Abstract
Purpose
Bone formation and healing are diminished in experimental type I diabetes. The present study investigated whether controlled local release of a Bone Morphogenetic Protein (rhBMP-2) stimulates bone defect healing in diabetes, due to its anabolic effects on bone.
Material and Methods
Bilateral experimental circular bone defects were created in the temporal bones of 64 BALB/cByJ mice. Defects were treated with acellular collagen sponges (ACS), 0.4 µg or 1.8 µg of rhBMP-2 per defect, while untreated defects served as controls. The effects of rhBMP-2 on calvaria defect healing over a 14 day healing period in diabetic and non-diabetic mice were determined histomorphometrically.
Results
Diabetes inhibited bone formation in both untreated and BMP-treated bone defects. Controlled local release of rhBMP-2 significantly stimulates bone formation in diabetic animals to near normal levels, and enhances bone regeneration in normal animals.
Conclusions
rhBMP-2 may be beneficial in treating deficient intramembranous bone formation in diabetes.
Keywords: diabetes, bone regeneration, rhBMP-2
INTRODUCTION
Undisturbed bone formation and regeneration are fundamental aspects of oral implantology. Successful osseointegration of metallic implants in native or regenerated bone are intrinsically dependent of normal bone formation1. Diabetes mellitus is classified as a risk factor for implant treatment, and severe or poorly controlled diabetes mellitus has been suggested to be a contraindication for treatment with dental implants2,3, due to a substantial effect on successful implant osseointegration4. Diabetes mellitus has been closely associated with disorders in skeletal physiology and osseous healing process5 collectively referred to as “diabetic bone disease” or “diabetic osteopathy”6. characterized by osteopenia7–8, decreased bone mineral content9,10 and delayed fracture healing11. Osteopenia is likely to result in diminished bone formation, and studies have demonstrated diminished bone formation in experimental bone defects12,13, as well as delayed bone regeneration in extraction sockets14, inhibited or delayed peri-implant bone formation and osseous integration15–22, and reduced peri-implant bone density23 in diabetic animals. Reduced removal torque values have also been observed post-implantation24, evidencing the effect of experimental diabetes on the biomechanical properties of endosseous implants. The altered bone response found in diabetes has been associated with reduced osteoblastic activity25–31, related to diabetic osteopenia28. Clinical ramifications for such findings may be represented by an increased risk for implant failure in diabetic patients compared with healthy controls32,33. Moreover, a trend of increased early vs. late implant failure34 and increased failure rate after functional loading35,36 have also been reported.
Advanced glycation end products (AGE’s) cause many complications of diabetes37,38 apparently due to accumulation of high levels in the living tissues. This phenomenon is a link between numerous diabetic complications, through induction of marked changes in cellular and extracellular matrix components and has also been documented in bone tissues12,39. Administration of AGE’s to calvaria defects in normal mice inhibits bone healing in vivo, and mimics inhibited bone formation in diabetic animals12. The receptor for advanced glycation end products (RAGE) is present in osteoblasts, and elevated levels of RAGE are observed in healing tissues in diabetic animals12. AGE/RAGE interactions result in increased apoptosis of mesenchymal cells40, particularly primary rat calvaria and murine MC3T3-E1 osteoblasts41, and have the potential to affect the growth and function of osteoblasts and impaired organization and mineralization of extracellular matrix39,42–45. Collagen is a major protein of bone organic matrix and undergoes intra- and extra-cellular post-translational modifications in order to form a functional extracellular matrix45. Thus, lysyl oxidase-dependent collagen cross-linking is essential for bone strength46. Elevated glycation of collagen, which occurs in diabetes, interferes with Discoidin Domain Receptor-2 (DDR2) binding and activation, hence failing to maintain lysyl oxidase levels made by osteoblasts45. DDR2 binding and activation were disrupted by collagen glycation, pointing to an alternative mechanism for the diminished levels of lysyl oxidase and consequently low lysyl oxidase-derived cross-links in diabetic bone45. Taken together, these studies suggest that osteoblast differentiation may be inhibited in diabetes, and that reduced osteoblast differentiation could be a major mechanism that contributes to the observed reduced osteoblast function and reduced bone healing in diabetes. If inhibited osteoblast differentiation is the principal mechanism of inhibited diabetic bone formation, it follows that application of factors known to stimulate osteoblast differentiation47–48 could potentially reverse and normalize diminished bone healing in diabetes. To test this idea, an experiment was undertaken in which a factor known to promote osteoblast differentiation, rhBMP-247–48, was applied to calvaria defects in diabetic mice, and the effect on bone healing was evaluated. The goal of the current study was to assess whether the controlled local application of rhBMP-2 restores intramembranous bone healing to normal levels in diabetic animals.
MATERIALS AND METHODS
Diabetes induction and characterization
Experiments were performed in BALB/cByJ mice (Jackson Laboratories, MA, USA) as previously described1,12 in accordance with the Guidelines of the National Institute of Health (NIH) for the care and use of animals for experimental procedures. Male, eight-week old animals were maintained according to approved protocols (Boston University IACUC). Animals were kept with free access to tap water and NIH 31M mouse diet (5K52; Purina Mills; USA). Generation of diabetic and control non-diabetic animals was accomplished using the multiple low dose streptozotocin methodology. The number of animals per experimental group was 8 unless otherwise indicated.
