentrations in human BM, MP and IP were extracted. The ratio of ARV in BM to MP (BM : MP ratio) is accepted as the key index of BM transfer of drug;10 where this was not specifically stated, it was calculated from median MP and BM concentrations where these were provided. BM : MP and IP : BM ratios were only determined when MP, BM and IP concentrations were detectable (above the assay limit of quantification). Hence not all studies provided sufficient data for inclusion in the statistical analysis or presentation in the figures.
Statistical analysis
The individual studies identified in this review provided a median and IQR or single point estimates for drug concentrations and BM : MP ratios. Therefore, to provide a pooled estimate for the BM : MP and IP : BM ratios, it was necessary to assume that the ratios were normally distributed and that the median is approximately equal to the mean. Additionally, it is assumed that the IQR is four-thirds of the standard deviation, based on the fact that the standard normal has its 25th and 75th percentiles approximately two-thirds of a standard deviation away from zero.11
Having calculated an assumed mean and standard deviation for all relevant studies, pooled estimates of each outcome were obtained via the generalized inverse variance method. Studies without a spread measure were removed from the pooling exercise.
To estimate clinical relevance, BM concentrations were interpreted as the percentage of the recommended infant dose12 that would be ingested by a fully breast-fed infant. If both treatment and prophylactic doses were available, we used the PMTCT dose reflecting that which a neonate might typically be prescribed. Whilst not included in WHO guidelines, the FDA has approved efavirenz use in infants aged >3 months and weighing >3.5 kg under exceptional circumstances. Genotyping for CYP2B6 metabolizer status is strongly recommended with appropriate dose adjustment, but if not available a dose of 100 mg would be used for a 3.5 kg infant;13 this was used in our calculations. The standard assumption of 150 mL/kg/day milk intake was made. As studies did not summarize infant weights, simulations were made for infants weighing 2, 3, 4 and 5 kg. CIs for the percentages were calculated using standard methodology and once again pooled estimates were obtained using the generalized inverse variance method with zero percentages being excluded from the pooling. CIs were capped at 0 and 100 as true percentages are being considered, not changes in percentages.
Heterogeneity was estimated via the I2 statistic. It was not possible to calculate an I2 statistic where there is only a single study or where only one of several studies for a drug has a measure of spread.
Results
Description of studies
Twenty-four studies (19 full text and 5 conference proceedings) met the inclusion criteria and are summarized in Table 1. Figure 1 illustrates the information sources and search strategy. Fourteen were PK studies nested within PMTCT efficacy trials, 8 were observational PK studies and 2 were nested within early-phase clinical trials. Nineteen studies were conducted in sub-Saharan Africa.
Table 1.
Study population
| Study population |
Drugs taken by mother |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| study | country | design | inclusion criteria | exclusion criteria | size | duration of ART | NVP | ZDV | 3TC | other | Drugs taken by infant |
| Aizire46 | Uganda | observational | ARV naive, CD4 >250 cells/mm3 | multiple pregnancy; in a clinical trial; partner refusal | 120 pairs | single perinatal dose | X | not stated | |||
| Benaboud34 | Cote d'Ivoire | nested in RCT | not stated (within trial population) | not stated | 5 mothers | 7 days | X | FTC, TDF | not stated | ||
| Colebunders18 | Belgium | observational | on HAART | not stated | 10 mothers | until after BM sampling | X | X | X | NFV, IDV | ZDV single dose |
| Corbetta22 | Malawi | nested in RCT | CD4 >200 cells/mm3; infant birth weight >2 kg | not stated | 20 pairs | 7 days or throughout breastfeeding (RCT) | X | X | X | d4T, NFV | NVP single dose + ZDV/3TC 7 days |
| Corbett16 | Malawi | nested in RCT | CD4 >200 cells/mm3; on ARVs, infants HIV− | not stated | 30 pairs | delivery to end of breastfeeding | X | X | LPV/r | not stated | |
| Fogel32 | Malawi | nested in RCT | CD4 <250 cells/mm3 | not stated | 52 pairs | median 1.5 months at sampling time | d4T | NVP (one of three regimens in PEPI-Malawi trial) | |||
| Frank47 | Uganda | observational | ineligible for HAART | not stated | 62 pairs | single perinatal dose | X | NVP 2 mg/kg | |||
