Abstract
Objective
This systematic review evaluates the impact of aspirin on audiometric outcomes with respect to: (1) doses exceeding 325 mg daily, (2) doses of 325 mg daily or less, (3) studies applicable to the general populace, and (4) studies applicable to those with inflammatory conditions. It also assesses the impact of aspirin on (a) self-reported hearing loss, (b) noise-induced audiometric changes, and (c) the adverse otological effects of aminoglycoside therapy.
Data Sources
Computerized searches of MEDLINE, PubMed, Cochrane, and EMBASE databases were performed, updated through January 2014, and supplemented by manual searches and inquiries to topic experts.
Review Methods
A systematic review was performed according to an a priori protocol. Data extraction was performed by 2 independent parties and focused on relevant audiological measurements, potential confounders, and study design elements associated with risk of bias, including utilization of randomization, prospective/retrospective data collection, and incorporation of blinding.
Results
The 37 criterion-meeting studies included a combined total of 185,155 participants. Aspirin ingestion ≥1.95 g/d was associated with worse audiometric results (4–112 dB threshold shift); the effect was dose dependent and reversible in the short term. There were no audiometric data that confirm that long-term doses of 81 mg or 325 mg daily have no hearing consequences. Paradoxically, aspirin (in doses shown to be detrimental in isolation) had a protective effect when co-administered with intravenous gentamicin.
Conclusions
With the large-scale population utilization of aspirin for cardiovascular prophylaxis, the potential risks to hearing health should be considered for future longitudinal study, particularly given that short-term effects may be reversible.
Keywords: hearing loss, sensorineural hearing loss, aspirin, public health
Introduction
Aspirin (acetylsalicylic acid, ASA) is ingested at the remarkable rate of 40,000 metric tons (120 billion tablets) per year, making it 1 of the 3 most utilized drugs worldwide.1,2 Originally isolated from the bark of a willow tree, its antipyretic, analgesic, and anti-inflammatory properties have formed the foundation for its widespread use. In addition, this nonsteroidal anti-inflammatory agent now plays a noteworthy role in preventive regimens. More specifically, the United States Preventative Task Force recommends that prophylactic ASA be considered in males 45 to 79 years of age to obtain a potential 32% relative risk reduction for myocardial infarction. The Task Force also recommends that daily usage be considered in females age 55 to 79 years to effect a potential 17% relative risk reduction for stroke.3 Therefore, ASA is likely to remain among the most ubiquitous of medications in active use.
Hearing loss is the most common sensory disorder in the United States, and its prevalence is increasing.4,5 While 31.5 million American adults self-reported hearing loss in 2000, that number increased to 37 million in 2006.4 With audiometric decline in older patients and aging of the overall population, the prevalence of hearing loss is of concern.6 Given that such hearing loss often negatively impacts communication, academic performance, work productivity, and social integration, the Federal Interagency Workgroup has prioritized reducing hearing loss in the Healthy People 2020 objectives that elucidate the nation’s health care objectives,7–9 also noting that hearing loss may be caused by “sensitivity to certain drugs or medications,” among other causes.9
Since hearing loss and ASA utilization are both so common, patients frequently present with both on their dossier. In fact, among the 45- to 79-year-old population subset where daily prophylactic ASA is routinely considered, there is a 13% to 68% prevalence of hearing loss.10–13 While this population subset includes those with multiple cardiovascular risk factors whose presence would trump that of hearing-related health, ASA may also currently be utilized in those with limited cardiovascular risk but progressive and impactful sensorineural hearing loss. In these circumstances, it would behoove us to understand whether it would better serve our patients to withhold daily ASA either temporarily or until additional cardiovascular concern is raised. It would also behoove us to understand whether the potential otological impact of ASA is dose dependent, so that such considerations would not trigger the drug’s inappropriate cessation.
Conventional wisdom holds that high doses of ASA may be associated with hearing loss but that low doses do not cause adverse otological effects. At the outset of this systematic review, however, we knew of no studies that reported formal audiometric results to confirm the safety of longstanding low-dose ASA. Given the potential for widespread public health impact, as well as the frequency with which related clinical scenarios arise, we undertook a systematic review to evaluate the specific impact of ASA on the prevalence and pattern of hearing loss. Systematic reviews are distinct from traditional narrative reviews in that they are performed according to well-defined, rigorous procedures and provide a reproducible, thorough method to evaluate the current best evidence regarding a specific clinical question. As such, they often constitute the highest level of evidence available14–16 and form the foundation for significant documents such as clinical practice guidelines17,18 and global collaborative workgroups such as the Cochrane Collaboration. This systematic review evaluates the impact of ASA on audiometric outcomes with respect to: (1) doses exceeding 325 mg daily, (2) doses of 325 mg daily or less, (3) studies applicable to the general populace, and (4) studies applicable to those with inflammatory conditions. It also assesses the impact of aspirin on (a) self-reported hearing loss, (b) noise-induced audiometric changes, and (c) the adverse otological effects of aminoglycoside therapy.
Methods
Search Strategy
A combination of computerized and manual searches was performed to identify all relevant data. A computerized PubMed search of MEDLINE ranging from 1965 through January 2014 was performed, updating the search through the latter date. Articles mapping to the exploded medical subject heading hearing loss or containing hearing loss in the title were combined into one group. Medical subject headings anti-inflammatory agents, non-steroidal, ibuprofen, aspirin, or acetaminophen were exploded and the articles were collected into a second group. The 2 groups were then cross-referenced and limited to human studies in the English language. Parallel searches using similar terms were performed in EMBASE and the Cochrane Library. The combined searches yielded 356 references. Titles and abstracts were then evaluated according to the inclusion/exclusion criteria described in the following. Reference lists for relevant narrative reviews and criteria-meeting publications were searched manually for additional studies. Two parties from audiology and otolaryngology performed searches independently, blinded to each other’s results. Topic experts were also contacted to determine if additional studies or unpublished data could be identified. Titles and abstracts for all identified studies were reviewed, and 37 articles were ultimately included in the analysis (Figure 1).
Figure 1.
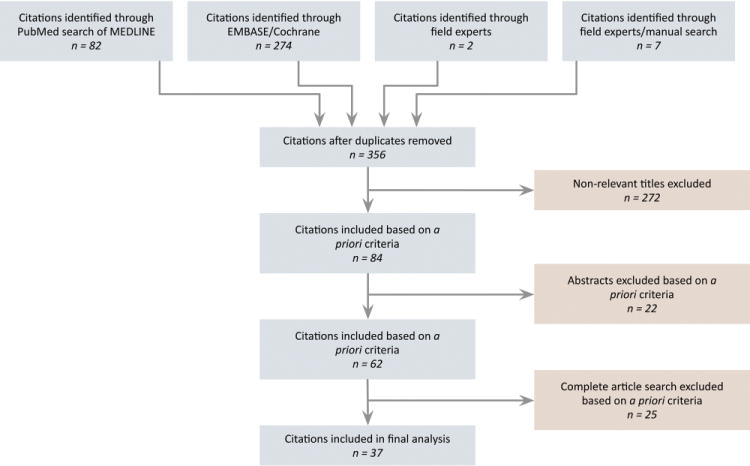
Flow diagram showing the stages of identification of studies by citation source.
Inclusion/Exclusion Criteria
Articles identified by the computerized and manual search strategy described previously were evaluated to meet these inclusion criteria: (1) patients of any age, ethnicity, health status, or socioeconomic background (patient population); (2) exposure to any ASA regimen of any dose, as confirmed via blood laboratory analysis, medical report, or self-report (intervention); (3) comparison to a control or non–ASA-exposed baseline data (comparison); (4) outcome measured in terms of sensorineural or mixed hearing loss via conventional audiometry, otoacoustic emissions (OAE), and/or psychophysical measurements; self-reported hearing assessments were included but tracked in a separate secondary group (outcomes); and (5) all study designs with all follow-up times in the search strategy timeframe were included (timeframe/studies). An additional discussion of the measures of hearing outcome is presented in Appendix 1 (available at www.otojournal.org).
Articles were excluded if (1) no hearing results were reported, (2) hearing results resulted in non-sensorineural hearing loss (eg, acute otitis media and otitis media with effusion), (3) hearing results were unclear regarding the type of audiometric criteria used to determine hearing loss, (4) a population of solely nonhearing patients were evaluated (nonapplicable denominator), and (5) they were abstracts without subsequent full manuscript publication.
Data Extraction
Data extraction focused on items relevant to the study results, potential sources of heterogeneity among those results, and study identification (author, year of publication, full reference citation). Extracted data included (1) number/percentage with maintenance, improvement, or deterioration of hearing thresholds; (2) number of subjects in each group; (3) specific drug regimen evaluated; (4) P value, confidence interval, standard error of the mean, proportions, or descriptive statistics reported; and (5) follow-up time. Data collection also included multiple potential sources of heterogeneity among studies: (a) dosing and duration of the drug regimen, (b) control regimen details if applicable, (c) audiological criteria used for stratification of data, (d) primary study endpoints, and (e) study design.19 In accordance with data demonstrating that specific “study quality” ranking scales may be misleading or give heterogeneous results,19–22 rather than utilizing a summary scale, we focused on evaluation of data quality by consistent factual description of individual elements of study design with specific attention to: whether randomization was performed, prospective/retrospective analysis, incorporation of blinding (participants and/or assessors), and whether hearing assessments were the primary outcome.
