Abstract
An appealing therapeutic target for AML is constitutively-activated, mutant FLT3, which is expressed in a subpopulation of AML patients and is generally a poor prognostic indicator in patients under the age of 65. There are currently several FLT3 inhibitors that are undergoing clinical investigation. However, the discovery of drug-resistant leukemic blast cells in FLT3 inhibitor-treated AML patients has prompted the search for novel, structurally diverse FLT3 inhibitors that could be alternatively used to circumvent drug resistance. Here, we provide an overview of FLT3 inhibitors under preclinical and clinical investigation, and we discuss mechanisms whereby AML cells develop resistance to FLT3 inhibitors, and the ways in which combination therapy could potentially be utilized to override drug resistance. We discuss how the cross-talk between major downstream signaling pathways, such as PI3K/PTEN/Akt/mTOR, RAS/Raf/MEK/ERK, and Jak/STAT, can be exploited for therapeutic purposes by targeting key signaling molecules with selective inhibitors, such as mTOR inhibitors, HSP90 inhibitors, or farnesyltransferase inhibitors, and identifying those agents with the ability to positively combine with inhibitors of FLT3, such as PKC412 and sunitinib. With the widespread onset of drug resistance associated with tyrosine kinase inhibitors, due to mechanisms involving development of point mutations or gene amplification of target proteins, the use of a multi-targeted therapeutic approach is of potential clinical benefit.
Keywords: stroma–related resistance, combination therapy, PKC412, sunitinib
1. Introduction
There are around 10,000 newly diagnosed acute myelocytic leukemia (AML) patients in the U.S. each year. This hematopoietic malignancy is characterized by aberrant proliferation of myeloid progenitor cells, coupled by a partial block in cellular differentiation (McKenzie et al., 2005). Permeation of bone marrow and peripheral blood with immature leukemic myeloblasts is the outcome of the abnormal survival advantage of leukemic cells, and causes such symptoms as bleeding, anemia, and infection.
Current therapies for AML often do not succeed because of therapy-induced mortality or drug resistance (Estey, 2001). The use of conventional chemotherapeutic agents as a single treatment approach is coupled to a low therapy-induced mortality, however a high risk of relapse due to drug resistance (Mathews and DiPersio, 2004). In contrast, allogeneic transplantation (alloBMT) has a high therapy-induced mortality, and yet a lower risk of relapse (Mathews and DiPersio, 2004). Due to the fact that alloBMT shows more promise in younger patients, it has an overall small impact on the majority of AML patients, who tend to be aged 65 and older (Witherspoon and Deeg, 1999).
In AML, the activation of signaling pathways results from a range of genetic modifications leading to mutation of signaling molecules, such as receptor tyrosine kinases. Approximately 30% of AML patients, as well as a portion of ALL patients, harbor a mutant form of the class III receptor tyrosine kinase, FLT3 (Fms-Like Tyrosine kinase-3; STK-1, human Stem Cell Tyrosine Kinase-1; or FLK-2, Fetal Liver Kinase-2) (Stirewalt and Radich, 2003). Internal tandem duplications (ITD) within the juxtamembrane domain represent the most common form of constitutively activated FLT3, occurring in approximately 20-25% of AML patients and in less than 5% of myelodysplastic syndrome (MDS) patients (Nakao et al., 1996; Horiike et al., 1997; Kiyoi et al., 1998; Kondo et al., 1999; Rombouts et al., 2000; Kelly et al., 2002). Indeed, a rapidly lethal myeloproliferative disorder in mice results from the in vivo transplantation of murine bone marrow cells infected with a FLT3-ITD-expressing retrovirus (Kelly et al., 2002).
Also identified in AML patients are point mutations within the “activation loop” of FLT3 (Yamamoto et al., 2001). For example, a missense mutation in the aspartic acid residue at position 835 is believed to induce the activation loop into an “activated” configuration.. Additional, albeit less prevalent, mutation in the kinase domain include N841I (Jiang et al., 2004) and Y842C (Kindler et al., 2005).
Generally, the existence of a FLT3 mutation translates into a poorer prognosis in both disease-free survival and overall survival (Mattison et al., 2007). In fact, patients harboring both a nucleophosmin 1 (NMP1) mutation, which is typically a positive prognostic indicator, and mutant FLT3 tend to have poorer outcomes (Mattison et al., 2007).
There are several inhibitors of FLT3 currently in clinical trials, and a number of novel inhibitors under preclinical investigation. However, the FLT3 inhibitors tested thus far clinically generally induce only partial and transient responses in patients when used as single agents. This suggests a need for the development of novel agents conferring higher potency and/or less toxicity that can either be used effectively as single agents or that can be effectively combined with other agents to suppress disease progression and prolong the lifespan of patients.
In addition to identifying and developing potent FLT3 inhibitors representative of novel and unique structural classes, there is a push toward gaining a better understanding of the mechanisms underlying drug resistance in AML. Clinical trial data with tyrosine kinase inhibitors show that while the peripheral blood of patients responds well, bone marrow responds less well. Stromal cells have been implicated in this mode of resistance, as they provide viability signals to leukemic cells that protect them from the effects of the inhibitor. Other mechanisms of drug resistance include the emergence of point mutations in the target protein, and deregulation of signaling molecules associated with apoptotic signaling leading to a survival advantage in leukemic cells.
There are several strategies that may be effective in preventing relapse due to the emergence of point mutations in target proteins, as well as in overcoming drug resistance believed to be caused by stromal-mediated viability signals or deregulation of apoptotic signaling. These include the combined use of more than one FLT3 inhibitor, providing their interaction with FLT3 signaling or the FLT3 protein target is distinct enough for the two inhibitors to synergize. Alternatively, FLT3 inhibitors can be combined with small molecule inhibitors that interact with key components of major signaling pathways that play a significant role in AML. Finally, FLT3 inhibitors can be combined with standard chemotherapy as an approach to achieve maximum efficacy in patients.
2. Classes of FLT3 inhibitors
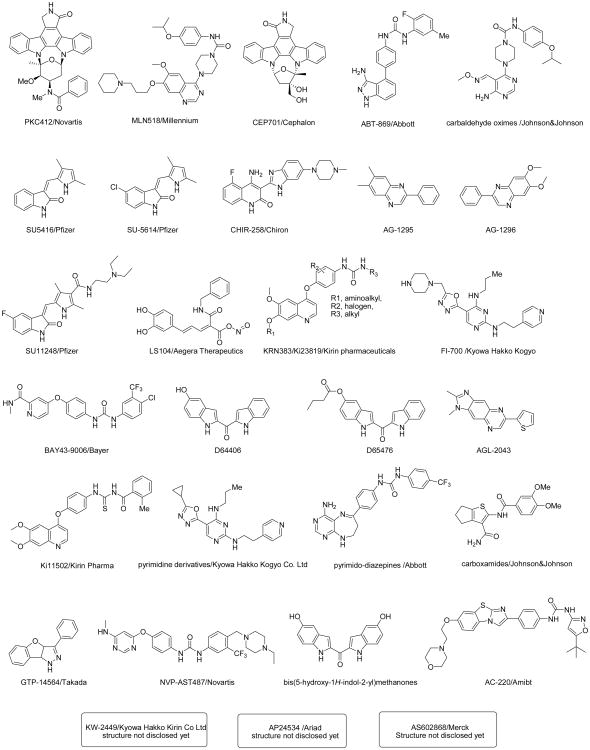
The structural classes of prominent FLT3 inhibitors in clinical trials or under preclinical investigation are shown in Figure 1. The N-indolocarbazole PKC412 (midostaurin; N-benzoyl-staurosporine; Novartis Pharma AG) is one of several FLT3 inhibitors that is undergoing clinical testing, and which is currently in late-stage clinical trials. PKC412 is a broad spectrum, orally bioavailable inhibitor of FLT3, as well as platelet-derived growth factor β (PDGFRβ), c-KIT, and c-FMS (Weisberg et al., 2002). In preclinical studies, PKC412 caused cell cycle arrest and induced apoptosis in mutant FLT3-positive cells by directly inhibiting the activity of the FLT3 kinase, with an IC50 of approximately 0.01 μM in Ba/F3-FLT3-ITD cells, and inhibits FLT3-ITD phosphorylation with an IC50 of 30 nM (Weisberg et al., 2002). PKC412 induces apoptosis in mutant FLT3-expressing cells with no significant effect on cell cycle progression, however causes G2 arrest without apoptosis in wild-type FLT3-expressing cells (Odgerel et al., 2008).