| Giuliano24 | Mozambique | observational | on any PMTCT regimen | not stated | 40 women | 28 weeks gestation–1 month post-partum | X | X | X | NVP 2 mg/kg | |
| Kunz20 | Uganda | nested in RCT | not stated (trial population) | on HAART | 62 pairs | single perinatal dose | X | NVP 2 mg/kg | |||
| Mirochnick48 | USA and Puerto Rico | Phase I | ARV naive; enrolled at >34 weeks gestation | intercurrent illness; significant fetal anomaly; lab abnormalities | 3 pairs | single perinatal dose | X | NVP 2 mg/kg | |||
| Mirochnick25 | Kenya | nested in RCT | not stated (trial population) | not stated | 67 pairs | from ∼34 weeks gestation through 6 months breastfeeding | X | X | X | NVP 2 mg/kg | |
| Mirochnick49 | Malawi + Brazil | nested in RCT | not stated (trial population) | previous TDF; condition that might affect PK | 25 mothers | single perinatal dose | TDF | TDF 4 mg/kg on days 1, 3 and 5 | |||
| Moodley29 | South Africa | nested in RCT | ARV naive | multiple pregnancy; significant fetal anomaly; lab abnormalities | 20 pairs | from ∼38 weeks gestation until 1 week post-partum | X | X | 3TC 4 mg/kg bd alone or with ZDV 2 mg/kg qds for 1 week | ||
| Musoke50 | Uganda | Phase I/II study | ARV naive; enrolled at >34 weeks gestation | intercurrent illness; significant fetal anomaly; lab abnormalities | 21 pairs | single perinatal dose | X | NVP 2 mg/kg | |||
| Olagunjua28 | Nigeria | observational | on EFV-based ARV; exclusively breastfeeding | not stated | 51 pairs | not stated | X | EFV, FTC, TDF | not stated | ||
| Palombi23 | Malawi | nested in RCT | not stated (trial population) | lab abnormalities | 66 pairs | from 25 weeks gestation until end of breastfeeding | X | X | X | d4T, LPV/r | NVP 2 mg/kg |
| Rezk31 | Malawi | nested in RCT | on HAART | not stated | 60 women | not stated | X | X | X | d4T, NFV, LPV/r | not stated |
| Ruffa17 | Haiti | observational | not stated | not stated | 6 women | single dose | X | no | |||
| Schneider27 | Rwanda | nested in RCT | >45 days since delivery | not stated | 13 pairs | from ∼28 weeks gestation until 6 months post-partum | X | X | X | EFV | NVP 2 mg/kg stat + ZDV 4 mg/kg for 7 days |
| Shapiro45 | Botswana | nested in RCT | not stated (trial population) | not stated | 50 pairs | from 24–36 weeks gestation, throughout breastfeeding | X | X | X | ABC, LPV/r | NVP 6 mg, ZDV 4 mg/kg bd for 4 weeks |
| Shapiro30 | Botswana | nested in RCT | on HAART | not stated | 20 pairs | >6 weeks | X | X | X | ZDV 4–6 mg tds | |
| Spencera15 | USA | observational | on HAART | not stated | 7 women | not stated | X | X | ATV | not stated | |
| Spencera14 | USA | observational | stable on HAART; undetectable viral load | not stated | 9 women | post-partum days 1–14 | ETR | not stated | |||
| Weidle51 | Kenya | nested in RCT | not stated (trial population) | not stated | 26 pairs | 34 weeks gestation to 6 months post-partum | NFV | not stated | |||
NVP, nevirapine; EFV, efavirenz; ETR, etravirine; ZDV, zidovudine; 3TC, lamivudine; d4T, stavudine; TDF, tenofovir; FTC, emtricitabine; ABC, abacavir; ATV, atazanavir; IDV, indinavir; LPV/r, ritonavir-boosted lopinavir; NFV, nelfinavir; RCT, randomized controlled trial; bd, twice daily dosing; tds, three times daily dosing; qds, four times daily dosing; stat, single dose.
aConference proceeding.
Figure 1.
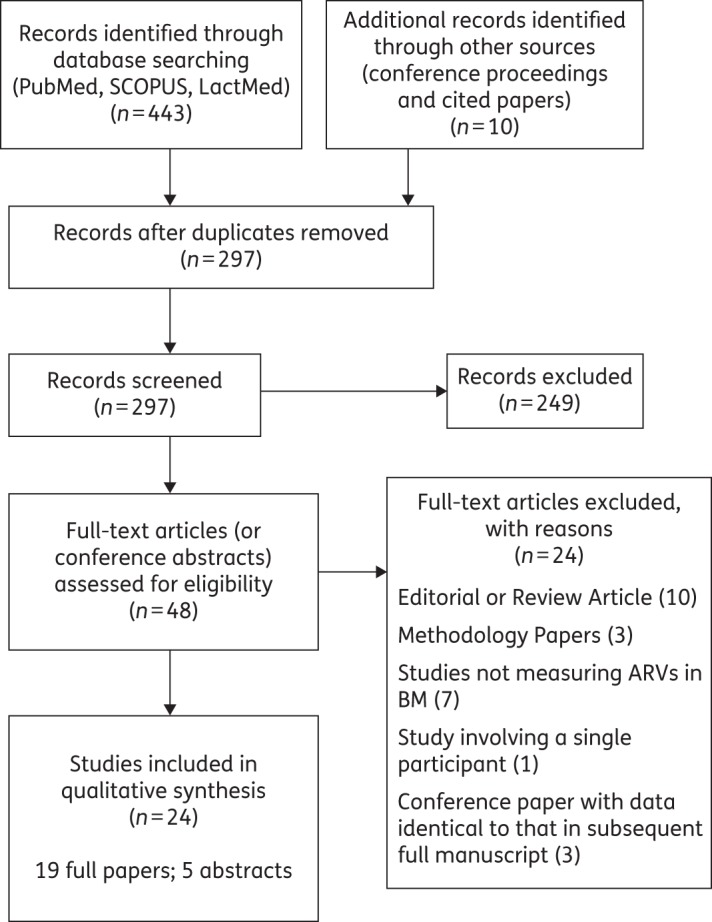
Information sources and search strategy.
The individual ARVs analysed in breastfeeding mothers were nevirapine (16 studies), zidovudine (12 studies), lamivudine (12 studies), stavudine (4 studies), lopinavir (4 studies), ritonavir (4 studies), nelfinavir (4 studies), tenofovir (2 studies), efavirenz (2 studies), atazanavir (1 study), indinavir (1 study), abacavir (1 study) and etravirine (1 study).
Eight studies reported IP concentrations where the infants were not given the same drug as the mother. A further seven also reported IP concentrations where infants and mothers were receiving the same drug; here, MP and BM concentrations were retained and IP levels excluded, as drug exposures in the infant would reflect both orally administered drug and BM transfer, making it impossible to determine the contribution from the mother via BM. In two studies, infant blood sampling was performed at intervals over a period of several months; in this case, we included in the analysis only timepoints where the directly administered infant drug would have no longer been detectable based on the known ARV elimination half-life.
Study design
The PK study design, types of matrices analysed (MP, BM and IP) and the laboratory methods are described in Tables S1 and S2.
