Abstract
Background
Mandible (MB) and maxilla possess unique metabolic and functional properties and demonstrate discrete responses to homeostatic, mechanical, hormonal and developmental stimuli. Osteogenic potential of bone marrow stromal cells (BMSCs) differs between MB versus long bones (LB). Furthermore, MB versus LB derived osteoclasts (OCs) have disparate functional properties. Here, we explored the osteoclastogenic potential of rat MB versus LB marrow in vitro and in vivo under basal and stimulated conditions.
Methods
Bone marrow from rat MB and LB was cultured in osteoblastic or osteoclastic differentiation media. Tartrate resistant acid phosphatase (TRAP) staining, resorption pit assays, and real-time PCR were performed. Additionally, osmotic mini-pumps were implanted in animals, mandibles and tibiae were isolated and multinucleated cells (MNCs) were measured.
Results
MB versus LB marrow cultures differentiated with RANKL and M-CSF produced more TRAP+ multinucleated cells (MNCs) and greater resorptive area. To explore MB versus LB BMSC supported osteoclastogenesis, confluent BMSCs were cultured with parathyroid hormone (PTH), 1α,25-dihydroxyvitaminD3 (1,25D3), or PTH+1,25D3. 1,25D3 or PTH+1,25D3 treated LB BMSCs expressed significantly higher RANKL and lower OPG mRNA and increased RANKL:OPG ratio. When whole marrow was cultured with PTH+1,25D3, more TRAP+ MNCs were seen in LB versus MB cultures. Ultimately, rats were infused with PTH+1,25D3 and MB versus tibia MNCs were measured. Hormonal stimulation increased osteoclastogenesis in both MB and tibia. However, higher TRAP+ MNC numbers were observed in tibia versus MB under basal and hormonal stimulation.
Conclusions
Collectively, our data illustrate differences both on osteoclastogenic potential and OC numbers of MB versus LB marrow.
Keywords: Mandible, Tibia, Osteoclast, Bone Marrow, Stromal Cells
Similar to other craniofacial bones, the mandible (MB) and maxilla display developmental, functional and homeostatic properties distinct from the appendicular skeleton. The jaws arise from neural crest cells of neuroectoderm rather than mesoderm 1, and are formed primarily by intramembranous as opposed to endochondral ossification 2. 1,25-dihydroxyvitamin D3 (1,25D3) and parathyroid hormone (PTH) knockout mice demonstrate that MB mineralization is affected by 1,25D3 deficiency but remains unaltered by abolishment of PTH. In contrast long bones (LB) show effects in loss of both hormones 3. Similarly, in ovariectomized and malnutrition rodent models, the MB loses significantly less bone than proximal tibia 4. Skeletal diseases only affecting the jaws, such as periodontitis 5, cherubism 6, hyperparathyroid jaw tumor syndrome 7, and bisphosphonate-related osteonecrosis of the jaws (ONJ) 8, further support distinctive MB homeostasis.
An increased osteogenic potential of rodent and human MB versus LB bone marrow stromal cells (BMSCs) both in vitro and in vivo has been described 9–11. Furthermore, human mandibular or maxillary BMSCs show enhanced response to osteogenic differentiation factors and bone morphogenetic protein 2 compared to cells derived from iliac crest 10, 11.
Osteoclasts (OCs) are multinucleated, bone-resorbing cells whose differentiation and maturation requires macrophage stimulating factor (M-CSF) and receptor of activator of NFκB ligand (RANKL), cytokines supplied among others by BMSCs 12. Bone-site specific phenotypic and functional differences of osteoclasts have been proposed 13. OCs from calvariae versus LB show differential usage of proteinases 14, 15 and expression levels of the enzyme tartrate resistant acid phosphatases (TRAP) 15, 16. In addition, murine jaw and LB marrows have different osteoclastic potential in the presence of M-CSF and RANKL stimulation and exhibit distinctive shape and response to culturing substrates 17, 18.
BMSCs are important regulators of osteoclastogenesis. BMSCs produce osteoprotegerin (OPG), an osteoclast decoy receptor that competes with RANK for RANKL binding 19. Therefore, the RANKL:OPG ratio pivotally determines the direction of osteoclastogenesis. RANKL and OPG expression are modulated by local cytokines and systemic hormones such as PTH and 1,25D3 20, 21. Interestingly, basal RANKL:OPG ratio is higher in mouse jaw versus long bone cultures, suggesting differential ability of the marrow environment to support osteoclastogenesis 18. However, differential ability of MB versus LB BMSCs to support osteoclastogenesis in stimulated conditions is not well understood.
