Abstract
Between 2% and 12% of adults in the general population report experiencing psychotic-like symptoms, and there is suggestive evidence that these symptoms are associated with risk of schizophrenia and other forms of psychopathology. Older parental age is an established risk factor for schizophrenia, however few studies have attempted to extend this relationship to psychotic-like symptoms. Data come from the National Comorbidity Survey-Replication and analysis is restricted to a subset of respondents who completed questions on psychosis (N=924). Lifetime occurrence of six psychotic-like symptoms (i.e., see a vision others couldn’t see, hear voices others couldn’t hear) was assessed by self-report. These symptoms were combined into a single binary (any vs. none) variable and analyzed using logistic regression, accounting for the complex survey design. Models were adjusted for age, sex, race/ethnicity, socioeconomic status, marital status, birth order, and history of mood, anxiety, and substance use disorders. Approximately 9% (n=103) of respondents reported at least one psychotic-like symptom. In fully-adjusted models, paternal age was significantly associated with experiencing psychotic-like symptoms (X2 = 13.34, p = 0.010). Relative to respondents whose fathers were aged 25 to 29 at the time of their birth, those with fathers aged >35 had 2.12 times higher odds (95% Confidence Interval: 1.08 – 4.16) of psychotic-like symptoms. There was no relationship between maternal age (younger or older) and psychotic-like symptoms (X2 = 0.54, p = .909). Older paternal, but not maternal, age at birth is associated with psychotic-like symptoms in adult offspring.
Keywords: psychosis, parental age, epidemiology, population, risk factors
1. Introduction
Worldwide prevalence estimates of schizophrenia range from .5% to 1% (Centers for Disease Control and Prevention, 2013; Messias et al., 2007). One of the most distinct clinical features of schizophrenia is the experience of positive psychotic symptoms, including delusions and hallucinations (National Institute of Mental Health, 2014). Although schizophrenia is a rare condition, experiences of psychotic-like symptoms (PLS) are relatively common in the general population. The lifetime prevalence of experiencing PLS in the general adult population ranges from 1.5% to 12% when ascertained using interview-administered questionnaires (Eaton et al., 1991), and estimates from self-administered questionnaires are often higher (Peters et al., 1999; Verdoux et al., 1998). While it is unclear how PLS relate to schizophrenia, there is suggestive evidence that these symptoms increase the risk of developing psychotic disorders, including schizophrenia, later in life (Hanssen et al., 2005). If risk factors for schizophrenia are shared with non-pathological experiences such as PLS, this would support the hypothesis that these symptoms lie on a continuum of risk with psychotic disorders more generally (Kaymaz et al., 2012; Schultze-Lutter, 2009). Finally, as schizophrenia is a rare disorder, if these more common symptoms are an early indicator of increased risk then investigating psychotic experiences in the general population may inform targeted intervention efforts (DeVylder et al., 2014b; Kaymaz et al., 2012).
Studies as early as the 1950s indicated older paternal age at birth was related to schizophrenia (Johanson, 1958). In the decades since these initial investigations, both advanced maternal age (generally defined as ≥35 years) (Gregory, 1959) and paternal age (generally defined as ≥40, but with more robust findings age ≥55 years) (Gregory, 1959; Malaspina et al., 2001; Sorensen et al., 2014; Zammit et al., 2003) have been associated with elevated risk of psychosis. However, many studies indicate that the observed association between advanced maternal age and schizophrenia is largely confounded by advanced paternal age (Byrne et al., 2003; Hare and Moran, 1979). In contrast, when controlling for paternal age, studies generally indicate that younger maternal age (generally defined as <20 years) is associated with increased risk of psychosis (El-Saadi et al., 2004; McGrath et al., 2014). Finally, some studies report a “J-shaped” relationship between paternal age and schizophrenia, with younger paternal age (<20 years) associated with modestly elevated risk, and advanced paternal age (≥45 years) associated with substantially elevated risk, relative to typical-age (e.g., 25 – 29) (McGrath et al., 2014).
The mechanisms underlying this purported relationship between parental age and schizophrenia risk are not well understood. Several recent studies have implicated de novo germ cell mutations (Byrne et al., 2003; Malaspina et al., 2001; Malaspina et al., 2002; Sipos et al., 2004), whereas others argue that this association is largely a function of selection factors (e.g., individuals with a genetic predisposition, have schizotypal personality traits, or who are socially reclusive may not have the opportunity to conceive children, particularly their first child, until later in life) (Jaffe et al., 2014; Pedersen et al., 2014; Petersen et al., 2011). The mechanisms underlying the association between younger parental, particularly younger maternal age, and risk of schizophrenia in offspring are also unclear. Again, several studies have pointed to the possibility of de novo mutations due to immature spermatids or from low activity of DNA repair or antioxidant enzymes (Malaspina, 2001), however environmental factors young parents often face (i.e., financial, residential, and/or relationship instability) are also likely relevant (McGrath et al., 2014).
