Abstract
Many common inflammatory disorders are characterized by the infiltration of neutrophils across epithelial lined (mucosal) surfaces resulting in disruption of critical barrier function that protects from microbes and noxious agents. In such conditions, disease symptoms are complex but directly related to leukocyte effects on the barrier and epithelial cell function. It is now highly regarded that cellular factors such as cytokines and receptor–ligand interactions mediating adhesion of leukocytes to epithelial cells have potent effects on epithelial homeostasis, defined by coordinated proliferation, migration, differentiation, and regulated cell shedding. Certain cytokines, for example, not only alter leukocyte interactions with epithelia through changes in expression of adhesion molecules but also affect barrier function through alterations in the composition and dynamics of intercellular junctions. In particular, inflammation-induced loss of many tight junction molecules, in part, can account for dysregulated cellular proliferation, migration, survival, and barrier function. This review will highlight how neutrophils interact with epithelial cells with particular focus on adhesion molecules involved and signaling events that play roles in regulating mucosal homeostasis and pathobiology. A better understanding of these molecular events may provide new ideas for therapeutics directed at attenuating consequences of pathologic inflammation of mucosal surfaces.
Keywords: gastrointestinal system, immunopathology, inflammation, pathobiology
Introduction
Intestinal homeostasis, which is intimately linked to the regulation of barrier function, is dependent on coordinated control of epithelial cell proliferation, migration, differentiation, and cell turnover. These processes maintain a physical barrier between the gut lumen and the underlying tissues. The epithelium that lines the gut is the primary regulator of barrier function and as such comes in contact with a variety of foreign substances and organisms such as bacteria and viruses that can cause injury upon contact or invasion. Damage to the intestinal epithelium, along with a subsequent cascade of complex inflammatory queues, leads to the recruitment of acute inflammatory cells or polymorphonuclear leukocytes (PMNs) that play an active role in phagocytizing bacteria and viruses while releasing highly reactive oxidative species (ROS) to destroy invading microbes. However, a delicate balance in regulation of PMN function is crucial in preventing ongoing collateral damage to the epithelium under intense proinflammatory conditions. Following an inflammatory stimulus, PMNs cross the epithelium into the intestinal lumen in a process known as transepithelial migration. The mechanisms by which neutrophils achieve this is both complex and incompletely understood, although it is becoming increasingly clear that homeostatic tight junction (TJ) signaling is altered during this process (Kucharzik et al. 2001). Large increases in permeability of the epithelium during intense PMN transmigration enhance the exposure of leukocytes to foreign antigens and increase the severity of inflammation. Damage to the intestinal mucosa along with altered barrier function associated with sustained PMN transepithelial migration has been linked to a number of chronic inflammatory conditions such as ulcerative colitis (UC) and Crohn’s disease that are collectively referred to as inflammatory bowel disease (IBD). In fact, a hallmark feature of disease flares in IBD is PMN invasion of the intestinal epithelium leading to crypt abscess formation (Figure 1) and is associated with the release of a variety of proinflammatory cytokines, ROS, and proteases. A result of this dysregulated PMN accumulation and activation is ulceration, loss of mucus, rectal bleeding, and diarrhea (Marks and Segal 2008). This review highlights recent advances in our understanding of how PMN migration across mucosal epithelia is regulated and the consequences these effects have on epithelial homeostasis. A greater understanding of these events may ultimately provide ideas for new therapeutic strategies designed to mitigate the pathobiology of IBD.
Figure 1.
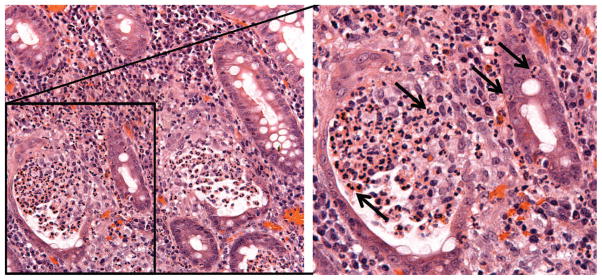
Crypt abscess with PMN infiltration. Colonic mucosal biopsy from an individual with ulcerative colitis showing massive PMN infiltration (arrows) into epithelial-lined crypts and formation of a crypt abscess (right). PMN = polymorphonuclear leukocyte.