PK design
For BM PK, 13 studies utilized a sparse PK sampling strategy (a single timepoint within a dosing interval), with sampling either within the first 6 weeks of life (6 studies) or at intervals up to 6 months of age (7 studies). Two studies undertook a rich PK profile on BM, sampling at 0, 2, 5, 8 and 24 h relative to dosing,14,15 and two further studies performed truncated rich PK analysis from 0 to 6 h post-dose.16,17 In seven studies, the PK design was not clear. Fifteen studies sampled all three matrices (MP, BM and IP), whereas 9 did not include infant concentrations. Twenty studies sampled the different matrices contemporaneously and 3 did not; in the final study, this was not clear (Table S1).
Methods to obtain and process BM
Nine studies provided detail on the clinical process of BM sampling (Table S1). Regarding the fraction of milk analysed, three studies reported the measurement of drug in BM with the lipid layer skimmed off, six reported measurement in whole BM (one of which specified that this was homogenized prior to extraction)18 and three studies considered both whole and skimmed BM. In the remaining 12 studies, the milk fraction was not specified (Table S2).
Quality of laboratory methods
We evaluated whether matrix-specific assay validation for BM was described, in accordance with FDA bioanalysis guidelines.19 The majority of methods were either LC-MS or HPLC with ultraviolet detection based; one study employed GC-MS. Two studies described the use of drug-free milk for use in assay optimization.20,21 Fourteen studies described assay validation or referenced validated methods. Fourteen studies specified the assay sensitivity in terms of the lower limit of quantification and eight studies specified the internal standard used (Table S2).
Disposition of ARVs into BM and infant
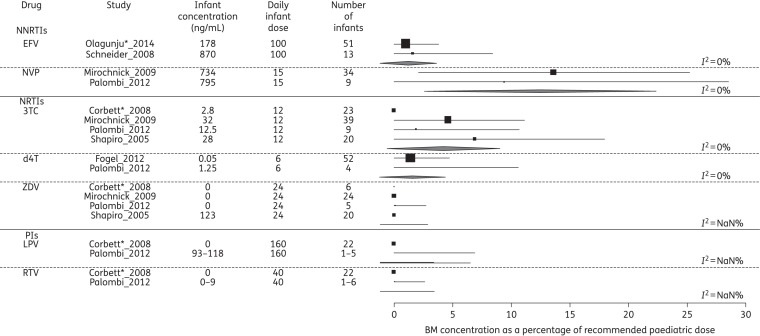
The ARV concentrations in MP and BM and the corresponding BM : MP ratios for each class of drug are summarized in Figures 2–4. Data on infant ARV concentrations resulting from BM exposure are summarized in Figure 5. Figure 6 illustrates the percentage of recommended infant dose ingested by a fully breast-fed 3 kg infant; results for other weights were similar and are not presented here.
Figure 3.
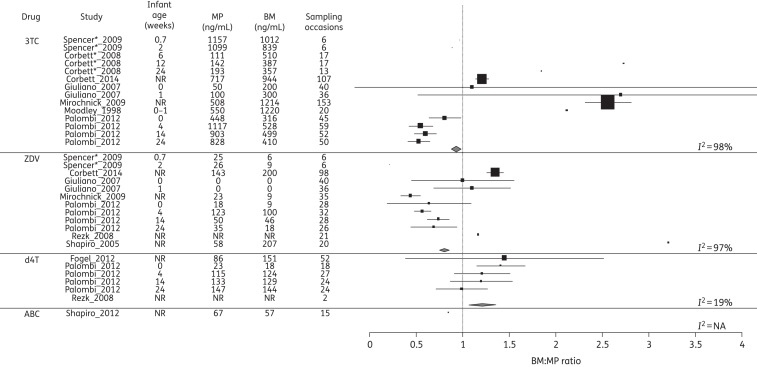
Forest plot of BM : MP ratios for NRTIs. Mean (SD) BM : MP ratios are illustrated for each drug. Where studies reported drug levels measured at different infant ages (representing different sampling times post-partum), these are represented as a separate line. The vertical line indicates a BM : MP ratio of 1, where BM and MP levels are equal. Pooled statistics are shown by the diamond and the I2 statistic is indicated. 3TC, lamivudine; ZDV, zidovudine; d4T, stavudine; ABC, abacavir; NR, not reported; NA, not available. *Conference proceeding.
Figure 2.
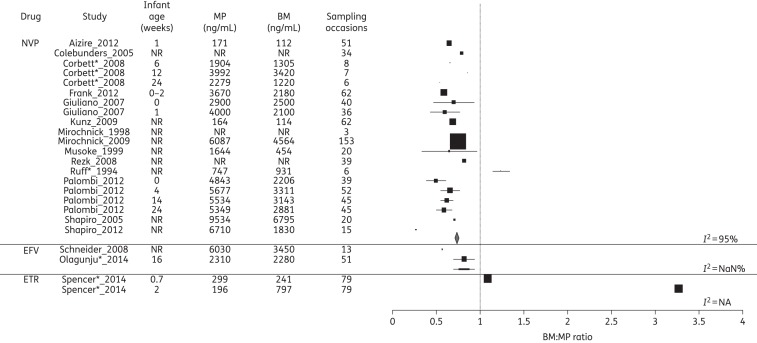
Forest plot of BM : MP ratios for NNRTIs. Mean (SD) BM : MP ratios are illustrated for each drug. Where studies reported drug levels measured at different infant ages (representing different sampling times post-partum), these are represented as a separate line. The vertical line indicates a BM : MP ratio of 1, where BM and MP levels are equal. Pooled statistics are shown by the diamond and the I2 statistic is indicated. NVP, nevirapine; EFV, efavirenz; ETR, etravirine; NR, not reported; NaN, not a number; NA, not available. *Conference proceeding.