The diabetic condition was characterized. Blood glucose (Accu-Check™ Advantage, Roche Diagnostics), and urine glucose levels (Multisix 10SG reagent strips, Bayer) were monitored twice weekly throughout the typical 33 day experimental periods, and diabetes onset on experimental day 12 was confirmed in all diabetic animals utilized (blood glucose levels of at least 250 mg/dl). Blood and urine glucose values for control animals were normal (100 – 110 mg/dl). Protein and ketones in the urine were assayed twice weekly and were not detected (Multisix 10SG reagent strips, Bayer). Levels of glycated hemoglobin in blood (Glyc-Affin® Ghb, Wallac, Inc., Ohio) and levels of insulin in serum (Linco Research, Inc., MO) were measured at sacrifice.
Experimental bone defects
Sixty-four animals were used. Half of the animals were made diabetic. Surgical procedures were performed one week following confirmation of diabetes (blood glucose >250 mg/dl; experimental day 18) and the animals received one 2.1 mm diameter lesion in each parietal bone. BMP-2 or buffer alone was applied to acellular collagen sponges (Genetics Institute) and discs (2 mm diameter) containing the specified amount of BMP-2 was applied to calvaria defects within two hours of preparation. Normal and diabetic animals were separated into each of the four treatment modalities to be tested resulting in a total of eight groups of animals: 1.8 µg of rhBMP-2 per defect (n=8), 0.4 µg of rhBMP-2 per defect (n=8), acellular collagen sponge loaded with vehicle only (n=8) and controls receiving no sponges (n=8). Flaps were sutured, post-operative care, histologic and histomorphometric analyses were performed. All the animals were sacrificed 14 days after the surgical procedure and the surgical areas were dissected free, fixed in formalin and processed for histologic and histomorphometric analyses.
Histologic and histomorphometric evaluation
Tissues were sectioned perpendicular to the plane of calvarial bone. The three most central sections of each defect were analyzed. Amounts and quality of regenerated bone tissue, cellularity, vascularization pattern and degree of inflammation were evaluated. Linear measurements were done by means of an image analysis system (ImagePro 4.0). Bone in-growth from the rims of the initial defects towards its center was quantified. Bone bridging was expressed as a percentage of the original defect width. Measurements made included: (a) the distance between the rims of the initial bone defect, (b) the distance between the rims of the remaining defect. The amount of bone bridging was calculated as in the formula: (a)–(b) / (a)×100. Area of regenerated bone, cartilage and residual ACS carrier present in the healed defects were measured from digitally-captured images of stained slides using the ImagePro 4.0 software. Boundaries of the features of interest were traced with a mouse-driven cursor on video images on the display monitor with a hand-held mouse. The area of the outlined image was then calculated electronically with the software package. Bone area was measured in three slides for each bone defect from each animal. The readings were averaged to obtain means for each bone defect, and both defects were averaged to obtain the mean for every animal, that was used as the unit for statistical analyses. Results were presented as means +/− standard deviation.
Statistics
The results of the biochemical measurements were analyzed with one-way ANOVA. Post-hoc analysis was performed with Student’s t test for ordinal variables and with Wilcoxon sign rank test for cardinal variables. A value for α of 95% or higher was used to declare statistical significance. The results of the histomorphometric measurements for bone bridging were analyzed with one-way ANOVA. Post-hoc analyses were carried out with Wilcoxon sign rank test. The results of the histomorphometric measurements for area determinations were analyzed with one-way ANOVA. Post-hoc statistical testing was performed using the Bonferroni method, and an α of 95% or higher was used to declare statistical significance.
RESULTS
All animals injected with STZ became diabetic by day 11, and results of characterization of diabetes in this model are summarized in Table 1. Data show that the diabetic state was not accompanied by extreme metabolic dysregulation. Calvaria defects after 14 days of healing demonstrated differences in healing as a function of diabetes. Bone bridging (Table 2) was inhibited by about 50% in diabetic animals compared to non-diabetic controls (Table 2; group 1 and group 2; p=0.004). The application of acellular collagen sponges (ACS) with adsorbed rhBMP-2 to bone defects in diabetic animals stimulated bone bridging and bone regeneration and was dose-dependent (Table 2). ACS without rhBMP-2 did not stimulate, but rather inhibited healing somewhat, thus demonstrating that stimulated healing depends directly on rhBMP-2. Representative micrographs of lesions treated with collagen carrier +/− BMP-2 are shown in Figure 1. Both doses were effective in stimulating diabetic bone healing, however, there was a tendency towards increased bone bridging in defects receiving the higher dose of rhBMP-2 (Table 2; group 3 and group 4; p= 0.05).
Table I.