Leukocyte Recruitment and Transmigration across the Intestinal Epithelium
Neutrophils, or PMNs, are highly mobile cells that circulate in the blood stream and exit blood vessels by moving between endothelial cells (paracellular route). While the details of neutrophil transendothelial migration are more clearly defined, much less is known about the underlying mechanisms regulating neutrophil transepithelial migration. Neutrophils are recruited along chemotactic gradients to the sites of intestinal mucosal infection or injury, which consist of a variety of stimuli that are released by the epithelium, such as hepoxilin A (hepA3), or by intestinal epithelial cells (IECs) and PMNs, as in the case for interleukin 8 (IL-8) and leukotriene B4 (Dias, Wallace, and Parsons 1992; Hammond et al. 1995; Mrsny et al. 2004). The release of similar chemoattractants by multiple cell types amplifies the intensity of the proinflammatory response and further reinforces leukocyte trafficking and recruitment upon early arrival of PMN. In response to host immune activation, numerous species of microbes have evolved mechanisms that take advantage of the host immune system to gain better access to host tissues or to evade the immune cells. For example, species of invasive Salmonella typhimurium and Shigella secrete peptides and toxins that also elicit or exacerbate PMN recruitment through the production of IL-8 (Lee et al. 2000). Furthermore, S. typhimurium invasion of IECs induces the activation of ezrin, also called cytovillin or villin-2, and is a protein tyrosine kinase substrate in microvilli that facilitates the apical expression of multidrug resistance–associated protein 2 (MRP2). Interestingly, MRP2 is an efflux pump for the neutrophil chemoattractant hepA3, which is a potent stimulus for transepithelial migration (Pazos et al. 2008). A number of other luminal agents are known to increase PMN transmigration. For example, studies have shown that bacterially derived n-formylated peptides such as formyl-methionyl-leucyl phenylalanine (fMLF) potently drive transepithelial migration of leukocytes in assay systems using monolayers of IECs cultured on permeable supports (Parkos et al. 1991). Thus, different environmental and host stimuli generate molecular signaling events that recruit leukocytes to the epithelium, leading to the development of a positive feedback loop that increases the levels of PMN transmigration into the gut lumen.
Molecular Basis of Leukocyte Transepithelial Migration
To cross epithelial barriers, PMNs engage the epithelium in complex binding and signaling processes that result in loosening of the barrier and facilitating the passage of neutrophils between the lateral surfaces of epithelial cells (Edens et al. 2002). In considering the molecular interactions between migrating PMN and the intestinal epithelium, it is important to note that the path of transmigration involves negotiating the paracellular or basolateral space between epithelial cells, which is of considerable length and contains formidable barriers at intercellular junctions composed of extracellular protein complexes that are anchored to the actin cytoskeleton (Anderson and Van Itallie 1995; Madara et al. 1992; Nusrat et al. 2000). Separating the apical or luminal aspect of IECs from basolateral domains that are in contact with subepithelial tissue or lamina propria is the TJ that is the central controller of epithelial barrier function. Thus, the TJ represents a key regulator of PMN transepithelial migration that must be crossed in order to reach the lumen of the intestine.
Neutrophils that have been recruited from the circulation must first bind to the basal surface of IECs in order to migrate across TJs into the lumen. It is well established that PMNs interact with the basal surface of epithelial cells with beta-2 integrin (CD11b/CD18) expressed at the neutrophil surface (Parkos et al. 1991; Zen et al. 2002), although the epithelial ligand is not known. However, it has been shown that not all transmigration events are dependent on β-2 integrin, which suggests that multiple binding partners may be involved and is an area of current research (Blake et al. 2004). After engaging β-2 integrin and moving into the paracellular space between IECs, CD47, a membrane-spanning glycoprotein expressed on the lateral surface of IECs and on neutrophils, interacts with neutrophil-expressed signal-regulatory protein-α (SIRP-α) to control the rate of PMN migration across the epithelium (Lee et al. 2010; Liu et al. 2005; Parkos, Colgon, Liang, et al. 1996).
Migration of PMN into the epithelial paracellular space places them in direct contact with desmosomes and adherens junctions containing structural proteins termed catenins and cadherins, which are critical regulators of cell junction formation and stability. Migrating PMN disrupt adherens junctions by releasing the protease elastase that facilitates transmigration (Ginzberg et al. 2001). A central adherens junction protein that is the target of neutrophil elastase is epithelial (E)-cadherin, which regulates epithelial barrier and homeostasis through the adapter proteins p120-catenin and β-catenin. Indeed, targeted knockout of p120-catenin in the small and large intestine of mice has a dramatic negative effect on intestinal barrier function, causing a substantial infiltration of neutrophils into the gut epithelium, a phenotype that is intriguingly similar to that observed in persons with IBD (Smalley-Freed et al. 2010).
The maze of TJ proteins that a PMN must next negotiate during transepithelial migration is highly complex, yet little is known about which proteins are adhesion receptors and which serve regulatory roles in this process through signaling functions (Colgan, Parkos, et al. 1993; Madara, Nash, and Parkos 1991; Nusrat et al. 1997; Parkos, Colgon and Madara 1994; Samonte et al. 2004). One TJ receptor–ligand pair for migrating PMN is epithelial expressed Coxsackie adenovirus receptor (CAR), which binds to PMN-expressed junctional adhesion molecule-like protein (JAM-L; Zen et al. 2005). Other trans-membrane epithelial TJ proteins that are candidate receptors include members of the claudin family, junctional adhesion molecules, and occludin. Many of these proteins not only serve to regulate epithelial barrier but also function as signaling molecules that fine-tune epithelial homeostasis through regulation of cell migration and proliferation.
