Abstract
Purpose
The purpose of this study is to develop and pilot an innovative behavioral intervention in adolescents with type 1 diabetes mellitus (T1DM) incorporating structured care of a pet to improve glycemic control.
Methods
Twenty-eight adolescents with A1C > 8.5% (69 mmol/mol) were randomly assigned to either the intervention group (care of a Betta splendens pet fish) or the control group (usual care). Adolescents in the intervention group were given instructions to associate daily and weekly fish care duties with diabetes self-management tasks including blood glucose testing and parent-adolescent communication.
Results
After 3 months the participants in the intervention group exhibited a statistically significant decrease in A1C levels (−0.5%) compared to their peers in the control group who had an increase in A1C levels (0.8%)(p = 0.04). The younger adolescents (ages 10–13) demonstrated a greater response to the intervention which was statistically significant (−1.5% vs. 0.6%, p = 0.04) compared with the older adolescents (ages 14–17).
Conclusions
Structured care of a pet fish can improve glycemic control in adolescents with T1DM, likely by providing cues to perform diabetes self-management behaviors.
Introduction
Adolescence is a vulnerable time for the deterioration of glycemic control in patients with type 1 diabetes mellitus (T1DM) due to the poor decision making, impulsivity, and sense of invincibility that characterize this time period and negatively affect diabetes self-care behaviors.1,2 Currently, most interventions aimed at enhancing self-management behaviors and glycemic control in the adolescent population are family-based such as behavioral family systems therapy and multisystemic therapy.3–6 They include multiple sessions covering various aspects of family dynamics (communication, problem-solving skills, psychotherapy) and reviewing diabetes medical care (management tasks, education). Unfortunately, factors like added cost, additional time obligation, and involvement of highly-trained mental health professionals make it difficult to incorporate these models into the routine outpatient clinic visit. In addition, recent meta-analysis suggest that these interventions have a modest effect on overall glycemic control.7 Consequently, there is a great need for innovative and acceptable strategies in this population that positively influence diabetes self-care management.
Associating medication administration with a regularly occurring activity or event (mealtime, wake-up, sleep) has been shown to enable medication adherence in elderly populations.8 The routine care of household pets involves repetitive, predictable activities such as feeding, walking, and grooming that are necessary for the welfare of the animal and enjoyable for the pet owner. Our hypothesis is that incorporating blood glucose monitoring and parental communication into the structured care of a pet fish will improve glycemic control in adolescents with T1DM by providing activity-based cues to perform diabetes self-care behaviors. Our intervention was designed to target the theoretical domain of behavior change related to the nature of the behavior with the goal of making diabetes self-care tasks automatic routine activities cued by the care of the fish9. A fish was chosen to minimize the burden of cost and pet care placed on families who agreed to participate in the study. The beneficial impact of pet ownership on human health has been investigated extensively and studies show that companion animals serve as moderators of stress, with beneficial influences on heart rate and blood pressure.10,11 They can also influence psychological health by ameliorating the effects of potentially stressful life events, reducing levels of anxiety, loneliness and depression, and enhancing feelings of autonomy, competence and self-esteem. 10,11 Unfortunately, few studies exploring the health benefits of pets were conducted in children, and none examined the impact of linking structured care of a fish with diabetes self-care behaviors on glycemic control in adolescents with T1DM or other chronic illnesses.
Research Design and Methods
Setting and participants
Adolescents 10–17 years old were recruited from a pediatric diabetes clinic at the Children’s Medical Center Dallas, a university-affiliated healthcare facility. Inclusion criteria included duration of T1DM for at least one year, A1C > 8.5%, and fluency in English. Exclusion criteria included: clinical or laboratory characteristics suggestive of type 2 diabetes mellitus, involvement in foster care, dual-home living situation, severe psychiatric disorders, developmental delay or cognitive impairment, or current responsible pet ownership on baseline questionnaire. Signed informed consent was obtained from a parent and written assent from the adolescent.
