Abstract
In this review we address primarily the role of ASICs in determining sensory signals from arterial baroreceptors, peripheral chemoreceptors, and cardiopulmonary and somatic afferents. Alterations in these sensory signals during acute cardiovascular stresses result in changes in sympathetic and parasympathetic activities that restore cardiovascular homeostasis. In pathological states, however, chronic dysfunctions of these afferents result in serious sympatho-vagal imbalances with significant increases in mortality and morbidity. We identified a role for ASIC2 in the mechano-sensitivity of aortic baroreceptors and of ASIC3 in the pH sensitivity of carotid bodies. In spontaneously hypertensive rats, we reported decreased expression of ASIC2 in nodose ganglia neurons and overexpression of ASIC3 in carotid bodies. This reciprocal expression of ASIC2 and ASIC3 results in reciprocal changes in sensory sensitivity of baro- and chemoreceptors and a consequential synergistic exaggeration sympathetic nerve activity. A similar reciprocal sensory dysautonomia prevails in heart failure and increases the risk of mortality. There is also evidence that ASIC heteromers in skeletal muscle afferents contribute significantly to the exercise pressor reflex. In cardiac muscle afferents of the dorsal root ganglia, they contribute to nociception and to the detrimental sympathetic activation during ischemia. Finally, we report that an inhibitory influence of ASIC2-mediated baroreceptor activity suppresses the sympatho-excitatory reflexes of the chemoreceptors and skeletal muscle afferents, as well as the ASIC1a–mediated excitation of central neurons during fear, threat, or panic. The translational potential of activation of ASIC2 in cardiovascular disease states may be a beneficial sympatho-inhibition and parasympathetic activation.
Keywords: ASIC, Baroreceptor, Chemoreceptor
1. Introduction
Cardiovascular homeostasis is essential for our survival and the maintenance of blood perfusion and oxygenation of our vital organs. During stressful hemodynamic and metabolic states such as blood loss, severe hypoxia, ischemia, acidosis, our autonomic sympathetic and parasympathetic nervous systems are reflexly regulated and exert powerful circulatory and respiratory adjustments to maintain optimal blood flow and oxygen delivery. The initiation of these reflexes occurs at the sites of mechano-baroreceptors, chemoreceptors and chemosensors on the sensory terminals of the carotid sinus nerves and aortic depressor nerves in the carotid sinuses, aortic arch and carotid bodies, and on the terminals of cardiac and pulmonary vagal and spinal afferents (Fig. 1). Alterations in arterial blood pressure, central blood volume, blood and tissue pH, pO2, and PCO2 and metabolites are transduced to generate action potentials that are transmitted through the petrosal, nodose, and dorsal root ganglia sensory neurons to the nucleus tractus solitarius (NTS) in the medulla. From the NTS, signals are relayed to central nuclei that include the caudal and rostral ventrolateral nuclei that contain sympathetic preganglionic neurons, and the dorsal motor nucleus of the vagus and nucleus ambiguus that contain parasympathetic preganglionic neurons.
Fig. 1.
Sensory afferents are powerful regulators of autonomic drive. Excitatory sensory afferents from the carotid bodies, from skeletal muscle and from the heart increase sympathetic nerve activity. Inhibitory sensory afferents from the carotid sinus baroreceptors, from the aortic depressor nerve and from vagal cardiopulmonary terminals inhibit sympathetic nerve activity and enhance parasympathetic activity.
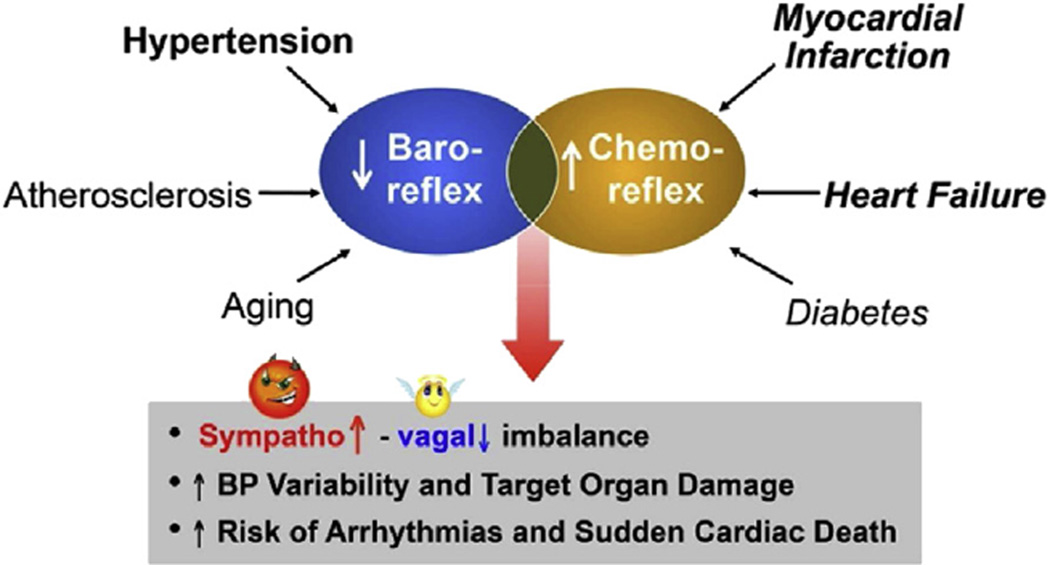
Dysfunction of specific sensory neuronal signals from diverse peripheral or central domains results in failure of autonomic responses to physiologic cardiovascular stresses such as occur with upright posture, dehydration, hypovolemia, hypoxia, acidosis, and metabolic changes with exercise as well as anger, fear or pain. In pathologic disease states, abnormalities of baroreceptor and chemoreceptor sensory neurons in particular result in serious sympatho-vagal imbalance and dysautonomia that are associated with significant increases in mortality and morbidity in heart failure, hypertension, myocardial infarction, and diabetes (Fig. 2).
Fig. 2.
Reciprocal sensory dysautonomia contributes to cardiovascular disease mortality. A decreased baroreceptor activity enhances sympathetic drive and sensitizes the chemoreceptor reflex which synergistically augments sympathetic activity even further. This combination of decreased baro and increase chemoreceptor activity has a disastrous outcome.
Many decades of work have contributed to our knowledge of the specific autonomic pathways that regulate the cardiovascular system, and we have made important inroads into understanding the specific hemodynamic and metabolic signals that activate the different receptors. However, it is only more recently that we have begun to identify the underlying mechanosensory and chemosensory molecules at the sensory nerve terminals that transduce these signals to initiate essential and specific neural reflexes.
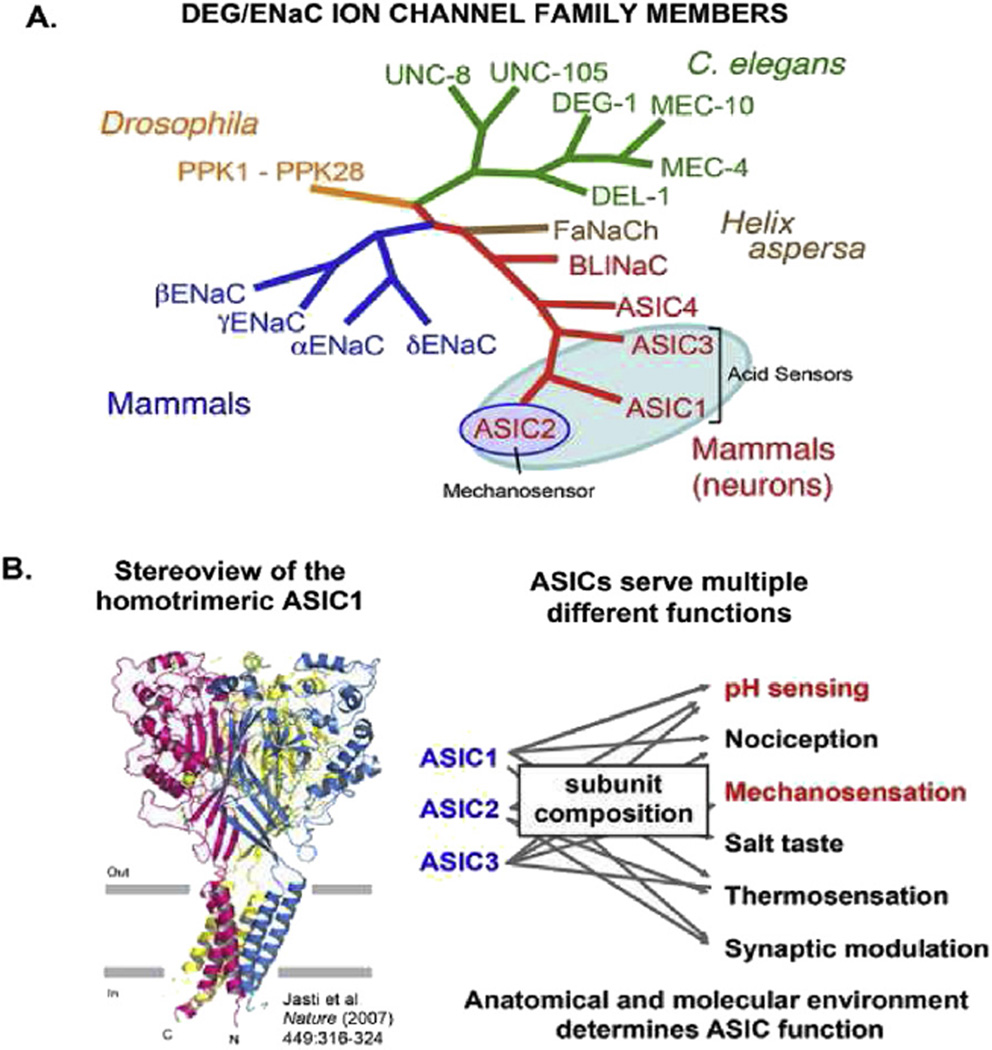
In this brief review, we will focus first on our work to identify the role of Acid-Sensing Ion Channels (ASICs), a sub-family of the Degenerin Epithelial Sodium Channels superfamily (DEG/ENaC) (Fig. 3) in the activation of two of the major domains of cardiovascular sensory signaling – the arterial baroreceptors and the carotid body chemoreceptors.