Figure 1. Chemical structures of FLT3 inhibitors (Schenone et al., 2008).
In a Phase Ib clinical trial, newly diagnosed AML patients were treated with 50 mg po bid PKC412 in simultaneous and sequential combinations with daunorubicin and cytarabine induction and high-dose cytarabine consolidation. Clinical responses were complete (CR) in 100% of mutant FLT3-positive patients, with transient and/or reversible side effects observed (Stone et al., 2005a). In a Phase II clinical trial, PKC412 was generally well-tolerated, with a decrease in peripheral blast counts observed in roughly a third of PKC412-treated relapsed/refractory AML patients, and a median response duration of 13 weeks (Stone et al., 2004; Stone et al., 2005b). The hematological response rate in advanced AML patients treated with PKC412 was similar to that of CML blast crisis patients receiving imatinib. PKC412 is currently in Phase III clinical trials.
The related, orally-administered indolocarbazole alkaloid CEP-701 (lestaurtinib; Cephalon), is an inhibitor of FLT3-ITD (Levis et al., 2002). CEP-701 has an IC50 of 5 nM against Ba/F3-FLT3-ITD cells, and inhibits FLT3-ITD phosphorylation with an IC50 of 3 nM (Levis et al., 2002). In addition to FLT3, protein targets for CEP-701 include TrkA and (vascular endothelial growth factor receptor) VEGFR (Levis et al., 2002). In early phase clinical trials, relapsed or refractory AML patients showed clinical responses of short duration to CEP-701 (Smith et al., 2003; Smith et al, 2004). A portion of patients showed complete inhibition of FLT3 autophosphorylation with no concomitant clinical response, while several others showed a decline in peripheral blood leukemic blasts to <5%. In a phase II trial for older patients with AML not considered fit for intensive chemotherapy, transient clinical responses were observed (Knapper et al., 2006). A Phase III trial has recently been completed for CEP-701, tested in relapsed AML patients harboring mutant FLT3 (this originally initiated as a Phase II trial). In addition, there is an ongoing phase III clinical trial investigating the effects of chemotherapy plus CEP-701 in the U.K. that is enrolling both mutant FLT3- and wt FLT3-harboring patients. CEP-701 has also been tested in Phase II clinical trials for prostate cancer, and is in Phase I clinical trials for high risk neuroblastoma.
Another class of FLT3 inhibitors includes the 3-substituted indolinones SU5416 and SU5614. SU5416 (semaxanib; SuGen) and SU5614 are both characterized inhibitors of FLT3 that also target c-KIT and VEGFR (Yee et al., 2002). SU5416 inhibits FLT3-ITD autophosphorylation with an IC50 of 100 nM and inhibits FLT3-ITD-positive cell proliferation with an IC50 of 250 nM. SU5614 inhibits FLT3-ITD autophosphorylation with an IC50 of 10 nM and inhibits FLT3-ITD-positive cell proliferation with an IC50 of 100 nM (Yee et al., 2002).
A multicenter Phase II clinical trial demonstrated SU5416 to inhibit FLT3 phosphorylation in refractory AML patients; incomplete clinical responses were observed in a portion of patients lasting from 1-5 months (Fiedler et al., 2003). SU5416 was investigated in another multicenter phase II study, and showed modest clinical activity as a single agent in refractory AML patients; overall median survival was 12 weeks, with grade 3 or 4 drug-related toxicities possibly attributable to drug formulation (Giles et al., 2003). SU5416 was shown in a U.S. phase II hematological malignancy trial to inhibit FLT3 phosphorylation in refractory AML patients; the majority of treated patients, however, failed to achieve a clinical response (O'Farrell et al., 2004).
The orally-administered indolinone derivative SU11248 (SU011248, sunitinib and Sutent; Pfizer) is equipotent against both FLT3-ITD and D835Y (Kancha et al., 2007). The growth of MV4-11 cells is inhibited by SU11248 with an IC50 of 10 nM (O'Farrell et al., 2003a). In addition to FLT3, SU11248 targets VEGFR2, PDGFRβ, and c-KIT. SU11248 was investigated against mutant FLT3-positive AML in Phase I clinical trials, with morphologic or partial responses of short duration observed (O'Farrell et al., 2003b; Fiedler et al., 2005). It is currently used to treat advanced kidney cancer (Vroling et al., 2009) and imatinib-resistant gastrointestinal stromal tumors (GIST) (Heinrich et al., 2008; Gajiwala et al., 2009).
Also tested in early clinical trials involving relapsed or refractory AML patients is the piperazinyl quinazoline MLN518 (tandutinib; CT53518; Millennium), a FLT3 inhibitor that also targets KIT and PDGFR, and which inhibits FLT3-ITD autophosphorylation with an IC50 of around 200 nM (Kelly et al., 2002). Phase I trials were carried out to test the safety and tolerance of oral doses of MLN518 in AML patients; MLN518 was also tested in combination with standard induction chemotherapy for treatment of patients with newly diagnosed AML. MLN-518 showed limited activity as a single agent against AML and myelodysplastic syndrome, however caused 90% complete remissions in patients with newly diagnosed AML when given in combination with cytarabine and daunorubicin (Cheng and Paz, 2008). A Phase II Study of MLN518 in patients with newly diagnosed AML who are considered ineligible for or who decline treatment with standard induction therapy was withdrawn prior to recruitment. MLN518 is in ongoing Phase II clinical trials for progressive prostate and bone metastases and glioblastoma. A Phase II study of MLN518 has been completed in patients with metastatic clear cell renal cell carcinoma, and a Phase I/II trial is recruiting for the testing of MLN518 against recurrent or progressive glioblastoma. A Phase II trial is recruiting for the testing of MLN518 in combination with bevacizumab against recurrent high-grade gliomas.
KW-2449, whose chemical structure is not yet disclosed, induces cell death in FLT3-ITD-positive cells and inhibits FLT3-ITD phosphorylation with an IC50 of 144 nM (Shiotsu et al., 2007; Shiotsu et al., 2008; Pratz et al., 2008a). Targets in addition to FLT3 include c-KIT and Aurora (Pratz et al., 2008a). KW-2449 was tested in a Phase I clinical trial, in which transient decreases in peripheral blast counts were observed (Cortes et al., 2008; Pratz et al., 2008a). KW-2449 is in Phase II clinical trials for FLT3-positive AML.
The N-(4-(3-Amino-1H-indazol-4-yl)phenyl)-N1-(2-fluoro-5-methylphenyl)urea ABT-869 inhibited mutant FLT3-positive MV-4-11 and MOLM-13 with an IC50 of around 4-6 nM and demonstrated anti-leukemia activity in vivo (Albert et al., 2006; Shankar et al., 2007). ABT-869 has also demonstrated in vivo activity against AML harboring wild-type FLT3 (Zhou et al., 2008a). Targets of ABT-869, in addition to FLT3, include PDGFR, KIT, and KDR (Shankar et al., 2007). ABT-869 is a multi-targeted inhibitor and is currently in Phase II clinical trials for metastatic breast cancer, advanced hepatocellular carcinoma, advanced colorectal cancer, and advanced renal cell carcinoma.
The benzimidalzole-quinoline CHIR-258 (TKI258; Chiron) inhibits FLT3-ITD phosphorylation with an IC50 of 1 nM and kills MV4-11 cells with an IC50 of 13 nM (Lopes de Menezes et al., 2005). Targets, in addition to FLT3, include KIT, FMS, VEGFR, and FGFR (Lopes de Menezes et al., 2005). The agent caused tumor regressions and killing of AML cells in bone marrow in subcutaneous and bone marrow engraftment leukemic xenograft models (Lopes de Menezes et al., 2005). CHIR-258, which shows promise as an anti-multiple myeloma agent (Trudel et al., 2005), has been enrolled in Phase I clinical trials including those for multiple myeloma, mixed solid tumors, and AML.
