Abstract
Objectives
Emerging data suggest that the combination of tacrolimus and the CCR5 antagonist maraviroc, both cytochrome P450-3A4 substrates, may be effective in preventing graft-versus-host disease in patients undergoing allogeneic HSCT. This study evaluated whether a pharmacokinetic interaction exists between these agents.
Methods
The study included 36 allogeneic HSCT recipients who received maraviroc + tacrolimus and 43 recipients of tacrolimus alone. We used a difference-in-differences analysis to examine the change in the concentration/dose ratios of tacrolimus after the discontinuation of maraviroc. In addition, we analysed the concentrations and dose requirements of tacrolimus in the two groups.
Results
There was no significant difference in tacrolimus concentration/dose ratios in patients receiving maraviroc + tacrolimus compared with tacrolimus alone. Upon discontinuation of maraviroc, the change in concentration/dose ratio was small and not significant relative to the control group, and the effect estimate was further attenuated after adjustment for confounders [−0.35 (ng/mL)/(mg/day); P = 0.46]. In addition, the change in mean tacrolimus dose after discontinuation of maraviroc was similar between the groups (0.12 mg/day; P = 0.56), as was the change in mean tacrolimus concentration (0.02 ng/mL; P = 0.97).
Conclusions
Our findings do not support a significant inhibitory effect of maraviroc on the metabolism of tacrolimus. These data demonstrate that this drug combination is safe and imply that the protective effect of maraviroc against graft-versus-host disease was not mediated through an increase in tacrolimus concentrations. These findings are important for the design of clinical trials that evaluate maraviroc in combination with cytochrome P450-3A4 substrates.
Keywords: pharmacology, transplantation, immunosuppression, chemokine receptors
Introduction
Pharmacokinetic interactions associated with calcineurin inhibitors are critical in patients undergoing solid organ transplantation (SOT) or HSCT owing to the narrow therapeutic index of these agents.1,2 The primary mechanism for drug–drug interactions (DDIs) related to calcineurin inhibitors is associated with an interference with the hepatic elimination of these agents. Calcineurin inhibitors are primarily eliminated by cytochrome P-450 (CYP) 3A4/5-mediated metabolism but many medications frequently used in transplant recipients either inhibit or induce these isoenzymes, resulting in either an increased risk of toxicity or a loss of efficacy.2 Although significant advances have been made in discerning clinically relevant pharmacokinetic interactions between standard transplant medications and calcineurin inhibitors, limited data are available regarding DDIs between novel therapeutic agents and calcineurin inhibitors in SOT and HSCT recipients.
Among the emerging agents of interest is maraviroc, a C-C chemokine receptor type 5 (CCR5) antagonist with promising therapeutic potential in both SOT and HSCT.3,4 Maraviroc is a known substrate of CYP3A4 and P-glycoprotein. Whether maraviroc also acts as an inhibitor of CYP3A4 remains unclear. A study in healthy subjects suggested that maraviroc had no clinically relevant effects on the pharmacokinetics of midazolam, a major CYP3A4 substrate commonly used for evaluating DDIs related to CYP3A4.5 A study evaluating whether a pharmacokinetic interaction exists between maraviroc and calcineurin inhibitors has never been performed. The only available data come from a recent case report published in JAC that described a 21% increase in tacrolimus exposure when combined with maraviroc in the treatment of a liver transplant recipient.6
Although maraviroc was initially developed as part of combination HAART for patients infected with CCR5-tropic HIV, emerging evidence has demonstrated CCR5 is also involved in lymphocyte migration and has been implicated in SOT rejection, autoimmune diseases and graft-versus-host disease (GVHD).7–9 We recently tested maraviroc as a prevention strategy against GVHD in patients undergoing allogeneic HSCT and found that the addition of maraviroc to tacrolimus and methotrexate resulted in a low rate of visceral GVHD.10 Thus, the goal of our analysis was to examine whether a pharmacokinetic interaction exists between maraviroc and tacrolimus in HSCT recipients.