Figure 4.
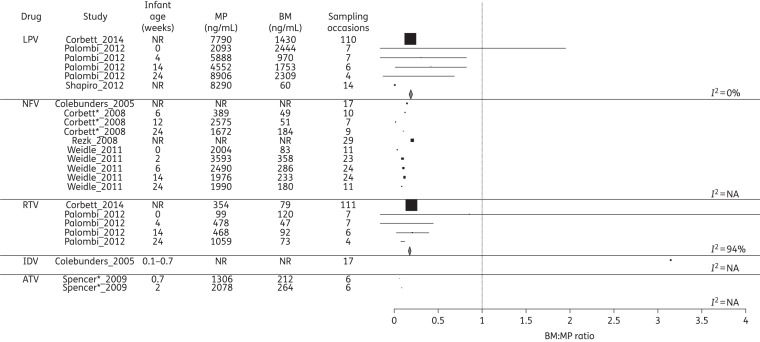
Forest plot of BM : MP ratios for PIs. Mean (SD) BM : MP ratios are illustrated for each drug. Where studies reported drug levels at different infant ages (representing different sampling times post-partum), these are represented as a separate line. The vertical line indicates a BM : MP ratio of 1, where BM and MP levels are equal. Pooled statistics are shown by the diamond and the I2 statistic is indicated. LPV, lopinavir; NFV, nelfinavir; RTV, ritonavir; IDV, indinavir; ATV, atazanavir; NR, not reported; NA, not available. *Conference proceeding.
Figure 5.
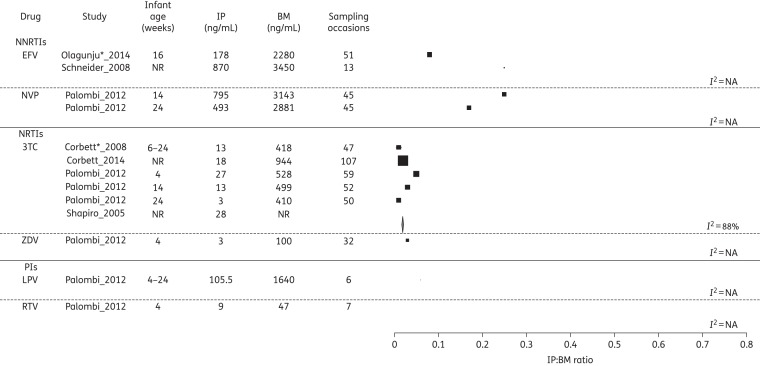
Forest plot of IP : BM ratios for all drugs where infant concentrations were detectable, grouped according to drug class. Where studies reported drug levels at different infant ages, these are represented as a separate line. Pooled statistics are shown by the diamond and the I2 statistic is indicated. EFV, efavirenz; NVP, nevirapine; 3TC, lamivudine; ZDV, zidovudine; LPV, lopinavir; RTV, ritonavir; NR, not reported; NA, not available. *Conference proceeding.
Figure 6.
‘Dose’ via BM to a fully breast-fed 3 kg infant, as a percentage of recommended paediatric dose. Pooled statistics are shown by the diamond and the I2 statistic is indicated. EFV, efavirenz; NVP, nevirapine; 3TC, lamivudine; d4T, stavudine; ZDV, zidovudine; LPV, lopinavir; RTV, ritonavir; NaN, not a number. *Conference proceeding.
NNRTIs
Seventeen studies reported on NNRTI levels in MP and BM, of which 14 measured nevirapine, 2 measured efavirenz and 1 measured etravirine.
Despite considerable heterogeneity (I2 = 95%), studies evaluating nevirapine transfer into BM consistently showed a BM : MP ratio of <1, with an overall pooled estimate of 0.73 (95% CI 0.71–0.76). Furthermore, three studies reported nevirapine levels in MP and BM at several timepoints post-delivery, demonstrating the BM : MP ratios to be relatively constant from 6 to 24 weeks post-partum, although numbers sampled at each timepoint were small.22–24 Eleven reporting IP nevirapine concentrations were confounded by administration of nevirapine to the infant. However, both Mirochnick et al.25 and Palombi et al.23 performed longitudinal analyses on MP, BM and IP; given the half-life of 30 h for nevirapine in infants from a similar population,26 by 3 months we presumed that detectable infant levels reflected BM transfer of drug. Both studies reported similar IP concentrations of ∼12% of MP at 14 weeks and <5% by 24 weeks.23,25
Both studies of efavirenz reported BM levels lower than plasma levels. The ratio of IP to BM was 0.24 in one study27 and 0.08 in the other.28 Etravirine was found to accumulate in the breast, with the BM : MP ratio rising from 1.09 to 3.27 between days 5 and 14 post-partum. Infant etravirine levels were not measured.
NRTIs
Fourteen studies reported NRTI levels in MP and BM, of which 10 measured lamivudine, 7 measured zidovudine, 3 measured stavudine, 2 measured tenofovir, 1 measured abacavir and 1 measured emtricitabine.
Seven of 10 studies showed lamivudine accumulation in the breast, with a median BM : MP ratio of >1, although the variability both within and between studies was notable (I2 = 98%). The remaining three studies23,29,45 found that lamivudine BM : MP ratios were <1. The overall pooled estimate was 0.93 (95% CI 0.89–0.98). The majority of studies15,16,23–25 demonstrated the BM : MP ratios were constant from delivery throughout post-partum. However, in studies that performed intensive PK sampling,15,16 there was evidence of differential PK within the MP and BM compartments; a slower elimination rate of lamivudine was observed in BM than in MP, which resulted in a gradual increase in the BM : MP ratio across the dosing interval. Breastfeeding infants also had measurable drug, with IP concentrations between 2% and 6% of MP.22,23,30 Infant lamivudine exposures and corresponding IP : BM ratios declined over the course of the post-partum period, in spite of a constant delivery of drug through BM, which potentially reflects maturation (or ontogeny) of the infant's metabolic clearance system. Two studies evaluated MP and BM levels of zidovudine and lamivudine at intervals up to 24 weeks post-partum,22,23 finding the BM : MP ratios of these drugs to remain constant over this time.