We hypothesize that the jaws have distinct basal and induced osteoclastogenic potential compared to the other skeletal sites. Such differential osteoclastogenic response could underlie, at least in part, the pathophysiologic mechanisms of diseases unique to the jaws. Here, we explored the osteoclastogenic potential of rat MB versus LB marrow in vitro and in vivo under basal and hormone treatment. To the best of our knowledge, this is the first report of investigating MB marrow osteoclastogenesis under stimulated conditions. Our data support differences both on osteoclastogenic potential and OC numbers of MB versus LB marrow.
MATERIALS & METHODS
Isolation and Culture of Mandible and Long-bone Marrow Cells
Animal approval and surgical procedures conformed to guidelines by the UCLA Chancellor’s Animal Research Committee. MB and LB marrow cells were isolated from one-month-old male Sprague-Dawley rats§, as previously described 9. Harvested whole marrow was pooled in a single suspension, and red blood cells were lysed by RBC Lysis Solutionll
For BMSC cultures, cells were cultured at a density of 1×106 cells/mL (5.3×105 cells/cm2) in α-MEM¶ supplemented with 10% FBS, and 1% antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin). After 6 days and upon confluency, suspension cells were discarded. Adherent BMSCs were cultured in fresh osteogenic differentiation media (α-MEM+10%FBS with 50μg/μl ascorbic acid and 4 mM β-glycerophosphate) with the addition of vehicle (veh), PTH (10nM), or 1,25D3 (10nM), which was replaced every 3–4 days. For whole marrow experiments, cells were cultured without the removal of suspension cells, and half of the media were replaced every 3–4 days.
Osteoclastogenesis
Bone marrow cell suspension in media supplemented with 25ng/ml rat M-CSF# were plated in 100mm culture dishes overnight. Non-adherent cells were plated at 1.5×105 cells/100μl in osteoclastogenic media [α-MEM+10%FBS, 50ng/ml M-CSF, 80ng/ml sRANKL#], which was replaced every 2–3 days. After 6 days, cells were fixed and stained for TRAP activity using the leukocyte acid phosphatase kit. TRAP+ multinucleated cells (≥3 nuclei) were counted under light microscope.
Bone Resorption Assay
Osteoclast precursors were cultured on calcium phosphate coated 16-well plates†† in α-MEM alone or osteoclastogenic media. After 10 days, cells were removed, and total resorption pit area was visualized by von Kossa stain. Using light microscope camera, image of each well was captured at 2x magnification. The total resorbed area of each well was measured using the cellSens® imaging software‡‡
RNA Isolation and Real-Time qPCR
Total RNA from BMSC culture was collected at days 7 and 14, peak osteogenic potential time points previously described 9 using Trizol§§ and used for qPCR, performed in triplicate for at least 3 independent experiments, with iQ SYBR Green supermixllll and rat gene-specific primers, including: RANKL (NM_057149) 5′-GGAGAGCGAAGACACAGAAGCACTAC-3′(forward), 5′-CGAGCCACGAACCTTCCAT-CATAGC-3′ (reverse); OPG (NM_012870) 5′-TGTCCCTTGCCCTGACTACTCTTATAC-3′(forward), 5′-CCTTCCTCACATTCGCACACTCG-3′ (reverse); and GAPDH (NM_017008) 5′-TTCAACGGCACAGTCAAGG-3′(forward), 5′-ATACTCAGCACCAGCATCAC-3′ (reverse). The maximum gene expression normalized to GAPDH was set at 100%, and the remaining expression levels were calculated as percent maximum induction. We elected to express gene levels as percent maximum instead of fold change to avoid large and artificial fluctuations of gene induction in cases were control levels were low.
In Vivo Assessment of Osteoclastogenesis
Eight male 3-month-old Sprague-Dawley rats§ were used. Subcutaneously implanted Alzet mini-osmotic pumps¶¶ continuously infused vehicle or 40 μg/kg/day hPTH (1-34)##, and 2 μg/kg/day 1,25D3** for 3 days. This time of PTH and 1,25D3 treatment has shown peak osteoclastic induction in vivo 22, 23. Animals were sacrificed at day 4, and mandible and tibia were fixed in 4% formaldehyde solution for 48 hours and stored in 70% ethanol.
Bones were decalcified in 14.5% EDTA (pH 7.2) for 4 weeks. Paraffin embedded 4-μm-thick coronal sections at the interproximal area between the first and second mandibular molars and cross sections of proximal tibial bones were stained with hematoxylin and eosin (H&E) and were digitally scanned using the Aperio XT automated slide scanner and the Aperio Imagescope version 11 software***. Osteoclasts (≥2 nuclei represent multinucreation, distingushing from mononucleated preosteoclasts 24) in contact with the bone surface and bone area were measured (ImageScope annotation tool) within the alveolar bone region of the mandible and the trabecular bone region of the proximal tibia.
Statistics
Data were expressed as mean ± standard error of the mean (SEM) from at least 3 independent experiments. Data among groups were compared with one-way analysis of variance (ANOVA) and statistical differences between groups were identified using Student’s t-test. A p<0.05 was considered significant.