Quantitative Data Analysis
An a priori plan was made to perform a quantitative meta-analysis of the following null hypotheses if the data were permissive: (1) ASA has no impact on the prevalence or pattern of sensorineural hearing loss and (2) any such impact is not dose dependent. However, due to large variation in study designs, audiometric criteria, affected frequency ranges, and reporting parameters, a pooled analysis was not appropriate.
If the primary study reported a statistical analysis, it was included in the extracted data. If the primary study did not describe a statistical comparison, when possible, post hoc calculations for the 95% confidence interval of the prevalence of hearing loss were calculated according to the standard binomial distribution (Stata 12.0, College Station, Texas).
Results
Study Characteristics
The 37 criterion-meeting studies included a total of 185,155 participants. Clinical and research audiometric evaluations included standard pure-tone thresholds and speech discrimination scores, OAE, and psychophysical measurements. Non-audiometric data consisted of self-reported hearing loss via survey results. There were 3 randomized controlled trials and 9 studies that employed blinding. The bulk of the data suggested ASA had a deleterious effect, but data from 2 randomized controlled trials demonstrated a protective effect as well.
Given the inherent variability in these studies’ patient populations, ASA exposures, and outcome measurements, we describe the results in categories. First, we present results measured by standard audiometry (ie, pure-tone averages, speech scores). Among these studies, we show data categorized by (1) ASA dosage (>325 mg/d, ≤325 mg/d) and (2) relevance to the general population or those with inflammatory conditions (eg, rheumatoid arthritis). Second, we briefly summarize the result of other audiometric outcomes, specifically OAE and psychophysical testing. Third, we describe the mixed results of studies of self-reported hearing symptoms, which include the studies with the largest sample sizes and longest follow-up periods; data are again categorized by the absence/presence of a focus on subjects with inflammatory conditions. Fourth, we evaluate the impact of ASA in the setting of other audiometrically detrimental exposures: noise and aminoglycosides.
Pure-tone Threshold Audiometry and Speech Discrimination Scores Associated with Aspirin in Subjects without Inflammatory Conditions
Doses exceeding 325 mg daily
Four prospective studies suggested that ASA had a deleterious audiometric effect when administered in doses of at least 1.95 g daily in patients without rheumatoid arthritis or connective tissue disease (Table 1).23–26 Three of these analyses compared audiometry after ASA ingestion to subjects’ baseline hearing, while 1 evaluated patients taking ASA for analgesia after tonsillectomy in comparison to controls. Two showed statistically significant worsening of audiometry (Table 2); the magnitude of loss ranged from 4 dB to 112 dB and was dose dependent in the single study that evaluated a range of doses on the same participants. Ingestion of less than 4 g per day was associated with pure-tone threshold worsening of 4.4 dB to 12.7 dB, while ingestion of 4 to 10 g per day was associated with changes of 15 dB to 112.5 dB. Speech discrimination scores were not evaluated. Two additional studies suggested a deleterious audiometric effect but did not report statistical analyses of their ASA-related findings (Table 3); in these instances, it was not that effects were present that were not statistically significant; a statistical analysis was simply not reported. In all 4 prospective analyses, the hearing loss was reversible. The maximum follow-up time in these studies was 3 weeks. There were also 7 case reports of patients unaffected by rheumatoid arthritis that described worsening audiometry with ASA; all instances except 1 improved after ASA was discontinued (Appendix 2, available at www.otojournal.org).27–32
Table 1.
Change in Mean Audiogram Results Post- versus Pre-ASA: Patients without Inflammatory Conditions.a
| Author, Year | 0.5 kHz | 1 kHz | 1.5 kHz | 2 kHz | 3 kHz | 4 kHz | 6 kHz | 8 kHz | Mean of 0.25, 0.5, 1,2,4, and 8 kHz |
Mean of 0.125–8 k Hz |
Speech Recognition Scores |
Reversibility of Hearing Loss |
Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdala, 2005b,23 | NR | NR | −12.7 (3.9 g/d) | NR | NR | NR | −14.3 | NR | NR | NR | NR | Yes | Comparison to baseline |
| Day, 1989b,25 | NR | NR | NR | NR | NR | NR | NR | NR | −4.38 (1.95 g/d) | NR | NR | Yes | Change from baseline; mean hearing loss in dB at each frequency examined |
| −11.56 (3.25 g/d) −46.56 (4.55 g/d) −112.5 (5.85 g/d) |
|||||||||||||
| Bonding, 197924 | NR | −15–40 (4 g/d) | NR | NR | NR | NR | NR | NR | NR | NR | NR | Yes | Flat hearing loss configurations |
| Johnsen, 198226 | NR | NR | NR | NR | NR | NR | NR | NR | NR | −10–15 (10 g/d) | NR | Yes |
Abbreviations:ASA, aspirin (acetylsalicylic acid); NR, not reported.
Negative numbers reflect worse hearing after ASA. For example, if the mean pre-ASA threshold was 10 dB and the mean post-ASA threshold was 40 dB, then the change in mean is −30 dB. Positive numbers conversely reflect better hearing after ASA. Numbers reported are audiometry in units of decibels (ASA dose).
Study demonstrated statistically significant effects.
Table 2.
Studies Demonstrating a Statistically Significant Deleterious Effect of ASA on Pure-tone Thresholds or Speech Discrimination in Patients without Inflammatory Conditions.
| Author, Year | Study Design (sample size) | Patient Description | ASA Regimen Evaluated | Hearing Evaluation | Follow-up Time | ASA Exposure Results | No ASA Exposure Results | Time from Exposure to Outcome | Additional Comments |
|---|---|---|---|---|---|---|---|---|---|
| Abdala, 200523 | Prospective cohort (n = 41, 10 of which were ASA exposed) | Adult ASA recipients: age 22–37 years (n = 10) Children with mild SNHL (n = 8) Healthy term–born neonates (n = 23) |
ASA 975 mg (3 tablets of 325 mg) Q6h for 4 d | Air and bone conduction audiogram Immittance testing DP-gram DPOAE |
Baseline, then 72 h and 96 h afterward | Mean audiometric threshold increase at 96 h: 12.7 dB at 1500 Hz, 14.3 dB at 6000 Hz. Significant correlation between serum level and audiometry (r = 0.75, P = .19) |
Subjects were compared to their own baseline | 72,96 h | Audiometric results were not directly correlated with DPOAE results |
| Day, 198925 | Prospective cohort, double-blinded (n = 8) |
Age 22–46 years with normal hearing at the outset Male healthy volunteers |
ASA in 4 serial scheduled doses: (1) 1.95 g/d (2) 3.25 g/d (3) 4.55 g/d (4) 5.85 g/d for 7 d each |
Pure-tone thresholds measured at 250–8000 Hz | On the seventh day of each treatment period | Mean HL per frequency (dB HL) 1.95g/d = 4.38 3.25g/d = 11.56 4.55g/d = 40.56 5.85g/d= 112.5 Mean Tinnitus intensity (dB HL) 1.95g/d = 25.50 3.25 g/d= 36.38 4.55 g/d= 51.00 5.85 g/d= 62.13 |
Subjects were compared to their own baseline. HL expressed as mean decrease from pretreatment thresholds in both ears | Effect present at 1 week | Hearing and tinnitus had a dose-dependent response to ASA level (P < .05). |
Abbreviations: ASA, aspirin (acetylsalicylic acid); DPOAE, distortion product otoacoustic emissions; HL, hearing loss; SNHL, sensorineural hearing loss.
Table 3.
Studies Suggesting a Potential Deleterious Effect of ASA on Pure-tone Thresholds and/or Speech Discrimination in Patients without Inflammatory Conditions.
| Author, Year | Study Design (sample size) | Patient Description | ASA Regimen Evaluated | Hearing Evaluation | Follow-up Time | ASA Exposure Results | No ASA Exposure Results | Time from Exposure to Outcome | Additional Comments |
|---|---|---|---|---|---|---|---|---|---|
| Bonding, 197924 | Prospective controlled study (n = 2l) |
ASA: Age 16–29 years, 4 males, 12 females undergoing tonsillectomy who utilized ASA for postoperative pain control Control: 5 non-ASA subjects All had normal hearing at the outset |
ASA 4 g every 24 h for 2–3 d | “Tested audiometrically and if a pure-tone SNHL was present, the patient’s CB at 1 kHz was determined,” estimations using loudness summation | Control audiometry and CB estimations were performed 2–3 weeks after HL developed | 15–40 dB HL at 1 kHz 56% (9/16) had a wider CB with temporary ASA induced HL |
Sensitivity curves remained unchanged in controls | Effect present after 2–3 d of treatment | Reversible |
| Johnsen, 198226 | Prospective cohort (n = 2) | 1 female, age 29 years with PT <15 dB HL audiometry 1 male, age 42 years with PT <20 dB HL AS and no response in AD |
ASA 10 g/d × 1 d | HL defined as > 15–20 dB HL at 125–8000 Hz Pure-tone thresholds |
Immediately before and after ingestion of ASA and again 2 days later | PT threshold was elevated to 25–30 dB HL and the response pattern of TEOAEs were altered | Subjects were compared to their own baseline | NR | Hearing thresholds returned to normal 48 h post exposure |
Abbreviations: AD, right ear;AS, left ear;ASA, aspirin (acetylsalicylic acid); CB, critical band; HL, hearing loss; NR, not reported; PT, pure tone; SNHL, sensorineural hearing loss;TEOAE, transient evoked otoacoustic emissions.