Regarding zidovudine, three studies reported lower concentrations in BM compared with MP,15,23,25 three described higher levels16,30,31 and the final study24 demonstrated wide interindividual variability in the BM : MP ratio. The overall pooled estimate was 0.80 (95% CI 0.76–0.85). Infant levels of zidovudine were determined in three studies.16,23,25 Zidovudine was measurable at the time of delivery (potentially a reflection of transplacental passage of drug), but was largely undetectable in IP after the neonatal period had passed.
Three studies report stavudine BM : MP ratios of >123,31,32 with a pooled estimate of 1.21 (1.07–1.36), which is significantly >1; however, infant concentrations approached the lower limit of quantification with a median value of zero.23,32 Two studies have measured tenofovir in BM. In one, tenofovir was measurable in the BM of only 4 out of 25 women sampled and neither study reported corresponding MP or IP concentrations.33,34 A single study measured emtricitabine in BM, but not MP. Abacavir had a reported BM:MP ratio of 0.85 in a single study, but BM, MP and IP concentrations were not reported.45
PIs
Eight studies reported on PI levels in BM. One study measured atazanavir, one indinavir, four lopinavir used in combination with low-dose ritonavir and four nelfinavir. In all cases, the low-dose ritonavir was used as a boosting agent for lopinavir. Overall penetration of these PIs into BM was low (<40%) relative to MP. Only indinavir was found to accumulate in BM and this study must be interpreted with caution since the samples were drawn from a single mother on five consecutive days.18 Of the five studies analysing infant concentrations of PIs, four reported levels below the limit of quantification. One study from Malawi was able to detect lopinavir and ritonavir in the plasma of breast-fed infants, at ∼2% of MP levels.23
Discussion
Although increasing numbers of countries have adopted WHO option B+, meaning that all pregnant and breastfeeding women will receive lifelong ART,2 understanding of the PK of transfer between mother and infant remains incomplete. Target concentrations have not been defined for any ARV in BM and many studies utilized an in vitro IC50 or IC95 with correction for protein binding. These approaches are not standardized and do not incorporate the active intracellular metabolites of NRTIs.35,36 Thus, we chose not to incorporate comparison against IC50 in the formal undertaking of this systematic review.
The NRTIs have higher and more variable BM penetration than the NNRTIs or PIs. Lamivudine has the highest accumulation in the BM and reaches detectable levels in the infant. Initially assumed to have unlikely clinical significance30 (exposure ∼5% of IC5037), more recent analysis of infants who acquired HIV through breastfeeding in the Kisumu Breastfeeding Study (KiBS) (mothers received zidovudine, lamivudine and either nevirapine or nelfinavir) found more than three-quarters to have resistance to both nevirapine and lamivudine.7 Further, amongst mothers who initiated triple therapy post-partum on the basis of clinical need in the PEPI-Malawi trial, almost one-third of HIV-positive infants were found to have multiclass drug resistance.8 The accumulation of zidovudine in BM was also highly variable between studies, but in most cases infant levels were undetectable, possibly attributed to the drug's rapid elimination half-life (∼1 h). Tenofovir and emtricitabine are measurable in BM, although the relationships with maternal and IP concentrations remain poorly defined; furthermore, tenofovir is not recommended for use in children aged <2 years. These findings have clinical and programmatic significance. Zidovudine and lamivudine remain components of first-line PMTCT and ARV regimens in low-resource settings and tenofovir and emtricitabine are increasingly used with implementation of WHO 2013 guidelines.38
Despite being a first-line ARV in PMTCT,38 only two studies assessed efavirenz concentrations in BM. These showed infant levels approach the minimum levels considered necessary for effective treatment in adults, reaching 13%27 and 8%39 of MP concentrations. No data about effective efavirenz levels necessary for protection of neonates by PMTCT are published. In part, this is a consequence of efavirenz having been contraindicated in pregnancy until 2012 due to teratogenicity concerns, which have recently been refuted by meta-analysis of exposed pregnancies;40 additionally, the drug is not recommended for use in children aged <3 years or weighing <10 kg except under exceptional circumstances and ideally with pharmacogenomic testing.13 Even with the extension to FDA licensing for young infants, current guidelines state it should not be used in infants aged <3 months. The studies by Schneider et al.27 and Olagunju et al.28 indicate that the ‘dose’ received by the breast-fed infant may reach 3.5% of the paediatric dose. The consequences of this in the neonatal period and the influence of maternal and infant pharmacogenomics on the concentrations reached warrant further evaluation.
The clinical relevance of ARV concentrations in BM is not fully understood. Whilst high levels have been correlated with reductions in the BM viral load, reducing the amount of virus to which the infant is exposed,24 differential accumulation of agents used within a triple-therapy regimen risks exposure to monotherapy within the compartment of the breast, potentially selecting resistant HIV strains. Furthermore, transfer of low levels of individual drugs to a breast-fed HIV-positive infant is associated with high rates of drug resistance as demonstrated in secondary analyses of both KiBS7 and PEPI-Malawi.8 Future studies should consider not only the levels of each individual drug, but also the optimal combination to be used to maximize benefits and reduce risks.