Patients and methods
Study population
We conducted a post hoc analysis of a prospective Phase I/II clinical trial that tested the safety and efficacy of adding maraviroc to a backbone GVHD prophylaxis regimen of tacrolimus/methotrexate in HSCT recipients. The trial results and methodology have been described in detail elsewhere.10 Briefly, the trial enrolled 38 patients presenting for HSCT at the Hospital of the University of Pennsylvania from June 2009 to March 2011. All patients received oral tacrolimus (0.06 mg/kg/day) in two divided doses beginning 2 days before HSCT, and the dose was adjusted to a target trough level of 5–15 ng/mL. In the treatment group, maraviroc was initiated 2 days before HSCT and continued until Day 30 post-HSCT. Of note, the trial included a maraviroc dose-finding component. Seven patients received 150 mg maraviroc twice daily and 31 received maraviroc 300 mg twice daily for the duration of the study. During the same time period, data were collected from an additional 43 control patients meeting the same study criteria who were not treated with maraviroc. The study was approved by the Institutional Review Board of the University of Pennsylvania, and the patients provided written informed consent.
Study variables
This analysis was conducted in patients with at least one weekly serum tacrolimus concentration available from Week 1 to Week 6 after HSCT. We collected data on tacrolimus dosing and serum concentration starting from 2 days prior to HSCT until Day 56 post-transplant. We also collected information on potential confounding variables present at baseline and during the follow-up. As tacrolimus dosing is rapidly tapered in the event of disease relapse, we censored the follow-up at the day of relapse. Baseline covariates included the patients' age, gender and weight. Time-varying covariates included treatments and outcomes that might affect the concentration/dose (C/D) ratios of tacrolimus through their effects on hepatic metabolism and/or gastrointestinal absorption, including exposure to azole antifungals or systemic corticosteroids, the development of acute Grade 2–4 gastrointestinal and/or liver GVHD, hepatic dysfunction (defined as a direct bilirubin, ALT or AST concentration >3 times the upper limit of normal), gastrointestinal dysfunction (defined as dysfunction requiring total parenteral nutrition or the occurrence of Grade 2–4 mucositis) and infectious complications. All the time-varying covariates were evaluated weekly. The severity of GVHD and mucositis was graded according to standard criteria.11,12
Endpoints
The effect of maraviroc exposure on the pharmacokinetics of tacrolimus was quantified by examining changes in the C/D ratios of tacrolimus. This endpoint has been shown to correlate with known predictors of the pharmacokinetics of tacrolimus in previous studies.13–15 An inhibition of tacrolimus metabolism by maraviroc would result in a higher serum concentration for any given dose. Accordingly, we would expect to observe a relative increase in the C/D ratio of tacrolimus during maraviroc exposure. Upon discontinuation of maraviroc, the inhibitory effect should dissipate and corresponding decreases in the C/D ratio would be expected. We examined these potential changes in the C/D ratio of tacrolimus in relation to maraviroc administration using a difference-in-differences analysis.16–19 This approach provides an implicit control of the baseline variables that may affect the pharmacokinetics of tacrolimus because each patient serves as their own control, thereby limiting confounding from time-invariant factors such as genetic polymorphisms. This method also accounts for the dynamic nature of the response to the dose of tacrolimus, as the dosage of tacrolimus required to maintain similar trough concentrations is known to decrease with time post-transplantation.20–23 Specifically, we compared the differences in the C/D ratios of tacrolimus between Week 2 and Week 6 in the maraviroc group with the difference in the C/D ratios between Week 2 and Week 6 in the control group. The timepoints for comparison were chosen a priori based on the terminal half-lives of tacrolimus and maraviroc (12–18 h and 14–18 h, respectively).24,25 Thus, we estimated that tacrolimus and maraviroc would reach steady-state by Week 2 after the initiation of treatment. Similarly, we anticipated Week 6 to be a sufficient time for a complete maraviroc washout to have occurred after discontinuation of the drug on Day 30 post-HSCT. To account for the possibility of the dose intensity of maraviroc affecting the C/D ratios of tacrolimus, we repeated the same analysis excluding patients who had received maraviroc 150 mg twice daily. Secondary endpoints included weekly mean tacrolimus concentrations and weekly mean tacrolimus dose requirements.