Study Design
The pilot and feasibility study was a randomized controlled trial comparing the effectiveness of the care of a pet fish (intervention group) with usual care (control group) on improving glycemic control. All participants (intervention and control groups) were encouraged to self-monitor blood glucose readings at least four times daily and review glucose trends on a blood glucose log with a parent at least once a week. A computerized random number generator was used to produce a 1:1 randomization schedule (O Gupta) and the sequence was concealed until participants were enrolled and interventions were assigned (O Gupta). The institutional review board at the University of Texas Southwestern Medical School approved the study protocol.
Intervention
At the time of enrollment, adolescents in the intervention group were given a fish bowl with equipment to care for the fish, a $5 gift card to purchase a fish (Betta splendens) from a local pet store, instructions for caring for a fish and recommendations to set up their fish bowl in their bedroom if possible. Adolescents in the intervention group were given written instructions at a 5th grade reading level regarding feeding their fish in the morning after waking and in the evening before bedtime, and they were instructed to check their blood glucose readings at those times. They were also instructed to change one-quarter of the water in the fish bowl once a week and to review their glucose logs with their caregiver at that time. All of the instructions were reviewed verbally with the participant and their family and they were aware of the intention to pair the twice daily and once weekly fish care activities with diabetes self-management tasks.
Measures
The primary outcome measure was change in A1C measured at baseline and a subsequent follow-up visit (typically three months after baseline visit). Secondary outcome measures included scores on generic (PedsQoL Generic Core 4.0 SF-15) and health-related (Diabetes Module 3.2) quality of life (QoL) surveys at baseline and a subsequent follow-up visit.12 Adolescents 13 years and older also completed the Self-Management of Type 1 Diabetes for Adolescents (SMOD-A) questionnaire.13 Data regarding the presence and care of a household pet were obtained at baseline and at the three month follow-up visit. Demographics and duration of T1DM were assessed for all participants. Zip code-based median annual household income was obtained from the 2010 US Census data (http://factfinder2.census.gov).
Statistical Analyses
Comparability of baseline characteristics between the groups was evaluated using the Student’s t-test for continuous variables and Fisher’s exact test for categorical values. Log transformations were used to normalize variables with positively skewed distributions. A repeated measures ANOVA analysis was used to examine differences in A1C, QoL or SMOD-A questionnaires over time from baseline to follow-up visit in participants with complete data. The study was designed to detect a ΔΔA1C of 1% (i.e. −0.5% vs. 0.5%) with a standard deviation (SD) of 0.9. Using a two-sided test with Type I error = 0.05, sample size calculation showed that 13 participants in each group provided 80% power. P < 0.05 was considered significant. Data were analyzed using Microsoft Excel 2010.
Results
Study Participants
Twenty-nine patients were recruited for this pilot study, 16 in the intervention group and 13 in the control group. However, one participant was excluded from the control group because she purchased a pet fish following her randomization into the Control Group. Therefore, subsequent data analysis was conducted using responses from the remaining twenty-eight participants. All participants had received standard diabetes education and training with a certified diabetes educator in our pediatric diabetes clinic. Fifteen (36%) of the youth were male and the mean age of the patient cohort was 14.2 ± 1.9 years. Baseline evaluation revealed no significant differences between the intervention and control groups for age, gender, race/ethnicity, diabetes duration, A1C at time of enrollment, presence of pets in home, socioeconomic status based on median household income of the participant’s zip code or scores on the QoL and SMOD-A questionnaires (Table 1).
Table 1.