The wide variability within and between studies may result from differences in sampling time relative to dose, differences in drug concentration assays and the statistical method used to report concentrations below the lower limit of quantification, in addition to biological differences between populations. However, it is noted that variability was particularly marked for the NRTIs. Intensive PK data suggest there is a possible lag in the elimination of lamivudine (and to a lesser extent zidovudine) from BM, as shown by a gradual increase in the BM : MP ratios over the course of the dosing interval. This may, in part, explain the extensive variation in NRTI BM : MP ratios across studies, as sparse samples are taken at different times relative to dosing.
The accumulation of PIs in BM is low. Protein binding is the strongest predictor of drug transfer in BM,41 which explains lower levels of PIs penetrating BM compared with the other classes of drug (∼30% protein binding for zidovudine and lamivudine compared with >90% for the PIs).42 Maternal covariates influencing the variability in BM elimination of ARVs are poorly defined, but may include clinical states affecting circulating plasma proteins, such as intercurrent illness and poor nutrition.
Limitations
Statistical limitations
There are a number of limitations to the analysis. A normal distribution is assumed when converting medians and IQRs to means and standard deviations and comparison of pooled estimates must be made with caution. Significant heterogeneity was observed with CIs that frequently do not overlap (Figures 2–5), necessitating restriction of analyses to the generalized inverse variance method (use of a random-effects model43 is an alternative approach).
We have presented a pooled estimate for every drug, which has an associated measure of spread. However, in some cases this means we are presenting a pooled estimate for a single study, which some may consider as not being meaningful. This is related to the limited data available.
The analysis of infant drug concentrations as a percentage of the recommended paediatric dose (Figure 6) relied on several assumptions. The source publications did not state infant weight and quantifying the volume of milk intake is challenging—we used the standard assumption of 150 mL/kg/day BM intake. Furthermore, most BM concentrations reflected a single timepoint during the dosing interval whereas the majority of mothers were taking a twice-daily regimen; our calculations assume a stable concentration of drug in BM throughout the dosing interval. Finally, we used an unlicensed efavirenz dose, which remains contraindicated in infants aged <3 months.
Limitations of reported methods
Although validated methods are essential for accurate and reliable drug concentration data,44 published clinical PK studies of ARVs in breastfeeding mother/infant pairs rarely report detail. Descriptions of sample collection, fraction of milk analysed, extraction method, type of internal standard, stability, matrix effects, recovery, accuracy and precision are frequently lacking.
The complexity of BM, particularly relating to variable lipid and protein content, requires that extraction methods are carefully validated. Among the studies reported here, most authors did not specify the milk fraction analysed or the extraction method used. This information is essential. Whereas Fogel et al.32 reported no significant difference in stavudine levels between whole and skimmed milk, Shapiro et al.45 noted significantly different recoveries of drug between skimmed and whole BM and consequently elected to report whole milk concentrations.30,45 Physicochemical principles influence the partitioning of drug into aqueous or lipid fractions of milk, but both are ingested by the infant and therefore whole milk is more likely to reflect the true clinical situation.
The majority of BM PK studies included in this review followed the stringent eligibility criteria of the PMTCT trial within which they were nested. This selection process, rather than recruitment under operational conditions, may have excluded women with covariates influencing BM elimination of ARVs.
Studies report BM concentrations of only 13 of the currently available ARVs. At the point of licensing, new drugs will not have been assessed in pregnant and breastfeeding populations. Whereas in well-resourced settings formula feeding is an option for mothers on these drugs, validated methodology is essential to enable investigation of BM elimination of drugs intended for widespread use in low-resource populations where prolonged breastfeeding is the norm.
To yield precise, high-quality data in an efficient manner, the proposed study of a novel ARV in breastfeeding mother/infant pairs can be informed by the findings of this review as summarized in Table 2.
Table 2.
Recommendations for future studies
| Component of study design | Ideal characteristics |
|---|---|
| Clinical | |
| population | population in whom the drug is to be used |
| study design | specific PK hypothesis or aims to be stated |
| sampling schedule | optimal sampling schedule to be considered from known PK of drugs |
| sample size | determined to enable estimation of PK parameters with a high degree of precision; rational basis from previous data (if available) |
| BM sampling | description of consistent, reproducible sampling method |
| time after birth | several timepoints relative to delivery; ideally the same mother/infant pairs followed longitudinally |
| time post-dose | sufficient timepoints to allow construction of an AUC for MP, BM and IP |
| documentation of timing | time of drug intake and PK sampling recorded |
| sampling of different matrices | contemporaneous sampling of MP and BM; validation of infant sampling in relation to maternal dose and recent feed |
| Laboratory | |
| assay validation | standards to be made up in donated BM to exclude matrix effect; use of internal standard labelled with a stable isotope |
| milk fraction | whole milk or clear justification for use of skimmed milk fraction with validation |
| BM storage | assay validation to include stability assessment |
| detection | highly sensitive assays such as HPLC-MS/MS to enable detection of low levels of drug |
| reporting | reporting of assay sensitivity to aid interpretation of BLQ levels |
| analysis | clear statement of method used to interpret BLQ levels |
BLQ, below the limit of quantification.
Conclusions
This systematic review reveals that the NRTI and NNRTI classes of ARVs are eliminated in the BM of HIV-positive women and transferred to breastfeeding infants, which may explain the development of drug-resistant HIV in infants where maternal ARVs have failed to prevent transmission. Available data are drawn from a small number of diverse studies employing laboratory methodology, which is frequently incompletely validated. Adoption of recent WHO guidelines will dramatically increase the use of ARVs in women of childbearing age in low-resource settings. Evaluation of the PK of both existing and novel drugs in breastfeeding mother/infant pairs is a matter of priority in order that best practice and safety can be ensured as novel regimens are rolled out.