Statistical analysis
Univariate comparisons of continuous data were assessed using the Student's t-test or Wilcoxon rank-sum test as appropriate. Categorical data were assessed using the χ2 test or Fisher's exact test as appropriate. The change in C/D ratio of tacrolimus over time was modelled with piecewise linear mixed-effects regression.26,27 We hypothesized that the C/D ratios of tacrolimus would change after the discontinuation of maraviroc. Accordingly, the mixed-effects model included a knot at Week 4, a term that allowed the trajectory in the C/D ratio of tacrolimus to differ between Weeks 1–4 and Weeks 5–8.27 Interaction terms between group and time variables were introduced to allow the trend in the C/D ratios of tacrolimus to differ between maraviroc-exposed and maraviroc-unexposed patients. Model estimates of the C/D ratios of tacrolimus at Week 2 and Week 6 were used to calculate the difference-in-differences values. Potential confounding was evaluated using a change-in-estimate approach.28,29 Variables were retained in the final model if they changed the difference-in-differences estimate by ≥10%. We additionally built separate mixed-effects models to evaluate the change in mean tacrolimus dose and mean tacrolimus concentration over time. A two-sided P value of ≤0.05 was considered significant for all analyses.
Results
Our patient population included 38 patients treated with maraviroc on a prospective clinical trial (Clinicaltrials.gov NCT00948753) and 43 contemporary control patients who underwent HSCT with the same conditioning regimen and similar supportive care medications but without maraviroc. Two patients were excluded from the maraviroc group due to missing data on the serum concentration of tacrolimus during Weeks 1–6. Within this cohort, complete concentration data for tacrolimus were available for 71/79 (90%) patients for all 8 weeks of the analysis. In the remaining eight patients (maraviroc n = 4; control n = 4), there were a total of 14 missing tacrolimus concentration values, which were confined to Weeks 7 and 8 of the study period.
The patients' characteristics are presented in Table 1. The treatment groups were well balanced at baseline. During the period of the analysis, the incidences of GVHD, infectious complications, relapse, organ dysfunction and mortality were similar. Nearly all patients received azole antifungal prophylaxis during the study period. The distribution of azole exposure during each week is described in Table S1 (available as Supplementary data at JAC Online). Voriconazole was administered more frequently in the maraviroc group than the control group (100% versus 83.7%; P = 0.014). Fluconazole use was less common and did not differ between the groups (11.1% versus 20.9%; P = 0.36). Corticosteroid exposure was also similar between the groups (19.4% versus 16.3%; P = 0.71).
Table 1.
Patient characteristics
| Maraviroc (n = 36) | Control (n = 43) | P | |
|---|---|---|---|
| Baseline characteristics | |||
| recipient age (years), median (range) | 62 (21–74) | 62 (27–75) | 0.77 |
| recipient male gender, n (%) | 21 (58) | 22 (51) | 0.52 |
| recipient weight (kg), median (range) | 79.5 (45–139) | 81.0 (53–122) | 0.45 |
| recipient BMI (kg/m2), median (range) | 27.5 (17.5–43.9) | 27.3 (18.6–36.8) | 0.39 |
| Complications by Day 56 | |||
| Grade 2–4 GVHD, n (%) | 3 (8) | 4 (9) | 1 |
| relapse, n (%) | 3 (8) | 4 (9) | 1 |
| infections, n (%) | 0.77 | ||
| none | 20 (56) | 22 (51) | |
| one | 11 (31) | 12 (28) | |
| two | 4 (11) | 3 (7) | |
| three | 1 (3) | 3 (7) | |
| gastrointestinal dysfunctiona, n (%) | 10 (28) | 11 (26) | 0.83 |
| hepatic dysfunctionb, n (%) | 6 (17) | 11 (26) | 0.34 |
| mortality, n (%) | 0 (0) | 1 (2) | 1 |
aDefined as requiring total parenteral nutrition or the occurrence of Grade 2–4 mucositis.
bDefined as direct bilirubin, ALT or AST concentrations >3 times the upper limit of normal.