RESULTS
Higher Number of TRAP+ MNCs and Increased Resorption of MB versus LB Marrow
To study the differences between the osteoclastogenic potential of rat MB versus LB marrow, non-adherent marrow cells were differentiated into mature OCs by M-CSF and RANKL. At day 6 of culture, TRAP+ MNCs were observed in both MB and LB cultures (white arrows, Fig. 1A, B). However, significantly more TRAP+ MNCs were seen in MB cultures (Fig. 1C). To verify that the observed TRAP+ MNCs were functional osteoclasts, osteoclast precursors were differentiated on calcium phosphate substrates. MB cultures showed increased resorptive pit formation (Fig. 1D, E) with significantly higher total resorbed area (Fig. 1F) than the LB cultures.
Figure 1.
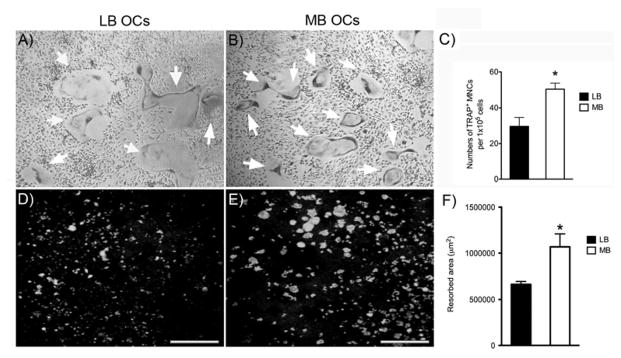
TRAP staining of cultures from long bone (A) and mandible (B), after 6 days of differentiation with M-CSF and RANKL. TRAP+ multinucleated cells (MNCs, indicated by white arrows) are shown at 4X magnification. C) Quantification of total TRAP+ MNCs (>3 nuclei) number formed by long bone and mandible marrow after 6 days of differentiation (mean +/− SEM). Von Kossa stain reveals resorptive pits formed by long bone (D) and mandible (E) marrow cultures after 10 days of differentiation in the presence of M-CSF and RANKL on calcium phosphate substrate. F) Quantification of total resorbed area formed in long bone and mandible cultures (mean +/− SEM). * p<0.05.
MB versus LB BMSCs Possess a Lower Osteoclastogenic Potential Under Hormonal Stimulation
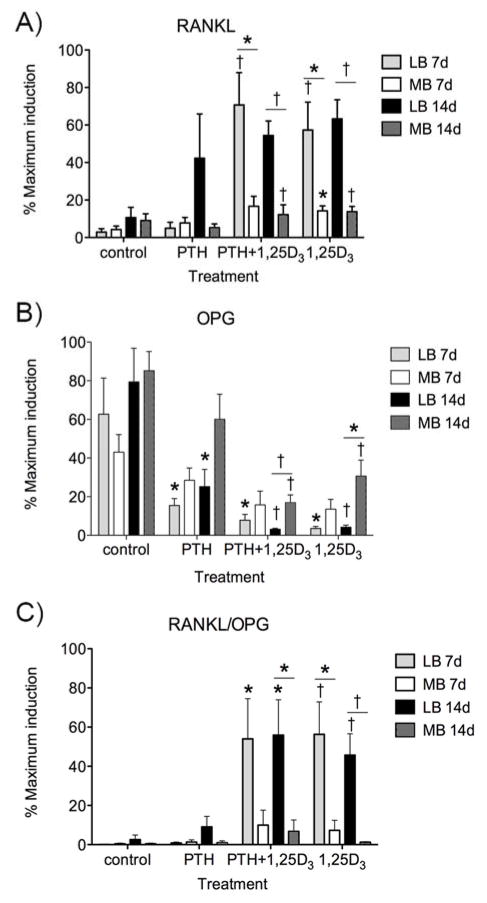
BMSCs support osteoclastogenesis through modulation of the RANKL-OPG system. Hormones that regulate bone homeostasis, such as PTH and 1,25D3 regulate production of osteoclast regulatory cytokines 21, 25. We thus explored RANKL and OPG expression in basal and hormone-stimulated conditions at 7 and 14 days of rat MB vs. LB BMSC cultures,. Basal RANKL and OPG mRNA expression was similar in MB versus LB BMSCs (Fig. 2A, B). However, in the presence of 1,25D3 alone or in combination with PTH, RANKL expression was higher in LB versus MB cultures at both 7 (p<0.05) and 14 days (p<0.01) (Fig. 2A). Under the same treatments, the inhibition of OPG expression was greater in LB versus MB BMSCs with PTH+1,25D3 (p<0.01) and 1,25D3 alone (p<0.05) at day 14 (Fig. 2B). Importantly, the RANKL:OPG ratio was substantially enhanced in LB versus MB BMSCs under 1,25D3 alone or in combination with PTH at 7 and 14 days of culture (Fig. 2C).