Doses of 325 mg daily or less
There were no studies that evaluated the impact of ASA doses of 325 mg or less per day. In fact, there were no studies that evaluated hearing outcomes with doses of less than 1.95 g per day in this more general patient population. Not only were there no data showing that these lower daily doses were not associated with either short-term or long-term audiometric effects, but there were also no data evaluating these dose ranges in subjects representative of the general population (ie, those not ingesting ASA for control of inflammatory conditions). Therefore, with regard to the prophylactic daily regimens administered at doses of 81 or 325 mg daily to the general population, there are currently no pure-tone audiometry or speech discrimination data on which to base decisions for hearing-related health.
Pure-tone Threshold Audiometry and Speech Discrimination Scores Associated with Aspirin in Subjects with Rheumatoid Arthritis and Connective Tissue Diseases
Doses exceeding 325 mg daily
Seven studies evaluated the impact of ASA on pure-tone averages and speech recognition (Table 4).33–39 One randomized, double-blind placebo-controlled study demonstrated that those receiving ASA had statistically significantly worse pure-tone thresholds, speech recognition, and word discrimination in background noise (Table 5). All subjects met diagnostic criteria for rheumatoid arthritis. The pharmacological reporting in this paper was somewhat vague in that it specified 325 mg doses during a 7-day interval but did not specify the frequency with which this dose was administered. An attempt was made to contact authors of this 1978 publication, but at the time of this writing, the dosing intervals and thus total daily ingestion remain unspecified (we received word that the 5 authors listed are either retired or deceased). The magnitude of audiometric worsening ranged from 6.8 dB to 26.8 dB, with more loss in the higher frequencies and at higher blood serum levels (Table 4); these findings were statistically significant. Drops in hearing were reversible and follow-up time was 7 days.
Table 4.
Change in Mean Audiogram Results Post- versus Pre-ASA: Patients with Rheumatoid Arthritis and Connective Tissue Diseases.a
| Author, Year | 0.25 kHz | 0.5 kHz | 1 kHz | 2 kHz | 3 kHz | 4 kHz | 6 kHz | 8 kHz | Speech Recognition Scores in Quiet | Reversibility of Hearing Loss | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jardini, 1978b,37 | −7.84 (BSL 5.79) −6.84 (BSL 11.44) |
−9.5 (BSL 5.79) −7.34 (BSL 11.44) |
−11.47 (BSL 5.79) −9.13 (BSL 11.44) |
−19.88 (BSL 5.79) −15.31 (BSL 11.44) |
NR | −21 (BSL 5.79) −19.16 (BSL 11.44) |
NR | −24 (BSL 5.79) −26.84 (BSL 11.44) |
−1.89% (BSL 5.79) −2.79% (BSL 11.44) |
Yes | Comparison of placebo to ASA thresholds; dose 325 mg × 7 d (dosing frequency NR). |
| Davis, 197934 | 11% (95% CI, 1.4%–34.7%) had new hearing loss (defined by “a threshold shift”) after ASA (3.6 g/d). 28% (95% CI, 9.7%–53.5%) of those with hearing loss had progression (defined by “a threshold shift”) after ASA (3.6 g/d). |
NR | Data were not reported in a measured frequency-specific fashion. | ||||||||
| Halla, 198835 | 17.9% (95% CI, 11.8%–25.4%) had audiometry worse than 25 dB after ASA administration (< 1 g/d to >4 g/d; dose-specific hearing results NR). | NR | |||||||||
| Myers, 196538 | In the group with normal hearing at the outset, “hearing loss ranged from 20 to 40 dB” after ASA (6–8 g/d). This HL was characterized in most cases as a bilateral, symmetrical threshold elevation at all frequencies. As the plasma salicylate level approached 30 mg/100 ml, a deficit between 30–40 dB was seen. |
Yes | |||||||||
| Heyworth, 197236 | 75% (95% CI, 42.8%–94.5%) had audiometry worse than 20 dB after ASA administration (specific dose per day NR). | Yes (4 of 9) | |||||||||
| Bernstein, 196733 | −25 to 45 (6–8 g/d) |
−25 to 45 (6–8 g/d) |
−25 to 45 (6–8 g/d) |
−25 to 45 (6–8 g/d) |
−25 to 45 (6–8 g/d) |
−25 to 45 (6–8 g/d) |
−25 to 45 (6–8 g/d) |
−25 to 45 (6–8 g/d) |
≥ −20% (5 out of 12 patients) (6–8 g/d) | Yes | Bekesy tracking revealed flat loss of 25dB to 45 dB at 0.125 kHz to 8 kHz. |
| Young, 198239 | 0 (6–9 g/d) | −1 (6–9 g/d) |
−2 (6–9 g/d) |
−1 (6–9 g/d) |
NR | 0 (6–9 g/d) |
NR | −9 (6–9 g/d) |
−4% (6–9 g/d) |
NR | Average thresholds of 5 subjects |
Abbreviations:ASA, aspirin (acetylsalicylic acid); BSL, blood serum level (mg/100 ml); NR, not reported.
Negative numbers reflect worse hearing after ASA. For example, if the mean pre-ASA threshold was 10 dB and the mean post-ASA threshold was 40 dB, then the change in mean is −30 dB. Positive numbers conversely reflect better hearing after ASA. Numbers reported are audiometry in units of decibels (ASA dose).
Study demonstrated statistically significant effects. Study is a randomized controlled trial.
Table 5.
Study Demonstrating a Statistically Significant Deleterious Effect of ASA on Pure-tone Thresholds or Speech Discrimination in Patients with Rheumatoid Arthritis and Connective Tissue Disorders.
| Author, Year | Study Design (sample size) | Patient Description | ASA Regimen Evaluated | Hearing Evaluation | Follow-up Time | ASA Exposure Results | No ASA Exposure Results | Time from Exposure to Outcome | Additional Comments |
|---|---|---|---|---|---|---|---|---|---|
| Jardini, 197837 | Randomized placebo-controlled study (n = 30) |
All diagnosed with RA ASA: 13 women, 2 men Age 19–69 years (mean S3) Placebo: 14 women, 1 man Age 29–76 (mean 51) |
ASA 325 mg (5 grain (1 grain = 65 mg) capsules) × 7 d (dosing frequency not specified) Lactose placebo × 7d |
PT air/bone conduction thresholds at 250–8000 Hz SRT;SDS in quiet (recorded CID W-22) and in noise (−4 dB competing SNR) |
Days 0, 3, and 7 | ASA group had significantly increased PT threshold shifts at 250–8000 Hz except at 2000 Hz as compared to control (P < .05). ASA group had significantly poorer SRTs (P < .05). ASA group had significantly worse discrimination in background noise (P < .05). |
Effect present at day 3 | Moderate BSL (35 mg/100 mL) may adversely affect auditory sensitivity. Thresholds recovered over the course of 1 week. |
|
Abbreviations: ASA, aspirin (acetylsalicylic acid); BSL blood serum level; CID W-22, word recognition list W-22 created by Central Institute for the Deaf; PT, pure tone; RA: rheumatoid arthritis; SDS, speech discrimination scores; SNR, signal to noise ratio; SRT, speech reception threshold.
The remaining 6 studies suggest worsening of audiometric thresholds, but the primary reports did not contain statistical analyses (Tables 6, 7). The magnitude of change in pure-tone thresholds ranged from 0 dB to 45 dB and crossed all measured frequencies (Table 4). In most instances, frequency-specific information was not reported. One of these studies evaluated speech discrimination33 and found that monaural testing was impacted more than binaural testing. When specified in these 6 studies, doses were typically high (3.6–9.0 g/d), but in 1 instance, data from those ingesting less than 1 g per day were merged with those taking more than 4 g per day.35 The longest follow-up times among these studies was 3 weeks.
Table 6.
Studies Suggesting a Potential Deleterious Effect of ASA on Pure-tone Thresholds and/or Speech Discrimination in Patients with Rheumatoid Arthritis and Connective Tissue Disorders.