Our analysis did not demonstrate a significant difference in tacrolimus C/D ratios in patients receiving maraviroc + tacrolimus compared with tacrolimus alone. The mean C/D ratio of tacrolimus increased over time in both patient groups, most of this occurring during Weeks 1–4 (Figure 1). During this period, the C/D ratios for tacrolimus were slightly higher in the maraviroc group compared with the control group. The pattern reversed in Weeks 5–8, with the C/D ratios in the maraviroc group falling below those of the control group. Although this pattern of change suggested an inhibitory effect of maraviroc on tacrolimus metabolism, the magnitude of the differences between the groups was small and not statistically significant [−0.49 (ng/mL)/(mg/day); 95% CI −1.39 to 0.4; P = 0.28], as reflected in the difference-in-differences analysis shown in Table 2. Of note, the value of the difference-in-differences estimate is negative because of the increasing trajectory of the tacrolimus C/D ratio over time. The effect estimate was attenuated after adjusting for the patient's weight, GVHD and antifungal and corticosteroid exposure [−0.35 (ng/mL)/(mg/day); 95% CI −1.19 to 0.64; P = 0.46]. This shows that when adjusting for potentially confounding variables, the C/D ratio of tacrolimus remained similar in the two groups. To evaluate whether the dose of maraviroc influenced the C/D ratio of tacrolimus, we repeated the same analysis including only patients who received maraviroc 300 mg twice daily (n = 31). Consistent with the results observed in the overall study population, we did not observe a significant difference in the C/D ratio of tacrolimus between patients who received maraviroc 300 mg twice daily compared with controls [−0.4 (ng/mL)/(mg/day); 95% CI −1.38 to 0.58; P = 0.42].
Figure 1.
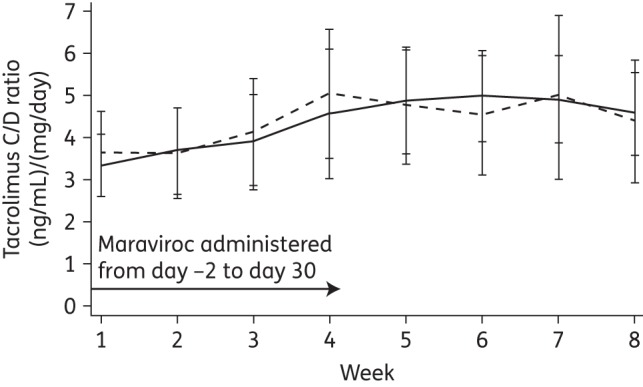
Change in the mean C/D ratio of tacrolimus over time: maraviroc versus control. The C/D ratios of tacrolimus for the maraviroc and control groups are represented by the broken line and the continuous line, respectively.
Table 2.