Fig. 3.
Evolutionary conservation of mammalian members of the DEG/ENac superfamily. A) Subunits of ENaC and ASICs subserve mechanosensitive and pH sensing functions in sensory terminals as ion channels of similar general topography. B) The channels consist of trimers each with 2 transmembrane domains, short intracellular terminals and a very large extracellular domain. The subunit composition determines the phenotype.
2. ASICs and arterial baroreceptors
2.1. ASIC2 is required for baroreceptor mechanosensation
Our first attempts to define the molecular determinants of mechanotransduction in baroreceptors started in the early 1990’s when we reported that gadolinium (Gd3+), which had been shown by several investigators to block mechanosensitive ion channels in different cell systems (Yang and Sachs, 1989; Zhou et al., 1991; Hansen et al., 1991; Sigurdson et al., 1992; Naruse and Sokabe, 1993), inhibited the mechanoelectrical transduction in rabbit carotid sinus baroreceptors (Hajduczok et al., 1994). Gd3+ also blocked the mechanically-activated Ca2+ transients and currents and the opening of single ion channels in isolated rat baroreceptor neurons (Sharma et al., 1995; Sullivan et al., 1997; Kraske et al., 1998). We learned that these channels were non-voltage gated and conducted Ca2+. However, the identity of these vertebrate mechanosensors remained elusive (Abboud, 1989; Lumpkin and Bautista, 2005; Vollrath et al., 2007). Studies of invertebrates began to shed light. Random chemical mutations in Caenorhabditis elegans that disrupted touch sensation and coordinated movements allowed the identification of several relevant genes, of which two appeared essential: Mec-4 and Mec-10 (Chalfie and Au, 1989; Chalfie et al., 1993; Driscoll and Chalfie, 1991; O’Hagan et al., 2005). With sequence homology, several new members of what became known as the DEG/ENaC ion channel superfamily were identified (Fig. 3). In addition, localization of the Drosophila melanogaster DEG/ENaC protein “pickpocket” to a subset of putative mechanosensitive neurons suggested that the mechanosensitive property of the family is evolutionary conserved (Adams et al., 1998).
2.1.1. Epithelial sodium channel
When the epithelial sodium channels (ENaC) subunits alpha-, beta-, gamma- were subsequently identified in mammals as members of the superfamily related to proteins involved in neurodegeneration (Canessa et al., 1993,1994; Lingueglia et al., 1993), we tested whether they were part of the mechanosensitive complex in baroreceptor neurons. We found that beta- and gamma-ENaC subunits, but not the alpha subunit, were expressed in the nodose ganglia and localized by immunofluorescence in the rat aortic arch and carotid sinus baroreceptor nerve terminals (Drummond et al., 1998). Moreover, in retrogradely-labeled and dissociated baroreceptor neurons the pharmacologic non-selective blocker of ENaC, amiloride, blocked the mechanically-stimulated Ca2+ transients and the depolarizations. Also, the increase in carotid sinus nerve activity of the isolated rabbit carotid sinus preparation and the associated reflex hypotension induced by increases in carotid sinus pressure were both abrogated by benzamil, the amiloride analog that inhibits DEG/ENaC channels (Drummond et al., 1998).
Whereas alpha-, beta-, gamma-ENaC are each required for the formation of constitutively open channels for epithelial Na+ transport (Snyder, 2002), we hypothesized that in the absence of alpha subunit, the beta-, gamma- subunits multimerize with an as yet unidentified subunit(s) in the baroreceptor nerve terminals to form a mechanosensory complex similar to the model of mechanotransduction in C. elegans whereby accessory proteins function to link MEC-4 and MEC-10 channels to the intracellular cytoskeleton and the extracellular matrix (Arnadóttir and Chalfie, 2010).
2.1.2. Acid-sensing ion channels
When new mammalian subfamily members of DEG/ENaC were identified in peripheral nerves in rat and in human brain (Price et al., 1996; Waldmann et al., 1996) and designated as the Acid-Sensing Ion Channels (ASICs) subfamily (Waldmann et al., 1997a), we became intrigued by the possibility that these members might also play a role in neurosensory transduction in baroreceptors (Bianchi and Driscoll, 2002; Krishtal, 2003; Wemmie et al., 2006).
Three ASIC genes and their spliced variants (ASIC1a, −1b, −2a, −2b, and −3) form homo- or heteromultimeric channels with different pH sensitivities (Benson et al., 2002; Kellenberger and Schild, 2002; Hesselager et al., 2004). ASIC1 and 3 homomers are most sensitive to low pH with half-maximal activation at ∼pH 6.5 (Waldmann et al., 1997a; Benson et al., 2002; Waldmann et al., 1997b), and are also involved in nociception in spinal sensory afferents and in pH sensitivity of carotid body glomus cells as we will show below. ASIC2a homomers, on the other hand, are the least pH sensitive (pH50 ≈ 4.5) and, interestingly, participate in the formation of channels that are inhibited by gadolinium (Babinski et al., 2000). When Price et al. (2000) discovered that the brain sodium channel BNC1 (later designated as ASIC2) contributed to cutaneous touch sensitivity, we tested the hypothesis that it contributes to mechanotransduction of arterial baroreceptors (Lu et al., 2009).
2.1.3. ASIC2 in arterial baroreceptors
We found a predominance of ASIC2b transcript in mouse nodose ganglia, which interestingly is an ASIC isoform that does not directly participate in acid-activated currents, being expressed along with transcripts for other subunits, −1a, −1b, −2a, and −3. Importantly, ASIC1, −2, and −3 colocalized by immunofluorescence in baroreceptor nerve terminals in the aortic arch (Lu et al., 2009) (Fig. 4).
Fig. 4.
Expression of ASICs in nodose neurons and of ASIC2 in baroreceptor endings. The relatively greater expression of ASIC2 RNA in the ganglia and the location of the protein in the baroreceptor nerve terminals supports the identity of ASIC2, which is the least acid sensitive ASIC, as a component of the mechanosensing complex.
To test for the contribution of ASIC2 to the mechanosensory properties of baroreceptors, we took advantage of genetically altered mice that had either targeted deletion (Price et al., 2000), or transgenic overexpression of ASIC2 driven by the panneuronal synapsin 1 promotor (Lu et al., 2009). First, in isolated nodose neurons from wild type mice, we found that mechanically induced depolarizations, by puffing extracellular buffer at 10 psi from a micropipette onto the cell, were significantly larger in retrogradely labeled aortic baroreceptors than those from non-labeled nodose neurons, and that ASIC2a mRNA levels as measured by single-cell RT-PCR were 3-fold greater in baroreceptors compared to non-labeled neurons. Moreover, mechanically-activated depolarizations were mostly absent from baroreceptor neurons from ASIC2 null mice. In contrast, mechanically activated depolarizations were greater and more sustained in neurons of transgenic ASIC2-overexpressing mice than those of wild type mice (Lu et al., 2009) (Fig. 5).
Fig. 5.
Mechanically-induced depolarizations of individual nodose baroreceptor neurons. The magnitude of depolarizations is significantly greater and more sustained in neurons from Tg mice overexpressing ASIC2 and significantly reduced or absent in neurons from ASIC2 null mice compared to those from wild type mice.
As a correlation to the above in vitro studies, we also tested the mechanosensory properties of baroreceptors in vivo by recording aortic depressor nerve activity (ADNA) induced by changes in arterial pressure in anaesthetized mice. In response to a rapid increase in blood pressure with phenylephrine infusion, ADNA rose abruptly to comparable levels in both wild type and ASIC2 null mice. The nerve activity then declined to a steady-state, however, the plateau of ADNA was significantly lower in ASIC2 null than wild type mice, even despite a higher arterial pressure in the null mice (Fig. 6). Together, these data suggest that ASIC2 is required for normal mechanosensation of baroreceptor neurons, and suggests that it may be part of a pressure-sensing channel within these cells (Lu et al., 2009).
Fig. 6.
Action potentials of aortic depressor nerve during phenylephrine induced increases in arterial pressure. The number of spikes and the % of maximum voltage measured during the sustained levels of elevated pressure is reduced by more than 50% in the ASIC2 null mice compared to wild type mice despite the higher arterial pressure in the KO mice.