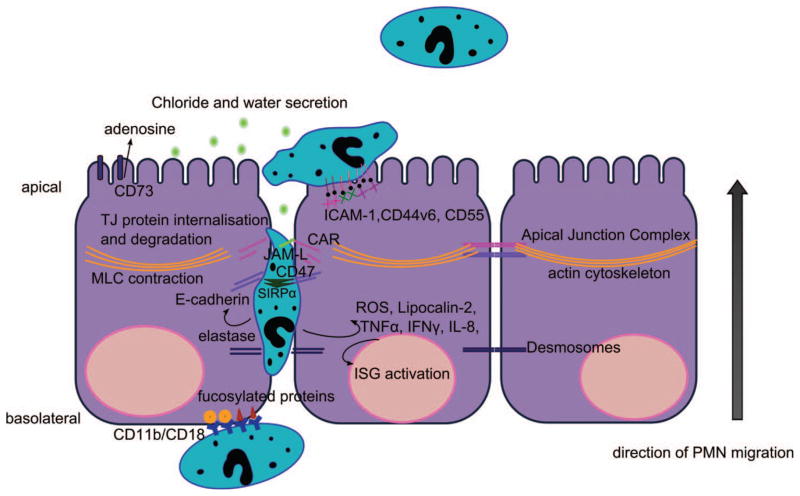
After crossing TJs, PMN arrive at the apical or luminal surfaces of the epithelium. Interestingly, it is now appreciated that during inflammation, there is upregulation of PMN adhesion molecules on the apical epithelial surface in the gut. One key adhesion molecule that is expressed at very low levels under normal conditions but is dramatically upregulated on the apical surface of IECs after exposure to inflammatory cytokines is intercellular adhesion molecule-1 (ICAM-1; Parkos, Colgon, Diamond et al. 1996). While this expression pattern would suggest that ICAM-1-based adhesion occurs only after PMN have migrated across the epithelium, recent evidence indicates that such binding events occur and may be functionally relevant. Specifically, there is now a report that ligation of ICAM-1 on the apical epithelial surface results in enhanced wound repair in vitro (Sumagin, Nusrat, and Parkos 2013). Another adhesive interaction at the level of postmigration that is now recognized involves CD44v6. Similar to ICAM-1, the v6 variant of CD44 is only expressed at significant levels on the apical epithelial surface under inflammatory conditions (Brazil et al. 2010). Here, PMN interactions with this molecule result in the shedding of CD44v6 that is required for migrated PMN to detach from the epithelium. Inhibition of this detachment with specific antibodies results in the accumulation of PMN on the apical surface of the epithelium (Brazil et al. 2010; Brazil, Louis, and Parkos 2013). In addition, it has been shown that CD55 (also called decay-accelerating factor [DAF]) participates in the process of PMN release from the apical surface (Lawrence et al. 2003; Louis et al. 2005). These antiadhesive forces are likely to be in direct opposition to the proadhesive forces produced by ICAM-1, thus raising the possibility that the balance of ICAM-1 and DAF/CD44v6 activity might play an important role in crypt abscess formation, which are collections of crypt epithelial–associated PMN. A summary of these steps and the molecules involved in both UC and Crohn’s disease are illustrated in Figure 2.
Figure 2.
Molecular interactions of neutrophils with epithelial cells during transepithelial migration. PMNs are recruited to the intestinal epithelium following extravasation from the local microcirculation. They subsequently migrate across the subepithelial space (lamina propria) and adhere to the basal aspect of IECs through interactions between CD11b/CD18 on neutrophils and fucosylated receptors on the epithelium. PMNs transmigrate across the epithelium in the paracellular space that is facilitated through binding interactions with cell surface receptors and releasing molecules that disrupt the apical junction complex. PMN migration across tight junctions (TJs) is regulated, in part, by interactions between PMN-expressed junctional adhesion molecule like protein (JAML) and coxsackie and adenovirus receptor (CAR). PMN migration across epithelial TJs results in increased permeability and enhanced access of microbes to the subepithelial space. PMN migration across TJs is also associated with the activation of ROS production, TLR signaling cascades, interferon-stimulated genes (ISGs), and the production of inflammatory cytokines. After migration across TJs, PMN interacts with the luminal or apical membrane of IECs via ICAM-1, CD44v6, and CD11b/CD18, resulting in, as of yet, incompletely defined signaling events, in addition to stimulating water efflux by induction of electrogenic Cl− secretion, through the release of 5′AMP, which is converted into adenosine by apically localized CD73. PMNs = polymorphonuclear leukocytes; IEC = intestinal epithelial cell; ROS = reactive oxidative species; TLR = toll-like receptor; AMP = adenosine monophosphate; ICAM-1 = intercellular adhesion molecule-1.