Baseline characteristics of adolescents with type 1 diabetes by randomization condition
| Intervention (Pet Fish) | Control (Usual Care) | P-value | |
|---|---|---|---|
| n | 16 | 12 | |
| Baseline A1C (%) | 10 ± 1.12 | 10.4 ± 1.5 | 0.4 |
| Baseline A1C (mmol/mol) | 86 ± 9.9 | 90 ± 14 | 0.4 |
| Age (years) | 14.3 ± 2 | 14 ± 1.9 | 0.63 |
| Male (%) | 43 | 25 | 0.43 |
| Race (%) | |||
| Caucasian | 56 | 33 | 0.28 |
| African American | 44 | 58 | 0.45 |
| Other | 0 | 9 | 0.43 |
| Hispanic (%) | 7 | 9 | 1 |
| Time since DM diagnosis (years) | 7.2 ± 4.5 | 5.4 ± 4.3 | 0.3 |
| No pets at home (%) | 56 | 67 | 0.7 |
| Pet at home without subject responsibility (%) | 43 | 33 | 0.7 |
| Zip code based annual household income (k dollars) | 54.8 ± 20.2 | 51.8 ± 22.7 | 0.7 |
| Peds QL - Generic Core (4.0 SF-15) | |||
| Physical Health Summary Score | 79.4 ± 15.6 | 77.2 ± 20.1 | 0.7 |
| Psychosocial Health Summary Score | 74.4 ± 15.5 | 70.8 ± 19.2 | 0.56 |
| Total Score | 76.2 ± 13.7 | 72.9 ± 15.7 | 0.56 |
| Peds QL - Diabetes Module (3.2) | |||
| Diabetes | 62.5 ± 14 | 55 ± 16 | 0.19 |
| Treatment I | 73.4 ± 16.9 | 67.1 ± 15 | 0.3 |
| Treatment II | 73.7 ± 14 | 75 ± 21 | 0.8 |
| Worry | 62.1 ± 19.3 | 62.8 ± 26.9 | 0.9 |
| Communication | 73.3 ± 18 | 74.5 ± 23 | 0.9 |
| Self Management of Diabetes in Adolescents | |||
| Collaboration with Parents | 15.9 ± 8.6 | 22.4 ± 9.5 | 0.14 |
| Diabetes Care Activities | 24.5 ± 5.2 | 29.4 ± 5.5 | 0.06 |
| Diabetes Problem Solving | 12.7 ± 4.4 | 11.4 ± 4 | 0.5 |
| Diabetes Communication | 13.9 ± 5.9 | 16.1 ± 8 | 0.5 |
| Goals | 13.4 ± 3.5 | 14.4 ± 3.1 | 0.5 |
Data shown as mean ± SD
Effects of Intervention
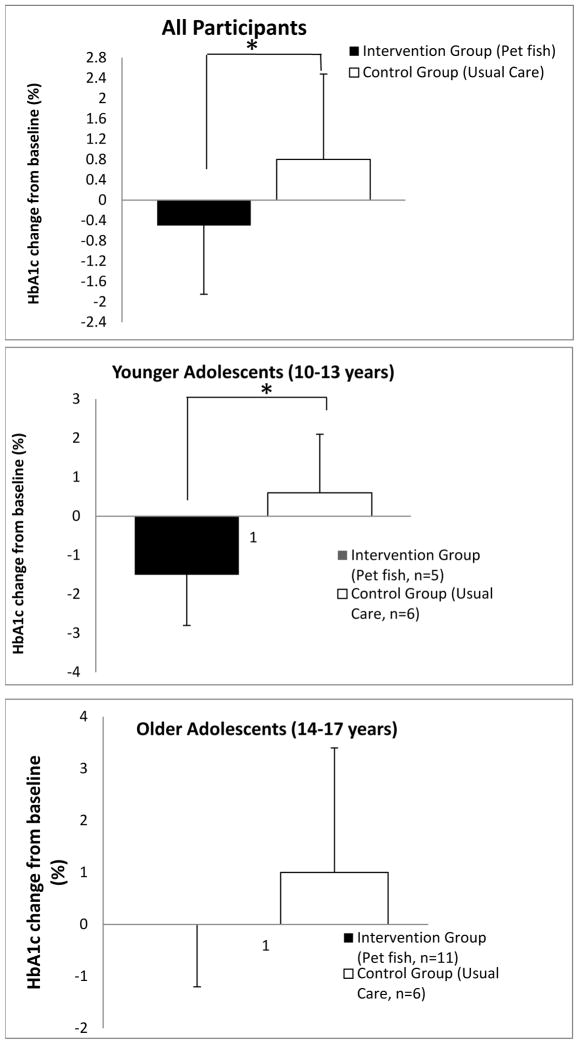
At the subsequent follow-up appointment, the participants in the intervention group exhibited a statistically significant decrease in A1C levels (−0.5%) compared with their peers in the control group who had an increase in A1C levels (0.8%)(p = 0.04) (Fig 1a). The raw effect size was −1.3% (95% CI −0.08, −2.52). While the intention is to have patients in poor diabetes control seen every two to three months, some follow-up appointments occurred after a longer duration of time due to cancellations and rescheduling of appointments. There was no difference in length of time to follow-up appointments, however, between the intervention group (3.1 months ± 1.5) and the control group (3.3 months ± 2.8)(median ± SD). The younger adolescents (ages 10–13 years) demonstrated a greater response to the intervention which was statistically significant (−1.5% vs. 0.6%, p = 0.04)(Fig 1b) compared with the older adolescents (ages 14–17, Fig 1c). The raw effect size was −2.1% (95% CI −0.16, −4.04). Differences in A1C by gender were not assessed due to the low number of males in the control group. No significant effects were observed for the Peds QoL modules (Generic and Diabetes) or the subscales on the SMOD-A questionnaire (data not shown). However, in the intervention group there was a trend towards improvement in the response to the question on the SMOD-A questionnaire that directly pertained to reviewing blood glucose readings with parents (Q8. My parents and I look together at the record of my blood sugar readings to make adjustments, 0.5 vs. 1.1, p = 0.09).