Difference-in-differences analysis of mean C/D ratio of tacrolimusa
| Group | Week 2 C/Db (95% CI) | Week 6 C/Db (95% CI) | Difference: Week 6 − Week 2b (95% CI) | P |
|---|---|---|---|---|
| Maraviroc | ||||
| unadjusted | 3.87 (3.02–4.72) | 4.59 (3.51–5.67) | 0.72 (0.03–1.40) | 0.04 |
| adjustedc | 2.21 (0.94–3.50) | 2.99 (1.51–4.49) | 0.78 (0.06–1.49) | 0.03 |
| Control | ||||
| unadjusted | 3.65 (2.87–4.43) | 4.87 (3.89–5.86) | 1.21 (0.58–1.84) | <0.001 |
| adjustedc | 2.26 (1.10–3.42) | 3.39 (2.03–4.75) | 1.13 (0.46–1.78) | 0.01 |
| Difference-in-differences | ||||
| unadjusted | −0.49 (−1.39 to 0.40) | 0.28 | ||
| adjusted | −0.35 (−1.19 to 0.64) | 0.46 | ||
aIn the first step of the difference-in-differences calculation, intragroup changes in C/D ratio are estimated: the values in Week 2 are subtracted from those in Week 6. In the second step, the change from baseline is compared in each group: the Week 6 − Week 2 difference in the control group is subtracted from the Week 6 − Week 2 difference in the maraviroc group. The results presented here are those obtained before and after adjusting for confounding variables.
bUnits are expressed as (ng/mL)/(mg/day).
cAdjusted for weight, GVHD, antifungal exposure and corticosteroid exposure.
In addition, our findings did not reveal any differences in the weekly tacrolimus dose requirements over time in the two groups. Consistent with the observed trajectory of an increasing C/D ratio of tacrolimus over time, the dosing requirements decreased significantly over time in both groups, as depicted in Figure S1. When adjusting for the patient's weight, GVHD and antifungal and corticosteroid exposure, the weekly mean tacrolimus dose decreased less during Weeks 5–8 in the maraviroc group compared with the control group, but the magnitude of the difference was small and non-significant (0.12 mg/day; 95% CI −0.29 to 0.55; P = 0.56).
We also compared the weekly mean tacrolimus concentrations in the two groups (Figure S2). The mixed-effects model estimate shows that the mean tacrolimus concentrations were 0.82 ng/mL lower in the maraviroc group throughout the study period (P = 0.03). However, the difference between the groups was constant over time, revealing no variations between the groups after the maraviroc had been discontinued (0.02 ng/mL; 95% CI −0.89 to 0.85; P = 0.97). These results demonstrate that tacrolimus concentrations were not influenced by exposure to maraviroc.
Discussion
In this study, we observed no significant differences in the C/D ratios of tacrolimus in patients receiving maraviroc + tacrolimus compared with tacrolimus alone. Although a trend towards a lower dose–response of tacrolimus was observed upon discontinuation of maraviroc, the change in tacrolimus C/D ratio was small and non-significant relative to the control group. In addition, our analysis did not demonstrate a significant variation in the dose requirements or concentrations of tacrolimus after the cessation of maraviroc. To the best of our knowledge, this is the first study to directly examine whether a pharmacokinetic interaction exists between tacrolimus and maraviroc.
The primary strength of our analysis lies in using data from a prospective clinical trial in a clinically relevant population. Our two study groups were treated on a single transplant protocol, received a uniform set of supportive care measures and were well-balanced at baseline. We included data on important potential confounding variables, including measures of organ function over time and concomitant medications with known interactive potential.2,25 After adjusting for confounding variables, the difference-in-differences estimate was attenuated, further supporting the absence of a clinically important DDI between maraviroc and tacrolimus.
Maraviroc was originally developed as part of combination HAART for patients infected with CCR5-tropic HIV infection.30,31 In addition to regulating the entry process of HIV into the host cells, the results of several pre-clinical studies have demonstrated that CCR5 serves as a critical mediator for lymphocyte recruitment to tissues that are involved in GVHD.32–35 Thus, our group evaluated the protective effect against GVHD of adding maraviroc to tacrolimus and methotrexate in 38 HSCT recipients enrolled on a Phase I/II study. The cumulative incidence of Grade 2–4 acute GVHD was low, at 14.7% on Day 100 and 23.6% on Day 180. Remarkably, no cases of acute gastrointestinal or liver GVHD were observed before Day 100.10 One of the objectives of this post hoc analysis was to evaluate whether the protective effect of maraviroc against GVHD was mediated through a possible increase in tacrolimus concentration. Importantly, the mean tacrolimus concentrations were lower in the maraviroc group relative to the control group during the first 56 days after transplant. These data confirm that the protective effect of maraviroc against GVHD was not mediated through an increase in tacrolimus concentrations.