| Author, Year | Study Design (sample size) | Patient Description | ASA Regimen Evaluated | Hearing Evaluation | Follow-up Time | ASA Exposure Results | No ASA Exposure Results | Time from Exposure to Outcome | Additional Comments |
|---|---|---|---|---|---|---|---|---|---|
| Halla, 198835 | Prospective controlled study (n = 316) |
Exposure:ASA/salycilate for RA (n = 134,20–88 y, mean 58.3) Controls: healthy volunteers (n = 182,20–61 y, mean 34.3) |
“Regular” ASA use for > 1 w prior to study (≤l g–≥4 g/d) Controls received no ASA. |
HL defined as >25 dB HL pure-tone thresholds | NR | 17.9% (24/134) had abnormal audiometry (post hoc 95% CI, 11.8%–25.4%) 71% (17/24) with abnormal audio had salicylate level > 1.42 mmol/1 |
No audiometric data were collected on the control group. | NR | Primary goal to determine if hearing symptoms could be used to titrate NSAID doses in RA patients Consecutive NSAID patients |
| Davis, 197934 | Cohort within a randomized controlled double-blind trial (n = 40) |
Age 22–68 years with RA | ASA 900 mg Q6h for 1 y or Pirprofen 200 mg Q6h for 1 y after a single blind placebo washout |
HL defined as a “threshold shift” | Prior to active drug, 20 w after, and at the study conclusion | 10% (2/18) of ASA subjects had new HL. 28% (5/18) of ASA group had progression of HL. Post hoc 95% CI, 1.4%–34.7% (new), 9.7%–53.5% (progression). |
Subjects were followed from their baseline | NR | 67% (12/18) of ASA subjects had either a CHL or SNHL upon entering the study. Hearing loss was a secondary outcome. 5 subjects withdrew due to Gl effects, urticarial rash, SNHL, and serum SGOT elevations. |
| Myers, 196538 | Prospective cohort (n = 25) |
Ages 13–78 years with RA or other connective-tissue diseases | ASA 6–8 g/d and magnesium aluminum hydroxide | Pure-tone thresholds measured at 250–8000 Hz and word discrimination tests | Audiogram was usually repeated 1 week after acute intoxication | 84% (21/25) had reversible bilateral symmetric HL; post hoc 95% CI, 63.4%–95.5%. | Subjects were compared to their own baseline | Effect present at 1 week | The threshold was associated with plasma salicylate level. Characteristic loss was 30–40 dB threshold worsening. |
| Heyworth, 197236 | Prospective cohort (n = 33) |
Age 26–83 years with RA 36% (12/33) had abnormal hearing thresholds in at least 1 frequency as baseline |
ASA (dosage NR) | HL defined as >20 dB below age-group threshold of Hinchecliffe’s presbycusis model. | Baseline then several days following cessation of ASA | 75% (9/12) had SNHL(95% CI, 42.8%–94.5%) 25% (3/12) had conductive HL |
Subjects were compared to their own baseline | NR | 11% (1/9) had Meniere’s. 11% (1/9) had acoustic trauma. 75% (9/12) noted hearing worsened after RA onset. Air/bone conduction; impedance, acoustic reflex, Fowler’s loudness balance test |
| Bernstein, 196733 | Prospective cohort (n = 12) |
Age 23–68 years with rheumatoid arthritis | ASA 6–8 g daily until patient subjectively reported hearing loss | HL defined as >25 dB on Bekesy tracking | 4 days | 100% (12/12) had HL that ranged from 25–45 dB. 42% (5/12) had SDS decreased ≥20%. |
Subjects were compared to their own baseline | NR | The magnitude of HL appeared to be related to the plasma salicylate level. HL was reversible. |
| Young, 198239 | Prospective cohort (n = 5) |
Age 22–38 years with normal hearing; 2 females had rheumatoid arthritis, 1 male had tendonitis. | ASA 4 × 2.24 mmol (1.6 g) Q5h × 1 d (n = 2) ASA 3 × 2.24 mmol (1.2 g) Q5h × 1 d (n=l) ASA 3 × 2.24 (1.2 g) mmol Q4h × 1 d (n = 2) |
HL defined as a shift in audiometric thresholds | 24 h and again within 24 h- 1 week period after first session | SDS decrease with −8dB SNR (16.4% difference from mean pre-ASA condition) |
Subjects were compared to their own baseline | NR | Results suggest that aspirin is capable of creating a loss of speech discrimination only when the signal is presented under very adverse listening conditions. |
Abbreviations:ASA, aspirin (acetylsalicylic acid); CHL, conductive hearing loss; Gl, gastrointestinal; HL, hearing loss; NR, not reported; NSAID, nonsteroidal anti-inflammatory drugs; RA, rheumatoid arthritis; SDS, speech discrimination scores; SGOT, serum glutamic oxaloacetic transaminase; SNHL, sensorineural hearing loss; SNR, signal to noise ratio; SRT, speech reception threshold.
Table 7.
Studies Describing the Impact of ASA on Self-reported Hearing Loss in Populations without Inflammatory Conditions.
| Author, Year | Study Design (sample size) | Patient Description | ASA Evaluation | Hearing Evaluation | Follow-up Time | ASA Exposure Results | Time from Exposure to Outcome | Additional Comments |
|---|---|---|---|---|---|---|---|---|
| Curhan, 201250 | Prospective observational cohort study (n = 62,261) Nurses Health Study II |
Age 31–48 years All women |
ASA Frequency of use categorized as < 1, 1,2–3,4–5, or ≥6 d/w |
Self-reported response to survey | Survey every 2 years from 1995 to 2009 | Multivariate adjusted analysis RR 1.07 (95% CI,0.97–1.18) for 2–3 d/w 0.98 (95% CI, 0.87–1.11) for 4–5 d/w 1.00 (95% CI, 0.94–1.07) for >6 d/w |
NR | Excluded if tinnitus ≥2 d/w or malignancy (non-melanoma skin cancer) |
| Curhan, 201051 | Prospective observational cohort study (n = 26,917) Health Professionals Study |
Age 40–74 years All men |
ASA Regular use defined as 2 or more times per week. |
Self-reported HL in response to survey question, | Survey every 2 years beginning in 1986 | Age and multivariable-adjusted HR 1.12 (95% CI, 1.04–1.20) for 2+ times/w |
NR | Excluded if malignancy (non-melanoma skin cancer) |
| Miller, 197852 | Prospective cohort study of consecutive inpatients (n = 35,931) BCDSP |
Male and female hospitalized medical patients in 22 hospitals in the US and abroad |
Long-acting ASA tablets: 0.09% (32/35,931) Plain ASA tablets: 7.3% (2613/35,931) |
“Clinically diagnosed deafness” | Data accumulated 1966–1975, other timeframes NR | Patients with “deafness”: Long-acting ASA: 28% (9/32) Plain ASA: 0.3% (9/2613) Patients without “deafness”: Long acting ASA: 72% (23/32) Plain ASA: 99.7% (2604/2613) Mean daily dose of ASA in patient with deafness was higher than in those without deafness (P < .005) |
NR | Patients who received buffered and enteric-coated ASA tablets were excluded. |
| Miller, 197753 | Prospective cohort study of consecutive inpatients (n = 26,294) BCDSP |
Hospitalized medical patients in 22 sites (US and abroad) | Plain ASA tablets (<0.6–4.8 g/d): 9.1% (2391/26294) | “Clinically diagnosed deafness” | Data from 1966 to 1977, other timeframes NR | 0.3% (8/2391) reported deafness 0.8% (20/2391) reported tinnitus |
NR | Patients who received buffered and enteric-coated ASA tablets were excluded. |
| Porter, 197754 | Prospective cohort study of consecutive inpatients (n = 32,812) BCDSP |
Patients in medical wards | ASA | Subjective report of “deafness” | NR | Reported “deafness” in 1.1% (33/2974) exposed to ASA Dose-dependent response suspected: <600 mg: 0% (0/312) 600–899 mg: 0.13% (3/2273) 900–1199mg: 4% (12/269) 1200+ mg: 15% (18/120) |
NR | Duration of “deafness”: 1 permanent, 26 reversible, 5 unknown |
Abbreviations: ASA, aspirin (acetylsalicylic acid); BCDSP, Boston Collaborative Drug Surveillance Program; HL, hearing loss; HR, hazard ratios using Cox proportional hazards regression models; NR, not reported; RR, relative risk.
Doses of 325 mg daily or less
There were no studies that specifically evaluated the impact of long-term low dose ASA in patients with rheumatoid arthritis or connective tissue diseases. While the aforementioned randomized controlled trial may have included patients taking 325 mg of ASA daily,37 any data specific to that total daily dose was merged with data from those who may have been taking more. Likewise, there was 1 cohort study whose study population included those ingesting less than 1 g daily but provided no audiometric results specific to the lower dose range. Therefore, among those with rheumatoid arthritis and connective tissue diseases, there are likewise no dose-specific data to guide audiometric decisions with regard to patients taking either 81 mg or 325 mg daily.
Aspirin-Associated Otoacoustic Emissions and Psychophysical Measurements
While formal pure-tone audiometry and speech scores are the most immediately interpretable metric for hearing loss, related results may also be quantified via evaluation of OAE or psychophysical measurements. Eight small studies evaluated the impact of ASA on OAE: 1 prospective placebo-controlled, double-blinded crossover study40 and 7 prospective cohort studies.23,41–46 These reports suggested a deleterious effect and are described in further detail in Appendix 3 (available at www.otojournal.org). Similarly, 5 prospective studies assessed the impact of ASA on psychophysical measurements, all of which suggested an adverse effect on hearing (Appendix 4, available at www.otojournal.org).24,36,47–49 When specified, follow-up times in these 13 studies was less than 3 weeks.
Self-reported Evaluation of Hearing Results Associated with Aspirin
The studies with the most impressive sample sizes and follow-up times focused on self-reported hearing loss; 7 studies had mixed results (Tables 8, 9).35,50–55 The largest prospective observational cohort study was conducted through the Nurses’ Health Study II and evaluated 62,261 adult women’s self-reported hearing loss and ASA consumption.50 Subjects responded to survey questions: “Do you have a hearing problem?” (no/mild/moderate/severe) and “If so, at what age did you first notice a change in hearing?” Subjects were grouped by self-reported frequency of analgesic use as assessed by questionnaire every 2 years from 1995 to 2009. Women who reported tinnitus >2 days per week were excluded from the analysis. Self-reported hearing results showed no association between ASA and hearing loss, regardless of the frequency of use (multivariate relative risk [RR] 1.07 [95% CI, 0.97–1.18] for 2 to 3 d/w; 0.98 [95% CI, 0.87–1.11] for 4 to 5 d/w; 1.00 [95% CI, 0.94–1.07] for ≥6 d/w). The results are limited to the frequency and duration of ASA and omit numeric dose information. A subanalysis of the present study using 2001 as the baseline evaluated “low-dose” versus “regular-dose” ASA and revealed no association between frequency of “low-dose” or “regular-dose” ASA and the risk of hearing loss. While “low-dose” and “high-dose” were not specifically defined within the report, review of the distributed questionnaires suggested that low-dose therapy was described as 100 mg or less per day. As these were self-reported ingestions and hearing perceptions, the participants were not blinded. These findings were not corroborated with formal audiometry.