2.2. ASIC2 is required for baroreflex function and participates in the regulation of blood pressure
Given the contribution of ASIC2 to mechanosensation of baroreceptors, we tested if baroreflex function and regulation of blood pressure was altered in mice lacking ASIC2.
As anticipated with a reduction in baroreceptor signaling, the deletion of ASIC2 resulted in the hemodynamic consequences of increased sympathetic activity, hypertension and tachycardia.
2.2.1. Telemetry in awake mice
Using chronically implanted telemeters in free moving home-caged mice, we found ASIC2 null mice demonstrated diurnal elevations in blood pressure and heart rate that were significantly enhanced compared to wild type mice, and these occurred despite an unexpected decrease in their locomotor activity, which would be expected to reduce their arterial pressure (Fig. 7).
Fig. 7.
Abnormal circadian oscillations of arterial pressure, heart rate and motor activity in awake ASIC2 null mice. The 24 h continuous recordings by telemetry show hypertension, and tachycardia despite reduced motor activity in ASIC2 null mice compared to WT mice. This hypertensive phenotype is ascribed to the suppressed baroreceptor and augmented chemoreceptor reflexes.
Measurements of baroreceptor sensitivity, based on the reciprocal relation of changes in pressure and heart rate, was also reduced in awake ASIC2 null mice. A sympatho-vagal imbalance was also evident, with an enhanced drop in heart rate with propranolol (β-adrenergic receptor blocker), a diminished tachycardia with atropine (muscarinic acetylcholine receptor antagonist), and a greater decrease in blood pressure with ganglion blockade - all reflecting a greater sympathetic activity and lesser parasympathetic control (Fig. 8).
Fig. 8.
Sympatho-vagal imbalance of cardiovascular homeostasis in conscious ASIC2 null mice. The greater bradycardia and greater drop in arterial pressure with propranolol and ganglion blockage respectively reflects an exaggerated sympathetic drive and the lesser tachycardia with atropine reflects a suppressed parasympathetic. This imbalance is a source of end-organ damage and greater mortality.
2.2.2. Bilateral carotid occlusion reflex
As a final test of baroreflex function, we studied the effects of bilateral carotid artery occlusion in mice breathing 100% oxygen to eliminate the contribution of activation of carotid chemoreceptors. Bilateral carotid occlusion (BCO) causes a pressor response, which is partly buffered by the increased activity of the ADN as arterial pressure rises. After section of the ADNs, the carotid occlusion reflex caused a significantly greater increase in pressure in the wild type than in ASIC2 null mice, reflecting the fact that ADN activity and its buffering capacity during the pressor response were much lower in the null mice, and confirms the impaired nerve activity seen with the direct ADN recordings (Lu et al., 2009).
We ascribe these changes in autonomic activity to the deletion of ASIC2 from the afferent arm of the baroreflex - leading to a reduced capacity to buffer increases in blood pressure and heart rate, and to suppress sympathetic activity. However, ASIC2 is also widely expressed in the CNS and perhaps in the central pathways of the baroreflex (Price et al., 2014), and so its central deletion may have also impaired the reflex responses. To test for this possibility, we electrically stimulated the aortic depressor nerve downstream from the baroreceptor complex, and found the resultant hypotension and bradycardia was indistinguishable in wild type and ASIC2 null mice.
These data, as well as the expression of ASIC2 in baroreceptor sensory endings, and the altered baroreceptor properties in ASIC2 null mice, all suggest that the diminished baroreflex is due to loss of ASIC2 at the baroreceptor nerve terminals (Lu et al., 2009).
3. ASICs and peripheral chemoreceptors
3.1. ASICs contribute to the chemoreceptor reflex
The bilateral common carotid occlusion (BCO) reflex results in a significant increase in arterial pressure which is usually ascribed to the unloading of arterial baroreceptors and removal of their restraining, inhibitory influence on sympathetic drive and arterial pressure. What is often unrecognized is the contribution of carotid chemoreceptor activation in response to the ischemic hypoxia of carotid bodies during the carotid occlusion (Iturriaga et al., 1988). That contribution under normal physiologic states is small, but it increases significantly with aging and can be quantified by having the animal breathe 100% O2 which eliminates the chemoreceptor component of pressor response (Fig. 9). As shown in Fig. 9, that contribution increases in animal models of cardiovascular disease (e.g., in the ApoE knock out mouse) as the baroreceptor contribution decreases.
Fig. 9.
Relative contributions of the baroreceptor and the chemoreceptor reflexes to the pressor response to bilateral carotid occlusion (BCO). The difference in pressor responses to BCO on 21% O2 and 100% O2 represents the chemoreceptor contribution since 100% O2 eliminates the chemoreceptor activity. The pathologic mouse model of ApoE knock out (B) has a heightened chemoreceptor activity and a suppressed baroreceptor component of the BCO pressor response compared to control mice (A).
In the studies described above in the ASIC2−/− mice, we were interested in the contribution of the baroreflex during carotid occlusion and so the mice were breathing 100% O2 to eliminate the chemoreceptor component of the carotid occlusion reflex (Iturriaga et al., 1988). When we measured the pressor response to BCO in ASIC2 null mice breathing room air we found it considerably larger than when they were breathing 100% oxygen. Surprisingly, the chemoreceptor component was significantly enhanced in ASIC2 null mice breathing room air while the baroreflex component was reduced significantly (Sabharwal et al., 2005) (Fig. 10). This reciprocal increase in the chemoreceptor reflex was puzzling.
Fig. 10.
Contribution of ASIC1, 2, and 3 to the carotid artery occlusion (CAO) pressor response. In wild type mice the pressor response to CAO reflects a predominant baroreceptor contribution (blue). In contrast, the deletion of ASIC2 caused a reduction in the baroreceptor contribution (blue) and a pronounced increase in the chemoreceptor reflex (red). This pattern is similar to that seen in the ApoE KO mouse. Additional deletion of ASIC1 and 3 essentially eliminated the chemoreceptor reflex (red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Thus, following the identification of ASIC2 as an important component of the baroreceptor complex and our observation that the carotid occlusion (BCO) pressor response in the ASIC2 null mice is caused by an exaggerated chemoreceptor component, we wondered whether additional deletion of the more pH sensitive subunits ASIC1 and ASIC3 would suppress that response. Early findings in ASIC2 null mice showed the increased chemoreceptor component of the BCO pressor response was actually eliminated with the additional deletions of ASIC1 and 3 (Sabharwal et al., 2005).
3.2. ASICs function as pH sensors in glomus cells of the carotid body
The above results lead us to further explore the role of ASICs within the carotid bodies. The carotid bodies serve as the major peripheral chemoreceptors sensing alterations in arterial blood oxygen, carbon dioxide, and pH. Within the carotid bodies, type 1 glomus cells are the chemosensitive cells activated by hypoxemia, hypercapnia, and acidosis. Depolarization of glomus cells leads to synaptic activation of adjacent carotid nerve endings that elicit both hyperventilation and sympathetic activation. We hypothesized that ASICs might serve as pH sensors in glomus cells. RT-PCR revealed a relatively high expression of ASIC3 and ASIC1b mRNA in rat carotid bodies, compared to a lower expression of ASIC1a, −2a, and 2b. Immunofluorescence revealed ASIC1 (antibody detects both −1a and −1b) and ASIC3 in clusters within glomus cells, with negligible expression of ASIC2 (Tan et al., 2007) (Fig. 11).
Fig. 11.
Expression of ASICs RNAs in carotid bodies and immunofluorescence of ASIC proteins in clusters of glomus cells. Immunofluorescence of acid sensing ASIC1b and 3 indicates that they are more prominently expressed in carotid bodies than the more mechanosensitive ASIC2. The latter is the more predominant ASIC in nodose ganglia, neurons. The immunofluorescent panels show the co-expression of ASIC1 and ASIC2 in one section and co-expression of ASIC1 and ASIC3 in another. Here is also ASIC2 fluorescence is negligible compared to ASIC1 and ASIC3.
Although we recognize the limitations of quantitative expression comparisons between ASIC subunits, these findings are in contrast to our results in nodose ganglion neurons, where ASIC2 subunits were predominant (Lu et al., 2009). These contrasting expression patterns lend support to the idea that different ASIC channels serve remarkably different functions within different sensory terminals.