While parental age is a well-established risk factor for the development of schizophrenia, few studies have attempted to extend these findings to PLS. The handful of studies that have investigated this relationship have found weak or null associations between parental age and elevated risk of PLS (Vreeker et al., 2013; Zammit et al., 2008). However, these reports have important limitations, including relatively young samples that have not fully passed through developmental risk period for delusions and hallucinations (Vreeker et al., 2013; Zammit et al., 2008), as well as examining only recent (e.g., past 6 months), not lifetime, occurrence of these symptoms (Zammit et al., 2008).
Building on this research, the aim of this study is to examine the relationship between parental age and risk of PLS in a nationally-representative sample of adults.
2. Methods
2.1. Data
Data come from the National Comorbidity Survey Replication (NCS-R), a nationally representative cross-sectional household survey of adults aged ≥18 conducted between 2001–2003. 9,282 respondents completed Part I of the survey, and the Part I sample was approximately 72% Non-Hispanic White, 12% Non-Hispanic Black, and 52% female; approximately one-third of respondents were aged 18–34 or aged 35–49, 21% were aged 50–64, and 16% were aged 65 and older. Part I was administered to all respondents, and included the World Health Organization Composite International Diagnostic Interview (WHO-CIDI), a fully-structured diagnostic instrument administered by lay interviewers (Kessler and Ustun, 2004). To reduce participant burden, only a subset of respondents (N=5,692) were asked Part II of the survey, which included assessments of risk factors. Additional details of the study design are described elsewhere (Kessler et al., 2004). This analysis is limited to respondents who had complete data on parental age at birth and psychotic-like symptoms (N= 924); the sample size is due to skip patterns in the survey that restricted the number of respondents who were asked all relevant items.
The NCS-R was approved by the IRB at the University of Michigan and all respondents provided informed consent.
2.2. Exposure
Maternal and paternal age at the time of respondents’ birth were assessed by self-report. Consistent with prior studies and accounting for the range of these variables in the sample (Byrne et al., 2003; El-Saadi et al., 2004; Malaspina et al., 2001; McGrath et al., 2014; Petersen et al., 2011), maternal and paternal ages were categorized as 13–19 years, 20–24 years, 25–29 years, 30–34 years old, and ≥35 years. Due to a limited number of respondents with mothers in the oldest age group (n=41), the ≥35 and 30 – 34 categories were collapsed. The 25–29 year age group was used as the reference group for analysis for both maternal and paternal age.
2.3. Outcome
Lifetime occurrence of six PLS were assessed by self-report using the WHO-CIDI Non-Affective Psychosis (NAP) screener: “Did you…” 1) Ever see a vision others couldn’t see, 2) Ever hear voices others couldn’t hear, 3) Ever have a mind control experience, 4) Ever feel your mind taken over by strange forces, 5) Ever experience communication attempts from strange voices, and 6) Ever experience an unjust plot to harm you or have people follow you. Respondents were instructed to only report on symptoms that were experienced while they were alert (e.g., not while sleeping, dreaming, or under the influence of drugs and alcohol). Each item was assessed as a dichotomous (ever/never) variable, which were then combined into a single dichotomous variable indicating any symptoms vs. no symptoms for analysis.
2.4. Confounders
Covariates included age at interview (in years), sex, race, education, household income, marital status, birth order, and psychiatric disorders. Race was categorized as White (reference group), Black, and other (predominantly Hispanic). Education was dichotomized as less than high school (reference group) vs. high school graduate or more. Household income categorized <$30,000/year (reference group), $30,000-$60,000/ year, and >$60,000/year based on the distribution of this variable in the sample. Marital status was dichotomized as currently married (reference group) vs. not currently married (e.g., divorced, widowed, never married).
Prior reports have found that risk of schizophrenia was only associated with paternal age for first-born children of older fathers, but not later-born children of older fathers (Petersen et al., 2011), and thus birth order was included as a covariate as a continuous variable indicated by the number of full siblings older than the respondent (e.g., only children and first-born=1, one older sibling=2, etc.).
PLS are more common among individuals with a history of psychopathology, including depressive, anxiety, and substance use disorders (DeVylder et al., 2014a; Johns et al., 2004; Stochl et al., 2014). Thus we included these lifetime history of these conditions, assessed according to DSM-IV criteria using the WHO-CIDI, as covariates. Depressive disorders included major depressive disorder (with hierarchy) and dysthymia (with hierarchy). Anxiety disorders included generalized anxiety disorder (with hierarchy), social phobia, panic disorder, and posttraumatic stress disorder. Substance use disorders included both drug and alcohol abuse and dependence.