Functional Consequences of Leukocyte–Epithelial Interactions
PMNs can affect epithelial barrier function either directly through receptor–ligand interactions or indirectly by the release of paracrine molecules. Naturally, when migrating PMNs cross TJs, there is a physical breach of the barrier that has been shown in many investigations (Colgan, Parkos, et al. 1993; Madara, Nash, and Parkos 1991; Parkos et al. 1992; Parkos, Colgon, and Madara 1994; Parkos et al. 1991). In addition, it is now apparent that the physical movement of PMNs through the epithelium initiates a cascade of protein signaling that ultimately leads to increases in permeability (Kucharzik et al. 2001) that result in exposure of the underlying lamina propria to luminal foreign antigens and toxins that exacerbate inflammation. Exposure to bacterial antigens activates toll-like receptor (TLR)-mediated signaling that is important in eliminating bacterial infections but can have negative consequences on the surrounding tissue. To counter this, a powerful antimicrobial protein called lipocalin-2, also known as PMN gelatinase-associated lipocalin (NGAL), is produced and released by PMNs and the epithelium, following TLR3 stimulation in IECs (Ostviks et al. 2013). This has important consequences as it can aid in bacterial clearance, although increased levels of lipocalin-2 can promote cancer (Rodvold, Mahadevan, and Zanetti 2012). Lipocalin-2 is now considered a biomarker for disease activity in IBD (Ostviks et al. 2013).
While direct adhesive interactions between PMN and many TJ proteins have not yet been shown, it is now well established that epithelial inflammation and PMN transepithelial migration are associated with alterations in the expression levels of several TJ proteins which, in turn, have pronounced effects on the barrier and homeostasis. Proinflammatory cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) that are expressed and released abundantly during IBD, have been shown to have dramatic effects on the internalization of TJ proteins such as occludin, JAM-A, and claudins (Bruewer et al. 2003; Kucharzik et al. 2001). As mentioned above, PMNs can also amplify proinflammatory signals and, as an example, in the early stages of PMN transmigration, TNF-α also causes an increase in the expression of ADAM17 (A Disintegrin And Metalloproteinase domain 17, also called tumor necrosis factor-?-converting enzyme) within the epithelium, an enzyme that catalyzes the conversion of TNF-α into its active form (Cesaro et al. 2009), thus reinforcing a positive feedback loop. Furthermore, expression of the TJ protein JAM-A is decreased under inflammatory conditions in the intestine and its loss has been shown to result in not only a leaky barrier but also alterations in epithelial cell proliferation and migration (Laukoetter et al. 2007; Mandell et al. 2005; Monteiro et al. 2013; Nava et al. 2011; Severson et al. 2009). Additionally, downregulation of occludin expression during PMN transmigration (Kucharzik et al. 2001) by proteosomal degradation (Coeffier et al. 2010) appears to be mediated through IL-18, which results in enhanced paracellular permeability and facilitated PMN migration (Lapointe and Buret 2012). Thus, while TJs form important physical roles in barrier function, they also play an important role in homeostasis through complex signaling events that can be drastically modified by inflammatory conditions and PMN transmigration.
As highlighted above, PMN migration across epithelial barriers has been shown to have direct and indirect effects on barrier function and epithelial homeostasis. Epithelial barrier, in addition to being regulated by transmembrane TJ proteins, is controlled by tension in an apically associated actin–myosin contractile ring whereby increased contraction results in enhanced permeability. Interestingly, when migrating PMNs interact with the intestinal epithelium, it is now recognized that alterations in permeability occur secondary to signaling events that result in the contraction of the apical actomyosin ring. Regulation of this contractile ring is complex and mediated through phosphorylation of myosin light chain (MLC) by MLC kinase and ρ-associated protein kinase-2 (ROCK-2). Indeed, when PMN receive signals to migrate across the intestinal epithelium, initial contact with the basal epithelial surface results in protease-activated receptor activation and signaling events, generating the phosphorylation of MLC, contraction of the actomyosin ring, increased permeability, and enhanced PMN transepithelial migration (Chin et al. 2008).
Even after PMNs reach the lumen of the gut, they continue to have potent effects on epithelial function. PMNs release copious amounts of 5′ adenosine monophosphate (AMP) that are acted on by an apically expressed 5′ ectonucleotidease termed CD73 that converts it into adenosine. Adenosine is able to activate cyclic adenosine monophosphate (cAMP) pathways in the epithelium, resulting in electrogenic Cl− secretion into the lumen, which is the basis for secretory diarrhea, a significant problem in people with IBD. Thus, while much attention has been drawn to important chemoattractants such as small peptides and bacterial toxins, ion concentration across intestinal barriers also have profound effects on PMN migration and gut homeostasis.
An additional indirect consequence of PMN transmigration in the gut is alteration in gene expression profiles, some of which indefinitely alter gene expression in the gut even after recovery and remission of UC (Planell et al. 2013). Many of the genes whose expression is altered by the downstream consequences of PMN transmigration encode cytokines or immune response effectors such as IL-8, nuclear factor kappa beta (NF-κβ), or signal transducer and activator of transcription (STAT) proteins. The altered expression of these genes leads to broad changes in physiology and increased inflammatory responses. A change in the expression of genes encoding TJ proteins has also been documented. For example, the expression of different genes encoding claudin proteins has been shown to occur in different tissues of the gastrointestinal tract, and at different levels, based on the inflammatory condition of IBD patients (Lameris et al. 2013). Interestingly, microRNA profiles from patients with UC or patients with Crohn’s disease have also been shown to be significantly different than healthy controls (Iborra et al. 2013), indicating that a global change in gene transcription is associated with chronic PMN transmigration. In addition, microRNA regulation of STAT3 is downregulated in the colon of pediatric UC patients, which increases STAT3 protein expression (Koukos et al. 2013). Thus, the consequences of uncontrolled PMN transepithelial migration can be drastic on gene expression, protein localization, and function, as well as cell and tissue function that ultimately culminate in a disarray of intestinal homeostasis and chronic illness.