Figure 2.
Effect of parathyroid hormone (PTH, 10 nM) and 1,25 dihydroxyvitaminD3 (1,25D3, 10 nM), alone or in combination, on the expression of RANKL (A), OPG (B) and RANKL:OPG ratio (C) by long bone versus mandible BMSCs cultured in osteogenic media for 7 or 14 days. mRNA levels were normalized to GAPDH and expressed as percentage maximum expression (mean +/− SEM). For control, vehicle treated BMSCs were cultured in osteogenic media for 7 or 14 days. * p<0.05, † p<0.01.
LB versus MB Whole Marrow Generates More TRAP+ MNCs Under Hormonal Induction
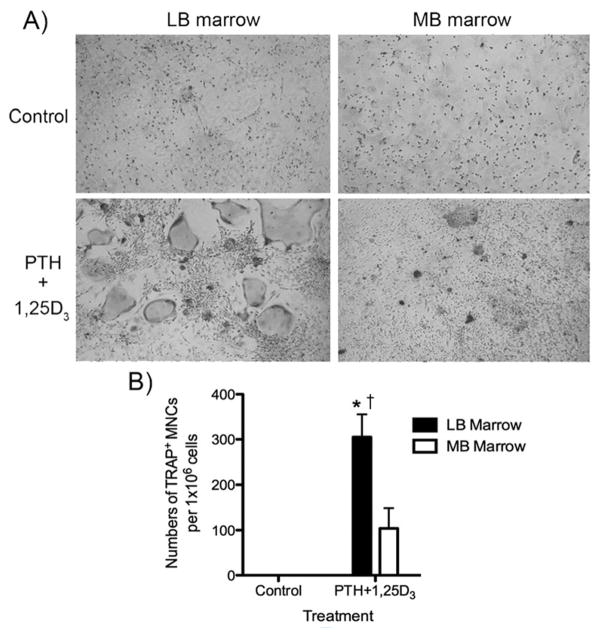
Our data showed that although rat MB marrow has a higher osteoclastogenic potential in the presence of M-CSF and RANKL, hormonally stimulated LB BMSCs express higher levels of RANKL and RANKL:OPG ratio that would favor increased osteoclastogenesis. To evaluate the osteoclastogenic ability of rat MB versus LB marrow, we cultured whole marrow, under basal and PTH+1,25D3 treatment. TRAP staining revealed TRAP+ MNCs in both LB and MB culture under PTH+1,25D3 stimulation but not at baseline (Fig. 3A). Quantitatively, LB marrow cultures contained significantly more TRAP+ MNCs compared to MB culture (Fig. 3B).
Figure 3.
A) TRAP staining of long bone versus mandible whole marrow under control or PTH + 1,25D3 treatment after 7 days. B) Quantification of TRAP+ MNC numbers in long bone or mandible marrow cultures (mean +/− SEM). * p<0.05 compared to veh treated control, † p<0.05 compared to PTH + 1,25D3 treated group.
Higher Basal and PTH+1,25D3-Stimulated MNC Number in Tibia versus Mandible in Rats in Vivo
To evaluate basal and hormonal-stimulation of osteoclast formation in vivo, mini-osmotic pumps containing either vehicle or PTH+1,25D3 were subcutaneously implanted for 3 days in adult rats. Then mandible and tibia were decalcified, and H&E staining was performed on sections from the mandibular alveolar bone in the interproximal area between the first and second molars (Fig. 4A) and the trabecular bone of the proximal tibia metaphyseal area (Fig. 4D). MNCs abutting the bone, presumably representing osteoclasts, were observed at baseline and under PTH+1,25D3 stimulation in both tibia and mandible (Fig. 4 B, C, E, F, green arrows). At baseline more osteoclasts were observed in tibia versus mandible. PTH+1,25D3 significantly increased osteoclast numbers in both tibia and mandible. Importantly, greater numbers of MNCs were observed under hormonal stimulation in tibia compared to mandible (Fig. 4G).
Figure 4.
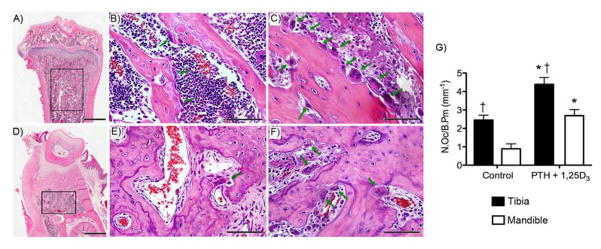
H&E sections at 2X (A, D), and 40X (B, C, E, F) of tibia (A, B, C) and mandible (D, E, F). Sprague-Dawley rats were continuously infused with vehicle (B and E) or PTH + 1,25D3 (C and F) for 3days. G) MNCs abutting bone surface (indicated by green arrows) were measured within the trabecular bone of tibia and mandible (black rectangular outline, A and D). * p<0.05 compared to veh treated control, † p<0.05 compared to mandible.