2.5. Analysis
We examined differences in demographic characteristics for respondents who reported at least one PLS as compared to those who reported none using Rao-Scott Chi-square tests for categorical variables and t-tests for continuous variables. We also examined gender differences in the experiences of specific psychotic-like symptoms using this approach. Logistic regression was used to assess the relationship between parental age and PLS. Separate models were fitted for maternal age, paternal age, and finally maternal and paternal age in the same model. Two sets of regression models were fit. In the first set, models were adjusted for demographic characteristics and birth order. In the second set, lifetime history of depressive, anxiety, and substance use disorders were included as additional covariates. Point estimates and standard errors were analyzed using survey procedures in SAS (v9.4) to account for the complex sampling design. The C-statistic, an index of model predictive capability (e.g., the degree to which the model correctly classifies observations according to the outcome), was used to compare models performance. The C-statistic ranges from 0.5 to 1 and values >0.7 indicate adequate predictive capability (Hosmer and Lemeshow, 2000).
3. Results
Nearly 9% (n=103) of respondents reported experiencing at least one PLS (Table 1). Of those who reported experiencing at least one symptom, mean paternal age was 26.50 and mean maternal age was 23.56. Of those who reported never experiencing any symptoms, mean paternal age was 27.12 and mean maternal age was 24.61. Paternal age and maternal were highly correlated (r = 0.81, p < .001). Respondents who reported at least one PLS were more likely to be female (X2 = 8.67, p = .003) and more likely to identify as Black or other race/ethnicity (X2 = 14.46, p < .001) relative to those who reported no PLS. Respondents who reported at least one PLS had lower income (X2 = 11.94, p = .003) and less education (X2 = 9.05, p = .003) than respondents who reported no symptoms. Respondents who reported experiencing at least one PLS were also more likely to have lifetime DSM-IV depressive disorders (X2 = 9.68, p = .002) and anxiety disorders (X2 = 14.48, p = <.001) than respondents who reported experiencing no symptoms. Overall, there were no significant differences in age, marital status, birth order, paternal age, maternal age, and history of substance use disorders between respondents who reported at least one symptom as compared to those who reported none.
Table 1.
Demographic characteristics of the National Comorbidity Study-Replication: 2001–2003
| Never experienced psychotic-like symptoms | Experienced at least one psychotic-like symptom | X2 or T, p-value | |
|---|---|---|---|
|
| |||
| N=924 | 821 | 103 | |
|
| |||
| Age (M, SE) | 32.26 (0.52) | 31.80 (1.40) | 1.73, .086 |
|
| |||
| Gender | |||
| Male | 379 (51.34) | 31 (30.83) | 8.67, .003 |
| Female | 442 (48.66) | 72 (69.17) | |
|
| |||
| Race | |||
| White | 615 (75.24) | 60 (48.67) | 14.46, <.001 |
| Black | 80 (10.41) | 21 (20.95) | |
| Other | 126 (14.34) | 22 (30.38) | |
|
| |||
| Household income | |||
| <$30k | 192 (23.70) | 45 (47.25) | 11.94, .003 |
| $30–60k | 241 (28.62) | 35 (25.69) | |
| >$60k | 388 (47.68) | 23 (27.06) | |
|
| |||
| Education | |||
| <12 years | 72 (10.30) | 19 (26.75) | 9.05, .003 |
| 12+ years | 749 (89.70) | 84 (73.25) | |
|
| |||
| Marital Status | |||
| Married | 456 (52.36) | 49 (48.05) | 0.30, .586 |
| Not Married | 365 (47.64) | 54 (51.95) | |
|
| |||
| Birth Order (M, SE) | 2.04 (0.05) | 1.91 (0.12) | 0.93, .352 |
|
| |||
| Paternal Age (years) | |||
| 13–19 | 62 (5.51) | 9 (8.80) | 8.39, .078 |
| 20–24 | 227 (30.91) | 35 (34.06) | |
| 25–29 | 270 (31.07) | 33 (27.61) | |
| 30–34 | 178 (22.70) | 14 (10.90) | |
| ≥35 | 84 (9.82) | 12 (18.64) | |
|
| |||
| Maternal Age (years) | |||
| 13–19 | 129 (14.83) | 27 (23.21) | 2.89, .409 |
| 20–24 | 318 (40.97) | 37 (34.12) | |
| 25–29 | 209 (24.82) | 20 (22.15) | |
| ≥ 30 | 165 (19.38) | 19 (20.52) | |
|
| |||
| Lifetime DSM-IV Diagnosis | |||
| Depressive disorder | 202 (14.67) | 39 (27.83) | 9.68, .002 |
| Anxiety disorder | 260 (18.99) | 50 (38.57) | 14.48, <.001 |
| Substance use disorder | 165 (13.97) | 26 (20.71) | 2.54, .111 |
Values are N (weighted percentage) unless otherwise noted.