PMN Effects on Barrier Recovery
Despite the deleterious effects of migrating PMNs on barrier function, it is now clear that these innate immune cells also play important roles in barrier recovery. The production of Lipoxin A4, resolvin E1 (which associates with the leukotriene B4 receptor; Arita et al. 2007), and protectin D1 from PMNs decreases their subsequent recruitment and increases apoptosis (Colgan, Serhan, et al. 1993 Schwab et al. 2007). Apoptosis, induced through either necrosis or engulfment by macrophages, is an important process that occurs in order to initiate inflammation resolution, although necrosis can result in the secretion of molecules such as proteases and antiapoptotic cytokines that impact on the epithelium (Ina et al. 1999). In an experimental model of IBD, treatment with resolvin E1 was found to be beneficial in clearing inflammation through the inhibition of proinflammatory cytokines IL-12 and TNF-α and reducing PMN infiltration (Arita et al. 2005). Typically in IBD persons, a deficiency of PMNs induced death ultimately leads to a continuation of the PMN-induced positive feedback loops that further the progression of intestinal inflammation (Brannigan et al. 2000).
Recently it has been shown that PMNs can help restore epithelial barrier homeostasis by enhancing cell proliferation and migration through the release of IL-22 (a member of the IL-10 cytokine family), upon activation with IL-23 (Brand et al. 2006). However, the high increase in IL-22 levels, which are observed in Crohn’s disease (Schmechel et al. 2008), does not only come from neutrophils but other immune cells such as natural killer cells. When IL-22 binds to its receptor, STAT3 induction leads to the production of antimicrobial peptides (Brand et al. 2006; Schmechel et al. 2008; Zindl et al. 2013) that have also been shown to provide a degree of protection against colitis in a mouse model (Zindl et al. 2013). The complexity of production and function of IL-22 has been recently reviewed and highlights this cytokine as an active area of research in therapeutic intervention for IBD (Mizoguchi 2012).
Pharmacologics and Immune Therapies for IBD
The complexity of IBD makes targeted therapy a challenge and this is exemplified by the availability of numerous treatments that are often only effective for patient subtypes or certain individuals. Clearly, as the immune response plays a key role in IBD, it is not surprising that the immune system has been the target of numerous pharmacologic agents. For example, infliximab is an anti-TNF-α compound that has been shown to be effective in the treatment of Crohn’s disease (Nahar et al. 2003). Second-generation TNF-α antagonists such as adalimumab and certolizumab pegol have also become available, but not all patients have responded to these treatments (Schmidt et al. 2009). Other more general immune suppressants like azathioprine have also been used but with limited effectiveness for treating Crohn’s disease (Chande, Tsoulis, and MacDonald 2013; Panes et al. 2013). However, the prevention of leukocyte trafficking, and thus the downstream consequences of their transmigration through the gut epithelium, seems to be the most ideal target, given the significant contribution this phenomenon has on IBD. Along with β-1 and -7 integrins, the α-4 integrin on leukocytes plays a key role in their trafficking, and recent efforts toward targeting this receptor has produced the α-4 integrin antagonist, GSK223618A, which has showed some promising therapeutic effects on dextran sodium sulfate (DSS)-induced colitis in mice (Murphy et al. 2010). The α-4 integrin monoclonal antibody natalizumab that blocks leukocyte migration into the tissue has also been used effectively in clinical trials (Schmidt et al. 2009). Thus, natalizumab treatment offers promise that PMN transepithelial migration is the underlying factor that causes symptomatic disease and not the recruitment of leukocytes to the epithelium (Kucharzik et al. 2005).
Research Gaps and Future Directions
While targeted therapy has proven useful in treating some cases of IBD, many patients still remain unresponsive to therapy. In fact, the majority of cases in IBD do not have a clear link to genetic defects where their etiology is complex and multifactorial. Cases of UC with increased PMN infiltration and lack of response to targeted TNF-a treatment, has proven challenging in preventing ulceration, healing damaged tissue, and promoting resolution of inflamed and damaged tissue. While leukocyte trafficking leads to the symptoms and pathophysiology observed in IBD, the complexity of the disease makes it difficult to demonstrate that this phenomenon is the cause or the likely consequence of immune system dysregulation. Thus, a major obstacle in understanding and treating IBD is the delineation of the multifaceted causes and symptoms of individual IBD cases, along with the use of the appropriately targeted therapy for that individual’s IBD profile.