The biaryl urea compound sorafenib (BAY 43-9006, Nexavar; Bayer), which was initially developed as a RAF inhibitor and shows activity against VEGFR-2, VEGFR-3, PDGFRβ, and KIT, was also recently shown to have activity against FLT3-ITD and D835G (Zhang et al., 2008; Lierman et al., 2007; Auclair et al., 2007). Sorafenib inhibits FLT3-ITD more potently than D835Y (Kancha et al., 2007); it inhibits FLT3-ITD phosphorylation with an IC50 of 2.8 nM and inhibits growth of MV4-11 cells with an IC50 of 0.88 nM (Auclair et al., 2007). Sorafenib was tested in a Phase I clinical trial for patients with refractory or relapsed AML and reduced the percentage of leukemia blasts in the bone marrow and peripheral blood of FLT3-ITD-positive AML patients (Zhang et al., 2008). Sorafenib has been FDA-approved for the treatment of advanced renal cell carcinoma and unresectable hepatocellular carcinoma; it is currently in clinical trials for imatinib- and sunitinib-resistant GIST.
The hydroxystyryl-acrylonitrile LS104 inhibits FLT3-ITD activity and is cytotoxic against mutant FLT3-expressing cells (Kasper et al., 2008). Recently, a Phase I clinical trial enrolling patients with refractory/relapsed hematologic malignancies commenced for LS104.
AP24534 (Ariad) is a multi-targeted kinase inhibitor that inhibits the proliferation of mutant FLT3-positive cells with an IC50 of 13 nM, and that inhibits mutant FLT3 phosphorylation with an IC50 of 1 nM (Rivera et al., 2008). Other targets of AP24534 include c-KIT and FGFR (Rivera et al., 2008). AP24534 is in Phase I clinical trials for CML and other hematologic malignancies.
Reports of other FLT3 inhibitors in preclinical development include the quinoxaline AG1295, which was specifically cytotoxic to FLT3-ITD-positive AML blasts (Levis et al., 2001); the quinoxaline AG1296, selectively kills mutant FLT3-positive cell lines and primary AML cells, and inhibits FLT3-ITD autophosphorylation with an IC50 of approximately 1 μM (Tse et al., 2001; Tse et al., 2002); the (5-hydroxy-1H-2-indolyl)(1H-2-indolyl)-methanone D64406 and the 5-butanoate-1H-2-indolyl)(1H-2-indolyl)-methanone D-65476, which displays an IC50 of around 0.2-0.3 μM against TEL-FLT3-transfected Ba/F3 cells (Teller et al., 2002); the tricyclic quinoxaline AGL2043 (Gazit et al., 2003); the 1-phenyl-3-H-8-oxa-2,3-diaza-cyclopenta[a]inden GTP-14564, which inhibits FLT3-ITD-expressing Ba/F3 cells at a concentration of 1 μM (Murata et al., 2003); the quinoline urea Ki23819, which has been shown to be effective against FLT3-ITD-expressing human cell lines (Komeno et al., 2005); KRN383 , which inhibits FLT3-ITD autophosphorylation with an IC50 of less than 5.9 nM, D835Y autophosphorylation with an IC50 of 43 nM, and proliferation of FLT3-ITD-positive cells with an IC50 of less than 2.9 nM and shows in vivo activity against FLT3-ITD-positive leukemia (Nishiyama et al., 2006); the 2,4,5-trisubstituted pyrimidine, FI-700, which inhibits FLT3 kinase activity with an IC50 of 20 nM, inhibits the growth of MV4-11 cells with an IC50 of 14 nM, and displays in vivo anti-leukemia activity (Kiyoi et al., 2007); the quinoline Ki11502, which inhibits the proliferation of mutant FLT3-positive MV4-11 and MOLM13 with an IC50 of 0.5-0.6 μM and an IC50 of 37.54 nM against FLT3 kinase (Nishioka et al., 2008); 5-(1,3,4-oxadiazol-2-yl)pyrimidine derivatives, which show efficacy when administered orally in a MOLM-13 xenograft model (Ishida et al., 2008); the N,N′-diphenyl urea NVP-AST487, which selectively targets mutant FLT3 kinase activity, is able to override PKC412 resistance in vitro and synergizes with chemotherapeutic agents against mutant FLT3-positive cells, and inhibits the growth of FLT3-ITD-expressing cells in vivo (Weisberg et al., 2008b); the bis(1H-indol-2-yl)methanone compound102, which overrides resistance to PKC412, including PKC412 resistance due to mutated residue N676 in FLT3, and which synergizes with chemotherapeutic agents (Mahboobi et al., 2006; Heidel et al., 2009). Other structural classes of FLT3 inhibitors include pyrimido-diazepines (Gracias et al., 2008), 4-amino-6-piperazin-1-yl-pyrimidine-5-carbaldehyde oximes (Gaul et al., 2007), and the 2-acylaminothiophene-3-carboxamides (Patch et al., 2006).
2. Clinical resistance to FLT3 inhibition
Drug resistance occurs in approximately 30% of FLT3-ITD-positive AML patients. While small molecule inhibitors of FLT3 are showing promise clinically for AML, thus far none has elicited sustained cytogenic responses as a single agent. For instance, quantitative measurement of FLT3 inhibition in patients treated with KW-2449 in a phase I trial showed that inhibition of FLT3 occurred transiently to less than 20% of baseline (Pratz et al., 2008a). It is possible that such incomplete and only temporary inhibition of FLT3 can be generalized to other FLT3 inhibitors under investigation and may be a primary reason for their limited clinical effectiveness (Chu and Small 2009).
Possible mechanisms of drug resistance include:
Acquired point mutations in the molecular targets (Shah et al., 2002; Cools et al., 2003). For example, resistance to PKC412 in patients has been attributed to pre-existing or acquired mutations in the kinase domain of FLT3 (Heidel et al., 2006).
Other mechanisms include up-regulation of the anti-apoptotic protein, MCL-1, which is induced by a non-juxtamembrane ITD that has integrated into the beta-2 sheet of the first kinase domain (FLT3_ITD627E) (Brietenbuecher et al., 2009).
Similarly, over-expression of survivin and enhanced activation of STAT signaling pathways has been found to mediate resistance to the FLT3 inhibition (Zhou et al., 2009), as has over-expression of anti-apoptotic proteins of the BCL2 family, which can be overcome by the novel BH3 mimetic, ABT-737 (Kohl et al., 2007).
The potential importance of apoptosis-related signaling molecules in relation to FLT3 signaling is exemplified as well by the finding that constitutive activation of FLT3 is responsible for IKK activation, and both kinases are believed to act in the same anti-apoptotic pathway (Grosjean-Raillard et al., 2008). AS602868, which is an inhibitor of both IkappaB kinase-2 (IKK2) and FLT3, kills mutant FLT3-positive cells (Griessinger et al., 2007).
There is considerable interest in investigating the contribution of the leukemia microenvironment to drug resistance of leukemic stem cells. Clinical studies of patients with advanced AML receiving FLT3 kinase inhibitors revealed a delayed or marginal decrease in bone marrow blasts, in contrast to a significant decrease in peripheral blasts. The survival of CD34+CD38-CD123+ leukaemic stem and progenitor cells was actually enhanced, as opposed to inhibited, by FLT3 inhibition in a defined “niche-like” in vitro microenvironment (Mony et al., 2008). Other studies have shown small-molecule CXCR4 inhibitors to be effective in enhancing chemotherapy- and FLT3 inhibitor-induced apoptosis of bone marrow stroma-protected AML cells in vitro and in vivo, implicating a causal relationship between chemokine receptor CXCR4 and stroma-derived factor 1alpha (SDF-1α) interaction and drug resistant leukemia (Zeng et al., 2006; Nervi et al., 2008; Zeng et al., 2008).
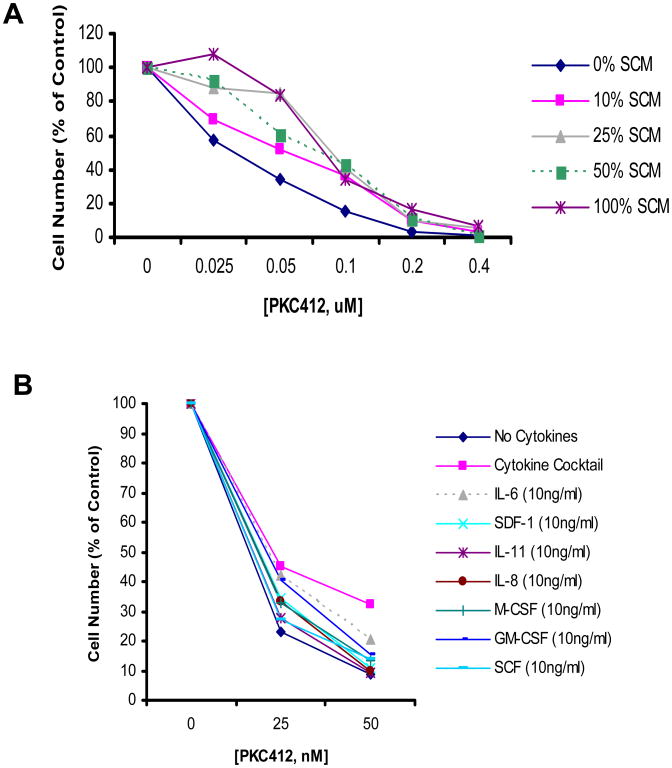
Specific stromal-secreted cytokines have been implicated in the protection of CML cells from cytotoxic, targeted agents (Weisberg et al., 2008a). We have found that the same panel of cytokines secreted in high concentration by the HS-5 human stromal cells are able to mimic the cytoprotective effect of stromal-conditioned media and can protect FLT3-ITD-positive cells from the inhibitory effects of PKC412 (Weisberg et al., unpublished data, Figure 2).