Table 2 describes the frequencies of specific PLS and total counts of symptoms overall and stratified by sex. The most commonly reported symptoms were visual (6.02%, n = 72) and auditory (4.03%, n = 45) hallucinations. Experiencing mind control, mind takeover, communication attempts from strange forces, and an unjust plot to harm were much less common (0.25%, 0.23%, 0.52%, and 0.81%, respectively). Overall, women were more likely than men to report any symptoms (12.14% vs. 5.52%, X2 = 8.67, p = .003), however there were gender differences in the specific type of symptoms reported. Women were more likely to report experiencing visions (8.67% vs. 3.31%, X2 = 8.98, p = .003), hearing voices (5.43% vs. 2.60%, X2 = 3.45, p = .063), and experiencing unjust plots to harm (1.14% vs. 0.47%, X2 = 1.74, p = .187), but men were more likely to report experiencing mind control (0.28% vs. 0.23%, X2 = 0.03, p = .860), mind takeover (0.28% vs. 0.19%, X2 = 0.131, p = .717), and communication attempts from strange forces (0.59% vs. 0.45%, X2 = 0.14, p = .710). Additionally, women were more likely to report experiencing two or more symptoms relative to men (3.23%, n = 22 of women vs. 1.22%, n = 7 of men, X2 = 10.19, p = .006).
Table 2.
Distribution of specific psychotic-like symptoms by gender
| Overall | Men | Women | |
|---|---|---|---|
|
| |||
| N | 924 | 410 | 514 |
|
| |||
| Age (M, SE) | 32.21 (0.48) | 31.71 (0.70) | 32.71 (0.69) |
|
| |||
| Specific Psychotic-Like Symptoms | |||
| See visions | 72 (6.02) | 18 (3.31) | 54 (8.67) |
| Hear voices | 45 (4.03) | 12 (2.60) | 33 (5.43) |
| Mind control | 3 (0.25) | 2 (0.28) | 1 (0.23) |
| Mind takeover | 4 (0.23) | 3 (0.28) | 1 (0.19) |
| Communication attempts | 7 (0.52) | 4 (0.59) | 3 (0.45) |
| Plot to harm | 9 (0.81) | 3 (0.47) | 6 (1.14) |
|
| |||
| Count of Symptoms | |||
| None | 821 (91.14) | 379 (94.48) | 442 (87.86) |
| One | 74 (6.62) | 24 (4.30) | 50 (8.91) |
| Two or More | 29 (2.24) | 7 (1.22) | 22 (3.23) |
Values are N (weighted percentage) unless otherwise noted.
Table 3 shows the results of two adjusted logistic regression models predicting experience of at least one PLS from paternal age. In Model 1, paternal age was significantly associated with reporting at least one psychotic-like symptom (X2 = 10.48, p = 0.033). Although the confidence bands included one, compared to respondents whose fathers were aged 25 to 29 years at the time of their birth, respondents with fathers in the oldest age group (aged ≥35 years) (OR (Odds Ratio): 1.89, 95% CI (Confidence Interval): 0.97–3.67) were more likely to experience PLS. Men and respondents with an income of >$30,000 were significantly less likely to report experiencing symptoms. Additionally adjusting these estimates for maternal age attenuated the effect of paternal age overall to marginal significance (X2 = 6.53, p = .163). In contrast, there was no significant relationship between maternal age at birth and psychotic-like symptoms (X2 = 0.68, p = .877) (Supplemental Table 1). Maternal age findings were unchanged when paternal age was added as a covariate (X2 = 1.46, p = .692).
Table 3.
Logistic regression of paternal age and offspring report of psychotic-like symptoms
| Model 1 | Model 2 | |||
|---|---|---|---|---|
|
| ||||
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
|
| ||||
| Paternal Age | 0.033 | 0.010 | ||
| 13–19 | 1.28 (0.34– 4.80) | 1.08 (0.33–3.48) | ||
| 20–24 | 1.15 (0.52–2.56) | 1.28 (0.62–2.64) | ||
| 25–29 | 1.0 (ref) | 1.0 (ref) | ||
| 30–34 | 0.61 (0.25–1.53) | 0.63 (0.25–1.60) | ||
| ≥35 | 1.89 (0.97–3.67) | 2.12 (1.08–4.16) | ||
|
| ||||
| Age | 1.01 (0.99–1.04) | 0.335 | 1.01 (0.98–1.03) | 0.628 |
|
| ||||
| Gender | 0.002 | 0.008 | ||
| Male | 1.0 (ref) | 1.0 (ref) | ||
| Female | 2.27 (1.34–3.85) | 2.18 (1.23–3.88) | ||
|
| ||||
| Race | 0.063 | 0.004 | ||
| White | 1.0 (ref) | 1.0 (ref) | ||
| Black | 2.06 (0.91–4.70) | 2.86 (1.24–6.60) | ||
| Other | 2.49 (1.05–5.91) | 3.07 (1.36–6.91) | ||
|
| ||||
| Income | 0.036 | 0.059 | ||
| <30k | 1.0 (ref) | 1.0 (ref) | ||
| 30–60k | 0.55 (0.25–1.21) | 0.50 (0.22–1.15) | ||
| >60k | 0.35 (0.15–0.78) | 0.36 (0.16–0.84) | ||
|
| ||||
| Education | 0.109 | 0.117 | ||
| 0–11 years | 1.0 (ref) | 1.0 (ref) | ||
| 12+ years | 0.50 (0.21–1.17) | 0.49 (0.20–1.19) | ||
|
| ||||
| Marital Status | 0.139 | 0.058 | ||
| Married | 1.0 (ref) | 1.0 (ref) | ||
| Not Married | 0.64 (0.35–1.16) | 0.57 (0.32–1.02) | ||
|
| ||||
| Birth Order | 1.03 (0.88–1.22) | 0.693 | 1.04 (0.88–1.22) | 0.665 |
|
| ||||
| Lifetime DSM-IV Diagnosis | ||||
| Depressive disorder | 2.16 (1.33–3.52) | 0.002 | ||
| Anxiety disorder | 2.26 (1.45–3.52) | <0.001 | ||
| Substance use disorder | 1.61 (0.81–3.21) | 0.173 | ||
|
| ||||
| C-Statistic | 0.681 | 0.720 | ||
Odds Ratios are adjusted for all variables in the model. CI: Confidence Interval. Estimates and standard errors are weighted and corrected for the complex survey design.