Our findings have several important implications. Several prospective clinical trials are currently ongoing to confirm the role of maraviroc + tacrolimus for GVHD prophylaxis in HSCT recipients. Sponsored by the Blood and Marrow Transplant Clinical Trials Network, a multicentre clinical trial is comparing the protective effect against GVHD of maraviroc + tacrolimus with several other novel approaches in adult HSCT (Clinicaltrials.gov NCT02208037).36 The University of Pennsylvania and Cincinnati Children's Hospital Medical Center are conducting additional clinical trials to further characterize this combination for GVHD prophylaxis in adult (Clinicaltrials.gov NCT01785810) and paediatric (Clinicaltrials.gov NCT02167451) HSCT, respectively.37,38 In addition, maraviroc may have therapeutic potential in SOT as an absence of CCR5 has been associated with lower rates of graft rejection.39,40 Moreover, maraviroc is also being evaluated for a broad range of clinical indications beyond the field of transplantation, having demonstrated promising pre-clinical or clinical activity in breast cancer, gastric cancer, chronic liver disease and JC virus-associated immune reconstitution inflammatory syndrome.41–46 Given the expanding therapeutic potential of maraviroc, it is imperative to characterize its effect on CYP3A4. The results of our analysis do not reveal evidence of a significant inhibitory effect of maraviroc on tacrolimus metabolism and suggest that these two agents can be administered safely together. These findings are important for the design of future clinical trials that evaluate the administration of maraviroc in combination with tacrolimus or other CYP3A4 substrates.
Funding
This study was partly funded by grants from Pfizer and the Abramson Cancer Center at the University of Pennsylvania. Pfizer provided maraviroc drug supply and partial research support, but played no decision-making role in this study. We gratefully acknowledge the grant support of the National Marrow Donor Program (Amy Strelzer Manasevit Award for the study of post-transplant complications to R. R.), the National Cancer Institute (K23CA178202 to R. R.), the Conquer Cancer Foundation (Career Development Award to R. R.) and the National Institute of General Medical Sciences (T32GM075766 to T. A. M.).
Transparency declarations
None to declare.
Supplementary data
Acknowledgements
We thank Sean Hennessy of the University of Pennsylvania and Manoli Vourvahis at Pfizer for useful discussions.
References
- 1.Srinivas TR, Meier-Kriesche HU, Kaplan B. Pharmacokinetic principles of immunosuppressive drugs. Am J Transplant 2005; 5: 207–17. [DOI] [PubMed] [Google Scholar]
- 2.Glotzbecker B, Duncan C, Alyea E, et al. Important drug interactions in hematopoietic stem cell transplantation: what every physician should know. Biol Blood Marrow Transplant 2012; 18: 989–1006. [DOI] [PubMed] [Google Scholar]
- 3.Haim-Boukobza S, Balabanian K, Teicher E, et al. Blockade of CCR5 to protect the liver graft in HIV/HCV co-infected patients. J Hepatol 2013; 59: 613–5. [DOI] [PubMed] [Google Scholar]
- 4.Choi SW, Reddy P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat Rev Clin Oncol 2014; 11: 536–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abel S, Russell D, Whitlock LA, et al. Effect of maraviroc on the pharmacokinetics of midazolam, lamivudine/zidovudine, and ethinyloestradiol/levonorgestrel in healthy volunteers. Br J Clin Pharmacol 2008; 65: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufty NE, Gilleran G, Hawkins D, et al. Pharmacokinetic interaction of maraviroc and tacrolimus in a patient coinfected with HIV and hepatitis B following hepatic transplant due to hepatocellular carcinoma. J Antimicrob Chemother 2013; 68: 972–4. [DOI] [PubMed] [Google Scholar]