Concluding Remarks
The integrity of the epithelial barrier and intestinal homeostasis is intricately affected by PMN recruitment into the gut lumen. Precise immune regulation is required to control PMN transepithelial migration into areas of intestinal insult or injury to deliver destructive substances and eliminate infection while causing minimal deleterious effects within the mucosa.
Resolution of inflammation requires destruction of dead PMNs by macrophages and the repair of damaged tissue. A defect in the intrinsic control of PMN transepithelial migration and elimination can ultimately contribute to pathologic inflammatory conditions, as observed in IBD. Thus, aiming therapeutic strategies toward regulating PMN trafficking in the intestine stands to be an important target in treating IBD.
Acknowledgments
The author(s) received the following financial support for the research, authorship, and/or publication of this article: We acknowledge funding grants from the National Institute of Health; DK061379, DK072564, DK079392 and DK007771.
The authors would like to thank Dr. Goo Lee for providing images of intestinal crypt abscesses.
Abbreviations
- AMP
adenosine monophosphate
- cAMP
cyclic adenosine monophosphate
- CAR
Coxsackie adenovirus receptor
- DAF
decay-accelerating factor
- DSS
dextran sodium sulfate
- fMLF
formyl-methionyl-leucyl phenylalanine
- hepA3
hepoxilin A
- IBD
inflammatory bowel disease
- ICAM-1
intercellular adhesion molecule-1
- IECs
intestinal epithelial cells
- IFN-γ
interferon gamma
- IL
interleukin
- JAM-L
junctional adhesion molecule-like protein
- MLC
myosin light chain
- MRP2
multidrug resistance–associated protein 2
- NF-κβ
nuclear factor kappa beta
- NGAL
PMN gelatinase–associated lipocalin
- PMNs
polymorphonuclear leukocytes
- ROCK-2
ρ-associated protein kinase-2
- ROS
reactive oxidative species
- SIRP-α
signal-regulatory protein-α
- STAT
signal transducer and activator of transcription
- TJ
tight junction
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor alpha
- UC
ulcerative colitis
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–75. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–17. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–76. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake KM, Carrigan SO, Issekutz AC, Stadnyk AW. Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol. 2004;136:262–68. doi: 10.1111/j.1365-2249.2004.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2006;290:G827–38. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- Brannigan AE, O’Connell PR, Hurley H, O’Neill A, Brady HR, Fitzpatrick JM, Watson RWG. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock. 2000;13:361–66. doi: 10.1097/00024382-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Brazil JC, Lee WY, Kolegraff KN, Nusrat A, Parkos CA, Louis NA. Neutrophil migration across intestinal epithelium: Evidence for a role of CD44 in regulating detachment of migrating cells from the luminal surface. J Immunol. 2010;185:7026–36. doi: 10.4049/jimmunol.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil JC, Louis NA, Parkos CA. The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1556–65. doi: 10.1097/MIB.0b013e318281f54e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- Cesaro A, Abakar-Mahamat A, Brest P, Lassalle S, Selva E, Filippi J, Hebuterne X, Hugot JP, Doglio A, Galland F, Naquet P, Vouret-Craviari V, Mograbi B, Hofman PM. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1332–43. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2013;4:CD000545. doi: 10.1002/14651858.CD000545.pub4. [DOI] [PubMed] [Google Scholar]
- Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol. 2008;181:5702–10. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coeffier M, Gloro R, Boukhettala N, Aziz M, Lecleire S, Vandaele N, Antonietti M, Savoye G, Bole-Feysot C, Dechelotte P, Reimund JM, Ducrotte P. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol. 2010;105:1181–88. doi: 10.1038/ajg.2009.700. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. J Cell Biol. 1993;120:785–98. doi: 10.1083/jcb.120.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Serhan CN, Parkos CA, Delparcher C, Madara JL. Lipoxin a(4) modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias VC, Wallace JL, Parsons HG. Modulation of cellular phospholipid fatty acids and leukotriene B4 synthesis in the human intestinal cell (CaCo-2) Gut. 1992;33:622–27. doi: 10.1136/gut.33.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens HA, Levi BP, Jaye DL, Walsh S, Reaves TA, Turner JR, Nusrat A, Parkos CA. Neutrophil transepithelial migration: Evidence for sequential, contact-dependent signaling events and enhanced paracellular permeability independent of transjunctional migration. J Immunol. 2002;169:476–86. doi: 10.4049/jimmunol.169.1.476. [DOI] [PubMed] [Google Scholar]
- Ginzberg HH, Cherapanov V, Dong Q, Cantin A, McCulloch CA, Shannon PT, Downey GP. Neutrophil-mediated epithelial injury during transmigration: Role of elastase. Am J Physiol Gastrointest Liver Physiol. 2001;281:G705–17. doi: 10.1152/ajpgi.2001.281.3.G705. [DOI] [PubMed] [Google Scholar]