Funding
C. J. W. held an Academy of Medical Sciences Starter Grant for Clinical Lecturers investigating ARV drugs in breastfeeding Ugandan mother/infant pairs. C. J. W. commenced a Wellcome Trust Clinical Postdoctoral Fellowship (104422/Z/14/Z) to investigate PK of ARVs in breastfeeding mother/infant pairs on 1 February 2015.
Transparency declarations
None to declare.
Author contributions
C. J. W.: concept for review, study design and protocol development, data collection, data analysis and interpretation, preparation of figures and manuscript writing.
P. G.: input into all areas listed for C. J. W., with particular input into protocol development and methods.
L. J. B.: statistical analysis of drug levels.
S. H. K.: discussion of all elements of study design, data collection and interpretation, particularly where this was challenging. Input into manuscript.
L. J. E.: protocol development, literature searches, data collection and analysis and manuscript writing.
Supplementary data
References
- 1.WHO/UNAIDS/UNICEF. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access 2011. Geneva: WHO, 2011. http://www.who.int/hiv/pub/progress_report2011/en/. [Google Scholar]
- 2.WHO. Programmatic Update: Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Geneva: WHO, 2012. http://apps.who.int/iris/handle/10665/70892. [Google Scholar]
- 3.Achievements in public health. Reduction in perinatal transmission of HIV infection—United States, 1985–2005. MMWR Morb Mortal Wkly Rep 2006; 55: 592–7. [PubMed] [Google Scholar]
- 4.Mofenson LM. Protecting the next generation: eliminating perinatal HIV-1 infection. N Engl J Med 2010; 362: 2316–8. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. Report on the Global HIV/AIDS Epidemic. Geneva: UNAIDS, 2010. http://www.unaids.org/globalreport/Global_report.htm. [Google Scholar]
- 6.WHO. Guidelines on HIV and Infant Feeding 2010. http://www.who.int/maternal_child_adolescent/documents/9789241599535/en/.
- 7.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med 2011; 8: e1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogel J, Li Q, Taha TE, et al. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants. Clin Infect Dis 2011; 52: 1069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson GD. Using pharmacokinetics to predict the effects of pregnancy and maternal–infant transfer of drugs during lactation. Expert Opin Drug Metab Toxicol 2006; 2: 947–60. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. http://handbook.cochrane.org/.
- 12.WHO. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access. Recommendations for a Public Health Approach. Annexe E. Geneva: WHO, 2010. [PubMed] [Google Scholar]
- 13.NIH. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. Page 227, Table 2 http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf. [Google Scholar]
- 14.Spencer L, Liu S, Wang C, et al. Intensive etravirine PK and HIV-1 viral load in breast milk and plasma in HIV+ women. In: Abstracts of the Twenty-first Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2014. Abstract 891 Foundation for Retrovirology and Human Health, Alexandria, VA, USA. [Google Scholar]
- 15.Spencer L, Neely M, Mordwinkin N, et al. Intensive PK of zidovudine, lamivudine and atazanavir and HIV-1 viral load in breast milk and plasma in HIV+ women receiving HAART therapy. In: Abstracts of the Sixteenth Conference on Retroviruses and Opportunistic Infections, Montreal, 2009. Abstract 942 Foundation for Retrovirology and Human Health, Alexandria, VA, USA. [Google Scholar]
- 16.Corbett AH, Kayira D, White NR, et al. Antiretroviral pharmacokinetics in mothers and breastfeeding infants from 6 to 24 weeks post partum: results of the BAN Study. Antivir Ther 2014; 19: 587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruff A, Hamzeh F, Lietman P, et al. Excretion of zidovudine in human breast milk. In: Abstracts of Thirty-fourth Interscience Conference on Antimicrobial Agents and Chemotherapy, Orlando, FL, 1994. Abstract L11 American Society for Microbiology, Washington, DC, USA. [Google Scholar]
- 18.Colebunders R, Hodossy B, Burger D, et al. The effect of highly active antiretroviral treatment on viral load and antiretroviral drug levels in breast milk. AIDS 2005; 19: 1912–5. [DOI] [PubMed] [Google Scholar]
- 19.FDA. Guidance for Industry: Bioanalytical Method Validation 2001. http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf.