After accounting for history of DSM-IV diagnoses, (Model 2) paternal age was significantly associated with reporting at least one PLS (X2 = 13.34, p = 0.010). Compared to respondents whose fathers were aged 25–29 years at the time of their birth, respondents with fathers aged ≥35 years were significantly more likely to experience symptoms (OR: 2.12, 95% CI: 1.08–4.16). Although not statistically significant, respondents with fathers aged 30 to 34 were less likely to report experiencing symptoms (OR: 0.63, 95% CI: 0.25–1.60) compared to fathers aged 25 to 29 years at time of birth. Again, adjusting these estimates for maternal age attenuated the effect of paternal age overall to marginal significance (X2 = 7.33, p = .120) and there was no significant relationship between maternal age at birth and PLS(X2 = 0.54, p = .909) (Supplemental Table 1). Maternal age findings were unchanged when paternal age was added as a covariate (X2 = 1.68, p = .641).
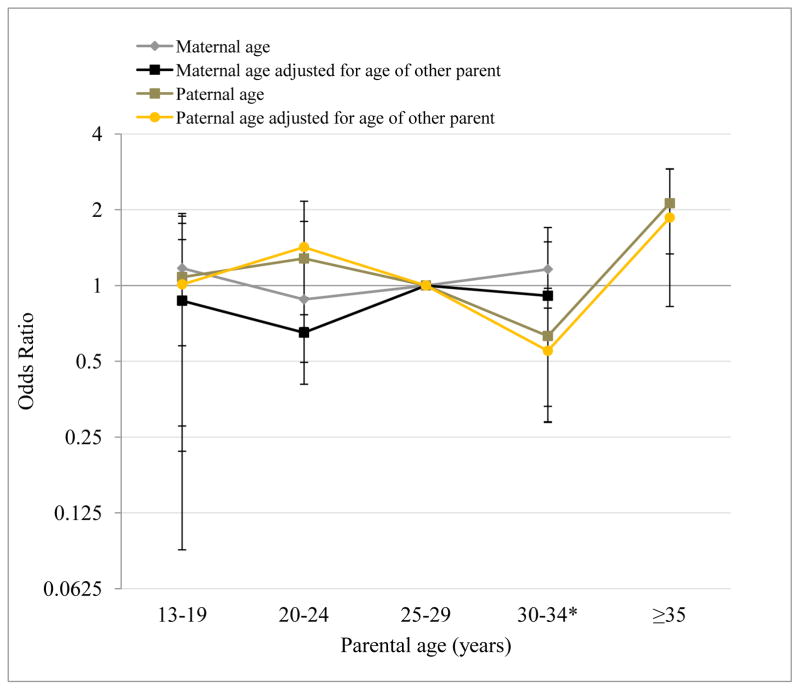
Figure 1 displays the relative odds of reporting at least one PLS by parental age categories, adjusting for all covariates in Model 2. The plot indicates that the relationship between paternal age and PLS is non-linear, providing suggestive evidence for a J-shaped relationship as hypothesized by prior reports.
Figure 1. Odds of experiencing one or more psychotic-like symptom by parental age.
Odds ratio and standard error estimates for the relationship between maternal and paternal age and experience of psychotic-like symptoms in adult offspring. National Comorbidity Study Replication (2001–2003) (N=924).
*30 to 34 age group includes ages 30 to 44 for maternal age.