- Hammond MEW, Lapointe GR, Feucht PH, Hilt S, Gallegos CA, Gordon CA, Giedlin MA, Mullenbach G, Tekampolson P. Il-8 induces neutrophil chemotaxis predominantly via type-I Il-8 receptors. J Immunol. 1995;155:1428–33. [PubMed] [Google Scholar]
- Iborra M, Bernuzzi F, Correale C, Vetrano S, Fiorino G, Beltran B, Marabita F, Locati M, Spinelli A, Nos P, Invernizzi P, Danese S. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin Exp Immunol. 2013;173:250–58. doi: 10.1111/cei.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ina K, Kusugami K, Hosokawa T, Imada A, Shimizu T, Yamaguchi T, Ohsuga M, Kyokane K, Sakai T, Nishio Y, Yokoyama Y, Ando T. Increased mucosal production of granulocyte colony-stimulating factor is related to a delay in neutrophil apoptosis in inflammatory bowel disease. J Gastroenterol and Hepatol. 1999;14:46–53. doi: 10.1046/j.1440-1746.1999.01807.x. [DOI] [PubMed] [Google Scholar]
- Koukos G, Polytarchou C, Kaplan JL, Morley-Fletcher A, Gras-Miralles B, Kokkotou E, Baril-Dore M, Pothoulakis C, Winter HS, Iliopoulos D. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology. 2013;145:842–52. doi: 10.1053/j.gastro.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T, Hudson JT, 3rd, Lugering A, Abbas JA, Bettini M, Lake JG, Evans ME, Ziegler TR, Merlin D, Madara JL, Williams IR. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54:1565–72. doi: 10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001–9. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lameris AL, Huybers S, Kaukinen K, Makela TH, Bindels RJ, Hoenderop JG, Nevalainen PI. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48:58–69. doi: 10.3109/00365521.2012.741616. [DOI] [PubMed] [Google Scholar]
- Lapointe TK, Buret AG. Interleukin-18 facilitates neutrophil transmigration via myosin light chain kinase-dependent disruption of occludin, without altering epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2012;302:G343–51. doi: 10.1152/ajpgi.00202.2011. [DOI] [PubMed] [Google Scholar]
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, Dermody TS, Nusrat A, Parkos CA. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–76. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence DW, Bruyninckx WJ, Louis NA, Lublin DM, Stahl GL, Parkos CA, Colgan SP. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. J Exp Med. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CA, Silva M, Siber AM, Kelly AJ, Galyov E, McCormick BA. A secreted Salmonella protein induces a proinflammatory response in epithelial cells, which promotes neutrophil migration. Proc Natl Acad Sci USA. 2000;97:12283–88. doi: 10.1073/pnas.97.22.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Weber DA, Laur O, Stowell SR, McCall I, Andargachew R, Cummings RD, Parkos CA. The role of cis dimerization of signal regulatory protein alpha (SIRPalpha) in binding to CD47. J Biol Chem. 2010;285:37953–63. doi: 10.1074/jbc.M110.180018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Soto I, Tong Q, Chin A, Buhring HJ, Wu T, Zen K, Parkos CA. SIRPbeta1 is expressed as a disulfide-linked homodimer in leukocytes and positively regulates neutrophil transepithelial migration. J Biol Chem. 2005;280:36132–40. doi: 10.1074/jbc.M506419200. [DOI] [PubMed] [Google Scholar]
- Louis NA, Hamilton KE, Kong T, Colgan SP. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. Faseb J. 2005;19:950–59. doi: 10.1096/fj.04-3251com. [DOI] [PubMed] [Google Scholar]
- Madara JL, Nash S, Parkos C. Neutrophil-epithelial cell interactions in the intestine. Adv Exp Med Biol. 1991;314:329–34. doi: 10.1007/978-1-4684-6024-7_22. [DOI] [PubMed] [Google Scholar]
- Madara JL, Parkos C, Colgan S, Nusrat A, Atisook K, Kaoutzani P. The movement of solutes and cells across tight junctions. Ann N Y Acad Sci. 1992;664:47–60. doi: 10.1111/j.1749-6632.1992.tb39748.x. [DOI] [PubMed] [Google Scholar]
- Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. J Biol Chem. 2005;280:11665–74. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- Marks DJ, Segal AW. Innate immunity in inflammatory bowel disease: A disease hypothesis. J Pathol. 2008;214:260–66. doi: 10.1002/path.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis. 2012;18:1777–84. doi: 10.1002/ibd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, Stehle T, Dermody TS, Schaefer SA, Hall RA, Nusrat A, Parkos CA. JAM-A associates with ZO-2, Afadin and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell. 2013;173:502–11. doi: 10.1091/mbc.E13-06-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsny RJ, Gewirtz AT, Siccardi D, Savidge T, Hurley BP, Madara JL, McCormick BA. Identification of hepoxilin A3 in inflammatory events: A required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci USA. 2004;101:7421–26. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, Moloney G, Macsharry J, Haynes A, Faivre E, Quinlan A, McLean PG, Lee K, O’Mahony L, Shanahan F, Melgar S, Nally K. Technical advance: Function and efficacy of an {alpha}4-integrin antagonist using bioluminescence imaging to detect leukocyte trafficking in murine experimental colitis. J Leukoc biol. 2010;88:1271–78. doi: 10.1189/jlb.0909627. [DOI] [PubMed] [Google Scholar]