DISCUSSION
Mandible, a bone of the orofacial complex, possesses unique metabolic and functional properties and demonstrates discrete responses to homeostatic, mechanical and developmental stimuli 26. Bone diseases such as cherubism 6, hyperparathyroid jaw tumor syndrome 7, and ONJ 8, 27, 28 affect the jaws while sparing the remaining skeleton. Differences in the osteogenic potential of LB versus MB BMSCs suggest a skeletal site specific BMSC response to various stimuli during bone remodeling and healing 10, 29. In this study, we examined potential differences on the osteoclastogenic ability between mandible and long bone under basal and stimulated conditions. Exploring mandibular osteoclastogenic potential under basal and hormonal regulation is significant, since it would provide valuable understanding of jaw bone homeostasis and might lead to better target approaches for bone conditions that selectively affect the face.
We first investigated the in vitro potential of rat MB versus LB marrow cells to form osteoclasts in the presence of M-CSF and RANKL. MB marrow significantly generated more TRAP+ MNCs. We tested marrow osteoclastogenesis at 6 days of culture 30. To test whether differences in TRAP+ MNC numbers result in increased resorption, we utilized a calcium phosphate substrate as a neutral culturing surface. Increased osteoclast formation was mirrored by larger total resorbed area generated by MB osteoclasts in comparison to LB cultures. Our findings suggested an increased osteoclastic potential of MB marrow.
Previous reports utilizing mouse derived MB and LB marrow found higher osteoclast numbers in LB marrow cultures at earlier time points, although at later time points no difference was observed. A difference in the MB versus LB marrow cellular composition, with monocytes and myeloid blasts as principal cells in the jaw and long bones respectively, could have accounted for the observed difference at the earlier time points 18. Interestingly, differences in osteoclast number between MB and LB appear to depend on the substrate the cells are cultured on. More osteoclasts were observed in LB marrow when cells were cultured in plastic or bone slices, while the contrary was true when cells were cultured on dentin slices 17. Utilization of rats versus mice as well as experimental differences in preparing and culturing marrow cells could account for the disparate findings in our study, where we observed difference in osteoclastogenic potential between the two skeletal sites, and other studies, where no differences were observed18.
Since we observed a higher osteoclastic potential of rat MB marrow when M-CSF and RANKL was added to the culture, we sought to investigate whether there is a difference in the ability of MB versus LB BMSCs to support osteoclast formation. Such differences should not be surprising, since diverse function of BMSCs from various skeletal sites, including the jaws, have been reported for humans, and rodents 9, 10, 31. BMSCs can support osteoclastogenesis through the production of RANKL and OPG. Furthermore, systemic hormones such as PTH and 1,25D3 induce osteoclast formation by, among others, altering RANKL and OPG expression and thus regulating RANKL:OPG ratio 20, 21, 32. 1,25D3 alone or in combination with PTH, induced RANKL expression in both MB and LB BMSCs. However, the RANKL induction in LB cells was significantly greater than that in MB cells. PTH, 1,25D3, and PTH+1,25D3 significantly attenuated OPG expression in LB BMSCs. Only PTH+1,25D3 inhibited OPG expression in MB BMSCs at 14 days of culture. Importantly, the inhibition of OPG expression in LB BMSCs was more pronounced. This differential RANKL induction and OPG inhibition resulted in an elevated RANKL:OPG ratio of LB BMSCs under stimulated conditions, suggesting a disparate response of LB versus MB BMSCs to hormonal stimulation.
The ability of cells along the osteoblastic lineage to support osteoclastogenesis has been explored 33. Although immature versus differentiated osteoblasts exhibit a strong potential to support osteoclast formation and differentiation in vitro 34, the vital role of osteocytes in regulation of osteoclast formation in vivo at baseline, as well as during unloading, is well established 35, 36. In our experiments, we studied RANKL and OPG expression at two culture points, representing different osteoblastic potential of marrow stromal cells 9. Basal RANKL levels were higher at later time points of culture, suggesting increased ability for support of osteoclastogenesis of more differentiated stromal cells. However, PTH and 1, 25D3 induced RANKL at a similar level for all time points.