4. Discussion
Advanced paternal age at birth, but not maternal age, is associated with greater likelihood of experiencing PLS in adult offspring. Our findings provide suggestive evidence for the hypothesized “J-shaped” relationship between paternal age and PLS, similar to that reported by McGrath and colleagues (2014) for schizophrenia, with the offspring of fathers aged 30 to 34 at the least risk and offspring of the oldest fathers (≥35) at the greatest risk compared to fathers aged 25 to 29. The overall association between paternal age and offspring symptoms was attenuated after accounting for maternal age. Consistent with prior reports (Byrne et al., 2003; Sorensen et al., 2014; Zammit et al., 2003), neither younger nor older maternal age was significantly related to offspring report of PLS after accounting for paternal age.
Our findings are broadly in line with epidemiologic work on paternal age and risk of schizophrenia, which has generally found that advanced paternal, but not advanced maternal, age is associated with elevated risk for schizophrenia (Byrne et al., 2003; El-Saadi et al., 2004; Hare and Moran, 1979; McGrath et al., 2014; Petersen et al., 2011; Sorensen et al., 2014; Zammit et al., 2003). Our results are consistent with large Scandinavian population-based studies using registry data that have found that maternal age is not related to psychotic symptomology after accounting for paternal age (Byrne et al., 2003; Sorensen et al., 2014; Zammit et al., 2003).
Results should be interpreted in light of study limitations. First, we were unable to control for paternal history of psychiatric disorders and parental age at birth of first-born (only birth order), allowing the possibility of residual confounding. Secondly, our measure of PLS is not a clinical diagnosis, and it is unresolved how these symptoms are related to risk of clinically-significant psychopathology. In their review of psychotic experiences in the general population, Stip and Letourneau (2009) suggest that psychosis may be a part of the typical human experience and only qualifies as an illness when it causes functional impairment. Furthermore, the recent interest in dimensional as opposed to diagnostic approaches to psychopathology more generally, including psychosis (Cuthbert and Insel, 2010), may be informative for both understanding the neurobiology as well as promote early detection and intervention of psychosis (Schultze-Lutter, 2009). Our finding that history of depressive, anxiety, and substance use disorders were associated with higher odds of PLS suggests these symptoms may have implications for clinical intervention, consistent with several prior reports (DeVylder et al., 2014a; Johns et al., 2004; Stochl et al., 2014).
There is also the potential for measurement error, particularly underreporting, of PLS (DeVylder and Hilimire, 2014; Kelleher et al., 2011). Experiences of psychotic-like symptoms were determined using the WHO-CIDI NAP screener, which validation studies have demonstrated greatly reduce false positive reports compared to screeners used in previous epidemiologic surveys (Kendler et al., 1996; Kessler et al., 2005). However, individuals with a history of schizophrenia are less likely to participate in survey research, resulting in non-response bias (Allgulander, 1989; Kessler et al., 2005). As long as this measurement error is non-differential with respect to parental age our estimates would be biased toward, rather than away, from the null. In addition, our sample only had a small number of offspring with fathers ≥45 years old, and thus we could not examine this relationship with precision for this group (McGrath et al., 2014). This study also has a number of strengths. Respondents were drawn from a large, population-based sample and our weighted estimates allowed us to infer how parental age is related to PLS in the US adult population as a whole. We were able to account for important confounders, including birth order and psychiatric comorbidity, which have been implicated by prior research.
While our findings are consistent with a growing body of research that points to advanced paternal age as a risk factor for schizophrenia (McGrath et al., 2014), more research is needed to understand how PLS are related to these severe forms of psychopathology. Others have suggested that these symptoms may lie on a continuum with psychotic disorders (Krabbendam et al., 2005; Stip and Letourneau, 2009), however without additional information (e.g., family history of psychosis, assessment of schizotypal or borderline personality) we cannot directly test this hypothesis in our data. Our findings do suggest that such an investigation is worthwhile; because PLS are substantially more common than schizophrenia, population-based investigations of the predictors and correlates of these symptoms may provide another avenue to identify risk factors for schizophrenia rather than relying only on clinically-identified cases from registry data.
Supplementary Material
Acknowledgments
This research used the National Comorbidity Survey Replication (NCS-R) data set. The NCS-R is part of the Collaborative Psychiatric Epidemiology Surveys (CPES). The Inter-university Consortium for Political and Social Research (Ann Arbor, Michigan) is responsible for the preparation, organization, and access of the public use of this data.
Funding
B. Mezuk is supported by a grant from the National Institute of Mental Health (K01-MH093642). The NCS-R was supported by the national Institute of Mental Health (U01-MH60220) with supplemental support from the National Institute of Drug Abuse, the Substance Abuse and Mental Health Services Administration, the Robert Wood Johnson Foundation (044708) and the John W. Alden Trust.
Footnotes
Author contributions
J. Foutz developed the concept for the paper, conducted the analysis, and wrote the first draft of the manuscript. B. Mezuk supervised the analysis and edited the manuscript. Both authors approve the final version of this paper for submission.
Disclosure
The authors have no conflicts of interest to disclose.