Figure 1.
Change of A1C level in (a) all participants, (b) younger participants, (c) older participants. The graph shows the mean change in A1C (%) at endpoint for the Intervention group (black bars) vs. Control group (white bars). Data are represented as mean ± SD. (*, P < 0.05).
Discussion
The present study assessed the feasibility and effect of an innovative behavioral intervention on glycemic control in adolescents with poorly controlled T1DM. Our findings revealed a statistically significant change in A1C at 3 months for the participants that received the intervention (a pet fish with instructions for pairing fish care with diabetes self-management tasks) compared with their peers in the control group (usual care). The advantage for the behavioral intervention was even more pronounced within the group of younger adolescents.
The design of the intervention allowed for the assessment of the impact of a novel, simple, and inexpensive adjunct to standard therapy. Participant and family satisfaction with the intervention was informally assessed at the follow-up visit. Most of the families reported that their adolescents enjoyed caring for the fish and it was not burdensome. One mother reported that their fish bowl gave off a bad odor despite frequent cleaning, and they were advised to place it in a cool location away from direct sunlight. Two participants needed replacement fish when their fish died during routine fish care, but there were no withdrawals from the intervention group for multiple fish deaths. Many participants in the control group were disappointed about not going home with a fish, but excited that they would receive their own fish at the completion of the study (one year from randomization).
It is not surprising that the intervention worked well for the younger adolescents. Early adolescence is characterized by a desire for independence, rule-following, and a greater interest in privacy, which the responsibility for caring for a fish located in the adolescents’ bedroom reflects.14 Parents, however, still serve as an important role model for these younger adolescents, and along with the visual reminder of the fish to the adolescent and indirectly to the parent, also may have played a role in the improved A1C by reminding these adolescents to check their blood glucose levels.14 During middle adolescence, this developmental stage is characterized by increasing independence from parents, a greater reliance on peer groups, and a desire to be normal.14 This desire to be like their peers may result in adolescents not checking their blood glucose level. Also, any parental reminders to check their blood glucose levels may have been ignored, given their growing independence.