- Nahar IK, Shojania K, Marra CA, Alamgir AH, Anis AH. Infliximab treatment of rheumatoid arthritis and Crohn’s disease. Ann Pharmacother. 2003;37:1256–65. doi: 10.1345/aph.1C039. [DOI] [PubMed] [Google Scholar]
- Nava P, Capaldo CT, Koch S, Kolegraff K, Rankin CR, Farkas AE, Feasel ME, Li L, Addis C, Parkos CA, Nusrat A. JAM-A regulates epithelial proliferation through Akt/beta-catenin signalling. EMBO Rep. 2011;12:314–20. doi: 10.1038/embor.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A, Parkos CA, Liang TW, Carnes DK, Madara JL. Neutrophil migration across model intestinal epithelia: Monolayer disruption and subsequent events in epithelial repair. Gastroenterology. 1997;113:1489–500. doi: 10.1053/gast.1997.v113.pm9352851. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, Innis-Whitehouse W, Eastburn KK, Madara JL. Tight junctions are membrane microdomains. J Cell Sci. 2000;113:1771–81. doi: 10.1242/jcs.113.10.1771. [DOI] [PubMed] [Google Scholar]
- Ostviks AE, Granlund AV, Torp SH, Flatberg A, Beisvag V, Waldum HL, Flo TH, Espevik T, Damas JK, Sandvik AK. Expression of toll-like receptor 3 is enhanced in active inflammatory bowel disease and mediates the excessive release of Lipocalin 2. Clin Exp Immunol. 2013;173:502–11. doi: 10.1111/cei.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panes J, Lopez-Sanroman A, Bermejo F, Garcia-Sanchez V, Esteve M, Torres Y, Domenech E, Piqueras M, Gomez-Garcia M, Gutierrez A, Taxonera C, Sans M. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterol. 2013;145:766–74. doi: 10.1053/j.gastro.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J Cell Biol. 1992;117:757–64. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Diamond MS, Nusrat A, Liang TW, Springer TA, Madara JL. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: Consequences for CD11b/CD18-mediated interactions with neutrophils. Mol Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Liang TW, Nusrat A, Bacarra AE, Carnes DK, Madara JL. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437–50. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkos CA, Colgan SP, Madara JL. Interactions of neutrophils with epithelial cells: Lessons from the intestine. J Am Soc Nephrol. 1994;5:138–52. doi: 10.1681/ASN.V52138. [DOI] [PubMed] [Google Scholar]
- Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium—Dependence on a Cd11b Cd18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–12. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Siccardi DJ, Mumy KL, Bien JD, Louie S, Shi HN, Gronert K, Mrsny RJ, McCormick BA. Multidrug resistance-associated transporter 2 regulates mucosal inflammation by facilitating the synthesis of hepoxilin A3. J Immunol. 2008;181:8044–52. doi: 10.4049/jimmunol.181.11.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planell N, Lozano JJ, Mora-Buch R, Masamunt MC, Jimeno M, Ordas I, Esteller M, Ricart E, Pique JM, Panes J, Salas A. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut. 2013;62:967–76. doi: 10.1136/gutjnl-2012-303333. [DOI] [PubMed] [Google Scholar]
- Rodvold JJ, Mahadevan NR, Zanetti M. Lipocalin 2 in cancer: When good immunity goes bad. Cancer Lett. 2012;316:132–38. doi: 10.1016/j.canlet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Samonte VA, Goto M, Ravindranath TM, Fazal N, Holloway VM, Goyal A, Gamelli RL, Sayeed MM. Exacerbation of intestinal permeability in rats after a two-hit injury: Burn and Enterococcus faecalis infection. Crit Care Med. 2004;32:2267–73. doi: 10.1097/01.ccm.0000145579.66001.05. [DOI] [PubMed] [Google Scholar]
- Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, Paschos E, Lohse P, Goke B, Brand S. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14:204–12. doi: 10.1002/ibd.20315. [DOI] [PubMed] [Google Scholar]
- Schmidt KJ, Buning J, Jankowiak C, Lehnert H, Fellermann K. Crohn’s targeted therapy: Myth or real goal? Curr Drug Discovery Technol. 2009;6:290–98. doi: 10.2174/157016309789869083. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916–25. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824–35. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumagin RNP, Nusrat A, Parkos CA. Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal Immunology. 2013 doi: 10.1038/mi.2013.106. (in press, manuscript ID MI-13-233.R1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. J Immunol. 2002;169:5270–78. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- Zen K, Liu Y, McCall IC, Wu T, Lee W, Babbin BA, Nusrat A, Parkos CA. Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Mol Biol Cell. 2005;16:2694–703. doi: 10.1091/mbc.E05-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindl CL, Lai JF, Lee YK, Maynard CL, Harbour SN, Ouyang W, Chaplin DD, Weaver CT. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc Natl Acad Sci USA. 2013;110:12768–73. doi: 10.1073/pnas.1300318110. [DOI] [PMC free article] [PubMed] [Google Scholar]