3.2.1.Proton-induced depolarization of glomus cells
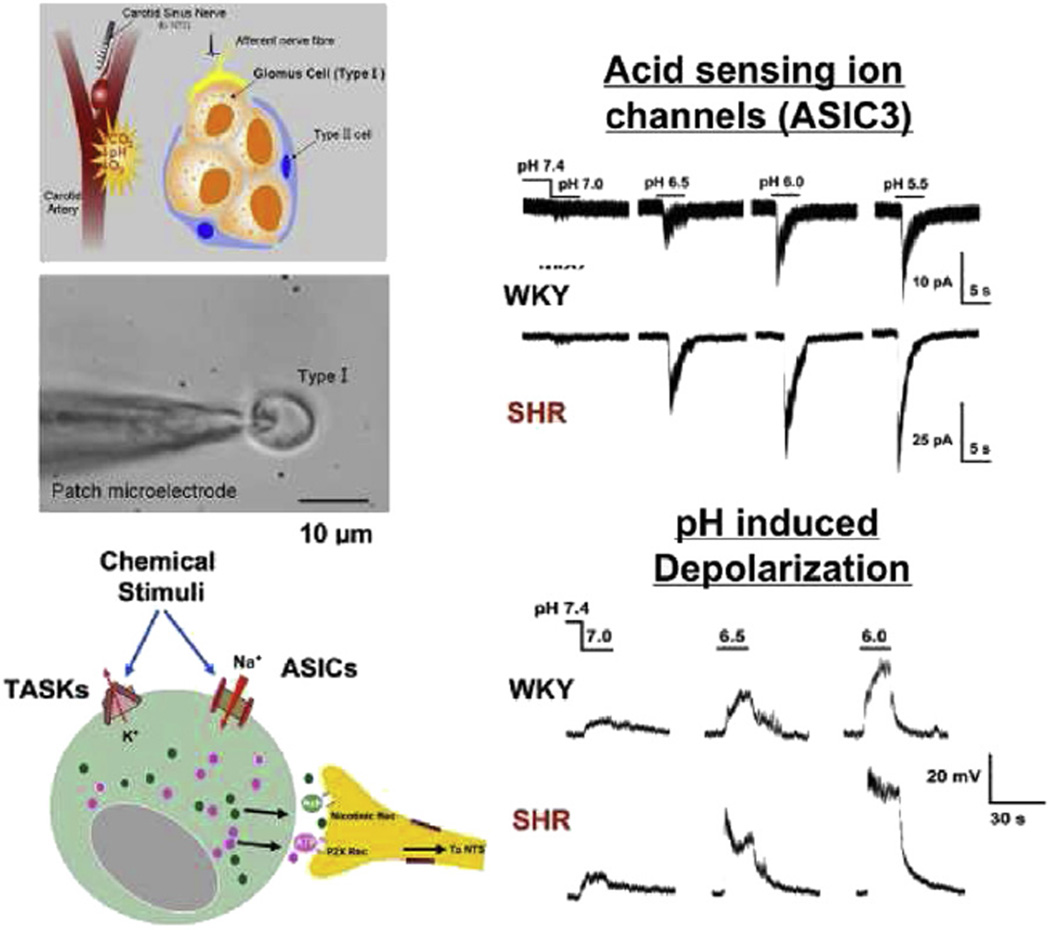
We performed electrophysiological studies (whole-cell patch clamp) on isolated glomus cells and found that exposure to low pH evoked rapid transient inward currents and depolarizations with the characteristic fast-gating kinetics of ASICs, followed by more sustained responses (Tan et al., 2007) (Fig. 12). The transient responses were blocked selectively by amiloride, and not by the TRPV1 antagonist capsazepine, the BK channel blocker iberiotoxin, nor psalmotoxin venom, a blocker of ASIC1a homomeric channels (Tan et al., 2007).
Fig. 12.
Electrophysiologic recordings in patch-clamped glomus cells from SHR vs. WKY carotid bodies. The schematics portray the carotid body, a cluster of glomus cells and the ion channels with release of transmitters from the glomus cell into the synaptic cleft. Patch-clamped type 1 glomus cells reveal inward currents and depolarizations in response to graded acid pHs that are significantly greater in SHR vs. WKY. The rapid initial depolarizations followed by the more sustained response reflect the opening of ASIC Na+ channels and closure of the TASK K+ channels respectively.
The transient acid sensitive currents in glomus cells were significantly facilitated in Ca2+-free solution and by lactate. Others have reported that ASICs are facilitated by a decrease in extracellular [Ca2+], as well as extracellular lactate, and the mechanism involves both a reduction in cation block of the channel as well as an increase in pH sensitivity (Immke and McCleskey, 2003, 2001; Zhang et al., 2006). This unique capacity of ASICs to synergistically integrate responses to H+ and lactate makes their presence in the carotid bodies particularly important as pH sensors that mediate the peripheral chemoreceptor response to metabolic acidosis associated with tissue ischemia and maximal exercise (Prabhakar and Peng, 2004; Prabhakar, 2006; Rausch et al., 1991; Kobayashi et al., 1996).
Although the desensitization rate of ASICs may limit their proton sensing capacity, many substances are known to modulate that desensitization (Lingueglia et al., 2006; Yagi et al., 2006) and sustained currents through ASIC3 ion channels have been reported at the modest pH changes that occur during myocardial ischemia (Yagi et al., 2006).
In addition to our characterization of the early responses in glomus cells as caused by opening of non-voltage gated ASIC-like Na+ currents, we found that the pharmacology of the sustained pH responses was consistent with the closure of another non-voltage gated tandem-p-domain acid-sensitive K+ channel (TASK), a finding also described by others (Buckler et al., 2000). pH sensing within glomus cell probably also involves other sensors including voltage-gated Ca2+ activated K+ channels (Peers, 1990) and L-type Ca2+ channels (Summers et al., 2002).
3.2.2. Selective sensitivity of glomus cells to hypoxia vs. acidosis
The pronounced morphological heterogeneity of glomus cells suggested to us that there may be a functional heterogeneity with respect to their responses to hypoxia and acidosis among them (Lu et al., 2013). We found that isolated clusters of glomus cells from rat carotid bodies were selectively more sensitive to either hypoxia (PO2 = 15 mm Hg) or to acidosis at pH 6.8 (Lu et al., 2013). We were able to recapitulate that uncoupling and reciprocity in response to acidosis and hypoxia or cyanide by genetically altering ASIC3 expression in mice.
In the transgenic ASIC3 overexpressing mouse, most clusters of glomus cells had increased sensitivity to pH and reduced cyanide sensitivity. While the converse occurred in ASIC3 null mice, this selective sensory transduction of glomus cells suggests the presence of afferents that would activate specific reflex responses to either acidosis or to hypoxia (Lu et al., 2013) and thus provide more optimal homeostatic responses to either of these two different sensory signals.
At this point, we can only speculate that overexpression of ASIC3 may be linked to the inhibition of BK or other K+ channels (which is also be inhibited by hypoxia), thereby explaining at least in part, the enhanced response to pH and the reciprocal reduced response to hypoxia in glomus cells of ASIC3 Tg mice. The mechanism involved in the inhibition of BK by ASIC is reviewed below.
4. Alterations in ASIC expression in the sensory dysautonomia of cardiovascular disease states
In 1974 we reported an occlusive central interaction between the baroreceptor and the chemoreceptor reflexes (Heistad et al., 1974, 1975) whereby the chemoreceptor responses were significantly enhanced when baroreceptor activity was reduced and vice versa. We later confirmed that interaction in humans (Somers et al., 1991) by showing that the increased sympathetic nerve activity during apnea and hypoxia was prevented by a neck suction device that activates carotid sinus baroreceptors.
The pathological relevance of this reciprocal reflex abnormality is evident in several animal models and in humans with heart failure, hypertension, myocardial infarction, and obstructive sleep apnea, causing excessive sympathetic activity and decreased parasympathetic activity that contribute to increased mortality and morbidity (Zucker et al., 2007; Schultz et al., 2007; Somers et al., 1988; Abboud, 2010; Abboud and Kumar, 2014). Our more recent work that defines ASICs as molecular determinants of these abnormal reflexes at the level of sensory terminals in baro- and chemoreceptors is intriguing (Lu et al., 2009; Sabharwal et al., 2005; Tan et al., 2007). We believe that molecular determinants at the sites of signal transduction involving ASICs may account for the reciprocal sensitivities and contribute synergistically to the excessive sympathetic nerve activity. A reduced expression of ASIC2 in baroreceptors and enhanced expression of ASIC3 in glomus cells would explain this sensory reciprocity.
4.1. Evidence of ASIC-mediated sensory dysautonomia in genetic hypertension
We wondered whether in an established model of genetic hypertension, namely the spontaneously hypertensive rat (SHR), known to have impaired baroreceptor and enhanced chemoreceptor reflexes (Fazan et al., 1999; Fukuda et al., 1987), if such reciprocal changes in expression of ASIC2 and ASIC3 occur.