Figure 2. Stromal-mediated cytoprotection of FLT3-ITD-expressing cells.
(A) Approximately 2-day (55 hr) treatment of human MOLM14 cells (express FLT3-ITD) with PKC412 in the presence and absence of different concentrations of HS-5 stromal conditioned media (media conditioned by stroma cells for 7 days). Cell viability assessed by trypan blue exclusion and drug-treated groups presented as percent of each individual control (0% SCM, 10% SCM, 25% SCM, 50% SCM, 100% SCM). (B) Three-day treatment of MOLM14 cells with PKC412 in the presence of cytokine cocktail or individual cytokines.
These findings point toward the existence of a protective environment, or stromal-mediated chemoresistance. Such resistance could potentially be overcome by treatment with other agents, for example those interfering with apoptotic signaling (Weisberg et al., 2007). In addition, it may be possible to use the level of minimal residual disease as a short-term end point that could help assess the efficacy of targeted therapies like FLT3 inhibitors (Hess et al., 2009).
3. Combination therapy
The FLT3 inhibitors tested thus far generally induce only partial and transient responses in patients when administered as single agents. Thus, there is a need for the discovery and development of novel, more efficacious and less toxic inhibitors of FLT3 that could potentially be used effectively as single agents. There is also a need to test these, as well as FLT3 inhibitors under investigation, in combination with other therapeutics already in clinical use for leukemia.
Detection of drug-resistant leukemic blast cells in AML patients undergoing PKC412 therapy has led to such a search for novel, structurally diverse inhibitors of FLT3 that, if used in combination with conventional anti-leukemic agents, could potentially be successful in preventing the development of drug resistance. For example, SU11248 exhibits additive-to-synergistic inhibitory effects on FLT3-ITD-expressing cells when combined with cytarabine or daunorubicin (Yee et al., 2004). PKC412 synergizes with cytarabine, doxorubicin, idarubicin, mitoxantrone, etoposide, 4-hydroperoxy-cyclophosphamide and vincristine against mutant FLT3-positive cells, yet is antagonistic with cytarabine, doxorubicin, idarubicin, mitoxantrone, and etoposide against FLT3 mutant-negative leukemias (Mollgard et al., 2008; Furukawa et al., 2007). ABT-869 synergizes with cytarabine and doxorubicin against mutant FLT3-expressing cells (Zhou et al., 2008b).
3.1. Combination of FLT3 inhibitors with chemotherapy
The pharmacodynamic interrelationship between FLT3 inhibitors and chemotherapy in general seems to be sequence-dependent: the use of a FLT3 inhibitor prior to chemotherapy often results in antagonism, whereas the use of a FLT3 inhibitor following chemotherapy often translates into synergy (Pratz and Levis, 2008b). For example, CEP-701 is synergistic with cytarabine (Ara-C), daunorubicin, mitoxantrone, and etoposide, respectively, when administered simultaneously or immediately following their administration (Levis et al., 2004; Knapper et al., 2006; Mead et al., 2008). The simultaneous combination of NVP-AST487 with Ara-C, doxorubicin, or PKC412 results in additive to synergistic effects (Weisberg et al., 2008b). The simultaneous administration of NVP-AST487 with Ara-C or doxorubicin leads to the strongest positive combination effect, which is similar to and comparable with sequential administration of Ara-C or doxorubicin 24 hr prior to administration of NVP-AST487 (Weisberg et al., 2008b). However, the administration of NVP-AST487 24 hr prior to either Ara-C or doxorubicin results in a slightly weaker combination effect as compared to the other regimens (Weisberg et al., 2008b). These findings suggest that FLT3 inhibitors could potentially be used in combination with standard chemotherapeutic agents currently in use for AML, and that the addition of inhibitors of FLT3 to AML chemotherapy regimens could potentially lead to improved clinical responses.
3.2. Combination of FLT3 inhibitors with other signaling inhibitors
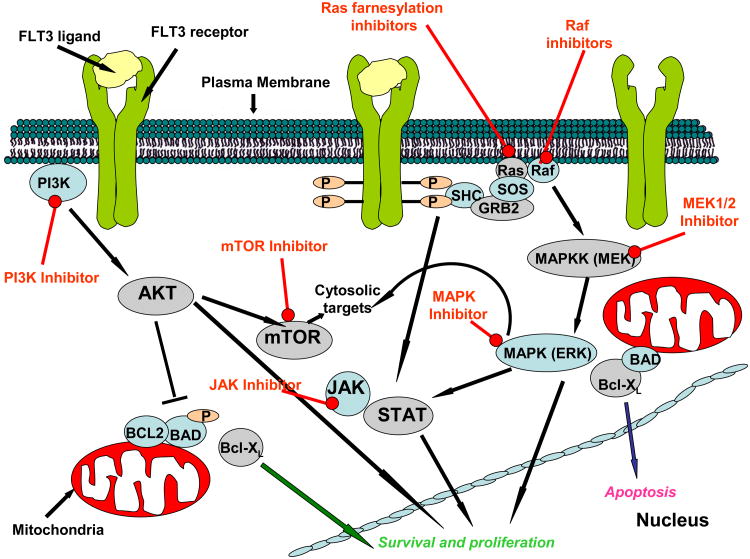
Activating mutations in receptor tyrosine kinases like FLT3 represent obvious and key therapeutic targets when considering treatment strategies for AML. However, another salient-and related- feature of the malignancy that confers survival advantages is the common deregulation of one or more of the three signaling pathways: PI3K//PTEN/Akt/mTOR, RAS/Raf/MEK/ERK, and Jak/STAT, each frequently activated by mutations in upstream genes (Kornblau et al., 2006). The redundancy and simultaneous/cross-activation between the three pathways warrants consideration of the use of a multi-targeted therapeutic strategy, or the use of more than one type of signaling inhibitor (Figure 3).
Figure 3. Inhibition of signaling components of the PI3K/PTEN/Akt/mTOR, RAS/Raf/MEK/ERK, and Jak/STAT pathways.
AKT, the downstream effector of PI3K, is activated through phosphorylation in the majority of cases of AML (Xu et al., 2003; Min et al., 2004; Tazarri et al., 2004; Grandage et al., 2005). Since FLT3-ITD mutations have been associated with AKT activation (Brandts et al., 2005), it has been suggested that the PI3K/AKT signaling pathway represents a critical, and shared, downstream target of these oncogenes. Wild-type FLT3, in response to ligand, activates pathways typical for type III tyrosine kinase receptors, including the PI3K//PTEN/Akt/mTOR, RAS/Raf/MEK/ERK and Jak/STAT pathways (LoPiccolo et al., 2008). Of these pathways, STAT5 is activated by signaling from FLT3-ITD (Y589 and Y591) and this is required for transformation in vivo (Rocnik et al., 2006). Notably, the activity of PKC412 against mutant FLT3-expressing cells is enhanced the novel dual PDK-1/PI3K inhibitor, BAG956, (Novartis Pharma AG) (Weisberg et al., 2008c).
Another important downstream effector of the PI3K/Akt signaling pathway, which mediates the effects of FLT3, is the highly conserved mammalian target of rapamycin (mTOR) (Giles and Albitar, 2005). Inhibition of mTOR with rapamycin inhibits proliferation of cells from patients with AML and FLT3 mutations (Recher et al., 2005). Of relevance, the combination of PKC412 and rapamycin is synergistic against cells expressing both PKC412-sensitive and PKC412-resistant mutant FLT3 (Mohi et al., 2004). In addition, the rapamycin derivative, RAD001, enhances the anti-leukemic activity of sunitinib (Ikezoe et al., 2006).