The sponsors had no role in the design, analysis, or interpretation of the findings. The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allgulander C. Psychoactive drug use in a general population sample, Sweden: Correlates with perceived health, psychiatric diagnoses, and mortality in an automated record-linkage study. Am J Public Health. 1989;79(8):1006–1010. doi: 10.2105/ajph.79.8.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M, Agerbo E, Ewald H, Eaton WW, Mortesen P. Parental age and risk of schizophrenia. Arch Gen Psychiat. 2003;60:673–678. doi: 10.1001/archpsyc.60.7.673. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: The NIMH Research Domain Criteria Project. Schizophr Bull. 2010;36(6):1061–1062. doi: 10.1093/schbul/sbq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVylder JE, Burnette D, Yang LH. Co-occurrence of psychotic experiences and common mental health conditions across four racially and ethnically diverse population samples. Psychol Med. 2014a;44:3503–3513. doi: 10.1017/S0033291714000944. [DOI] [PubMed] [Google Scholar]
- DeVylder JE, Hilimire MR. Screening for psychotic experiences: social desirability biases in a non-clinical sample. Early Interv Psychiatry. 2014 doi: 10.1111/eip.12161. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- DeVylder JE, Oh HY, Corcoran CM, Lukens EP. Treatment seeking and unmet need for care among persons reporting psychosis-like experiences. Psychiatric Services. 2014b;65(6):774–780. doi: 10.1176/appi.ps.201300254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Romanoski A, Anthony J, Nestadt G. Screening for psychosis in the general population with self-report interview. J Nerv Ment Dis. 1991;179(11):689–693. doi: 10.1097/00005053-199111000-00007. [DOI] [PubMed] [Google Scholar]
- El-Saadi O, Pedersen CB, McNeil TF, Saha S, Welham J, O’Callaghan EO, Cantor-Graae E, Chant D, Mortensen PB, McGrath J. Paternal and maternal age as risk factors for psychosis: findings from Denmark, Sweden and Australia. Schizophr Res. 2004;67:227–236. doi: 10.1016/S0920-9964(03)00100-2. [DOI] [PubMed] [Google Scholar]
- Gregory I. Factors influencing first admission rates to Canadian mental hospitals: III; an analysis by education, marital status, country of birth, religion, and rural-urban residence, 1950–1952. Can J Psychiat. 1959;4:133–151. doi: 10.1177/070674375900400209. [DOI] [PubMed] [Google Scholar]
- Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J. The incidence and outcome of subclinical psychotic experiences in the general population. Brit J Clin Psychol. 2005;44:181–191. doi: 10.1348/014466505X29611. [DOI] [PubMed] [Google Scholar]
- Hare EH, Moran PA. Raised parental age in psychiatric patients: evidence for the constitutional hypothesis. Brit J Psychiat. 1979;134:169–177. doi: 10.1192/bjp.134.2.169. [DOI] [PubMed] [Google Scholar]
- Health and Education: Mental Health Information. [Accessed April 27, 2014];National Institute of Mental Health website. http://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml#part4.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2. John Wiley & Sons; New York: 2000. [Google Scholar]
- Jaffe AE, Eaton WW, Straub RE, Marenco S, Weinberger DR. Paternal age, de novo mutations and schizophrenia. Mol Psychiatry. 2014;19:274–275. doi: 10.1038/mp.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson E. A study of schizophrenia in the male. Acta Psychiat Scand. 1958;33(suppl 125):7–107. [PubMed] [Google Scholar]
- Johns LC, Cannon M, Singleton N, Murray RM, Farrell M, Brugha T, Bebbington P, Jenkins R, Meltzer H. Prevalence and correlates of self-reported psychotic symptoms in the British population. Br J Psychiatry. 2004;185:298–305. doi: 10.1192/bjp.185.4.298. [DOI] [PubMed] [Google Scholar]
- Kaymaz N, Drukker M, Lieb R, Wittchen HU, Werbeloff N, Weiser M, Lataster T, van Os J. Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42:2239–2253. doi: 10.1017/S0033291711002911. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Harley M, Murtagh A, Cannon M. Are screening instruments valid for psychotic-like experiences? A validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull. 2011;37(2):262–269. doi: 10.1093/schbul/sbp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gallagher TJ, Abelson JM, Kessler RC. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. Arch Gen Psychiatry. 1996;53:1011–1031. doi: 10.1001/archpsyc.1996.01830110060007. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Chiu WT, Demler O, Heeringa S, Hiripi E, Jin R, Pennell BE, Walters EE, Zasalvsky A, Zheng H. The US National Comorbidity Survey Replication (NCS-R): design and field procedures. Int J Methods Psychiatr Res. 2004;13(2):69–92. doi: 10.1002/mpr.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Birnbaum H, Demler O, Falloon IRH, Gagnon E, Guyer M, Howes MJ, Kendler KS, Shi L, Walters E, Wu EQ. The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R) Biol Psychiatry. 2005;58:668–767. doi: 10.1016/j.biopsych.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Ustun TB. The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbendam L, Myin-Germeys I, Bak M, van Os J. Explaining transitions over the hypothesized psychosis continuum. Aust N Z J Psychiatry. 2005;39:180–186. doi: 10.1080/j.1440-1614.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- Malaspina D. Paternal factors and schizophrenia risk: de novo mutations and imprinting. Schizophr Bull. 2001;27(3):379–393. doi: 10.1093/oxfordjournals.schbul.a006882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Friedman J, Yale S, Goetz D, Goetz R, Harlap S, Gorman J. Paternal age and sporadic schizophrenia: Evidence for de novo mutations. Am J Med Genet. 2002;114(3):299–303. doi: 10.1002/ajmg.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, Susser ES. Advancing paternal age and the risk of schizophrenia. Arch Gen Psychiatry. 2001;58:361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, Pedersen CB. A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry. 2014;71(3):301–309. doi: 10.1001/jamapsychiatry.2013.4081. [DOI] [PubMed] [Google Scholar]
- Mental Health: Burden of Mental Illness. [Accessed October 16, 2013];Centers for Disease Control and Prevention website. http://www.cdc.gov/mentalhealth/basics/burden.htm. Updated October 4, 2013.