Biochemical and biometric measurements of diabetic and non-diabetic animals treated with rhBMP-2 and control treatments obtained on experimental day 33
| Parameter | Non-diabetic | Diabetic |
|---|---|---|
| Blood glucose (mg/dl) | 126.2 ± 11.1 | 498.3 ± 64.2* |
| GHb A1c (ng/ml) | 6.58 ± 0.7 | 14.6 ± 1.6* |
| Food (g/day/animal) | 6.1 ± 1.9 | 12.1 ± 3.1* |
| Weight (g) | 29.3 ± 0.4 | 26.12 ± 0.9* |
Measurements were performed on experimental day 33. Results were presented as means +/− standard deviation. Data from 32 diabetic and 32 non-diabetic animals are presented. Glucose and Glycated hemoglobin levels were obtained from whole blood preparations. Diabetic animals are hyperglycemic. Significantly elevated GHb A1c levels documented sustained hyperglycemia in diabetic animals. Diabetic animals exhibited reduced body weight, despite being significantly hyperphagic as expected and consistent with previous findings (8).
(* p <0.05; un-paired t-test or Mann-Whitney).
Table II.
Histomorphometric analyses of bone bridging and area of regenerated bone in calvarial defects from diabetic and non-diabetic animals treated with rhBMP-2.
| Group number and name | Bone bridging (% +/− SD) | Bone area (mm2 +/− SD) |
|---|---|---|
| 1. Diabetic (no ACS) | 23.28 +/− 11.2 | 0.14 ± 0.009 |
| 2. Normal (no ACS) | 47.64 +/− 24.02 | 0.19 ± 0.01 |
| 3. Diabetic + 0.4 µg BMP-2 | 87.09 +/− 24.58 | 0.47 +/− 0.19 |
| 4. Diabetic + 1.8 µg BMP-2 | 100 +/− 0 | 0.75 +/− 0.17 |
| 5. Normal + 0.4 µg BMP-2 | 100 +/− 0 | 0.66 +/− 0.19 |
| 6. Normal + 1.8 µg BMP-2 | 100 +/− 0 | 1.01 +/− 0.25 |
| 7. Diabetic (+ ACS) | 14. 25 +/− 3.27 | 0.09 +/− 0.008 |
| 8. Normal (+ACS) | 24.56 +/− 10.46 | 0.12 +/− 0.008 |
Bone bridging measurements were made from serial sections (4µm), and were stained with H&E or Masson’s Trichrome. Specimens from the center of the defects were analyzed. Linear measurements of bone in-growth from the rims of the initial defects towards its center were quantified after 14 days of healing. Bone bridging was expressed as a percentage of the total defect width. (B) Area of regenerated bone. Area of regenerated bone was measured from digitally-captured images of stained slides. Boundaries of the features of interest were traced with a mouse-driven cursor on video images on the display monitor with a hand-held mouse. The area of the outlined image was then calculated electronically with the software package. Bone area was measured in three slides for each bone defect from each animal (n = 8). The readings were averaged to obtain means for each bone defect, and both defects were averaged to obtain the mean for every animal, that was used as the unit for statistical analyses. Results were presented as means +/− standard error.