Osteoclast formation depends on both the availability of osteoclastic precursors, as well as on appropriate signals that will promote their differentiation and fusion into mature multinucleated osteoclasts. To compare the ability of rat LB versus MB marrow to form MNC TRAP+ cells, whole marrow in the absence of added RANKL and M-CSF was cultured in the presence of hormonal stimulation. LB marrow generated significantly greater TRAP+ MNCs, reinforcing the higher osteoclastogenic ability of LB marrow under PTH+1,25D3 treatment. Ultimately we evaluated basal and hormonal stimulation of osteoclast formation in vivo. Interestingly, higher MNC numbers were identified in tibia versus mandible in control animals, indicating that baseline bone homeostasis favors increased osteoclast presence that might reflect higher basal bone remodeling in long bones. Hormonal treatment significantly induced osteoclast formation in both skeletal sites with a higher overall osteoclast number in tibia, similar to the in vitro findings of PTH+1,25D3 increase in whole marrow MNCs.
Overall, we detected distinct osteoclastogenic ability of rat MB versus LB marrow. MB marrow appeared to posses more basal osteoclast precursors. Alternatively MB preosteoclasts could demonstrate increased proliferation under M-CSF and RANKL stimulation. However, LB marrow cells responded to hormonal treatment with enhanced RANKL gene expression and RANKL:OPG ratio, and with increased osteoclast numbers. These observations point to discrete and complex regulation of jaw versus appendicular bone homeostasis. Our findings have potential implications for pharmacologic treatment of periodontal bone loss. Osteoclast activation is central in the pathogenesis of periodontitis 37–39. Pharmacologic inhibitors of osteoclast function or differentiation attenuate bone loss in animal models of periodontitis and have been entertained as potential interventions for periodontal disease 40–45. Systemic hormones and inflammatory cytokines play key role in osteoclast formation and activation 38, 43, 46, 47. Indeed, association of systemic diseases that show increased osteoclast activity with periodontal bone loss has been explored 48, 49, 51. Our results suggest a bone-site specific regulation of osteoclast activation. Combined with other published data that show bone-site specific differences in osteoclastic function 13, 14, 17, potential specific inhibitors of alveolar bone osteoclast activation and/or function could be developed. Such pharmacologic interventions would be important in regulating periodontal bone loss, while sparing bone homeostasis in the remaining of the skeleton. Alternatively, osteoclastic inhibitors for the rest of the skeleton but not the jaws would be valuable for management of bone metabolic diseases, such as osteoporosis, or bone cancer, while reducing the risk for ONJ.
CONCLUSION
In summary, our data demonstrate a diverse osteoclastogenic capacity of rat MB versus LB marrow. Although MB marrow appeared to posses intrinsically more osteoclast precursors, LB BMSCs showed enhanced response to hormonal increase of RANKL expression and RANKL:OPG ratio. Furthermore, PTH and 1,25D3 induced higher TRAP+ MNC numbers in whole marrow of LB versus MB. Paralleling these findings, tibia showed higher osteoclasts at basal and PTH+1,25D3 stimulated conditions. The diverse osteoclastic potential of the MB versus other skeletal sites could explain, in part, the differential response of the jaws to mechanical, hormonal and nutritional signals, as well as to antiresorptive therapies.
Acknowledgments
This work was funded by NIH/NIDCR (Bethesda, MD) funding R01 DE019465 and K02 DE021444, while TC was supported by T32 DE007296.
Footnotes
Charles River, Wilmington, MA
BioLegend®, San Diego, CA,
Mediatech, Herndon, VA,
Prepotech, Rocky Hill, NJ
Sigma-Aldrich, St. Louis, MO
BioCoat ™ Osteologic ™ MultiTest Slides, BD Biosciences, Bedford, MA
Olympus, Center Valley, PA
Invitrogen, Carlsbad, CA
Bio-Rad, Hercules, CA
model 1003D, Alza Corp., Palo Alto, CA
Bachem, Inc., Torrance, CA
Aperio Technologies, Inc., Vista, CA
DISCLOSURES
Dr. Tetradis has served as a paid consultant for and has received grant support from Amgen Inc, Thousand Oaks, CA. All other authors state that they do not have any conflicts of interest.