Table 8.
Studies Describing the Impact of ASA on Self-reported Hearing Loss in Patients with Rheumatoid Arthritis.
| Author, Year | Study Design (sample size) | Patient Description | ASA Evaluation | Hearing Evaluation | Follow-up Time | ASA Exposure Results | Time from Exposure to Outcome | Additional Comments |
|---|---|---|---|---|---|---|---|---|
| Huskisson, 197474 | Prospective double-blind controlled study (n = 60) | Outpatients with classical or definite RA | ASA 6 g/d: (n = 31) Fenoprofen 2.4 g/d: (n = 29) |
Weekly for 4 weeks, monthly up to end of the twenty-fourth week of treatment | Patients assessed for side effects (mild, moderate, severe) | Week 1 : ASA: 66% (19/29) reported tinnitus/deafness Week 24: ASA:4I%reported tinnitus/deafness Fenoprofen: 3% (1/29) reported tinnitus/deafness Difference between Fenoprofen and ASA:χ2 = 7.50, P < .01 |
1 week | 3 patients didn’t complete study. |
| Halla, 198835 | Prospective controlled study (n = 316) | Exposure: ASA/salycilate for RA (n= 134,20–88 y, mean 58.3) Controls: healthy volunteers (n = 182,20–61 years, mean 34.3) |
“Regular” ASA use for > 1 w prior to study (≤1–≥4 g/d) Controls received no ASA. |
HL defined as >25 dB HL pure-tone thresholds | NR | ASA: 31% (42/134) had subjective hearing loss Control: 28% (51/182) had subjective hearing loss P = NS, post hoc |
NR | Primary goal to determine if hearing symptoms could be used to titrate NSAID doses in RA patients Consecutive NSAID patients |
Abbreviations: ASA, aspirin (acetylsalicylic acid); HL, hearing loss; NR, not reported; NSAID, nonsteroidal anti-inflammatory drugs; RA, rheumatoid arthritis.
Table 9.
Studies Evaluating Whether Aspirin Potentiates the Effect of Noise on Hearing.
| Author, Year | Study Design (sample size) | Patient Description | NSAID Evaluated | Hearing Evaluation | Follow-up Time | NSAID Exposure Results | No NSAID Exposure Results | Time from Exposure to Outcome | Additional Comments |
|---|---|---|---|---|---|---|---|---|---|
| Lindgren, 198656 | Prospective cohort (n = 8) | Age mean 19.3 years, male normal hearing (250 Hz–8 kHz) at the outset, exposed to noise (one-third octave band filtered noise with 2 kHz CF at 105 dB SPL for 10 min) | ASA 1 g 1 h prior to noise exposure in 5 sessions Control: no ASA |
2–8 kHz Bekesy sweep frequency audiometry | Baseline, 1 minute after noise exposure | TTS reduced in the fourth session only. Over all sessions, ASA had no effect on the TTS. | Subjects compared to their own baseline. | NR | Originally 10 subjects: 1 subject withdrew, 1 subject’s data lost to computer failure |
| McFadden, 198475 | Prospective crossover study (n = 11) | 11 males, 19–23 years old with normal hearing | ASA 3.9 g/d × 4d All sessions with intensity necessary for 10 min 2500 Hz tone to produce ~12 dB of TTS |
>5 dB shift in audiometric thresholds, as measured by a 2-interval forced choice method | 4 days | ASA with exposure to intense noise produced HL ~10–15 dB greater than that produced by exposure to the intense sound alone. | Subjects were compared to their own baseline and to their own performance in other drug regimens | 2.8 min | Moderate doses of ASA may increase risk of noise-induced hearing loss. |
| McFadden, 198357 | Prospective cohort (n = 4) | 4 volunteers (age, gender unspecified) exposed to 10 min 2500 Hz tone at intensity necessary to produce 14 dB TTS at baseline | ASA 1.95 or 3.9 g/d × 0.5–2.75 d | Adaptive psychophysical method with interval forced choice | Baseline, during, and 2 d after exposure | Exposures that ordinarily produce 14 dB TTS produce 18–27dB instead: effect is dose and duration dependent | Subjects were compared to their own baseline | 0–48 h | All hearing losses were reversed at 24–48 h after the exposure |
Abbreviations: ASA, aspirin (acetylsalicylic acid); CF, center frequency; HL, hearing loss; NR, not reported; NSAID, nonsteroidal anti-inflammatory drugs; SPL, sound pressure level;TTS, temporary threshold shift.
The second recent prospective observational cohort study was conducted through the Health Professionals Follow-up Study and evaluated 26,917 adult male patients with regard to ASA usage regularly (≥2×/w) or non-regularly (≤2×/w).51 Self-reported hearing loss was measured in response to the survey questions: “Have you been professionally diagnosed with hearing loss?” or “Do you feel you have a hearing loss?” The survey was given every 2 years beginning in 1986. The characterization of ASA usage is limited to the frequency and duration and does not describe specific dose information. The multivariate-adjusted hazard ratio revealed a statistically significant increase in risk of self-reported hearing loss with twice weekly consumption of ASA (1.12 [95% CI, 1.04–1.20]), in contrast to the results from the Nurses’ Health Study II. Since these were self-reported medications and symptoms, the participants were not blinded. The pure-tone thresholds and speech scores associated with the self-reported results were not investigated.
Also focusing on self-reported hearing symptoms were 3 prospective evaluations of consecutive inpatients from a program spanning 22 hospitals in the US and abroad. Self-reported hearing results were described in ASA exposed patients (<0.6–>4.8 g/d), but not the unexposed or pre-exposed, so no relative risks were reported.52–54 All demonstrated at least some percentage (0.3%–28%) of subjective hearing loss among ASA consumers. Again, these patients were aware of their ASA ingestion status and audiometry was not simultaneously reported.
Two studies evaluated the impact of ASA on self-reported hearing in rheumatoid arthritis patients.19,32 One was a prospective, double-blind, controlled study, which evaluated ASA (6 g/d) in comparison to fenoprofen (2.4 g/d) and found that the ASA group was significantly more likely to report tinnitus or hearing problems than the fenoprofen group.32 The other study prospectively evaluated subjects with “regular” ASA usage for rheumatoid arthritis in comparison to healthy controls.35 There was no significant difference between groups. This was the only study to describe concomitant self-reported and audiometric results in the setting of ASA usage. The self-reported prevalence of hearing loss was mildly higher than the audiometrically demonstrated sensorineural changes (31% vs 28%) in the ASA group. No audiometric data were obtained in the control group.
Impact of Aspirin on Hearing Changes after Noise Exposures
Three small studies evaluated whether concurrent ASA ingestion potentiates the hearing effect seen by noise with mixed results (Table 10).47,56,57 One study determined that noise resulted in worse temporary threshold shifts with ASA than without (18–27 dB vs 14 dB) and recommended that ASA should be avoided prior to loud exposures.57 The second study also supported a worse effect with ASA, while the third study suggested that ASA had no synergistic effect with noise.56
Table 10.
Studies with Audiometric Data Showing ASA Confers Protection from Gentamicin-Associated Hearing Loss.
| Author, Year | Study Design (sample size) | Patient Description | NSAID Evaluated | Hearing Evaluation | Follow-up Time | ASA Results | Placebo Results | Time from Exposure to Outcome | Additional Comments |
|---|---|---|---|---|---|---|---|---|---|
| Sha, 200659; Chen, 200760 | Randomized double-blind placebo-controlled trial (n = 195) | ASA: 76 males and 13 females Placebo: 95 males and 11 females scheduled for gentamicin 80–160 mg intravenous twice per day |
ASA 1 g Q8h ×14 d | Pure-tone audiograms; HL defined as 15 dB or more at 6 and 8k Hz | Day 1 of treatment, day of discharge, and 5–7 w after beginning treatment | 3% (3/89) had HL ≥ 15 dB at 6000 Hz and at 8000 Hz P = .013 |
13% (14/106) had HL ≥ 15 dB at 6000 Hz and at 8000 Hz | Not further specified | Severity of illness requiring gentamicin may have been a theoretical confounder. |
| Behnoud, 200958 | Randomized double-blind controlled trial (n = 60) | Age > 18 years, scheduled gentamicin 80 mg 3 times intravenous per day Exclusion criteria: preexisting HL, systemic disease, and pregnant |
Experimental group: ASA 1.5 g/d × 7 d Control group: given placebo |
PTA and SDS; HL defined as threshold shift of > 15 dB at 6 and 8 kHz | Baseline, 8, and 15 days after beginning treatment | 3% (1/30) had HL ≥ 15 dB at 4000 Hz (P = .001) 3% (1/30) had HL ≥ 15 dB at 8000 Hz (P = .0014) |
36% (11/30) had HL≥ 15 dB at 4000 Hz (P = .001) 20% (6/30) had HL ≥ 15 dB at 8000 Hz P=.0014) |
8, 15 days | ASA was also associated with better PTAs in other frequencies. Patients had similar gender, age, weight, at baseline. |
Abbreviations: ASA, aspirin (acetylsalicylic acid); HL, hearing loss; NSAID, nonsteroidal anti-inflammatory drugs; PTA, pure-tone average; SDS, speech discrimination scores.