- 7.Fischerder M, Luckow B, Hocher B, et al. CC chemokine receptor 5 and renal-transplant survival. Lancet 2001; 357: 1758–61. [DOI] [PubMed] [Google Scholar]
- 8.Jaksch M, Remberger M, Mattsson J. Increased gene expression of chemokine receptors is correlated with acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2005; 11: 280–7. [DOI] [PubMed] [Google Scholar]
- 9.Cravens PD, Lipsky PE. Dendritic cells, chemokine receptors and autoimmune inflammatory disease. Immunol Cell Biol 2002; 80: 497–505. [DOI] [PubMed] [Google Scholar]
- 10.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med 2012; 367: 135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–8. [PubMed] [Google Scholar]
- 12.Peterson DE, Bensadoun RJ, Roila F. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol 2011; 22: 78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anglicheau D, Flamant M, Schlageter MH, et al. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant 2003; 18: 2409–14. [DOI] [PubMed] [Google Scholar]
- 14.Stratta P, Quaglia M, Cena T, et al. The interactions of age, sex, body mass index, genetics, and steroid weight-based doses on tacrolimus dosing requirement after adult kidney transplantation. Eur J Clin Pharmacol 2012; 68: 671–80. [DOI] [PubMed] [Google Scholar]
- 15.Hashida T, Masuda S, Uemoto S, et al. Pharmacokinetic and prognostic significant of intestinal MDR1 expression in recipients of living-donor liver transplantation. Clin Pharmacol Ther 2001; 69: 308–16. [DOI] [PubMed] [Google Scholar]
- 16.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Casual Inference. Boston, MA: Houghton Mifflin, 2002. [Google Scholar]
- 17.Harris AD, Bradham DD, Baumgarten M, et al. The use and interpretation of quasi-experimental studies in infectious diseases. Clin Infect Dis 2004; 38: 1586–91. [DOI] [PubMed] [Google Scholar]
- 18.Kristensen SR, Meacock R, Turner AJ, et al. Long-term effect of hospital pay for performance on mortality in England. N Engl J Med 2014; 371: 540–8. [DOI] [PubMed] [Google Scholar]
- 19.Patel MS, Volpp KG, Small DS, et al. Association of the 2011 ACGME resident duty hour reforms with mortality and readmissions among hospitalized Medicare patients. JAMA 2014; 312: 2364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu RH, Lee PH, Tsai MK. Clinical influencing factors for daily dose, trough level, and relative clearance of tacrolimus in renal transplant recipients. Transplant Proc 2000; 32: 1689–92. [DOI] [PubMed] [Google Scholar]
- 21.Pou L, Brunet M, Andres I, et al. Influence of posttransplant time on dose and concentration of tacrolimus in liver transplant patients. Transpl Int 1998; 11 Suppl 1: S270–1. [DOI] [PubMed] [Google Scholar]
- 22.Undre NA, Schafer A. Factors affecting the pharmacokinetics of tacrolimus in the first year after renal transplantation. European Tacrolimus Multicentre Renal Study Group. Transplant Proc 1998; 30: 1261–3. [DOI] [PubMed] [Google Scholar]
- 23.Christiaans M, van Duijnhoven E, Beysens T, et al. Effect of breakfast on the oral bioavailability of tacrolimus and changes in pharmacokinetics at different times posttransplant in renal transplant recipients. Transplant Proc 1998; 30: 1271–3. [DOI] [PubMed] [Google Scholar]
- 24.Selzentry (Package Insert). Research Triangle Park, NC: ViiV Healthcare, 2014. [Google Scholar]
- 25.Jacobson P, Ng J, Ratanatharathorn V, et al. Factors affecting the pharmacokinetics of tacrolimus (FK506) in hematopoietic cell transplant (HCT) patients. Bone Marrow Transplant 2001; 28: 753–8. [DOI] [PubMed] [Google Scholar]
- 26.Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics 1982; 38: 963–74. [PubMed] [Google Scholar]