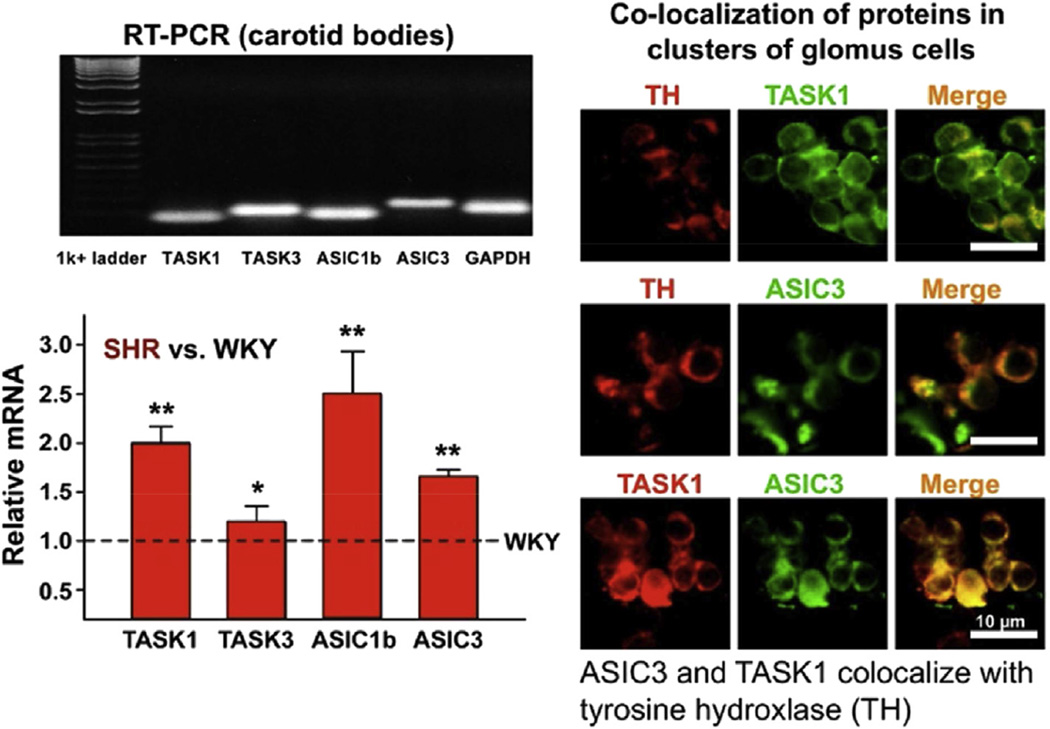
Our preliminary results indicate that a significant number of isolated baroreceptor nodose neurons from hypertensive SHR did not depolarize with mechanical stimulation and the expression of ASIC2a was significantly reduced in their nodose ganglia compared to Sprague-Dawley and Wistar-Kyoto (WKY) rats (Lu et al., 2007; Snitsarev et al., 2005) (Figs. 13 and 14). On the other hand the pH-dependent, amiloride-sensitive inward currents and early depolarizations in isolated glomus cells from carotid bodies of young SHR were significantly enhanced and associated with significant increases in expression of ASIC1 and ASIC3 RNA and ASIC3 protein compared to WKY. Immunofluorescence in clusters of glomus cells revealed ASIC3 and TASK antibodies co-localized along with tyrosine hydroxylase the specific marker of glomus cells (Tan et al., 2010). Thus, the reciprocal expression of ASIC2 and 3 in nodose neurons and carotid bodies respectively (Lu et al., 2007; Tan et al., 2010) may account for the decrease in mechanosensitivity of baroreceptors and increase in pH-sensitivity of chemoreceptors (Fig. 15).
Fig. 13.
Mechanosensitivity of DiI labeled aortic baroreceptor nodose neurons correlates with ASIC2 expression. Schematics show the labeling, isolation, and patch clamping of the neurons and the single-cell RT-PCR measurements. DiI labeled baroreceptor (BR) neurons have larger depolarizations during puffing saline at 10 psi than non-BR neurons and significantly greater ASIC2a mRNA levels.
Fig. 14.
ASIC2-dependence of mechanically induced depolarization of nodose neurons. A) Failure of nodose neurons of SHR to depolarize with mechanical stimulation correlates with a significant reduction in ASIC2a in their nodose ganglia compared with WKY. The reduction in ASIC2a seen on the western blot of the ganglia in SHR is selectively targeted to the ganglia and is not seen in the brain of SHR compared to WKY. B) The ASIC2a mRNA level in baroreceptor (DiI labeled) vs. non-baroreceptor neurons which are very high in WKY are dramatically reduced in SHR.
Fig. 15.
Expression of ASICs and TASK ion channels in carotid bodies and clusters of glomus cells of SHR vs. WKY. A greater expression of RNAs is seen in SHR carotid bodies vs. WKY, which is compatible with the greater acid sensitivity seen in SHR vs. WKY glomus cell. The co-localization of ASIC3 and TASK1 and the co-localization of each with TH (tyrosine hydroxylase) reflects their distribution in glomus cells Type 1.
4.2. Other mechanisms involving ASICs contribute to reciprocal excitability of baro- and chemoreceptors
In addition to changes in the number of ASIC channels expressed, two other mechanisms involving ASICs may contribute to the decreased excitability of baroreceptors or increased pH sensitivity of chemoreceptors in SHR. One is ASIC inhibition of BK+ potassium channels and the other is the heteromeric composition of the ASIC channels.
4.2.1. ASIC/BK+ interaction inhibits BK+
For example, we noted that the nodose neurons of SHR had a relatively greater negative resting membrane potential (−57.0 ± 2.9 mV) and were much less sensitive to depolarizing current injection than WKY rats (−45.9 ± 4.1 mV) (La Rovere et al., 1998). An unexpected inhibitory interaction between ASICs and Maxi-K (BK, the Ca2+ sensitive K+ channels) may account for these abnormalities. We found that when ASIC and BK are co-expressed in close association in the cell membrane ASICs inhibit BK currents (Petroff et al., 2008,2012). The reduced expression of ASIC2 in SHR nodose neurons may disinhibit or enhance BK activity that may cause the more negative resting membrane potential and a greater suppression of mechanically-induced depolarization. Iberiotoxin (100 nM), a selective inhibitor of Maxi-K+ channels, shifted the RMP from [2243]57 mV to [2243]46 mV in SHR neurons and restored their excitability with depolarizing currents but had no effect on WKY neurons where the RMP remained unchanged by iberiotoxin (Snitsarev et al., 2005).
4.2.2. Heteromultimeric composition of the channel determines pH sensitivity
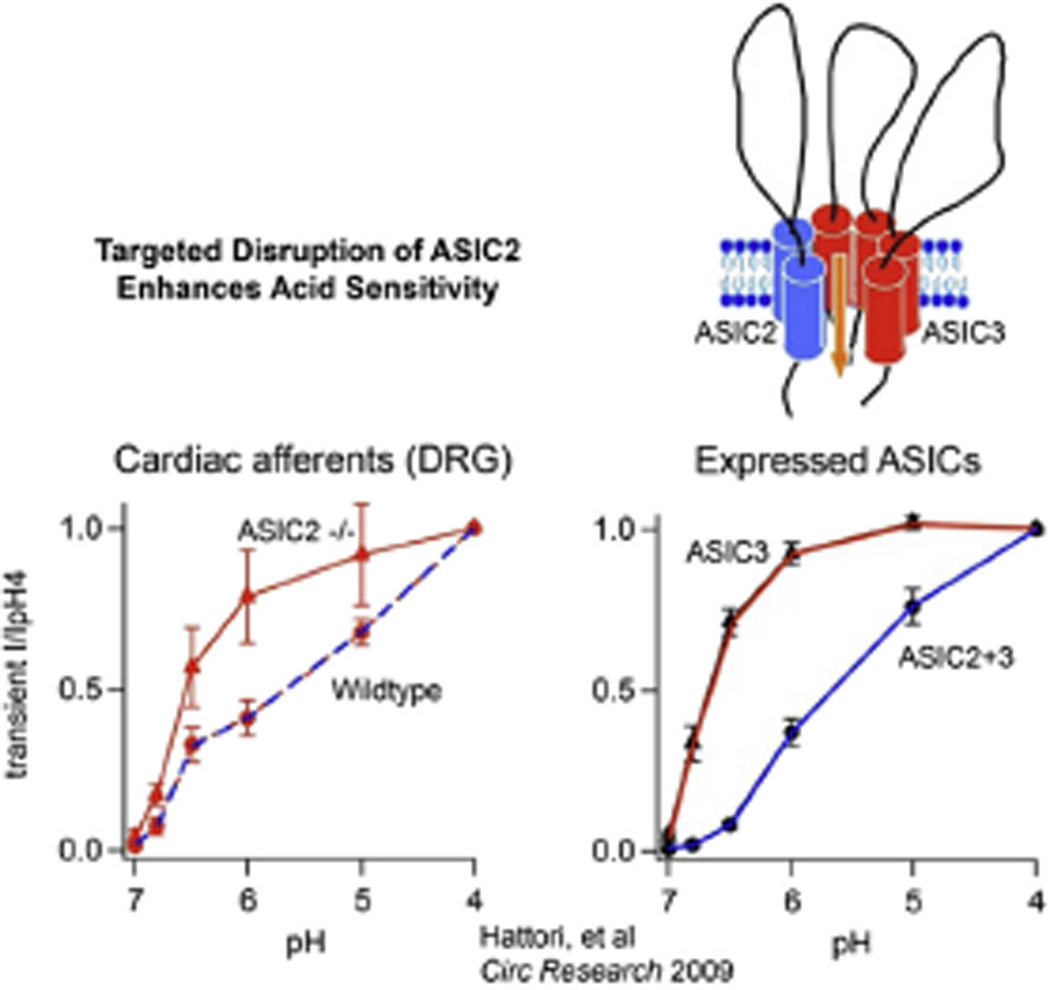
Our current understanding of the role of ASICs in sensory signaling is the fact that the heteromultimeric composition of the channels and the differential expression of the various subunits define their pH sensitivity and possibly their phenotype as a mechano or chemosensitive channel. As shown by Hattori et al., the heterologous expression of ASIC3 in COS-7 cells resulted in greater pH sensitivity and faster activation kinetics than the expression of ASIC2 (Hattori et al., 2009). More importantly, and with greater relevance to our results in the ASIC2 null mice as well as the increased pH sensitivity of glomus cells in SHR, the coexpression of ASIC2 and ASIC3 results in a heteromultimeric channel with lower pH sensitivity that is enhanced significantly once ASIC2 expression is reduced or deleted (Fig. 16).