Protective Effects of Aspirin When Co-Administered with Aminoglycoside Therapy
Remarkably, 2 of the most rigorous studies of the impact of ASA on audiometric outcomes showed that it had a protective effect on hearing, although when co-administered with an aminoglycoside. Two randomized double-blind placebo-controlled trials showed ASA significantly mitigated the hearing effects of intravenous gentamicin (Table 11).58,59 The larger of these (n = 195) evaluated the impact of ASA on pure-tone audiometry (6000, 8000 Hz) in adults receiving intravenous gentamicin therapy.59,60 Randomization was effective; age, sex, weight, and pretreatment audiometry were balanced between groups. The treatment group received ASA 1 g every 8 hours (3 g/d) for 14 days, while the control group received placebo on the same schedule. The ASA group had significantly fewer patients with high frequency hearing thresholds worse than 15 dB after treatment (3% vs 13%, P = .013).
Table 11.
Study Designs: Audiometric and Self-reported Outcomes.
| Author, Year | Randomized | Prospective | Blinding of Participants | Blinding of Assessors/Outcome Measurement | Hearing Is the Primary Outcome |
|---|---|---|---|---|---|
| Pure-tone threshold audiometry and speech discrimination scores associated with aspirin in subjects without inflammatory conditions | |||||
| Abdala, 200523 | — | x | — | — | x |
| Day, 198925 | — | x | x | x | x |
| Bonding, 197924 | — | x | — | — | x |
| Johnsen, 198226 | — | x | — | — | x |
| Pure-tone threshold audiometry and speech discrimination scores associated with aspirin in subjects with rheumatoid arthritis and connective tissue diseases | |||||
| Jardini, 197837 | x | x | x | x | x |
| Halla, 198835 | — | x | — | — | x |
| Davis, 197934 | — | x | x | x | — |
| Myers, 196538 | — | x | — | — | x |
| Heyworth, 197236 | — | x | — | — | x |
| Bernstein, 196733 | — | x | — | — | x |
| Young, 198239 | — | x | — | — | x |
| Self-reported evaluation of hearing results associated with aspirin in populations without inflammatory conditions | |||||
| Curhan, 201250 | — | x | — | — | x |
| Curhan, 201051 | — | x | — | — | x |
| Miller, 197852 | — | x | — | — | x |
| Miller, 197753 | — | x | — | — | x |
| Porter, 197754 | — | x | — | — | — |
| Self-reported evaluation of hearing results associated with aspirin in patients with rheumatoid arthritis | |||||
| Huskisson, 197474 | — | x | x | x | — |
| Halla, 198835 | — | x | — | — | x |
| Impact of aspirin on hearing changes after noise exposures | |||||
| Lindgren, 198656 | — | x | — | — | x |
| McFadden, 198475 | — | x | — | — | x |
| McFadden, 198357 | — | x | — | — | x |
| Protective effects of aspirin when co-administered with aminoglycoside therapy | |||||
| Sha, 200659; Chen, 200760 | x | x | x | x | x |
| Behnoud, 200958 | x | x | x | x | x |
The smaller trial (n = 60) evaluated the impact of ASA on pure-tone audiometry in normal-hearing adults scheduled for intravenous gentamicin treatment.58 Randomization resulted in similar sex, age, weight, and pure-tone thresholds in the experimental and control groups at the outset. The treatment group received 500 mg ASA every 8 hours (1.5 g/d) for 7 days, while the control group received placebo on the same schedule. Pure-tone audiometry and speech discrimination scores were tested at day 0, day 8, and day 15. Hearing results were again better in the ASA group (3.3% vs 20%–36% with thresholds >15 dB; P = .001 at 4000 Hz, P = .04 at 8000 Hz).
This protective effect of 1.5 to 3.0 g daily in the setting of gentamicin therapy in these 2 randomized studies appears paradoxical in the setting of the data demonstrating statistically significant worsening in pure-tone audiometry after administering similar doses of ASA alone.
Study Designs and Risk of Bias
This systematic review uncovered reports of a variety of study designs (Tables 12, 13). Randomized controlled trials were limited to 3 double-blinded studies in which hearing results were the primary outcome; 1 demonstrated a statistically significantly worse audiometric results in patients with rheumatoid arthritis who received at least 325 mg daily, while the other 2 showed a statistically significant protective effect when 1.5 to 3.0 g daily are administered to those undergoing intravenous gentamicin therapy. Nine studies employed blinding and hearing results were the primary outcome in the vast majority of studies.
Table 12.
Study Designs: Otoacoustic Emissions and Psychophysical Measurements.
| Author, Year | Randomized | Prospective | Blinding of Participants | Blinding of Assessors/Outcome Measurement | Hearing Is the Primary Outcome |
|---|---|---|---|---|---|
| Otoacoustic emissions associated with aspirin | |||||
| Brown, 199376 | — | x | x | x | x |
| Abdala, 200523 | — | x | — | — | x |
| Parazzini, 200541 | — | x | — | — | — |
| McFadden, 198446 | — | x | — | — | — |
| Long, 198842 | — | x | — | — | — |
| Rao, 201143 | — | x | — | — | — |
| Wier, 198944 | — | x | — | — | — |
| Hall, 200145 | — | x | — | — | — |
| Psychophysical measurements associated with aspirin | |||||
| Beveridge, 199649 | — | x | x | x | x |
| Carlyon, 199348 | — | x | x | x | x |
| McFadden, 198475 | — | x | — | — | x |
| Bonding, 197924 | — | x | — | — | x |
| McFadden, 198446 | — | x | — | — | x |
Discussion
The data uncovered by this systematic review were multifaceted. First, results confirmed conventional wisdom regarding high-dose ASA therapy; the preponderance of data suggested a deleterious effect when administered in doses at or exceeding 1.95 g daily in both those with and without rheumatoid arthritis and connective tissue disorders. Studies with statistically significant results described a magnitude of impact ranging from 4 dB to 112 dB, which was both dose-dependent and reversible, and follow-up periods were limited to less than a month.
Second, there were no studies that evaluated audiometry results in relation to ASA daily dosages of 81 or 325 mg. In addition, studies with follow-up times exceeding 20 weeks were limited to those with self-reported hearing outcomes without audiometric corroboration. There are no data reporting pure-tone thresholds or speech scores in patients undergoing long-term low-dose ASA therapy, and it is possible that those who seek medical care more frequently may be more likely to both take ASA and be aware of hearing deficits due to surveillance bias.61 No data exist to confirm conventional wisdom that low dose ASA administration over long periods does not have an impact on audiometry. In addition, it is unknown whether any such potential effect would still be reversible after an extended course, as the bulk of studies demonstrating reversibility have follow-up times of less than 1 month.
Third, the data regarding the concomitant exposure of ASA and other elements detrimental to hearing showed somewhat paradoxical results. While the data regarding simultaneous noise exposure were mixed, the data from randomized, placebo-controlled, double-blind trials with formal audiometry as the primary outcome measure demonstrated a protective effect of ASA on gentamicin ototoxicity at doses of 1.5 to 3.0 g daily.
Clinical Implications of the Results
Clinically, these findings suggest that with regard to hearing, ASA therapy should be tailored to specific clinical scenarios. In 1 scenario, a hearing-impaired patient ingesting ≥1.95 g of ASA daily would be expected to have an audiometric benefit from stopping the medication as the effects are typically reversible, at least in short-term studies. In a second, more common scenario of a patient with hearing loss or at risk of hearing loss who is ingesting 81 or 325 mg daily for the foreseeable future, there are no data to guide decisions regarding ASA usage; at this point, there are no data to support a trial off the medication to assess for a potentially reversible effect, so the proven cardiovascular protective effects would take precedent. In the less common third scenario where a patient requires gentamicin treatment, there is level 1 evidence to support the co-administration of ASA for improved audiometric results.
Multiple studies demonstrate that the effect of ASA on hearing is reversible, particularly when administered over a short timeframe.24,26–31,33,35,37,44,47,54,57 However, the duration of studied ASA therapy and follow-up times after ASA cessation in many instances are limited. Therefore, it remains unclear whether regimens of longer duration retain that reversible effect.
Potential Protective Effects of ASA on Hearing
While data suggest that ASA doses of 1.5 to 3 g daily worsen hearing when administered in isolation, when the same regimens are given with gentamicin, ASA becomes protective. This apparently paradoxically protective effect of ASA may occur through an antioxidant mechanism, which protects the outer hair cells from aminoglycoside-released reactive oxygen species that would otherwise subsequently result in apoptotic cochleotoxicity.60 Aminoglycosides displace calcium from its binding sites resulting in a restriction of calcium dependent physiological mechanisms in the inner ear, which impairs natural antioxidant generation.58 Additional research into the related NF-κB pathway may provide future insight into the protective mechanism.62 One wonders whether a study of ASA administration concomitant with intratympanic gentamicin for Meniere’s disease might also someday demonstrate a hearing-protective effect.
The ASA/gentamicin randomized controlled trials also raise the question of whether there might be other instances where ASA utilization might be of benefit. Accordingly, the potential for ASA to protect from cisplatin-related ototoxicity is under investigation in a randomized, phase II, double-blind, placebo-controlled, 2-arm trial.59,63,64
In addition, in a related topic not without controversy, there are mixed results regarding the impact of cardiovascular disease on hearing loss.65–69 Since ASA is recommended for cardiovascular prophylaxis,70,71 if there is a true association between cardiovascular disease and hearing loss, then ASA might be indirectly protective of hearing in some patients through preventive effects.