- 27.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd edn. New York: Wiley, 2011. [Google Scholar]
- 28.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989; 129: 125–37. [DOI] [PubMed] [Google Scholar]
- 29.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993; 138: 923–36. [DOI] [PubMed] [Google Scholar]
- 30.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med 2008; 359: 1429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fatkenheuer G, Nelson M, Lazzarin A, et al. Subgroup analyses of maraviroc in previously treated R5 HIV-1 infection. N Engl J Med 2008; 359: 1442–55. [DOI] [PubMed] [Google Scholar]
- 32.Choi SW, Hildebrandt GC, Olkiewicz KM, et al. CCR1/CCL5 (RANTES) receptor-ligand interactions modulate allogeneic T-cell responses and graft-versus-host disease following stem-cell transplantation. Blood 2007; 110: 3447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serody JS, Burkett SE, Panoskaltsis-Mortari A, et al. T-lymphocyte production of macrophage inflammatory protein-1α is critical to the recruitment of CD8+ T cells to the liver, lung, and spleen during graft-versus-host disease. Blood 2000; 96: 2973–80. [PubMed] [Google Scholar]
- 34.Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5+CD8+ T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest 1999; 104: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murai M, Yoneyama H, Ezaki T, et al. Peyer's patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat Immunol 2003; 4: 154–60. [DOI] [PubMed] [Google Scholar]
- 36.Blood and Marrow Transplant Clinical Trials Network. BMT CTN Protocol 1203. https://web.emmes.com/study/bmt2/protocol/1203_protocol/1203_protocol.html. [DOI] [PMC free article] [PubMed]
- 37.University of Pennsylvania. Phase II Maraviroc for GVHD Prevention. Identifier: NCT01785810. http://clinicaltrials.gov/ct2/show/NCT01785810?term=maraviroc+transplant&rank=4.
- 38.Cincinnati Children's Hospital Medical Center. Maraviroc as GVHD Prophylaxis in Transplant Recipients. Identifier: NCT02167451 http://clinicaltrials.gov/ct2/show/NCT02167451?term=maraviroc+transplant&rank=1.
- 39.Heidenhaim C, Puhl G, Moench C, et al. Chemokine receptor 5Δ32 mutation reduces the risk of acute rejection in liver transplantation. Ann Transplant 2009; 14: 36–44. [PubMed] [Google Scholar]
- 40.Gao W, Faia KL, Csizmadia V, et al. Beneficial effects of targeting CCR5 in allograft recipients. Transplantation 2001; 72: 1199–205. [DOI] [PubMed] [Google Scholar]
- 41.Velasco-Velazquez M, Jiao X, De La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res 2012; 72: 3839–50. [DOI] [PubMed] [Google Scholar]
- 42.Lee E, Fertig EJ, Jin K, et al. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun 2014; 5: 4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mencarelli A, Graziosi L, Renga B, et al. CCR5 antagonism by maraviroc reduces the potential for gastric cancer cell dissemination. Transl Oncol 2013; 6: 784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ochoa-Callejero L, Perez-Martinez L, Rubio-Mediavilla S, et al. Maraviroc, a CCR5 antagonist, prevents development of hepatocellular carcinoma in a mouse model. PLoS One 2013; 8: e53992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Martinez L, Perez-Matute P, Aguilera-Lizarraga J, et al. Maraviroc, a CCR5 antagonist, ameliorates the development of hepatic steatosis in a mouse model of non-alcoholic fatty liver disease (NAFLD). J Antimicrob Chemother 2014; 69: 1903–10. [DOI] [PubMed] [Google Scholar]
- 46.Giacomini PS, Rozenberg A, Metz I, et al. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med 2014; 30: 486–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