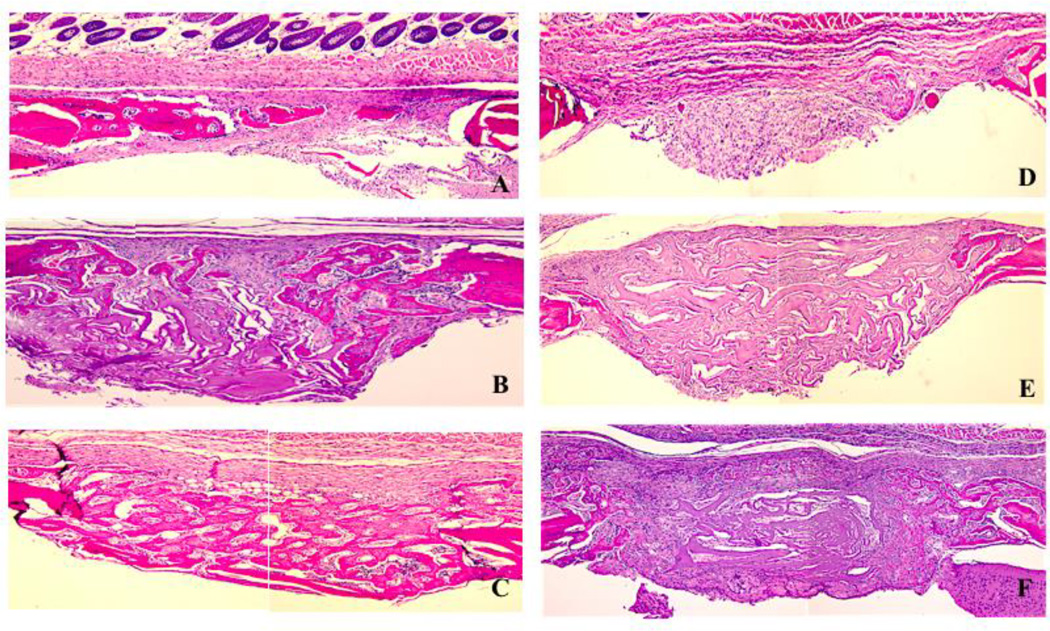
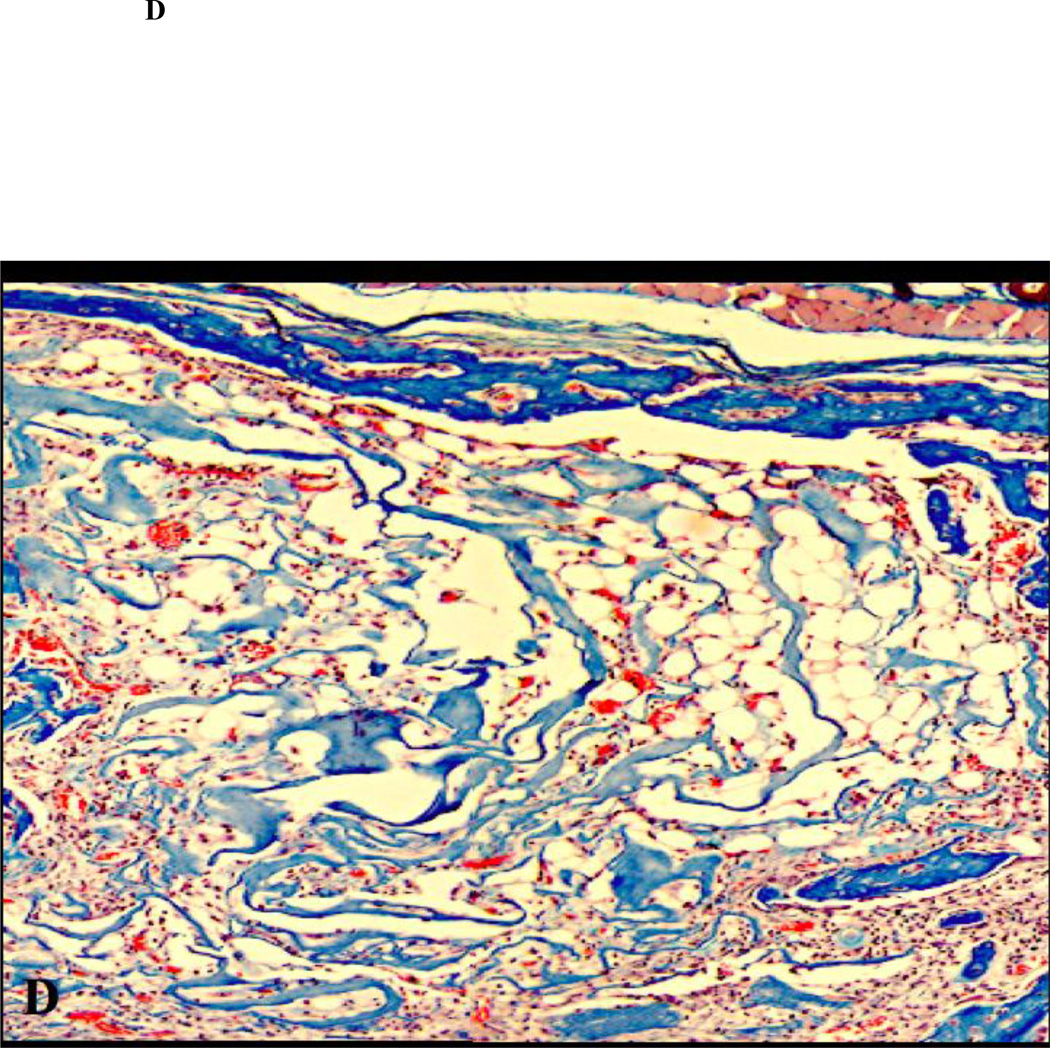
Figure 1.
Histology of calvarial defects harvested after 14 days of healing (H&E staining; original magnification 100×). (A) non-diabetic animal; (B) diabetic animal; (C) non-diabetic animal, ACS carrier; (D) diabetic animal, ACS carrier; (E) non-diabetic animal, ACS loaded with 1.8 µg rhBMP-2; (F) diabetic animal, ACS loaded with 1.8 µg rhBMP-2;
Non-diabetic animals exhibited a more robust response to rhBMP-2 as expected. For example, the defects in diabetic animals implanted with the lower dose of rhBMP-2 exhibited a tendency towards less bridging compared to non-diabetic animals implanted with either dose, further illustrating the inhibitory effect of type I diabetes on bone formation (Table 2; group 3 and group 5; p= 0.05). Application of rhBMP-2 significantly stimulated bone regeneration in non-diabetic animals compared to untreated non-diabetic controls with and without PBS-loaded ACS applied to the defects (Table 2; groups 5, 6 and 8; p< 0.001). All the defects in non-diabetic animals that were grafted with rhBMP-2 were completely bridged, irrespective of the dose applied (Table 2, groups 5 and 6; p>0.05). Bone bridging in defects in diabetic animals treated with the higher dose of rhBMP-2 was also complete and exhibited no significant difference compared to the bridging observed in normal animals treated with either dose (Table 2, groups 4–6). Differences in bridging in defects from non-diabetic and diabetic animals implanted with the ACS without rhBMP-2 were not significant (Table 2, groups 7 and 8; p=0.122), however, bridging in these groups was significantly less than in untreated non-diabetic animals (p=0.001). This indicates that unloaded ACS actually inhibits healing slightly in all animals. Taken together these data support that calvaria defect healing is inhibited in diabetes. rhBMP2 stimulates bone bridging in diabetic animals in a dose-dependent manner, and that the resulting bone bridging is complete and approaches the degree of bridging that occurs in normal animals treated with rhBMP2.