Several mechanisms of change may have been responsible for the observed improvement in A1C including enhanced self-efficacy with self-care behaviors, or a change in mood or perceived well-being which has been shown to directly relate to glycemic control.15,16 While we did not appreciate any changes in the quality of life or self-management tasks based on the responses to the QoL and SMOD-A surveys, this may be due to the small sample size as the study was not powered to detect small changes. Several alternative instruments are available for studying self-efficacy17 and self-care18 in adolescent patients, and may be more suitable for this type of study. Interestingly, the intervention group showed a trend towards increasing frequency in reviewing blood glucose readings with parents. Parental involvement has been shown to improve both glycemic control and adherence to self-care behaviors, particularly when the parents are closely engaged in monitoring their adolescent’s diabetes management tasks.19,20 In addition, other measures of mood, responsibility, conscientiousness, or altruism may be helpful in identifying the mediators of the positive effect that the pet fish had on glycemic control. Once the behaviors are established, they may be easier to maintain. Increased self-efficacy as a result of improved self-care may also serve to maintain these behaviors. However, it is possible that the effects of the intervention will not be sustainable after the initial three month period, and longer term measures of the intervention effects are in progress.
The present study has several limitations including small sample size. Our study did not formally document whether the adolescents directly cared for the fish and we did not assess other variables that may affect glycemic control such as direct measures of blood glucose monitoring frequency and other indices of compliance. We did not evaluate the clinician and parent burden of the behavioral intervention. The clinician was not blinded in our study, which theoretically could impact the glycemic outcome. The major strengths of our study are the randomization and use of a control group as well as the novelty, simplicity and low cost of our intervention. This is significant since it increases the availability of our intervention to multiple patient populations with various educational and economic resources. We were also able to study two groups of children that were relatively similar except for their randomization to the intervention or control groups. Therefore, factors that might affect glycemic control such as age at diagnosis of diabetes, diabetes duration, and socioeconomic status did not confound our findings.
In conclusion, our data show that care of a pet fish can lead to improved glycemic control in a cohort of adolescents with poorly controlled T1DM. Associating diabetes self-care tasks with routine, consistent daily activities may be another tool in the diabetes educator toolbox that can be used to enhance compliance and ultimately improve glycemic control. The identification of this successful behavioral intervention justifies conducting ongoing studies to validate these findings in a larger cohort for a longer follow-up period, pinpointing the mechanisms leading to the improvement, utilizing different household pets and monitoring the changes in health service utilization (emergency room visits and inpatient admissions for diabetic ketoacidosis).
Acknowledgments
This project was supported by grant 5-R03-HD071263-02, co-funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the WALTHAM Centre for Pet Nutrition, a division of Mars, Incorporated. Additional support was provided by a grant from Partnerships for Cure and a gift from the Dedman Family.