Inhibition of heat shock protein 90 (Hsp90), which chaperones mutant FLT3 but not wild-type FLT3, leads to disruption of the JAK/STAT, RAS/Raf/MEK/ERK, and PI3K/Akt signaling pathways, is effective in killing primary, mutant FLT3-positive AML cells (Shaer et al., 2008). Another novel agent, histone deacetylase inhibitor MS-275, inhibits the growth of mutant FLT3-positive cells with an IC50 of below 1 μM; this agent induces acetylation of Hsp90 in conjunction with ubiquination of FLT3, which leads to FLT3 degradation and disruption of signaling pathways mediated by ERK, Akt, and STAT5 (Nishioka et al., 2008a).
Clinical benefit could potentially arise from simultaneous inhibition of both FLT3 kinase activation and RAS/Raf/MEK/ERK signaling. For instance, inhibition of MEK1/2 kinases by the agent, AZD6244 (ARRY-142886), inhibits the proliferation of mutant FLT3-expressing cells and synergizes with the FLT3 kinase inhibitor, sunitinib (Nishioka et al., 2008b). In similar fashion, lonafarnib, a farnesyl-transferase inhibitor (FTI) positively combines with PKC412 against mutant FLT3-positive cells (Mollgard et al., 2008).
4. Conclusion
There is an urgent need for development of new treatment strategies that could lead to improved therapeutic efficacy in AML patients. Existing therapeutic approaches include the discovery and development of novel agents with unique structures conferring higher potency and selectivity toward FLT3 as a target. Such characteristics may allow for more complete inhibition of the FLT3 kinase protein target as compared to that of existing therapies in preclinical and clinical development. Elucidation of novel mechanisms of resistance that are associated with enhancement of leukemic cell survival, such as stromal-mediated chemoresistance and upregulation of anti-apoptotic signaling molecules, will warrant the testing and potential use of pro-apoptotic agents alone or in combination with FLT3 inhibitors. As a way to potentially suppress the emergence of point mutations in FLT3 conferring drug resistance, two different FLT3 inhibitors could be used together if the mechanism whereby cells develop resistance to each is different. Alternatively, FLT3 inhibitors can be tested for their ability to synergize with standard chemotherapeutic agents or inhibitors of the unifying MAP/MEK/ERK or PI3K/Akt signaling pathways in an attempt to optimize clinical responsiveness. All approaches represent steps toward potentially overcoming some of the existing challenges and obstacles in the therapy of AML, and continued research and progress in these areas should guide clinicians toward more effective treatment of their patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert DH, Tapang P, Magoc TJ, Pease LJ, Reuter DR, Wei RQ, Li J, Guo J, et al. Preclinical activity of ABT-869: a multitargeted receptor tyrosine kinase inhibitor. Mol Cancer Ther. 2006;5:995–1006. doi: 10.1158/1535-7163.MCT-05-0410. [DOI] [PubMed] [Google Scholar]
- Al Shaer L, Walsby E, Gilkes A, Tonks A, Walsh V, Mills K, Burnett A, Rowntree C. Heat shock protein 90 inhibition is cytotoxic to primary AML cells expressing mutant FLT3 and results in altered downstream signaling. Br J Haematol. 2008;141:483–493. doi: 10.1111/j.1365-2141.2008.07053.x. [DOI] [PubMed] [Google Scholar]
- Armstrong SA, Kung AL, Mabon ME, Silverman LB, Stam RW, DenBoer ML, Pieters R, Kersey JH, Sallan SE, Fletcher JA, Golub TR, Griffin JD, Korsmeyer SJ. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173–183. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, Zhang X, Wilkie D, Burd A, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. 2007;21:439–445. doi: 10.1038/sj.leu.2404508. [DOI] [PubMed] [Google Scholar]
- Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J, Buerger H, Muller-Tidow C, Choudhary C, McMahon M, Berdel WE, Serve H. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–9650. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- Breitenbuecher F, Markova B, Kasper S, Carius B, Stauder T, Bohmer FD, Masson K, Ronnstrand L, Huber C, Kindler T, Fischer T. Blood. 2009. A novel molecular mechanism of primary resistance to FLT3-kinase inhibitors in acute myeloid leukemia. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Paz K. Tandutinib, an oral, small-molecule inhibitor of FLT3 for the treatment of AML and other cancer indications. IDrugs. 2008;11:46–56. [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Chu SH, Small D. Mechanisms of resistance to FLT3 inhibitors. Drug Resist Updates. 2009;12:8–16. doi: 10.1016/j.drup.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, Kutok J, Clark J, Galinsky I, Griffin JD, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. New Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- Cools J, Mentens N, Furet P, Fabbro D, Clark JJ, Griffin JD, Marynen P, Gilliland DG. Prediction of resistance to small molecule FLT3 inhibitors: Implications for molecularly targeted therapy of acute leukemia. Cancer Res. 2004;64:6385–6389. doi: 10.1158/0008-5472.CAN-04-2148. [DOI] [PubMed] [Google Scholar]
- Cortes J, Roboz GJ, Kantarjian HM, Feldman EJ, Karp JE, Pratz KW, Rao NS, Akinaga S, Levis MJ. A phase I dose escalation study of KW-2449, an oral multi-kinase inhibitor against FLT3, Abl, FGFR1 and Aurora in patients with relapsed/refractory AML, ALL and MDS or resistant/intolerant CML. Blood. 2008 #2967 (Abstract) [Google Scholar]
- Estey EH. Therapeutic options for acute myelogenous leukemia. Cancer. 2001;92:1059–1073. doi: 10.1002/1097-0142(20010901)92:5<1059::aid-cncr1421>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Fiedler W, Mesters R, Tinnefeld H, Loges S, Staib P, Duhrsen U, Flasshove M, Ottmann OG, et al. A phase 2 clinical study of SU5416 in patients with refractory acute myeloid leukemia. Blood. 2003;102:2763–2767. doi: 10.1182/blood-2002-10-2998. [DOI] [PubMed] [Google Scholar]
- Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, Bello CL, Allred R, Manning WC, Cherrington JM, et al. A phase I study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105:986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Vu HA, Akutsu M, Odgerel T, Izumi T, Tsunoda S, Matsuo Y, Kirito K, Sato Y, Mano H, Kano Y. Divergent cytotoxic effects of PKC412 in combination with conventional antileukemic agents in FLT3 mutation-positive versus –negative leukemia cell lines. Leukemia. 2007;21:1005–1014. doi: 10.1038/sj.leu.2404593. [DOI] [PubMed] [Google Scholar]
- Gajiwala KS, Wu JC, Christensen J, Deshmukh GD, Diehl W, DiNitto JP, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci (USA) 2009;106:1542–1547. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul MD, Xu G, Kirkpatrick J, Ott H, Baumann CA. 4-Amino-6-piperazin-1-yl-pyrimidine-5-carbaldehyde oximes as potent FLT-3 inhibitors. Bioorg Med Chem Lett. 2007;17:4861–4865. doi: 10.1016/j.bmcl.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Gazit A, Yee K, Uecker A, Bohmer FD, Sioblom T, Ostman A, Waltenberger J, Golomb G, Banai S, Heinrich MC, Levitzki A. Tricyclic quinoxalines as potent kinase inhibitors of PDGFR kinase, Flt3 and Kit. Bioorg Med Chem. 2003;11:2007–2018. doi: 10.1016/s0968-0896(03)00048-8. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Albitar M. Mammalian target of rapamycin as a therapeutic target in leukemia. Curr Mol Med. 2005;5:653–661. doi: 10.2174/156652405774641034. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Stopeck AT, Silverman LR, Lancet JE, Cooper MA, Hannah AL, Cherrington JM, O'Farrell AM, et al. SU5416, a small molecule tyrosine kinase receptor inhibitor, has biologic activity in patients with refractory acute myeloid leukemia or myelodysplastic syndromes. Blood. 2003;102:795–801. doi: 10.1182/blood-2002-10-3023. [DOI] [PubMed] [Google Scholar]