- 20.Kunz A, Frank M, Mugenyi K, et al. Persistence of nevirapine in breast milk and plasma of mothers and their children after single-dose administration. J Antimicrob Chemother 2009; 63: 170–7. [DOI] [PubMed] [Google Scholar]
- 21.Rezk NL, White N, Kashuba AD. An accurate and precise high-performance liquid chromatography method for the rapid quantification of the novel HIV integrase inhibitor raltegravir in human blood plasma after solid phase extraction. Anal Chim Acta 2008; 628: 204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbett A, Martinson F, Rezk N, et al. Antiretroviral drug concentrations in breast milk and breastfeeding infants. In: Abstracts of the Fifteenth Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2008. Abstract 648 Foundation for Retrovirology and Human Health, Alexandria, VA, USA. [Google Scholar]
- 23.Palombi L, Pirillo MF, Andreotti M, et al. Antiretroviral prophylaxis for breastfeeding transmission in Malawi: drug concentrations, virological efficacy and safety. Antivir Ther 2012; 17: 1511–9. [DOI] [PubMed] [Google Scholar]
- 24.Giuliano M, Guidotti G, Andreotti M, et al. Triple antiretroviral prophylaxis administered during pregnancy and after delivery significantly reduces breast milk viral load: a study within the Drug Resource Enhancement Against AIDS and Malnutrition Program. J Acquir Immune Defic Syndr 2007; 44: 286–91. [DOI] [PubMed] [Google Scholar]
- 25.Mirochnick M, Thomas T, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother 2009; 53: 1170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirochnick M, Nielsen-Saines K, Pilotto JH, et al. Nevirapine concentrations in newborns receiving an extended prophylactic regimen. J Acquir Immune Defic Syndr 2008; 47: 334–7. [PubMed] [Google Scholar]
- 27.Schneider S, Peltier A, Gras A, et al. Efavirenz in human breast milk, mothers', and newborns' plasma. J Acquir Immune Defic Syndr 2008; 48: 450–4. [DOI] [PubMed] [Google Scholar]
- 28.Olagunju A, Siccardi M, Okafor O, et al. Pharmacogenetics of efavirenz excretion into human breast milk and transfer to breastfed infants. In: Abstracts of the Twenty-first Conference on Retroviruses and Opportunistic Infections, Boston, MA, 2014. Abstract 888 Foundation for Retrovirology and Human Health, Alexandria, VA, USA. [Google Scholar]
- 29.Moodley J, Moodley D, Pillay K, et al. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J Infect Dis 1998; 178: 1327–33. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro RL, Holland DT, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis 2005; 192: 720–7. [DOI] [PubMed] [Google Scholar]
- 31.Rezk NL, White N, Bridges AS, et al. Studies on antiretroviral drug concentrations in breast milk: validation of a liquid chromatography-tandem mass spectrometric method for the determination of 7 anti-human immunodeficiency virus medications. Ther Drug Monit 2008; 30: 611–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fogel JM, Taha TE, Sun J, et al. Stavudine concentrations in women receiving postpartum antiretroviral treatment and their breastfeeding infants. J Acquir Immune Defic Syndr 2012; 60: 462–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirochnick M, Kafulafula G, Kreitchmann R, et al. Pharmacokinetics (PK) of tenofovir disoproxil fumarate (TDF) after administration to HIV-1 infected pregnant women and their newborns. In: Abstracts of the Sixteenth Conference on Retroviruses and Opportunistic Infections, Montreal, 2009. Abstract T-137 Foundation for Retrovirology and Human Health, Alexandria, VA, USA. [Google Scholar]
- 34.Benaboud S, Pruvost A, Coffie PA, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d'Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother 2011; 55: 1315–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson PL, Kakuda TN, Kawle S, et al. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 2003; 17: 2159–68. [DOI] [PubMed] [Google Scholar]
- 36.Fletcher CV, Kawle SP, Kakuda TN, et al. Zidovudine triphosphate and lamivudine triphosphate concentration–response relationships in HIV-infected persons. AIDS 2000; 14: 2137–44. [DOI] [PubMed] [Google Scholar]
- 37.Coates JA, Cammack N, Jenkinson HJ, et al. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother 1992; 36: 733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva: WHO, 2013. http://www.who.int/hiv/pub/guidelines/arv2013/en/. [PubMed] [Google Scholar]
- 39.Acosta EP, Limoli KL, Trinh L, et al. Novel method to assess antiretroviral target trough concentrations using in vitro susceptibility data. Antimicrob Agents Chemother 2012; 56: 5938–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford N, Mofenson L, Kranzer K, et al. Safety of efavirenz in first-trimester of pregnancy: a systematic review and meta-analysis of outcomes from observational cohorts. AIDS 2010; 24: 1461–70. [DOI] [PubMed] [Google Scholar]
- 41.Abraham MH, Gil-Lostes J, Fatemi M. Prediction of milk/plasma concentration ratios of drugs and environmental pollutants. Eur J Med Chem 2009; 44: 2452–8. [DOI] [PubMed] [Google Scholar]
- 42.Boffito M, Back DJ, Blaschke TF, et al. Protein binding in antiretroviral therapies. AIDS Res Hum Retroviruses 2003; 19: 825–35. [DOI] [PubMed] [Google Scholar]
- 43.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–14. [DOI] [PubMed] [Google Scholar]
- 44.Begg EJ, Duffull SB, Hackett LP, et al. Studying drugs in human milk: time to unify the approach. J Hum Lact 2002; 18: 323–32. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro RL, Ribaudo H, Powis K, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS 2012; 26: 120–1. [DOI] [PubMed] [Google Scholar]
- 46.Aizire J, McConnell MS, Mudiope P, et al. Kinetics of nevirapine and its impact on HIV-1 RNA levels in maternal plasma and breast milk over time after perinatal single-dose nevirapine. J Acquir Immune Defic Syndr 2012; 60: 483–8. [DOI] [PubMed] [Google Scholar]
- 47.Frank M, Harms G, Kunz A, et al. Population pharmacokinetic analysis of a nevirapine-based HIV-1 prevention of mother-to-child transmission program in Uganda to assess the impact of different dosing regimens for newborns. J Clin Pharmacol 2013; 53: 294–304. [DOI] [PubMed] [Google Scholar]
- 48.Mirochnick M, Fenton T, Gagnier P, et al. Pharmacokinetics of nevirapine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Pediatric AIDS Clinical Trials Group Protocol 250 Team. J Infect Dis 1998; 178: 368–74. [DOI] [PubMed] [Google Scholar]
- 49.Mirochnick M, Taha T, Kreitchmann R, et al. Pharmacokinetics and safety of tenofovir in HIV-infected women during labor and their infants during the first week of life. J Acquir Immune Defic Syndr 2014; 65: 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musoke P, Guay LA, Bagenda D, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006). AIDS 1999; 13: 479–86. [DOI] [PubMed] [Google Scholar]
- 51.Weidle PJ, Zeh C, Martin A, et al. Nelfinavir and its active metabolite, hydroxy-t-butylamidenelfinavir (M8), are transferred in small quantities to breast milk and do not reach biologically significant concentrations in breast-feeding infants whose mothers are taking nelfinavir. Antimicrob Agents Chemother 2011; 55: 5168–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.