References
- 1.Silverstein JMD, et al. Care of Children and Adolescents With Type 1 Diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B, Ho J, Brackett J, Finkelstein D, Laffel L. Parental involvement in diabetes management tasks: Relationships to blood glucose monitoring adherence and metabolic control in young adolescents with insulin-dependent diabetes mellitus. Journal of Pediatrics. 1997;130:257–265. doi: 10.1016/s0022-3476(97)70352-4. [DOI] [PubMed] [Google Scholar]
- 3.Wysocki T, Harris MA, Greco P, Bubb J, Danda CE, Harvey LM, McDonell K, Taylor A, White NH. Randomized, controlled trial of behavior therapy for families of adolescents with insulin-dependent diabetes mellitus. J Pediatr Psychol. 2000 Jan-Feb;25(1):23–33. doi: 10.1093/jpepsy/25.1.23. [DOI] [PubMed] [Google Scholar]
- 4.Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, Sadler M, Mauras N, White NH. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006 Oct;31(9):928–38. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 5.Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control: a randomized controlled trial. Diabetes Care. 2005 Jul;28(7):1604–10. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BJ, Svoren B, Laffel L. Initiatives to promote effective self-care skills in children and adolescents with diabetes mellitus. Dis Manage Health Outcomes. 2007;15(2):101–108. [Google Scholar]
- 7.Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, Kimber A, Cradock S, McEvilly EA. Behavioral interventions for adolescents with type 1 diabetes: how effective are they? Diabetes Care. 2000 Sep;23(9):1416–22. doi: 10.2337/diacare.23.9.1416. [DOI] [PubMed] [Google Scholar]
- 8.Sanders MJ, Van Oss T. Using daily routines to promote medication adherence in older adults. Am J Occup Ther. 2013 Jan-Feb;67(1):91–9. doi: 10.5014/ajot.2013.005033. [DOI] [PubMed] [Google Scholar]
- 9.Michie S, Johnston M, Abraham C, Lawton R, Parker D, Walker A “Psychological Theory” Group. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care. 2005 Feb;14(1):26–33. doi: 10.1136/qshc.2004.011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells DL. The Effects of Animals on Human Health and Well-Being. Journal of Social Issues. 2009;65(3):523–543. [Google Scholar]
- 11.McNicholas J, et al. Pet ownership and human health: a brief review of evidence and issues. British Medical Journal. 2005;331(7527):1252–1254. doi: 10.1136/bmj.331.7527.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varni JW, Burwinkle TM, Jacobs JR, Gottschalk M, Kaufman F, Jones KL. The PedsQL in type 1 and type 2 diabetes: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales and type 1 Diabetes Module. Diabetes Care. 2003 Mar;26(3):631–7. doi: 10.2337/diacare.26.3.631. [DOI] [PubMed] [Google Scholar]
- 13.Schilling LS, Dixon JK, Knafl KA, Lynn MR, Murphy K, Dumser S, Grey M. A new self-report measure of self-management of type 1 diabetes for adolescents. Nurs Res. 2009 Jul-Aug;58(4):228–36. doi: 10.1097/NNR.0b013e3181ac142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Last accessed November 4, 2013]; http://www.brightfutures.org/bf2/pdf/pdf/AD.pdf.
- 15.Hermanns N, Scheff C, Kulzer B, Weyers P, Pauli P, Kubiak T, Haak T. Association of glucose levels and glucose variability with mood in type 1 diabetic patients. Diabetologia. 2007 May;50(5):930–3. doi: 10.1007/s00125-007-0643-y. [DOI] [PubMed] [Google Scholar]
- 16.Yi-Frazier J, Hilliard M, Cochrane K, Hood K. The Impact of Positive Psychology on Diabetes Outcomes: A Review. Psychology. 2012;(3):1116–1124. [Google Scholar]
- 17.Grossman HY, Brink S, Hauser ST. Self-efficacy in adolescent girls and boys with insulin-dependent diabetes mellitus. Diabetes Care. 1987 May-Jun;10(3):324–9. doi: 10.2337/diacare.10.3.324. [DOI] [PubMed] [Google Scholar]
- 18.La Greca AM, Swales T, Klemp S, Madigan S. Self care behaviors among adolescents with diabetes. Proceedings of the 9th Annual Sessions of the Society of Behavioral Medicine; 1988. p. A42. [Google Scholar]
- 19.Berg CA, Butler JM, Osborn P, King G, Palmer DL, Butner J, Murray M, Lindsay R, Donaldson D, Foster C, Swinyard M, Wiebe DJ. Role of parental monitoring in understanding the benefits of parental acceptance on adolescent adherence and metabolic control of type 1 diabetes. Diabetes Care. 2008 Apr;31(4):678–83. doi: 10.2337/dc07-1678. [DOI] [PubMed] [Google Scholar]
- 20.Ellis DA, Podolski CL, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: impact on regimen adherence in youth with type 1 diabetes. J Pediatr Psychol. 2007 Sep;32(8):907–17. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]