Bone bridging is a linear measurement and does not fully reflect the degree of accumulated healing bone. Thus, area measurements of new bone were performed in order to analyze more closely the ability of rhBMP2 to stimulate bone healing in diabetes. The exogenous application of rhBMP-2 significantly enhanced the area of regenerated bone in healing diabetic animals. Defects from diabetic animals receiving either dose of rhBMP-2 exhibited significantly more bone regeneration than untreated defects or ACS-implanted defects from both normal and diabetic animals (Table 2). Defects of diabetic animals treated with 1.8 µg of rhBMP-2 exhibited significantly bigger areas of bone regenerated than the ones treated 0.4 µg of rhBMP-2 (Table 2, group 3 and group 4; p= 0.002). Similar dose-dependent findings were observed in defects from normal animals, in which the higher rhBMP-2 dose yielded more bone regeneration than the smaller dose (Table 2; group 6 and group 5; p<0.001), characterizing a direct dose-response effect of rhBMP-2 implantation and area of bone regeneration in both normal and diabetic animals. Bone defects from normal animals implanted with rhBMP-2 exhibited significantly more bone regeneration than untreated defects and defects implanted with the ACS carrier (Table 2). Defects from non-diabetic animals implanted with 1.8 µg of rhBMP-2 exhibited significantly more area of regenerated bone than defects from diabetic animals implanted with either rhBMP-2 dose (Table 2). Defects from non-diabetic animals implanted with 0.4 µg of rhBMP-2 exhibited significantly more bone regeneration than diabetic animals treated with the same rhBMP-2 dose (Table 2, groups 5 and 3; p=0.03). No significant difference was found in the area of regenerated bone in ACS treated defects of normal and diabetic animals (Table 2; groups 7 and 8; p=0.7). Taken together, results suggest that rhBMP-2 stimulates bone healing in diabetic animals during 14 days of healing, but that the amount of new bone formed by area measurements is less than what forms in normal healing lesions stimulated with rhBMP-2.
Defects receiving rhBMP-2 implants exhibited some degree of residual ACS carrier populated by non-mineralized connective tissue, irrespective of the dose implanted, in both normal and diabetic animals. Data in Table 3 show quantitative histomorphometric analyses of the area of residual soft tissues within the healing defects treated with rhBMP-2. As shown, diabetic animals implanted with rhBMP-2 exhibited significantly decreased bone density than normal animals, due to inhibited osteogenesis and increased soft tissue area in the regenerated bone. The higher dose of rhBMP-2 elicited a more robust osteogenic response, and a lesser degree of soft tissue formation (Table 3 and Figure 2), partially compensating the effects of diabetes in bone formation.
Table III.
Histomorphometric analyses of area of regenerated bone density in calvarial defects from diabetic and non-diabetic animals treated with rhBMP-2.
| Group number and name | Soft tissues (mm2 +/− SD) |
Bone area (mm2 +/− SD) |
B Bone density (%+/− SD) |
|---|---|---|---|
| 1. Diabetic (+ ACS) | 0.74 +/− 0.09 | 0.14 ± 0.009 | 15.91 ± 3.21 |
| 2. Normal (+ ACS) | 0.62 +/− 0.13 | 0.19 ± 0.01 | 24.46 ± 8.21 |
| 3. Diabetic + 0.4 µg BMP-2 | 0.61 +/− 0.21 | 0.47 +/− 0.19 | 43.52 ± 16.73 |
| 4. Diabetic + 1.8 µg BMP-2 | 0.54 +/− 0.12 | 0.75 +/− 0.17 | 58.14 ± 13.26 |
| 5. Normal + 0.4 µg BMP-2 | 0.44 +/− 0.13 | 0.66 +/− 0.19 | 60.00 ± 26.31 |
| 6. Normal + 1.8 µg BMP-2 | 0.27 +/− 0.08 | 1.01 +/− 0.25 | 78.91 ± 14.17 |
Bone density measurements were made from serial sections (4µm), and were stained with H&E or Masson’s Trichrome. Specimens from the center of the defects were analyzed. Area measurements of bone in-growth from the rims of the initial defects towards its center and area of non-mineralized soft tissues (residual ACS + connective tissue) were quantified after 14 days of healing. Bone density was expressed as a percentage of the total defect width (area of regenerated bone + area of soft tissue). Results were presented as means +/− standard error.