- Messias E, Chen C, Eaton WW. Epidemiology of schizophrenia: Review of findings and myths. Psychiatr Clin North Am. 2007;30(3):323–338. doi: 10.1016/j.psc.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CB, McGrath J, Mortensen PB, Petersen L. The importance of father’s age to schizophrenia risk. Mol Psychiatry. 2014;19:530–531. doi: 10.1038/mp.2013.69. [DOI] [PubMed] [Google Scholar]
- Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: Introducing the PDI (Peters et al. Delusions Inventory) Schizophr Bull. 1999;25(3):553–576. doi: 10.1093/oxfordjournals.schbul.a033401. [DOI] [PubMed] [Google Scholar]
- Petersen L, Mortensen PB, Pedersen CB. Paternal age at birth of first child and risk of schizophrenia. Am J Psychiat. 2011;168(1):82–88. doi: 10.1176/appi.ajp.2010.10020252. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F. Subjective symptoms of schizophrenia in research and the clinic: The basic symptom concept. Schizophr Bull. 2009;35(1):5–8. doi: 10.1093/schbul/sbn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos A, Rasmussen F, Harrison G, Tynelius P, Lewis G, Leon DA, Gunnell D. Paternal age and schizophrenia: a population based cohort study. Brit Med J. 2004;329(7474):1070–1073. doi: 10.1136/bmj.38243.672396.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen HJ, Pedersen CB, Nordentoft M, Mortensen PB, Ehrenstein V, Petersen L. Effects of paternal age and offspring cognitive ability in early adulthood on the risk of schizophrenia and related disorders. Schizophr Res. 2014;160:131–135. doi: 10.1016/j.schres.2014.09.035. [DOI] [PubMed] [Google Scholar]
- Stip E, Letourneau G. Psychotic symptoms as a continuum between normality and pathology. Can J Psychiatry. 2009;54(3):140–151. doi: 10.1177/070674370905400302. [DOI] [PubMed] [Google Scholar]
- Stochl J, Khandaker GM, Lewis G, Perez J, Goodyer IM, Zammit S, Sullivan S, Croudace TJ, Jones PB. Mood, anxiety and psychotic phenomena measure a common psychopathological factor. Psychol Med. 2014 doi: 10.1017/S003329171400261X. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Verdoux H, Maurice-Tison S, Gay B, van Os J, Salamon R, Bourgeois ML. A survey of delusional ideation in primary-care patients. Psychol Med. 1998;28(1):127–134. doi: 10.1017/s0033291797005667. [DOI] [PubMed] [Google Scholar]
- Vreeker A, Schubart CD, Van Gastel WA, Kahn RS, Boks MP. Advanced paternal age and vulnerability to psychotic-like experiences in the offspring. Shizophr Res. 2013;143:74–76. doi: 10.1016/j.schres.2012.10.035. [DOI] [PubMed] [Google Scholar]
- Zammit S, Allebeck P, Dalman C, Lundberg I, Hemmingson T, Owen M, Lewis G. Paternal age and risk for schizophrenia. Brit J Psychiat. 2003;183:405–408. doi: 10.1192/bjp.183.5.405. [DOI] [PubMed] [Google Scholar]
- Zammit S, Horwood J, Thompson A, Thomas K, Menezes P, Gunnell D, Hollis C, Wolke D, Lewis G, Harrison G. Investigating if psychosis-like symptoms (PLIKS) are associated with family history of schizophrenia or paternal age in the ALSPAC birth cohort. Schizophr Res. 2008;104:279–286. doi: 10.1016/j.schres.2008.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.