Fig. 16.
Heteromeric expression of ASIC subunits define the degree of acid sensitivity. The expression system in the right panel shows that the absence of ASIC2 from the heteromer ASIC2/3 enhances the acid sensitivity markedly. Similarly the acid sensitivity of cardiac DRB neurons from ASIC2 null mice is significantly enhanced compared to wild type (left panel). This enhancement may account for the marked increase in chemoreceptor activity observed in mice with deletion of ASIC2.
A similar marked enhancement of pH sensitivity was found in cardiac dorsal root ganglion neurons of ASIC2 null mice compared to WT mice (Hattori et al., 2009). A comparable enhancement of low pH sensitivity of glomus cells when ASIC2 is deleted would explain the enhanced chemoreceptor component of the BCO pressor reflex in the ASIC2 null mice and its total suppression by the additional deletions of ASIC1 and 3 (Sabharwal et al., 2005). Thus, the reciprocal changes in sensory signals can be explained by the fact that disruption of the mechanosensitive ASIC2 subunit from the trimeric heteromer of an ASIC channel results in enhancement of its acid sensitivity through the remaining ASIC1 and/or ASIC3 subunits (Fig. 16).
One would conclude from all the foregoing that ASIC2 must be a major molecular sensor in the baroreceptor complex that determines the transduction of increase in arterial pressure and the effectiveness of the baroreflex, in addition to its role in the heteromeric ASIC channel that restrains acid sensitivity of the chemoreceptors. In its absence, a serious dysautonomia prevails and increases cardiovascular risk and mortality.
As mentioned above, impaired baroreceptor activity is prevalent in patients with several serious cardiovascular disease states (La Rovere et al., 1998; Mortara et al., 1997; La Rovere et al., 2001; Robinson et al., 2003; Lawrence et al., 1997). This work provides an understanding of a major pathophysiologic process at a molecular level and offers a rationale for the current interventions that include electrical stimulation of the carotid sinus nerve or the vagus nerve in humans with heart failure and hypertension.
5. ASICs in other neural pathways that influence cardiovascular homeostasis
Although we have focused on arterial baroreceptors and carotid chemoreceptors, ASICs most certainly modulate cardiovascular homeostasis via other neural pathways. Here we will briefly review their role in skeletal muscle afferents, cardiac afferents, and within the central nervous system.
5.1. Skeletal muscle afferents
Skeletal muscle has the capacity for high metabolic activity. During intense exercise, muscle cells generate and release protons, lactate, and other metabolites, such that the interstitial pH in human skeletal muscle can drop to the 6.7–7.0 range (Bangsbo et al., 1993; Street et al., 2001). These metabolic changes, as well as mechanical perturbations, are sensed by type III (thinly myelinated) or type IV (unmyelinated) skeletal muscle afferents. In turn, activation of muscle afferents during exercise evokes reflexes that increase blood pressure, heart rate, and ventilation predominantly by increasing sympathetic nerve activity (termed the ‘exercise pressor reflex’) (Kaufman and Hayes, 2002; McCloskey and Mitchell, 1972; Alam and Smirk, 1937).
Increasing evidence suggests that ASICs are important sensors in muscle afferents. First, ASICs are expressed in muscle afferents at higher levels than in cutaneous afferents, and they are activated in the narrow ranges of extracellular pH and other metabolites that occur during muscle ischemia and exercise (Molliver et al., 2005; Light et al., 2008). We found that ASIC-like currents are generated in ∼70% of labeled muscle afferents, and the subunit composition is a unique heteromeric channels consisting primarily of ASIC1a and −3 subunits, with a lesser contribution from ASIC2 subunits (Gautam and Benson, 2013). Second, ASICs are required for the development of normal muscle pain. Either genetic or pharmacological inhibition of ASICs attenuates hyperalgesia in mouse models of muscle pain (Sluka et al., 2003, 2007; Walder et al., 2011). Third, inhibition of ASICs significantly attenuates the exercise pressor reflex (Li et al., 2004; Gao et al., 2006; Hayes et al., 2007; Tsuchimochi et al., 2011). Hayes et al. showed that an ASIC antagonist, A-317567, injected into muscle inhibited the pressor responses to lactic acid injection by 75%, and to static muscle contraction by 60%, and yet had no effect on the pressor responses to passive stretch or capsaicin injection (Hayes et al., 2008). An exaggerated exercise pressor response with excessive sympathetic activity may contribute to exercise intolerance and contribute to the risk of adverse cardiac events in patients with heart failure (Piepoli et al., 1999; Sinoway and Li, 2005; Smith et al., 2006). Often the reason for this exaggerated exercise pressor response is the absence of the inhibitory influence of arterial baroreceptors and cardiopulmonary mechanoreceptors on the somatic excitatory afferent reflex which we observed in animals and humans (Thames and Abboud, 1979; Abboud et al., 1981). Recent data suggests that perturbations in ASIC3 in muscle afferents may play a role in the altered metaboreceptor component of the exercise pressor reflex in a rat model of heart failure (Xing et al., 2014).
5.2. Cardiac afferents
We have also shown that ASICs are also highly expressed in cardiac sensory neurons, particularly in those with the dorsal root ganglia (termed cardiac sympathetic afferents because they traverse within the cardiac sympathetic nerves) (Benson et al., 1999; Sutherland et al., 2001). In contrast to ASIC channels in skeletal muscle afferents, we have demonstrated that the ASIC channel in mouse cardiac afferents is a heteromer composed of ASIC2a and −3 subunits (Hattori et al., 2009). Besides serving as pain sensors during myocardial ischemia or infarction, cardiac sympathetic afferents trigger sympathoexcitation (Malliani et al., 1969; Minisi and Thames, 1991), and there is evidence to support their contribution to the detrimental sympathetic activation associated with cardiovascular disease states (Wang and Ma, 2000; Wu et al., 2008).
5.3. CNS emotional regulatory regions
While the peripheral sensory nervous system provides information about the state of the body, this information is integrated and modulated by autonomic CNS regions in the brainstem including the nucleus tractus solitarii and the motor output centers. While ASIC1 and −2 subunits are expressed throughout many regions of the brain (Wemmie et al., 2002; Alvarez de la Rosa et al., 2003), their expression and function within these important central autonomic regulatory regions is largely unexplored. On the other hand, ASICs have been shown to play important functions in higher forebrain regions, including the hippocampus and limbic system (Baron et al., 2002). In particular, ASICs have proven to be important for innate fear responses and acquired fear-conditioned behaviors, as well as modulating responses to other aversive stimuli (Price et al., 2014; Wemmie et al., 2003; Vralsted et al., 2011; Ziemann et al., 2009). It is well understood that these higher brain regions that control emotions and motivations play an important role in regulating autonomic output. Thus, it is highly likely that ASICs within the CNS participate in the maintenance of cardiovascular homeostasis.
An intriguing recent observation (Garfinkel et al., 2014) indicates that cognition, emotions and fear can be influenced by the cardiac cycle. Specifically the processing of fear stimuli may be affected by the activation of arterial baroreceptors during systole. Edwards et al. reported that the human nociceptive flexion reflex threshold is higher during systole than diastole (Edwards et al., 2002) and concluded that nociception can be attenuated by the bursts of afferent neural activity from arterial baroreceptors to brainstem. Thus, the inhibitory ASIC2 mediated mechanosensory activity from baroreceptors does not only suppress sympathetic activity directly and does so also indirectly by inhibiting the chemoreceptor ASIC3 mediated excitatory response as described above; it may in addition suppress the amygdala responses to fear, threat or panic mediated by ASIC1a.
6. Summary
First, in humans and in animal models of cardiovascular diseases, the exaggerated sympathetic drive is a function not only of loss of the inhibitory influence of baro- and mechanosensory afferents but also a simultaneously increased activity of the excitatory chemoreceptors: a state we refer to as reciprocal sensory dysautonomia.
Second, nonvoltage-gated ion channels of the ASIC subfamily of the evolutionally conserved DEG/ENaC superfamily are important components of the mechano- and chemotransduction mechanisms. ASIC2, the least acid-sensitive, is an important mechanosensing molecule in baroreceptors, and ASIC3 is important in the rapid transduction of acid sensitivity by chemoreceptors.
Third, deletion of ASIC2 results in a phenotype of hypertension with decreased baroreceptor sensitivity and vagal influence, increased chemoreceptor sensitivity, and sympathetic influence. The genetically hypertensive rat (SHR) replicates the ASIC2 disruption phenotype with exaggerated sympathetic nerve activity, decreased baroreceptor sensitivity, reduced ASIC2 expression in nodose ganglia, and enhanced chemoreceptor sensitivity and ASIC3 expression in carotid bodies.