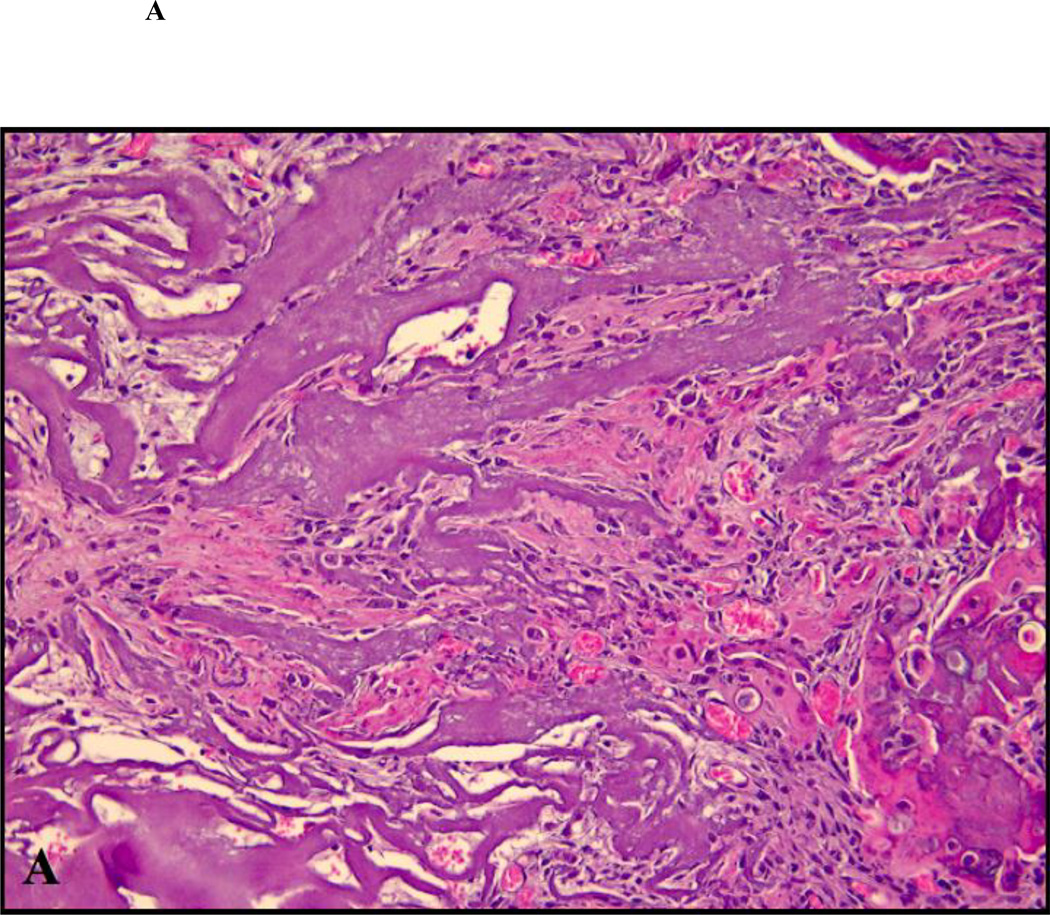
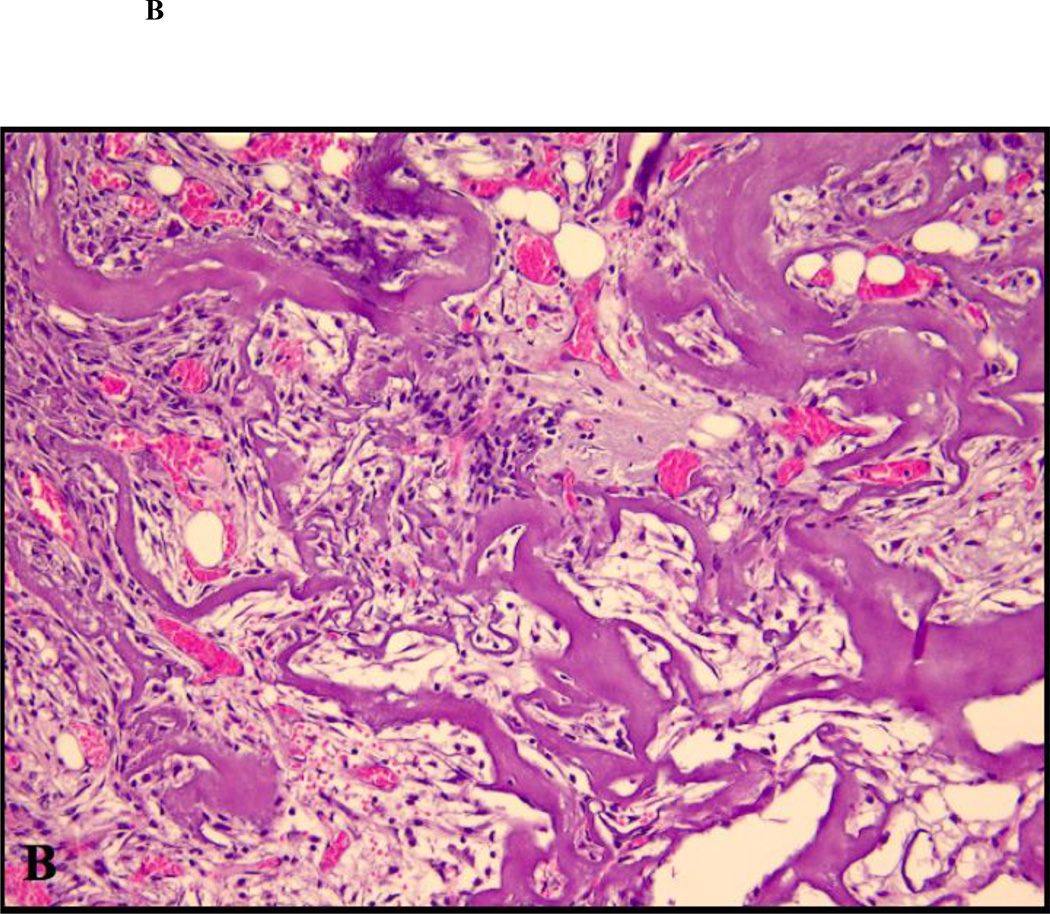
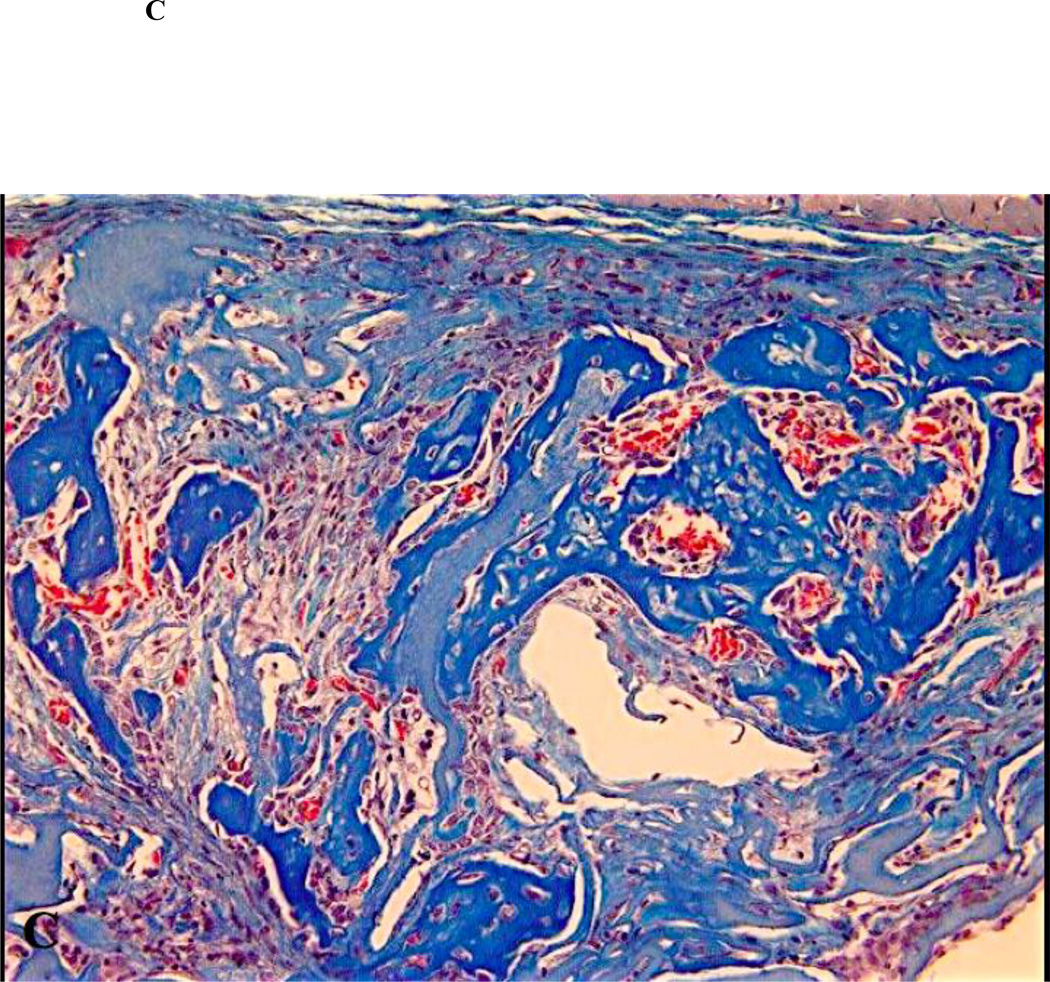
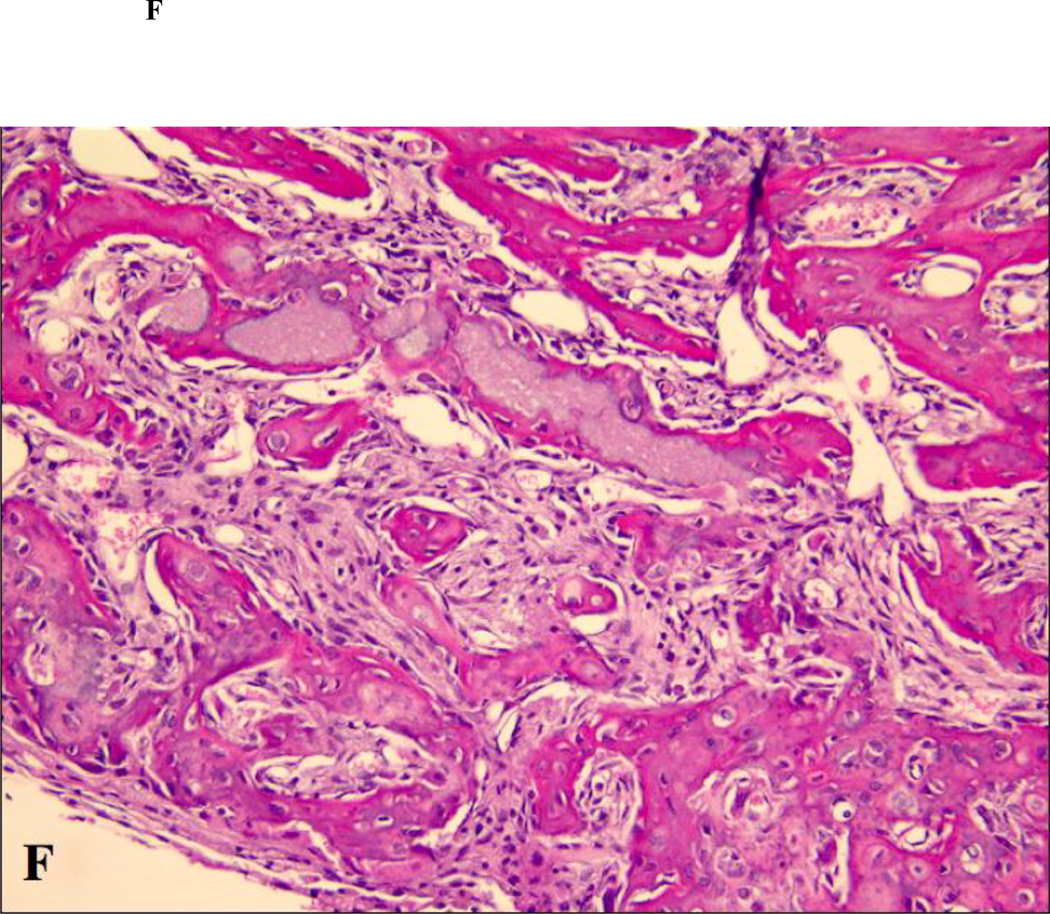
Figure 2.
Close up views of the center of the defects treated with ACS carrier with or without rhBMP-2. (A) non-diabetic animal, ACS carrier (H&E staining; original magnification 400×); (B) diabetic animal, ACS carrier (H&E staining; original magnification 400×); (C) non-diabetic animal, ACS carrier loaded with 0.4 µg rhBMP-2 (Masson trichrome staining; original magnification 400×); (D) diabetic animal, ACS loaded with 0.4 µg rhBMP-2 (Masson trichrome staining; original magnification 400×); (E) non-diabetic animal, ACS loaded with 1.8 µg rhBMP-2 (Masson trichrome staining; original magnification 400×); (F) diabetic animal, ACS loaded with 1.8 µg rhBMP-2(H&E staining; original magnification 400×).
DISCUSSION