Potential Additional Effect Modifiers of the Impact of ASA on Hearing
The results of large prospective observational cohorts suggest that ASA may have a differential effect in males and females. These data are, however, currently based on self-reported hearing assessments without audiometric confirmation. Nonetheless, they were large-scale analyses with a decade of follow-up. Of note, patients with tinnitus more than twice weekly were removed from the female analysis, potentially removing patients with associated hearing loss in either the exposed or unexposed group. Future studies may help elucidate whether a true gender effect exists based on audiometric testing.
Similarly, some data suggest that eye color or an associated feature may alter the ASA-associated effect; dark-eyed subjects may show larger ASA-related threshold shifts than light-eyed listeners.47 Understanding any such effect modifiers may ultimately provide insight to allow for more selective counseling of individual patients.
Risk of Bias
The 3 randomized controlled trials and the preponderance of data as a whole suggest that ASA has an impact on hearing, either detrimental with higher doses in isolation or protective when co-administered with gentamicin. When considering these largely positive findings, the risk of publication bias must be considered72,73; it is possible that studies with negative findings were simply less likely to advance to publication.
Conclusions
ASA ingestion of ≥1.95 g per day is associated with worse audiometric results (4–112 dB threshold shift), and data suggest that the effect is dose-dependent and reversible in the short term. There are no audiometric data that confirm that long-term doses of 81 mg or 325 mg daily have no hearing consequences and no data to suggest that these doses should be stopped in the setting of hearing loss. Paradoxically, ASA (in doses shown to be detrimental in isolation) has a protective effect when co-administered with intravenous gentamicin. With the large-scale population utilization of aspirin for cardiovascular prophylaxis, the potential risks to hearing health should be considered for future longitudinal study, particularly given that short-term effects may be reversible.
Supplementary Material
Acknowledgments
J.J.S. would like to thank Peter H. Stone, MD, professor of medicine, Harvard Medical School and director, Vascular Profiling Research Group, Brigham and Women’s Hospital, for his critical review of the manuscript. JJS would also like to thank the Creating Healthcare Excellence through Education and Research network and Thomas Y. Lin for support during preparation of the manuscript. M.E.K. would like to thank Garrick C. Horn for support during preparation of the manuscript.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
This article was presented at the 2014 AAO-HNSF Annual Meeting & OTO EXPO; September 21–24, 2014; Orlando, Florida.
Author Contributions
Meghann Elizabeth Kyle, acquisition of data, analysis and interpretation of data, drafting and editing manuscript, final approval; James C. Wang, acquisition of data, analysis and interpretation of data, contributing to manuscript, final approval; Jennifer J. Shin, analysis and interpretation of data, acquisition of data, drafting and editing manuscript, final approval.
Disclosures
Competing interests: Jennifer J. Shin receives royalties from book publications: Evidence-based Otolaryngology (Springer International, 2008, 2010), Otolaryngology Prep and Practice (Plural Publishing, 2013).
Sponsorships: None.
Funding source: None.
Supplemental Material
Additional supporting information may be found at http://otojournal.org/supplemental.
References
- 1.Warner TD, Mitchell JA. Cyclooxygenase-3 (COX-3): filling in the gaps toward a COX continuum? Proc Natl Acad Sci U S A. 2002;99:13371–13373. doi: 10.1073/pnas.222543099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 3.Calonge NPD, DeWitt TG. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 4.Pleis JR, Lethbridge-Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Vital Health Stat. 2007;10:1–153. [PubMed] [Google Scholar]
- 5.Shargorodsky J, Curhan SG, Curhan GC, Eavey R. Change in prevalence of hearing loss in US adolescents. JAMA. 2010;304:772–778. doi: 10.1001/jama.2010.1124. [DOI] [PubMed] [Google Scholar]
- 6.Bainbridge KE, Wallhagen MI. Hearing loss in an aging American population: extent, impact, and management. Annu Rev Public Health. 2014;35:139–152. doi: 10.1146/annurev-publhealth-032013-182510. [DOI] [PubMed] [Google Scholar]
- 7.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg. 2014;150:378–384. doi: 10.1177/0194599813518021. [DOI] [PubMed] [Google Scholar]
- 8.Lin FR. Hearing loss in older adults: who’s listening? JAMA. 2012;307:1147–1148. doi: 10.1001/jama.2012.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federal-Interagency-Workgroup. Hearing and other sensory or communication disorders, Healthy People 2020. http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=20. Published 2012. Accessed September 29, 2014.
- 10.Walling A, Dickson G. Hearing loss in older adults. Am Fam Physician. 2012;85:1150–1156. [PubMed] [Google Scholar]
- 11.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171:1851–1852. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gates GA, Cooper JC, Jr, Kannel WB, Miller NJ. Hearing in the elderly: the Framingham cohort, 1983–1985. Part I. Basic audiometric test results. Ear Hear. 1990;11:247–256. [PubMed] [Google Scholar]
- 13.Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurootol. 2007;12:345–358. doi: 10.1159/000106478. [DOI] [PubMed] [Google Scholar]
- 14.Shin JJ, Randolph GW, Rauch SD. Evidence-based medicine in otolaryngology, part 1: the multiple faces of evidence-based medicine. Otolaryngol Head Neck Surg. 2010;142:637–646. doi: 10.1016/j.otohns.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Shin JJ, Stinnett SS. Introduction to Evidence-Based Medicine, Levels of Evidence, and Systematic Reviews. New York: Springer; 2008. [Google Scholar]
- 16.Crowther M, Lim W, Crowther MA. Systematic review and meta-analysis methodology. Blood. 2010;116:3140–3146. doi: 10.1182/blood-2010-05-280883. [DOI] [PubMed] [Google Scholar]
- 17.Rosenfeld RM, Shiffman RN. Clinical practice guidelines: a manual for developing evidence-based guidelines to facilitate performance measurement and quality improvement. Otolaryngol Head Neck Surg. 2006;135(suppl 4):S1–28. doi: 10.1016/S0194-5998(06)03094-4. [DOI] [PubMed] [Google Scholar]
- 18.Haynes RB. Of studies, syntheses, synopses, summaries, and systems: the “5S” evolution of information services for evidence-based health care decisions. ACP J Club. 2006;145:A8. [PubMed] [Google Scholar]
- 19.Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe D. Of apples and oranges, file drawers and garbage: why validity issues in meta-analysis will not go away. Clin Psychol Rev. 1997;17:881–901. doi: 10.1016/s0272-7358(97)00056-1. [DOI] [PubMed] [Google Scholar]
- 21.Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18:12–18. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- 22.Hartling L, Ospina M, Liang Y, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ. 2009;339:b4012. doi: 10.1136/bmj.b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdala C. Effects of aspirin on distortion product otoacoustic emission suppression in human adults: a comparison with neonatal data. J Acoust Soc Am. 2005;118:1566–1575. doi: 10.1121/1.1985043. [DOI] [PubMed] [Google Scholar]
- 24.Bonding P. Critical bandwidth in patients with a hearing loss induced by salicylates. Audiology. 1979;18:133–144. doi: 10.3109/00206097909072627. [DOI] [PubMed] [Google Scholar]
- 25.Day RO, Graham GG, Bieri D, et al. Concentration-response relationships for salicylate-induced ototoxicity in normal volunteers. Br J Clin Pharmacol. 1989;28:695–702. doi: 10.1111/j.1365-2125.1989.tb03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsen NJ, Elberling C. Evoked acoustic emissions from the human ear. I. Equipment and response parameters. Scand Audiol. 1982;11:3–12. doi: 10.3109/01050398209076194. [DOI] [PubMed] [Google Scholar]
- 27.Janssen T, Boege P, Oestreicher E, Arnold W. Tinnitus and 2f1–f2 distortion product otoacoustic emissions following salicylate overdose. J Acoust Soc Am. 2000;107:1790–1792. doi: 10.1121/1.428578. [DOI] [PubMed] [Google Scholar]
- 28.Jordan CE. Case history: asking the right questions. Ear Hear. 1991;12:363–364. [PubMed] [Google Scholar]
- 29.Koegel L., Jr Ototoxicity: a contemporary review of aminoglycosides, loop diuretics, acetylsalicylic acid, quinine, erythromycin, and cisplatinum. Am J Otol. 1985;6:190–199. [PubMed] [Google Scholar]
- 30.Jarvis JF. A case of unilateral permanent deafness following acetylsalicylic acid. J Laryngol Otol. 1966;80:318–320. doi: 10.1017/s0022215100065270. [DOI] [PubMed] [Google Scholar]