- Gracias V, Ji Z, Akritopoulou-Zanze I, Abad-Zapatero C, Huth JR, Song D, Hajduk PJ, Johnson EF, Glaser KB, et al. Scaffold oriented synthesis. Part 2: Design, synthesis and biological evaluation of pyrimido-diazepines as receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2008;18:2691–2695. doi: 10.1016/j.bmcl.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Grandage VL, Gale RE, Linch DC, Khwaja A. PI3-kinase/Akt is constitutively active in primary acute myeloid leukaemia cells and regulates survival and chemoresistance via NF-kappaB, Mapkinase and p53 pathways. Leukemia. 2005;19:586–594. doi: 10.1038/sj.leu.2403653. [DOI] [PubMed] [Google Scholar]
- Griessinger E, Imbert V, Lagadec P, Gonthier N, Dubreuil P, Romanelli A, Dreano M, Peyron JF. AS602868, a dual inhibitor of IKK2 and FLT3 to target AML cells. Leukemia. 2007;21:877–885. doi: 10.1038/sj.leu.2404614. [DOI] [PubMed] [Google Scholar]
- Grosjean-Raillard J, Ades L, Boehrer S, Tailler M, Fabre C, Braun T, De Botton S, Israel A, Fenaux P, Kroemer G. Flt3 receptor inhibition reduces constitutive NFkappaB activation in high-risk myelodysplastic syndrome and acute myeloid leukemia. Apoptosis. 2008;13:1148–1161. doi: 10.1007/s10495-008-0243-4. [DOI] [PubMed] [Google Scholar]
- Heidel F, Lipka DB, Mirea FK, Mahboobi S, Grundler R, Kancha RK, Duyster J, Naumann M, Huber C, Bohmer FD, Fischer T. Bis(1H-indol-2-yl)methanones are effective inhibitors of FLT3-ITD tyrosine kinase and partially overcome resistance to PKC412A in vitro. Br J Haematol. 2009;144:865–874. doi: 10.1111/j.1365-2141.2008.07567.x. [DOI] [PubMed] [Google Scholar]
- Heidel F, Solem FK, Breitenbuecher F, Lipka DB, Kasper S, Thiede MH, Brandts C, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107:293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5322–5325. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CJ, Feller N, Denkers F, Kelder A, Merle PA, Heinrich MC, Harlow A, Berkhof J, Ossenkoppele GJ, Waisfisz Q, Schuurhuis GJ. Correlation of minimal residual disease cell frequency with molecular genotype in patients with acute myeloid leukemia. Haematologica. 2009;94:46–53. doi: 10.3324/haematol.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiike S, Yokota S, Nakao M, Iwai T, Sasai Y, Kaneko H, Taniwaki M, Kashima K, Fujii H, Abe T, Misawa S. Tandem duplications of the FLT3 receptor gene are associated with leukemic transformation of myelodysplasia. Leukemia. 1997;11:1442–1446. doi: 10.1038/sj.leu.2400770. [DOI] [PubMed] [Google Scholar]
- Ikezoe T, Nishioka C, Tasaka T, Yang Y, Komatsu N, Togitani K, Koeffler HP, Taguchi H. The antitumor effects of sunitinib (formerly SU11248) against a variety of human hematologic malignancies: enchancement of growth inhibition via inhibition of mammalian target of rapamycin signaling. Mol Cancer Ther. 2006;5:2522–2530. doi: 10.1158/1535-7163.MCT-06-0071. [DOI] [PubMed] [Google Scholar]
- Ishida H, Isami S, Matsumura T, Umehara H, Yamashita Y, Kajita J, Fuse E, et al. Novel and orally active 5-(1,3,4-oxadiazol-2-yl)pyrimidine derivatives as selective FLT3 inhibitors. Bioorg Med Chem Lett. 2008;18:5472–5477. doi: 10.1016/j.bmcl.2008.09.031. [DOI] [PubMed] [Google Scholar]
- Jiang J, Paez JG, Lee JC, Bo R, Stone RM, DeAngelo DJ, Galinsky I, Wolpin BM, et al. Identifying and characterizing a novel activating mutation of the FLT3 tyrosine kinase in AML. Blood. 2004;104:1855–1858. doi: 10.1182/blood-2004-02-0712. [DOI] [PubMed] [Google Scholar]
- Kancha RK, Grundler R, Peschel C, Duyster J. Sensitivity toward sorafenib and sunitinib varies between different activating and drug-resistant FLT3-ITD mutations. Exp Hematol. 2007;35:1522–1526. doi: 10.1016/j.exphem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kasper S, Breitenbuecher F, Hoehn Y, Heidel F, Lipka DB, Markova B, Huber C, Kindler T, Fischer T. The kinase inhibitor LS104 induces apoptosis, enhances cytotoxic effects of chemotherapeutic drugs and is targeting the receptor tyrosine kinase FLT3 in acute myeloid leukemia. Leuk Res. 2008;32:1698–1708. doi: 10.1016/j.leukres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Kelly LM, Liu Q, Kutok JL, Williams IR, Boulton CL, Gilliland DG. FLT3 internal tandem duplication mutations associated with human acute myeloid leukemias induce myeloproliferative disease in a murine bone marrow transplant model. Blood. 2002;99:310–318. doi: 10.1182/blood.v99.1.310. [DOI] [PubMed] [Google Scholar]
- Kelly LM, Yu JC, Boulton CL, Apatira M, Li J, Sullivan CM, Williams I, Amaral SM, Curley DP, et al. CT53518, a novel selective FLT3 antagonist for the treatment of acute myelogenous leukemia (AML) Cancer Cell. 2002;1:421–432. doi: 10.1016/s1535-6108(02)00070-3. [DOI] [PubMed] [Google Scholar]
- Kindler T, Breitenbuecher F, Kasper S, Estey E, Giles F, Feldman E, Ehninger G, Schiller G, Klimek V, Nimer SD, et al. Identification of a novel activating mutation (Y842C) within the activation loop of FLT3 in patients with acute myeloid leukemia (AML) Blood. 2005;105:335–340. doi: 10.1182/blood-2004-02-0660. [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Shiotsu Y, Ozeki K, Yamaji S, Kosugi H, Umehara H, Shimizu M, Arai H, Ishii K, Akinaga S, Naoe T. A novel FLT3 inhibitor FI-700 selectively suppresses the growth of leukemia cells with FLT3 mutations. Clin Cancer Res. 2007;13:4575–4582. doi: 10.1158/1078-0432.CCR-07-0225. [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, Naoe T. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, Clark R, Levis MJ, Small D. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- Knapper S, Mills KI, Gilkes AF, Austin SJ, Walsh V, Burnett AK. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: the induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006;108:3494–3503. doi: 10.1182/blood-2006-04-015487. [DOI] [PubMed] [Google Scholar]
- Kohl TM, Hellinger C, Ahmed F, Buske C, Hiddemann W, Bohlander SK, Spiekermann K. BH3 mimetic ABT-737 neutralizes resistance to FLT3 inhibitor treatment mediated by FLT3-independent expression of BCL2 in primary AML blasts. Leukemia. 2007;21:1763–1772. doi: 10.1038/sj.leu.2404776. [DOI] [PubMed] [Google Scholar]
- Komeno Y, Kurokawa M, Imai Y, Takeshita M, Matsumura T, Kubo K, Yoshino T, et al. Identification of Ki23819, a highly potent inhibitor of kinase activity of mutant FLT3 receptor tyrosine kinase. Leukemia. 2005;19:930–935. doi: 10.1038/sj.leu.2403736. [DOI] [PubMed] [Google Scholar]
- Kondo M, Horibe K, Takahashi Y, Matsumoto K, Fukuda M, Inaba J, Kato K, Kojima S, Matsuyama T. Prognostic value of internal tandem duplication of the FLT3 gene in childhood acute myelogenous leukemia. Med Pediatr Oncol. 1999;33:525–529. doi: 10.1002/(sici)1096-911x(199912)33:6<525::aid-mpo1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kornblau SM, Womble M, Qiu YH, Jackson CE, Chen W, Konopleya M, Estey EH, Andreeff M. Simultaneous activation of multiple signal transduction pathways confers poor prognosis in acute myelogenous leukemia. Blood. 2006;108:2358–2365. doi: 10.1182/blood-2006-02-003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, Smith BD, Jones-Bolin S, Ruggeri B, Dionne C, Small D. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- Levis M, Pham R, Smith BD, Small D. In vitro studies of a FLT3 inhibitor combined with chemotherapy: sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- Levis M, Tse KF, Smith BD, Garrett E, Small D. A FLT3 tyrosine kinase inhibitor is selectively cytotoxic to acute myeloid leukemia blasts harboring FLT3 internal tandem duplication mutations. Blood. 2001;98:885–887. doi: 10.1182/blood.v98.3.885. [DOI] [PubMed] [Google Scholar]