At the molecular level, the reciprocal sensory signaling from baro and chemoreceptors seen in the ASIC2 null phenotype and in SHR may be partially explained by the fact that disruption of the mechanosensitive ASIC2 subunit from the trimeric heteromer of an ASIC channel results in enhancement of its acid sensitivity through the remaining ASIC1 and/or ASIC3 subunits.
Fourth, the inhibitory baroreceptor signal activated by ASIC2 predominates over and alleviates the exaggerated exercise pressor effects caused by skeletal muscle excitatory afferents activated by ASIC1a/or 3. A similar inhibitory influence of baroreceptors on excitatory central neurons (e.g. amygdala) may alleviate emotions of fear, threat and panic.
7. Conclusion and translational potential
The inhibitory restraint of ASIC2 mediated activation of baroreceptors on sympathetic activity is magnified by: 1) its inhibitory influence on the chemoreceptor and skeletal muscle ASIC1a/or 3 mediated excitatory reflexes, and 2) its inhibitory influence on ASIC1a mediated excitation of central neurons during emotional stress, fear, and panic.
Reciprocal changes in expression of ASICs in sensory neurons may account for the excessive mortality and morbidity of the consequential exaggerated sympathetic nerve activity in heart failure, hypertension, and myocardial infarction.
The development of drugs to sensitize the inhibitory ASIC2 mediated sensory signal of baroreceptors and suppress the excitatory ASIC1a/3 mediated afferent activation may help restore the autonomic balance. In the meantime the invasive electrical stimulation of the carotid sinus nerves and cervical vagus nerve to enhance the baroreceptor and inhibitory sensory afferent signals, and the surgical denervation of the carotid bodies currently attempted to suppress the chemoreceptor excitatory sensory input are undergoing clinical trials in hopeful anticipation.
Acknowledgment
We thank Arlinda LaRose and Sally Knipfer for assistance in preparing the manuscript and Shawn Roach in formatting the figures.
References
- Abboud FM. Ventricular syncope: is the heart a sensory organ? (Editorial) N. Engl. J. Med. 1989;320:390–392. doi: 10.1056/NEJM198902093200609. [DOI] [PubMed] [Google Scholar]
- Abboud FM. In search of autonomic balance: the good, the bad and the ugly. Am. J. Physiol. Regul. Physiol. 2010;298:R1449–R1467. doi: 10.1152/ajpregu.00130.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abboud FM, Kumar R. Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J. Clin. Investig. 2014;124:1454–1457. doi: 10.1172/JCI70420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abboud FM, Mark AL, Thames MD. Modulation of the somatic reflex by carotid baroreceptors and by cardiopulmonary afferents in animals and in humans. Circ. Res. 1981;48(Part II):I-131–I-137. [PubMed] [Google Scholar]
- Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J. cell Biol. 1998;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Smirk RF. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J. Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J. Physiol. 2003;546:77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Ann. Rev. Biophys. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- Babinski K, Catarsi S, Biagini G, Séguéla P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+ J. Biol. Chem. 2000;275:28519–28525. doi: 10.1074/jbc.M004114200. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Johansen L, Graham T, Saltin B. Lactate and H+ effluxes from human skeletal muscles during intense, dynamic exercise. J. Physiol. 1993;462:115–133. doi: 10.1113/jphysiol.1993.sp019546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J. Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circ. Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi L, Driscoll M. Protons at the gate: DEG/ENaC ion channels help us feel and remember. Neuron. 2002;234:337–340. doi: 10.1016/s0896-6273(02)00687-6. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horisberger J-D, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science. 1989;243:1027–1033. doi: 10.1126/science.2646709. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Driscoll M, Huang M. Degenerin similarities. Nature. 1993;361:504. doi: 10.1038/361504a0. [DOI] [PubMed] [Google Scholar]
- Driscoll M, Chalfie M. The mec-4 gene is a member of a family of Caenorhabditis elegans genes that can mutate to induce neuronal degeneration. Nature. 1991;349:588–593. doi: 10.1038/349588a0. [DOI] [PubMed] [Google Scholar]
- Drummond HA, Price MA, Welsh MJ, Abboud FM. A molecular component of the arterial baroreceptor mechanotransducer. Neuron. 1998;21:1435–1441. doi: 10.1016/s0896-6273(00)80661-3. [DOI] [PubMed] [Google Scholar]
- Edwards L, McIntyre D, Carroll D, Ring C, Martin U. The human nociceptive flexion reflex threshold is higher during systole than diastole. Psycho-physiology. 2002;39:678–681. doi: 10.1017.S0048577202011770. [DOI] [PubMed] [Google Scholar]
- Fazan PV, Junior FR, Salgado CH, Barreira AA. Morphology of aortic depressor nerve myelinated fibers in normotensive Wistar-Kyoto and spontaneously hypertensive rats. J. Auton. Nerv. Syst. 1999;77:133–139. [PubMed] [Google Scholar]
- Fukuda Y, Sato A, Trzebski A. Carotid chemoreceptor discharge responses to hypoxia and hypercapnia in normotensive and spontaneously hypertensive rats. J. Auton. Nerv. Syst. 1987;19:1–11. doi: 10.1016/0165-1838(87)90139-1. [DOI] [PubMed] [Google Scholar]
- Gao Z, Henig O, Kehoe V, Sinoway LI, Li J. Vanilloid type 1 receptor and the acid-sensing ion channel mediate acid phosphate activation of muscle afferent nerves in rats. J. Appl. Physiol. 2006;100:421–426. doi: 10.1152/japplphysiol.00659.2005. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Minati L, Gray MA, Seth AK, Dolan RJ, Critchley HD. Fear from the heart: sensitivity to fear stimuli depends on individual heartbeats. J. Neurosci. 2014;34:6573–6582. doi: 10.1523/JNEUROSCI.3507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 sub-units. FASEB J. 2013;27:793–802. doi: 10.1096/fj.12-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduczok G, Chapleau MW, Ferlic RJ, Mao HZ, Abboud FM. Gadolinium inhibits mechano-electrical transduction in rabbit carotid baroreceptors: implication of stretch-activated channels. J. Clin. Investig. 1994;94:2392–2396. doi: 10.1172/JCI117605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DE, Borganelli M, Stacy CP, Jr, Taylor LK. Dose-dependent inhibition of stretch-induced arrhythmias by gadolinium in isolated canine ventricles. Evidence for a unique mode of antiarrhythmic action. Circ Res. 1991;69:820–831. doi: 10.1161/01.res.69.3.820. [DOI] [PubMed] [Google Scholar]
- Hattori T, Chen J, Harding AM, Price MP, Lu Y, Abboud FM, Benson CJ. ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ. Res. 2009;105:279–286. doi: 10.1161/CIRCRESAHA.109.202036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J. Physiol. 2007;581:1271–1282. doi: 10.1113/jphysiol.2007.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1720–H1725. doi: 10.1152/ajpheart.00623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Abboud FM, Mark AL, Schmid PG. Interaction of baroreceptor and chemoreceptor reflexes: modulation of the chemoreceptor reflex by changes in baroreceptor activity. In: Brooks LA, Swanson J, editors. J. Clin. Investig. Vol. 53. 1974. pp. 1226–1236. with technical assistance of. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Abboud FM, Mark AL, Schmid PG. Effect of baroreceptor activity on ventilatory response to chemoreceptor stimulation. J. Appl. Physiol. 1975;39:411–416. doi: 10.1152/jappl.1975.39.3.411. [DOI] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, Ahring PK. pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J. Biol. Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat. Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- Iturriaga R, Alcayaga J, Zapata P. Contribution of carotid body chemoreceptors and carotid sinus baroreceptors to the ventilatory and circulatory reflexes produced by common carotid occlusion. Acta Physiol. Pharmacol. Latinoam. 1988;38:27–48. [PubMed] [Google Scholar]
- Kaufman MP, Hayes SG. The exercise pressor reflex clinical autonomic research. Clin. Auton. Res. 2002;12:429–439. doi: 10.1007/s10286-002-0059-1. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Sakakibara Y, Masuda A, Ohdaira T, Honda Y. Contribution of peripheral chemoreceptor drive in exercise hyperpnea in humans. Appl. Hum. Sci. 1996;15:259–266. doi: 10.2114/jpa.15.259. [DOI] [PubMed] [Google Scholar]
- Kraske S, Cunningham JT, Hajduczok G, Chapleau MW, Abboud FM, Wachtel RE. Mechanosensitive ion channels in putative aortic baroreceptor neurons. Am. J. Physiol. Heart Circ. Physiol. 1998;275:H1497–H1501. doi: 10.1152/ajpheart.1998.275.4.H1497. [DOI] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. ATRAMI Investigators Autonomic tone and reflexes after myocardial infarction. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- Lawrence IG, Weston PJ, Bennett MA, McNally PG, Burden AC, Thurston H. Is impaired baroreflex sensitivity a predictor or cause of sudden death in insulin-dependent diabetes mellitus? Diabet. Med. 1997;14:82–85. doi: 10.1002/(SICI)1096-9136(199701)14:1<82::AID-DIA290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Li J, Maile MD, Sinoway AN, Sinoway LI. Muscle pressor reflex: potential role of vanilloid type 1 receptor and acid-sensing ion channel. J. Appl. Physiol. 2004;97:1709–1714. doi: 10.1152/japplphysiol.00389.2004. [DOI] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J. Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993;318:95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, Deval E, Lazdunski M. FMRFamide-gated sodium channel and ASIC channels: a new class of ionotropic receptors for FMRFamide and related peptides. Peptides. 2006;27:1138–1152. doi: 10.1016/j.peptides.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Lu Y, Whiteis CA, Chapleau MW, Abboud FM. Decreased mRNA expression of ASIC2a in nodose sensory ganglia is associated with development of hypertension in SHR. Abstr. FASEB J. 2007;21:A1405. [Google Scholar]
- Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64:885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Whiteis C, Sluka KA, Chapleau MW, Abboud FM. Responses of body glomus cells to hypoxia and acidosis are uncoupled, and linked to ASIC3 expression. J. Physiol. 2013;591:919–932. doi: 10.1113/jphysiol.2012.247189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Bautista DM. Feeling the pressure in mammalian somato-sensation. Curr. Opin. Neurobiol. 2005;15:382–388. doi: 10.1016/j.conb.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliani A, Schwartz PJ, Zanchetti A. A sympathetic reflex elicited by experimental coronary occlusion. Am. J. Physiol. 1969;217:703–709. doi: 10.1152/ajplegacy.1969.217.3.703. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J. Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minisi AJ, Thames MD. Activation of cardiac sympathetic afferents during coronary occlusion. Evidence for reflex activation of sympathetic nervous system during transmural myocardial ischemia in the dog. Circulation. 1991;84:357–367. doi: 10.1161/01.cir.84.1.357. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol. Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–3458. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- Naruse K, Sokabe M. Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells. Am. J. Physiol. Cell. 1993;264:C1037–C1044. doi: 10.1152/ajpcell.1993.264.4.C1037. [DOI] [PubMed] [Google Scholar]
- O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- Peers C. Effect of lowered extracellular pH on Ca2(+)-dependent K+ currents in type I cells from the neonatal rat carotid body. J. Physiol. 1990;422:381–395. doi: 10.1113/jphysiol.1990.sp017990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff EY, Price MP, Snitsarev V, Gong H, Korovkina V, Abboud FM, Welsh MJ. Acid-sensing ion channels interact with and inhibit BK K+ channels. Proc. Natl. Acad. Sci. 2008;105:3140–3144. doi: 10.1073/pnas.0712280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroff E, Snitsarev V, Gong H, Abboud FM. Acid sensing ion channels regulate neuronal excitability by inhibiting BK potassium channels. Biochem. Biophys. Res. Commun. 2012;426:511–515. doi: 10.1016/j.bbrc.2012.08.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepoli M, Ponikowski P, Clark Al, Banasiak W, Capucci A, Coats AJ. A neural link to explain the “muscle hypothesis” of exercise intolerance in chronic heart failure. Am. Heart J. 1999;137:1050–1056. doi: 10.1016/s0002-8703(99)70361-3. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp. Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ. Peripheral chemoreceptors in health and disease. J. Appl. Physiol. 2004;96:359–366. doi: 10.1152/japplphysiol.00809.2003. [DOI] [PubMed] [Google Scholar]
- Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J. Biol. Chem. 1996;271:7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Price MP, Gong H, Parsons MG, Kundert JR, Reznikov LR, Bernardinelli L, Chaloner K, Buchanan GF, Wemmie JA, Richerson GB, Cassell MD, Welsh MJ. Localization and behaviors in null mice suggest that ASIC1 and ASIC2 modulate responses to aversive stimuli. Genes Brain Behav. 2014;13:179–194. doi: 10.1111/gbb.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch SM, Whipp BJ, Wasserman K, Huszczuk A. Role of the carotid bodies in the respiratory compensation for the metabolic acidosis of exercise in humans. J. Physiol. 1991;444:567–578. doi: 10.1113/jphysiol.1991.sp018894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TG, Dawson SL, Eames PJ, Panerai RB, Potter JF. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke. 2003;34:705–712. doi: 10.1161/01.STR.0000058493.94875.9F. [DOI] [PubMed] [Google Scholar]
- Sabharwal R, Chapleau MW, Price MP, Welsh MJ, Abboud FM. Molecular mechanisms of baro- and chemoreceptor activation: evidence that ASIC1 and ASIC3 contribute to chemoreceptor activation. Abstr. Circ. 2005;112:156. [Google Scholar]
- Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50:6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- Sharma RV, Chapleau MW, Hajduczok G, Wachtel RE, Waite LJ, Bhalla RC, Abboud FM. Mechanical stimulation increases intracellular calcium concentration in nodose sensory neurons. Neuroscience. 1995;66:433–441. doi: 10.1016/0306-4522(94)00560-r. [DOI] [PubMed] [Google Scholar]
- Sigurdson W, Ruknudin A, Sachs F. Calcium imaging of mechanically induced fluxes in tissue cultured chick heart: role of stretch-activated ion channels. Am. J. Physiol. Heart. 1992;262:H1110–H1115. doi: 10.1152/ajpheart.1992.262.4.H1110. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Li J. A perspective on the muscle reflex: Implications for congestive heart failure. J. Appl. Physiol. 2005;99:5–22. doi: 10.1152/japplphysiol.01405.2004. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Radhakrishnan R, Benson CJ, Eschol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp. Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- Snitsarev V, Iyer K, Whiteis CA, Chapleau MW, Abboud FM. Novel molecular defects in mechanosensitivity of aortic baroreceptor neurons from spontaneously hypertensive rats. Abstr. FASEB J. 2005;19:A607. [Google Scholar]
- Snyder PM. The epithelial Na+ channel: cell surface insertion and retrieval in Na+ homeostasis and hypertension. Endocr. Rev. 2002;23(2):258–275. doi: 10.1210/edrv.23.2.0458. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J. Clin. Investig. 1991;87:1953–1957. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. J. Physiol. 2001;537:993–998. doi: 10.1111/j.1469-7793.2001.00993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJ, Sharma RV, Wachtel RE, Chapleau MW, Waite LJ, Bhalla RC, Abboud FM. Non-voltage-gated Ca2+ influx through mechanosensitive ion channels in aortic baroreceptor neurons. Circ. Res. 1997;80:861–867. doi: 10.1161/01.res.80.6.861. [DOI] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar NR. CO(2) and pH independently modulate L-type Ca(2+) current in rabbit carotid body glomus cells. J. Neurophysiol. 2002;88:604–612. doi: 10.1152/jn.2002.88.2.604. [DOI] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl. Acad. Sci. U. S. A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZY, Lu Y, Whiteis CA, Benson CJ, Chapleau MW, Abboud FM. Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ. Res. 2007;101:1009–1019. doi: 10.1161/CIRCRESAHA.107.154377. [DOI] [PubMed] [Google Scholar]
- Tan ZY, Lu Y, Whiteis CA, Simms A, Paton JFR, Chapleau M, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and over-expression of ASIC and TASK channels before the onset of hypertension in SHR. Circ. Res. 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames MD, Abboud FM. Interaction of somatic and cardiopulmonary receptors in control of renal circulation. Am. J. Physiol. Heart Circ Physiol. 1979;237:H560–H565. doi: 10.1152/ajpheart.1979.237.5.H560. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J. Physiol. 2011;589:6173–6189. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath MA, Kwan KY, Corey DP. The micromachinery of mechano-transduction in hair cells. Annu. Rev. Neurosci. 2007;30:339–365. doi: 10.1146/annurev.neuro.29.051605.112917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vralsted VC, Price MP, Du J, Schnizler M, Wunsch AM, Ziemann AE, Welsh MJ, Wemmie JA. Expressing acid-sensing ion channel 3 in the brain alters acid-evoked currents and impairs fear conditioning. Genes Brain Behav. 2011;10:444–450. doi: 10.1111/j.1601-183X.2011.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain. 2011;152:2348–2356. doi: 10.1016/j.pain.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans . J. Biol. Chem. 1996;271:10433–10436. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, DeWeille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J. Biol. Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Wang W, Ma R. Cardiac sympathetic afferent reflexes in heart failure. Heart Fail. Rev. 2000;5:57–71. doi: 10.1023/A:1009898107964. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JHJ, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JHJ, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J. Neurosci. 2003;23:5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton. Neurosci. 2008;138:9–23. doi: 10.1016/j.autneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J, Li J. ASIC3 contributes to the blunted muscle metaboreflex in heart failure. Med. Sci. Sports Exerc. 2014 Jun 30; doi: 10.1249/MSS.0000000000000415. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ. Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science (Wash DC) 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Zhang P, Sigworth FJ, Canessa CM. Gating of acid-sensitive ion channel-1: release of Ca2+ block vs. allosteric mechanism. J. Gen. Physiol. 2006;127:109–117. doi: 10.1085/jgp.200509396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XL, Stumpf MA, Hock HC, Kung C. A mechanosensitive channel in whole cells and in membrane patches of the fungus Uromyces. Science (Wash DC) 1991;253:1415–1417. doi: 10.1126/science.1716786. [DOI] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, Irwin ED, Serdar DJ, Peuler JD, Rossing MA. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50:904–910. doi: 10.1161/HYPERTENSIONAHA.107.095216. [DOI] [PubMed] [Google Scholar]