- 31.Ramsden RT, Latif A, O’Malley S. Electrocochleographic changes in acute salicylate overdosage. J Laryngol Otol. 1985;99:1269–1273. doi: 10.1017/s0022215100098510. [DOI] [PubMed] [Google Scholar]
- 32.Naganawa S, Ishihara S, Iwano S, Sone M, Nakashima T. Detection of presumed hemorrhage in the ampullar endolymph of the semicircular canal: a case report. Magn Reson Med Sci. 2009;8:187–191. doi: 10.2463/mrms.8.187. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein JM, Weiss AD. Further observations on salicylate ototoxicity. J Laryngol Otol. 1967;81:915–925. doi: 10.1017/s0022215100067852. [DOI] [PubMed] [Google Scholar]
- 34.Davis JD, Struth AG, Turner RA, Pisko EJ, Ruchte IR. Pirprofen and aspirin in the treatment of rheumatoid arthritis. Clin Pharmacol Ther. 1979;25:618–623. doi: 10.1002/cpt1979255part1618. [DOI] [PubMed] [Google Scholar]
- 35.Halla JT, Hardin JG. Salicylate ototoxicity in patients with rheumatoid arthritis: a controlled study. Ann Rheum Dis. 1988;47:134–137. doi: 10.1136/ard.47.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heyworth T, Liyanage SP. A pilot survey of hearing loss in patients with rheumatoid arthritis. Scand J Rheumatol. 1972;1:81–83. doi: 10.3109/03009747209103000. [DOI] [PubMed] [Google Scholar]
- 37.Jardini L, Findlay R, Burgi E, Hinderer K, Agarwal A. Auditory changes associated with moderate blood salicylate levels. Rheumatol Rehabil. 1978;17:233–236. doi: 10.1093/rheumatology/17.4.233. [DOI] [PubMed] [Google Scholar]
- 38.Myers EN, Bernstein JM. Salicylate ototoxicity; a clinical and experimental study. Arch Otolaryngol. 1965;82:483–493. doi: 10.1001/archotol.1965.00760010485006. [DOI] [PubMed] [Google Scholar]
- 39.Young LL, Jr, Wilson KA. Effects of acetylsalicylic acid on speech discrimination. Audiology. 1982;21:342–349. doi: 10.3109/00206098209072749. [DOI] [PubMed] [Google Scholar]
- 40.Brown RD, Henley CM, Penny JE, Kupetz S. Link between functional and morphological changes in the inner ear—functional changes produced by ototoxic agents and their interactions. Arch Toxicol Suppl. 1985;8:240–250. doi: 10.1007/978-3-642-69928-3_36. [DOI] [PubMed] [Google Scholar]
- 41.Parazzini M, Hall AJ, Lutman ME, Kapadia S. Effect of aspirin on phase gradient of 2F1–F2 distortion product otoacoustic emissions. Hear Res. 2005;205:44–52. doi: 10.1016/j.heares.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Long GR, Tubis A. Modification of spontaneous and evoked otoacoustic emissions and associated psychoacoustic microstructure by aspirin consumption. J Acoust Soc Am. 1988;84:1343–1353. doi: 10.1121/1.396633. [DOI] [PubMed] [Google Scholar]
- 43.Rao A, Long GR. Effects of aspirin on distortion product fine structure: interpreted by the two-source model for distortion product otoacoustic emissions generation. J Acoust Soc Am. 2011;129:792–800. doi: 10.1121/1.3523308. [DOI] [PubMed] [Google Scholar]
- 44.Wier CC, Pasanen EG, McFadden D. Partial dissociation of spontaneous otoacoustic emissions and distortion products during aspirin use in humans. J Acoust Soc Am. 1988;84:230–237. doi: 10.1121/1.396970. [DOI] [PubMed] [Google Scholar]
- 45.Hall AJ, Lutman ME. The effect of aspirin on human cochlear amplifiers. Br J Audiol. 2001;35:115–164. [Google Scholar]
- 46.McFadden D, Plattsmier HS. Aspirin abolishes spontaneous otoacoustic emissions. J Acoust Soc Am. 1984;76:443–448. doi: 10.1121/1.391585. [DOI] [PubMed] [Google Scholar]
- 47.McFadden D, Plattsmier HS, Pasanen EG. Aspirin-induced hearing loss as a model of sensorineural hearing loss. Hear Res. 1984;16:251–260. doi: 10.1016/0378-5955(84)90114-x. [DOI] [PubMed] [Google Scholar]
- 48.Carlyon RP, Butt M. Effects of aspirin on human auditory filters. Hear Res. 1993;66:233–244. doi: 10.1016/0378-5955(93)90143-o. [DOI] [PubMed] [Google Scholar]
- 49.Beveridge HA, Carlyon RP. Effects of aspirin on human psychophysical tuning curves in forward and simultaneous masking. Hear Res. 1996;99:110–118. doi: 10.1016/s0378-5955(96)00091-3. [DOI] [PubMed] [Google Scholar]
- 50.Curhan SG, Shargorodsky J, Eavey R, Curhan GC. Analgesic use and the risk of hearing loss in women. Am J Epidemiol. 2012;176:544–554. doi: 10.1093/aje/kws146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curhan SG, Eavey R, Shargorodsky J, Curhan GC. Analgesic use and the risk of hearing loss in men. Am J Med. 2010;123:231–237. doi: 10.1016/j.amjmed.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller RR. Deafness due to plain and long-acting aspirin tablets. J Clin Pharmacol. 1978;18:468–471. doi: 10.1002/j.1552-4604.1978.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 53.Miller RR, Jick H. Acute toxicity of aspirin in hospitalized medical patients. Am J Med Sci. 1977;274:271–279. doi: 10.1097/00000441-197711000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Porter J, Jick H. Drug-induced anaphylaxis, convulsions, deafness, and extrapyramidal symptoms. Lancet. 1977;1:587–588. doi: 10.1016/s0140-6736(77)92011-6. [DOI] [PubMed] [Google Scholar]
- 55.Rigby MH, Parnes LS. Profound hearing loss associated with oxycodone-acetaminophen abuse. J Otolaryngol Head Neck Surg. 2008;37:E161–162. [PubMed] [Google Scholar]
- 56.Lindgren F, Axelsson A. Temporary threshold shift induced by noise exposure and moderate salicylate intake. Scand Audiol Suppl. 1986;26:41–44. [PubMed] [Google Scholar]
- 57.McFadden D, Plattsmier HS. Aspirin can potentiate the temporary hearing loss induced by intense sounds. Hear Res. 1983;9:295–316. doi: 10.1016/0378-5955(83)90033-3. [DOI] [PubMed] [Google Scholar]
- 58.Behnoud F, Davoudpur K, Goodarzi MT. Can aspirin protect or at least attenuate gentamicin ototoxicity in humans? Saudi Med J. 2009;30:1165–1169. [PubMed] [Google Scholar]
- 59.Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Huang WG, Zha DJ, et al. Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hear Res. 2007;226:178–182. doi: 10.1016/j.heares.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 61.Sica GT. Bias in research studies. Radiology. 2006;238:780–789. doi: 10.1148/radiol.2383041109. [DOI] [PubMed] [Google Scholar]
- 62.Lecain E, Omri B, Behar-Cohen F, Tran Ba Huy P, Crisanti P. The role of PKCzeta in amikacin-induced apoptosis in the cochlea: prevention by aspirin. Apoptosis. 2007;12:333–342. doi: 10.1007/s10495-006-0580-0. [DOI] [PubMed] [Google Scholar]
- 63.Pathak RK, Marrache S, Choi JH, Berding TB, Dhar S. The prodrug platin-a: simultaneous release of Cisplatin and aspirin. Angew Chem Int Ed Engl. 2014;53:1963–1967. doi: 10.1002/anie.201308899. [DOI] [PubMed] [Google Scholar]
- 64.EUCTR2012-001509-25-GB. COAST-Cisplatin Ototoxicity attenuated by Aspirin Trial A randomised, Phase II, double-blind, placebo-controlled, two arm Trial to establish whether Aspirin can reduce hearing loss/ototoxicity for patients receiving Cisplatin chemotherapy.
- 65.Helzner EP, Patel AS, Pratt S, et al. Hearing sensitivity in older adults: associations with cardiovascular risk factors in the health, aging and body composition study. J Am Geriatr Soc. 2011;59:972–979. doi: 10.1111/j.1532-5415.2011.03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hutchinson KM, Alessio H, Baiduc RR. Association between cardiovascular health and hearing function: pure-tone and distortion product otoacoustic emission measures. Am J Audiol. 2010;19:26–35. doi: 10.1044/1059-0889(2009/09-0009). [DOI] [PubMed] [Google Scholar]
- 67.Oron Y, Elgart K, Marom T, Roth Y. Cardiovascular risk factors as causes for hearing impairment. Audiol Neurootol. 2014;19:256–260. doi: 10.1159/000363215. [DOI] [PubMed] [Google Scholar]
- 68.Park S, Johnson MA, Shea Miller K, De Chicchis AR. Hearing loss and cardiovascular disease risk factors in older adults. J Nutr Health Aging. 2007;11:515–518. [PubMed] [Google Scholar]
- 69.Shargorodsky J, Curhan SG, Eavey R, Curhan GC. A prospective study of cardiovascular risk factors and incident hearing loss in men. Laryngoscope. 2010;120:1887–1891. doi: 10.1002/lary.21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aspirin prophylaxis for cardiovascular disease. U.S. Preventive Services Task Force. Am Fam Physician. 1989;40:117–120. [PubMed] [Google Scholar]
- 71.Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]
- 72.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 73.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 74.Huskisson EC, Wojtulewski JA, Berry H, Scott J, Hart FD, Balme HW. Treatment of rheumatoid arthritis with fenoprofen: comparison with aspirin. Br Med J. 1974;1:176–180. doi: 10.1136/bmj.1.5900.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McFadden D, Plattsmier HS, Pasanen EG. Temporary hearing loss induced by combinations of intense sounds and nonsteroidal anti-inflammatory drugs. Am J Otolaryngol. 1984;5:235–241. doi: 10.1016/s0196-0709(84)80033-2. [DOI] [PubMed] [Google Scholar]
- 76.Brown AM, Williams DM, Gaskill SA. The effect of aspirin on cochlear mechanical tuning. J Acoust Soc Am. 1993;93:3298–3307. doi: 10.1121/1.405714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