- Lierman E, Lahortiga I, Van Miegroet H, Mentens N, Marynen P, Cools J. The ability of sorafenib to inhibit oncogenic PDGFRbeta and FLT3 mutants and overcome resistance to other small molecule inhibitors. Haematologica. 2007;92:27–34. doi: 10.3324/haematol.10692. [DOI] [PubMed] [Google Scholar]
- Lopes de Menezes DE, Peng J, Garrett EN, Louie SG, Lee SH, Wiesmann M, Tang Y, et al. CHIR-258: A potent inhibitor of FLT3 kinase in experimental tumor xenograft models of human acute myelogenous leukemia. Clin Cancer Res. 2005;11:5281–5291. doi: 10.1158/1078-0432.CCR-05-0358. [DOI] [PubMed] [Google Scholar]
- LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updates. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahboobi S, Uecker A, Sellmer A, Cenac C, Hocher H, Pongratz H, Eichhorn E, et al. Novel bis(1H-indol-2-yl)methanones as potent inhibitors of FLT3 and platelet-derived growth factor receptor tyrosine kinase. J Med Chem. 2006;49:3101–3115. doi: 10.1021/jm058033i. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y, Kimura G, Fujii N, Omoto E, Harada M, Orita K. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23) Leukemia. 1997;11:1469–1477. doi: 10.1038/sj.leu.2400768. [DOI] [PubMed] [Google Scholar]
- Mattison RJ, Ostler KR, Locke FL, Godley LA. Implications of FLT3 mutations in the therapy of acute myeloid leukemia. Rev Recent Clin Trials. 2007;2:135–141. doi: 10.2174/157488707780599320. [DOI] [PubMed] [Google Scholar]
- McKenzie SB. Advances in understanding the biology and genetics of acute myelocytic leukemia. Clin Lab Sci. 2005;18:28–37. [PubMed] [Google Scholar]
- Mead AJ, Gale RE, Kottaridis PD, Matsuda S, Khwaja A, Linch DC. Acute myeloid leukaemia blast cells with a tyrosine kinase domain mutation of FLT3 are less sensitive to lestaurtinib than those with a FLT3 internal tandem duplication. Br J Haematol. 2008;141:454–460. doi: 10.1111/j.1365-2141.2008.07025.x. [DOI] [PubMed] [Google Scholar]
- Min YH, Cheong JW, Kim JY, Eom JI, Lee ST, Hahn JS, Ko YW, Lee MH. Cytoplasmic mislocalization of p27Kip1 protein is associated with constitutive phosphorylation of Akt or protein kinase B and poor prognosis in acute myelogenous leukemia. Cancer Res. 2004;64:5225–5231. doi: 10.1158/0008-5472.CAN-04-0174. [DOI] [PubMed] [Google Scholar]
- Mohi MG, Boulton C, Gu TL, Sternberg DW, Neuberg D, Griffin JD, Gilliland DG, Neel BG. Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogenic PTKs. Proc Natl Acad Sci(USA) 2004;101:3130–3135. doi: 10.1073/pnas.0400063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollgard L, Deneberg S, Nahi H, Bengtzen S, Jonsson-Videsater K, Fioretos T, Andersson A, Paul C Lehmann, S The FLT3 inhibitor PKC412 in combination with cytostatic drugs in vitro in acute myeloid leukemia. Cancer Chemother Pharmacol. 2008;62:439–448. doi: 10.1007/s00280-007-0623-4. [DOI] [PubMed] [Google Scholar]
- Mony U, Jawad M, Seedhouse C, Russell N, Pallis M. Resistance to FLT3 inhibition in an in vitro model of primary AML cells with a stem cell phenotype in a defined microenvironment. Leukemia. 2008;22:1395–1401. doi: 10.1038/leu.2008.125. [DOI] [PubMed] [Google Scholar]
- Murata K, Kumagai H, Kawashima T, Tamitsu K, Irie M, Nakajima H, Suzu S, Shibuya M, Kamihira S, Nosaka T, Asano S, Kitamura T. Selective cytotoxic mechanism of GTP-14564, a novel tyrosine kinase inhibitor in leukemia cells expressing a constitutively active Fms-like tyrosine kinase 3 (FLT3) J Biol Chem. 2003;278:32892–32898. doi: 10.1074/jbc.M210405200. [DOI] [PubMed] [Google Scholar]
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, Sonada Y, Fujimoto T, Misawa S. Internal tandem duplication of the FLT3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, Dipersio JF. Chemosensitization of AML following mobilization by the CXCR4 antagonist AMD3100. Blood. 2008 doi: 10.1182/blood-2008-06-162123. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama U, Yoshino T, Ozai M, Yoshioka R, Fujisawa M, Ogasawara Y, Kitahori M, et al. Antineoplastic effect of a single oral dose of the novel Flt3 inhibitor KRN383 on xenografted human leukemic cells harboring Flt3-activating mutations. Leuk Res. 2006;30:1541–1546. doi: 10.1016/j.leukres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Miwa A, Tasaka T, Kuwayama Y, Togitani K, Koeffler HP, Yokoyama A. Ki11502, a novel multitargeted receptor tyrosine kinase inhibitor, induces growth arrest and apoptosis of human leukemia cells in vitro and in vivo. Blood. 2008a;111:5086–5092. doi: 10.1182/blood-2007-06-098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Takeshita A, Taniguchi A, Komatsu N, Togitani K, Koeffler HP, Yokoyama A. Blockade of MEK/ERK signaling enhances sunitinib-induced growth inhibition and apoptosis of leukemia cells possessing activating mutations of the FLT3 gene. Leuk Res. 2008;32:865–872. doi: 10.1016/j.leukres.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Takeuchi S, Koeffler HP, Yokoyama A. MS-275, a novel histone deacetylase inhibitor with selectivity against HDAC1, induces degradation of FLT3 via inhibition of chaperone function of heat shock protein 90 in AML cells. Leuk Res. 2008;32:1382–1392. doi: 10.1016/j.leukres.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Odgerel T, Kikuchi J, Wada T, Shimizu R, Futaki K, Kano Y, Furukawa Y. The FLT3 inhibitor PKC412 exerts differential cell cycle effects on leukemic cells depending on the presence of FLT3 mutations. Oncogene. 2008;27:3102–3110. doi: 10.1038/sj.onc.1210980. [DOI] [PubMed] [Google Scholar]
- O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong IM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003a;101:3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- O'Farrell AM, Foran JM, Fiedler W, Serve H, Paquette RL, Cooper MA, Yuen HA, et al. An innovative phase I clinical study demonstrates inhibition of FLT3 phosphorylation by SU11248 in acute myeloid leukemia patients. Clin Cancer Res. 2003b;9:5465–5476. [PubMed] [Google Scholar]
- O'Farrell AM, Yuen HA, Smolich B, Hannah AL, Louie SG, Hong W, Stopeck AT, Silverman LR, et al. Effects of SU5416, a small molecule tyrosine kinase receptor inhibitor, on FLT3 expression and phosphorylation in patients with refractory acute myeloid leukemia. Leuk Res. 2004;28:679–689. doi: 10.1016/j.leukres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Pratz KW, Cortes J, Roboz GJ, Rao N, Arowojolu O, Stine A, Shiotsu Y, Shudo A, Akinaga S, Small D, Karp JE, Levis M. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2008 doi: 10.1182/blood-2008-09-177030. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratz K, Levis M. Incorporating FLT3 inhibitors into acute myeloid leukemia treatment regimens. Leuk Lymphoma. 2008;49:852–863. doi: 10.1080/10428190801895352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, Benzaquen D, Laurent G, Huguet F, Payrastre B. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105:2527–2534. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- Rivera VM, Xu Q, Berk L, Keats J, Wardwell S, Wang F, Shakespeare WC, Clackson T. Potent antitumor activity of AP24534, an orally active inhibitor of Bcr-Abl, Flt3 and other kinases, in both in vitro and in vivo models of acute myeloid leukemia (AML) Blood. 2008;112:1008–1009. Abstract. [Google Scholar]