References
- 1.Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson CM, Miclau T, Hu D, Alpern E, Helms JA. Common molecular pathways in skeletal morphogenesis and repair. Ann N Y Acad Sci. 1998;857:33–42. doi: 10.1111/j.1749-6632.1998.tb10105.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu H, Guo J, Wang L, et al. Distinctive anabolic roles of 1,25-dihydroxyvitamin D(3) and parathyroid hormone in teeth and mandible versus long bones. J Endocrinol. 2009;203:203–213. doi: 10.1677/JOE-09-0247. [DOI] [PubMed] [Google Scholar]
- 4.Mavropoulos A, Rizzoli R, Ammann P. Different responsiveness of alveolar and tibial bone to bone loss stimuli. J Bone Miner Res. 2007;22:403–410. doi: 10.1359/jbmr.061208. [DOI] [PubMed] [Google Scholar]
- 5.Beck J, Arbes S. Epidemiology of periodontal diseases. In: Newman M, Takei H, Carranza F, editors. Carranza’s Clinical Periodontology. Philadelphia: WB Saunders; 2011. pp. 79–94. [Google Scholar]
- 6.Ueki Y, Tiziani V, Santanna C, et al. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28:125–126. doi: 10.1038/88832. [DOI] [PubMed] [Google Scholar]
- 7.Simonds WF, James-Newton LA, Agarwal SK, et al. Familial isolated hyperparathyroidism: clinical and genetic characteristics of 36 kindreds. Medicine (Baltimore) 2002;81:1–26. doi: 10.1097/00005792-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 9.Aghaloo TL, Chaichanasakul T, Bezouglaia O, et al. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res. 2010;89:1293–1298. doi: 10.1177/0022034510378427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Osyczka AM, Damek-Poprawa M, Wojtowicz A, Akintoye SO. Age and skeletal sites affect BMP-2 responsiveness of human bone marrow stromal cells. Connect Tissue Res. 2009;50:270–277. doi: 10.1080/03008200902846262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 13.Everts V, de Vries TJ, Helfrich MH. Osteoclast heterogeneity: lessons from osteopetrosis and inflammatory conditions. Biochim Biophys Acta. 2009;1792:757–765. doi: 10.1016/j.bbadis.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Everts V, Korper W, Jansen DC, et al. Functional heterogeneity of osteoclasts: matrix metalloproteinases participate in osteoclastic resorption of calvarial bone but not in resorption of long bone. Faseb J. 1999;13:1219–1230. doi: 10.1096/fasebj.13.10.1219. [DOI] [PubMed] [Google Scholar]
- 15.Perez-Amodio S, Jansen DC, Schoenmaker T, et al. Calvarial osteoclasts express a higher level of tartrate-resistant acid phosphatase than long bone osteoclasts and activation does not depend on cathepsin K or L activity. Calcif Tissue Int. 2006;79:245–254. doi: 10.1007/s00223-005-0289-z. [DOI] [PubMed] [Google Scholar]
- 16.Zenger S, Ek-Rylander B, Andersson G. Long bone osteoclasts display an augmented osteoclast phenotype compared to calvarial osteoclasts. Biochem Biophys Res Commun. 2010;394:743–749. doi: 10.1016/j.bbrc.2010.03.063. [DOI] [PubMed] [Google Scholar]
- 17.Azari A, Schoenmaker T, de Souza Faloni AP, Everts V, de Vries TJ. Jaw and long bone marrow derived osteoclasts differ in shape and their response to bone and dentin. Biochem Biophys Res Commun. 2011;409:205–210. doi: 10.1016/j.bbrc.2011.04.120. [DOI] [PubMed] [Google Scholar]
- 18.de Souza Faloni AP, Schoenmaker T, Azari A, et al. Jaw and long bone marrows have a different osteoclastogenic potential. Calcif Tissue Int. 2011;88:63–74. doi: 10.1007/s00223-010-9418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Udagawa N, Nakamura I, Miyaura C, Takahashi N. Modulation of osteoclast differentiation by local factors. Bone. 1995;17:87S–91S. doi: 10.1016/8756-3282(95)00185-g. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Seedor JG, Rodan GA, Balena R. Endogenous calcitonin attenuates parathyroid hormone-induced cancellous bone loss in the rat. Endocrinology. 1995;136:788–795. doi: 10.1210/endo.136.2.7835311. [DOI] [PubMed] [Google Scholar]
- 23.Jobin JR, Bonjour JP. Compensatory renal growth: modulation by calcium PTH and 1,25-(OH)2D3. Kidney Int. 1986;29:1124–1130. doi: 10.1038/ki.1986.117. [DOI] [PubMed] [Google Scholar]
- 24.Escudero ND, Mandalunis PM. Influence of bisphosphonate treatment on medullary macrophages and osteoclasts: an experimental study. Bone marrow research. 2012;2012:526236. doi: 10.1155/2012/526236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagai M, Sato N. Reciprocal gene expression of osteoclastogenesis inhibitory factor and osteoclast differentiation factor regulates osteoclast formation. Biochem Biophys Res Commun. 1999;257:719–723. doi: 10.1006/bbrc.1999.0524. [DOI] [PubMed] [Google Scholar]
- 26.Sodek J, McKee MD. Molecular and cellular biology of alveolar bone. Periodontol 2000. 2000;24:99–126. doi: 10.1034/j.1600-0757.2000.2240106.x. [DOI] [PubMed] [Google Scholar]