- Rocnik JL, Okabe R, Yu JC, Lee BH, Giese N, Schenkein DP, Gilliland DG. Roles of tyrosine 589 and 591 in STAT5 activation and transformation mediated by FLT3-ITD. Blood. 2006;108:1339–1345. doi: 10.1182/blood-2005-11-011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts WJ, Blokland I, Lowenberg B, Ploemacher RE. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the FLT3 gene. Leukemia. 2000;14:675–683. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, Sawyers CL. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- Shankar DB, Li J, Tapang P, Owen J, Pease LJ, Dai Y, Wei RQ, Albert DH, et al. ABT-869, a multitargeted receptor tyrosine kinase inhibitor: inhibition of FLT3 phosphorylation and signaling in acute myeloid leukemia. Blood. 2007;109:3400–3408. doi: 10.1182/blood-2006-06-029579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenone S, Brullo C, Botta M. Small molecules ATP-competitive inhibitors of FLT3: a chemical overview. Current Medicinal Chemistry. 2008;15:3113–3132. doi: 10.2174/092986708786848613. [DOI] [PubMed] [Google Scholar]
- Shiotsu Y, et al. KW-2449, a novel multi-kinase inhibitor against FLT3, Abl, FGFR1 and Aurora suppresses the growth of AML both in vitro and in vivo. Blood. 2007;110:1832. abstract. [Google Scholar]
- Shiotsu Y, Kiyoi H, Tanizaki R, Minami Y, Akihiro A, Ishikawa Y, Ishii K, et al. KW-2449, a novel multi-kinase inhibitor, suppresses the growth of imatinib-resistant Ph+ leukemia including BCR-ABL/T315I both in vitro and in vivo. Blood. 2008;112:579. Abstract. [Google Scholar]
- Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, Murphy KM, Dauses T, Allebach J, Small D. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- Smith BD, Levis M, Beran M, Giles P, Russell BL, Hellriegel E, Dauses T, Allebach J, Small D. Single agent CEP-701, a novel FLT3 inhibitor, shows initial response in patients with refractory acute myeloid leukemia. Proc Am Soc Clin Oncol. 2003;22:779. Abstract. [Google Scholar]
- Stone RM, De Angelo J, Galinsky I, Estey E, Klimek V, Grandin W, Lebwohl D, et al. PKC412 FLT3 inhibitor therapy in AML: results of a phase II trial. Ann Hematol. 2004;83(Suppl 1):S89–90. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, Grandin W, Lebwohl D, Wang Y, Cohen P, Fox EA, Neuberg D, Clark J, Gilliland DG, Griffin JD. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005b;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- Stone RM, Fischer T, Paquette R, Schiller G, Schiffer CA, Ehninger G, et al. Phase 1B study of PKC412, an oral FLT3 kinase inhibitor, in sequential and simultaneous combinations with daunorubicin and cytarabine (DA) induction and high-dose cytarabine consolidation in newly diagnosed patients with AML. Blood. 2005a;106:404. Abstract. [Google Scholar]
- Stirewalt DL, Radich JP. The role of FLT3 in hematopoeitic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- Tazzari PL, Cappellini A, Grafone T, Mantovani I, Ricci F, Billi AM, Ottaviani E, Conte R, Martinelli G, Martelli AM. Detection of serine 473 phosphorylated Akt in acute myeloid leukaemia blasts by flow cytometry. Br J Haematol. 2004;126:675–681. doi: 10.1111/j.1365-2141.2004.05121.x. [DOI] [PubMed] [Google Scholar]
- Teller S, Kramer D, Bohmer SA, Tse KF, Small D, Mahboobi S, et al. Bis(1H-2-indolyl)-1-methanones as inhibitors of the hematopoietic tyrosine kinase Flt3. Leukemia. 2002;16:1528–1534. doi: 10.1038/sj.leu.2402630. [DOI] [PubMed] [Google Scholar]
- Trudel S, Li ZH, Wei E, Wiesmann M, Chang H, Chen C, Reece D, Heise C, Stewart AK. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood. 2005;105:2941–2948. doi: 10.1182/blood-2004-10-3913. [DOI] [PubMed] [Google Scholar]
- Tse KF, Allebach J, Levis M, Smith BD, Bohmer FD, Small D. Inhibition of the transforming activity of FLT3 internal tandem duplication mutants from AML patients by a tyrosine kinase inhibitor. Leukemia. 2002;16:2027–2036. doi: 10.1038/sj.leu.2402674. [DOI] [PubMed] [Google Scholar]
- Tse KF, Novelli E, Civin CI, Bohmer FD, Small D. Inhibition of FLT3-mediated transformation by use of a tyrosine kinase inhibitor. Leukemia. 2001;15:1001–1010. doi: 10.1038/sj.leu.2402199. [DOI] [PubMed] [Google Scholar]
- Vroling L, van der Veldt AA, de Haas RR, Haanen JB, Schuurhuis GJ, et al. Increased numbers of small circulating endothelial cells in renal cell cancer patients treated with sunitinib. Angiogenesis. 2009;12:69–79. doi: 10.1007/s10456-009-9133-9. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Banerji L, Wright RD, Barrett R, Ray A, Moreno D, Catley L, Jiang J, Hall-Meyers E, et al. Potentiation of antileukemic therapies by the dual PI3K/PDK-1 inhibitor, BAG956: effects on BCR-ABL and mutant FLT3-expressing cells. Blood. 2008c;111:3723–3734. doi: 10.1182/blood-2007-09-114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, Gilliland DG, Griffin JD. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Kung AL, Wright RD, Moreno D, Catley L, Ray A, Zawel L, Tran M, et al. Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells. Mol Cancer Ther. 2007;6:1951–1961. doi: 10.1158/1535-7163.MCT-06-0810. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Roesel J, Bold G, Furet P, Jiang J, Cools J, Wright RD, Nelson E, et al. Antileukemic effects of the novel, mutant FLT3 inhibitor NVP-AST487: effects on PKC412-sensitive and –resistant FLT3-expressing cells. Blood. 2008b;112:5161–5170. doi: 10.1182/blood-2008-02-138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg E, Wright RD, McMillin DW, Mitsiades C, Ray A, Barrett R, Adamia S, Stone R, Galinsky I, Kung AL, Griffin JD. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol Cancer Ther. 2008a;7:1121–1129. doi: 10.1158/1535-7163.MCT-07-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherspoon RP, Deeg HJ. Allogeneic bone marrow transplantation for secondary leukemia or myelodysplasia. Haematologica. 1999;84:1085–1087. [PubMed] [Google Scholar]
- Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- Yee KW, O'Farrell AM, Smolich BD, Cherrington JM, McMahon G, Wait CL, McGreevey LS, Griffith DJ, Heinrich MC. SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood. 2002;100:2941–2949. doi: 10.1182/blood-2002-02-0531. [DOI] [PubMed] [Google Scholar]
- Yee KW, Schittenheim M, O'Farrell AM, Town AR, McGreevey L, Bainbridge T, Cherrington JM, Heinrich MC. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3-ITD-positive leukemic cells. Blood. 2004;104:4202–4209. doi: 10.1182/blood-2003-10-3381. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Samudio IJ, Munsell M, An J, Huang Z, Estey E, Andreeff M, Konopleva M. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Mol Cancer Ther. 2006;5:3113–3121. doi: 10.1158/1535-7163.MCT-06-0228. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling X, Frolova O, Levis M, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2008 doi: 10.1182/blood-2008-05-158311. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, Estrov Z, Quintas-Cardama A, Small D, Cortes J, Andreeff M. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- Zhou J, Pan M, Xie Z, Loh SL, Bi C, Tai YC, Lilly M, Lim YP, et al. Synergistic antileukemic effects between ABT-869 and chemotherapy involve downregulation of cell cycle-regulated genes and c-Mos-mediated MAPK pathway. Leukemia. 2008;22:138–146. doi: 10.1038/sj.leu.2404960. [DOI] [PubMed] [Google Scholar]
- Zhou J, Bi C, Janakakumara JV, Liu SC, Chng WJ, Tay KG, Poon LF, Xie Z, et al. Enhanced activation of STAT pathways and overexpression of surviving confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 2009 doi: 10.1182/blood-2008-05-156422. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zhou J, Khng J, Jasinghe VJ, Bi C, Neo CH, Pan M, Poon LF, Xie Z, et al. In vivo activity of ABT-869, a multi-target kinase inhibitor, against acute myeloid leukemia with wild-type FLT3 receptor. Leuk Res. 2008a;32:1091–1100. doi: 10.1016/j.leukres.2007.11.025. [DOI] [PubMed] [Google Scholar]