- 27.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 28.Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws--2009 update. J Oral Maxillofac Surg. 2009;67:2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Aghaloo TL, Felsenfeld AL, Tetradis S. Osteonecrosis of the jaw in a patient on Denosumab. J Oral Maxillofac Surg. 2010;68:959–963. doi: 10.1016/j.joms.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradley EW, Oursler MJ. Osteoclast culture and resorption assays. Methods Mol Biol. 2008;455:19–35. doi: 10.1007/978-1-59745-104-8_2. [DOI] [PubMed] [Google Scholar]
- 31.Yamaza T, Ren G, Akiyama K, Chen C, Shi Y, Shi S. Mouse mandible contains distinctive mesenchymal stem cells. J Dent Res. 2011;90:317–324. doi: 10.1177/0022034510387796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin TJ, Ng KW. Mechanisms by which cells of the osteoblast lineage control osteoclast formation and activity. J Cell Biochem. 1994;56:357–366. doi: 10.1002/jcb.240560312. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54:258–263. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Jiang X, Delaney J, et al. Immature osteoblast lineage cells increase osteoclastogenesis in osteogenesis imperfecta murine. Am J Pathol. 2010;176:2405–2413. doi: 10.2353/ajpath.2010.090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 37.Bartold PM, Cantley MD, Haynes DR. Mechanisms and control of pathologic bone loss in periodontitis. Periodontol 2000. 2010;53:55–69. doi: 10.1111/j.1600-0757.2010.00347.x. [DOI] [PubMed] [Google Scholar]
- 38.Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. Journal of clinical periodontology. 2012;39:239–248. doi: 10.1111/j.1600-051X.2011.01810.x. [DOI] [PubMed] [Google Scholar]
- 39.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends in molecular medicine. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Pradeep AR, Sharma A, Rao NS, Bajaj P, Naik SB, Kumari M. Local drug delivery of alendronate gel for the treatment of patients with chronic periodontitis with diabetes mellitus: a double-masked controlled clinical trial. J Periodontol. 2012;83:1322–1328. doi: 10.1902/jop.2012.110292. [DOI] [PubMed] [Google Scholar]
- 41.Price U, Le HO, Powell SE, et al. Effects of local simvastatin-alendronate conjugate in preventing periodontitis bone loss. J Periodontal Res. 2012 doi: 10.1111/jre.12036. [DOI] [PubMed] [Google Scholar]
- 42.Sharma A, Pradeep AR. Clinical efficacy of 1% alendronate gel in adjunct to mechanotherapy in the treatment of aggressive periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83:19–26. doi: 10.1902/jop.2011.110206. [DOI] [PubMed] [Google Scholar]
- 43.Wada N, Maeda H, Yoshimine Y, Akamine A. Lipopolysaccharide stimulates expression of osteoprotegerin and receptor activator of NF-kappa B ligand in periodontal ligament fibroblasts through the induction of interleukin-1 beta and tumor necrosis factor-alpha. Bone. 2004;35:629–635. doi: 10.1016/j.bone.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Wada Y, Mizuno M, Tamura M. Enamel matrix derivative neutralized the effect of lipopolysaccharide on osteoprotegerin and receptor activator of nuclear factor kappa B ligand expression of osteoblasts. Archives of oral biology. 2009;54:306–312. doi: 10.1016/j.archoralbio.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Gokhale SR, Padhye AM. Future prospects of systemic host modulatory agents in periodontal therapy. Br Dent J. 2013;214:467–471. doi: 10.1038/sj.bdj.2013.432. [DOI] [PubMed] [Google Scholar]
- 46.Jiao Y, Darzi Y, Tawaratsumida K, et al. Induction of bone loss by pathobiont-mediated nod1 signaling in the oral cavity. Cell host & microbe. 2013;13:595–601. doi: 10.1016/j.chom.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res. 2007;86:306–319. doi: 10.1177/154405910708600403. [DOI] [PubMed] [Google Scholar]
- 48.Guiglia R, Di Fede O, Lo Russo L, Sprini D, Rini GB, Campisi G. Osteoporosis, jawbones and periodontal disease. Med Oral Patol Oral Cir Bucal. 2013;18:e93–99. doi: 10.4317/medoral.18298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Megson E, Kapellas K, Bartold PM. Relationship between periodontal disease and osteoporosis. International journal of evidence-based healthcare. 2010;8:129–139. doi: 10.1111/j.1744-1609.2010.00171.x. [DOI] [PubMed] [Google Scholar]
- 50.Martinez-Maestre MA, Gonzalez-Cejudo C, Machuca G, Torrejon R, Castelo-Branco C. Periodontitis and osteoporosis: a systematic review. Climacteric: the journal of the International Menopause Society. 2010;13:523–529. doi: 10.3109/13697137.2010.500749. [DOI] [PubMed] [Google Scholar]
- 51.de Smit MJ, Brouwer E, Vissink A, van Winkelhoff AJ. Rheumatoid arthritis and periodontitis; a possible link via citrullination. Anaerobe. 2011;17:196–200. doi: 10.1016/j.anaerobe.2011.03.019. [DOI] [PubMed] [Google Scholar]