Among the well-known metabolic consequences of diabetes, increased concern has been raised towards its deleterious effects in bone and mineral metabolism. This study demonstrates that rhBMP-2 partially corrects inhibited bone healing caused by Type I diabetes. The nature of bone healing in diabetic animals was qualitatively different to that found in non-diabetic animals. Standardized bone defects in non-diabetic animals healed predominantly with new bone, while bone defects in diabetic animals healed by a combination of bone, residual ACS and connective tissue.
Experimental studies have demonstrated a significant negative impact of diabetes in the alveolar bone biology49–51, including inhibited formation of the collagenous framework in the tooth extraction socket, delayed alveolar healing51, reduced bone formation and turnover in the alveolar wall surrounding the root50, reduction in osteocyte density and an increase in empty lacunar density was observed in the alveolar bone of diabetic animals49. Most of these changes are attributed to the osteopenic state of diabetic bone. Osteopenia is a complication of type 1 diabetes7 that results in reduced osteoblastic activity25–30 and decreased bone mineral content9–10 and is likely to result in diminished bone formation. Studies have demonstrated diminished bone formation in experimental bone defects1–12, as well as delayed bone regeneration in extraction sockets14, and inhibited osseous integration of implants18 and higher outcome variability and increased rate of infectious complications following Guided Bone Regeneration procedures52 in type I diabetic animals, thus illustrating a possible negative effect of diabetes on bone healing and implant therapy. Interestingly, it has been demonstrated that alterations in the extracellular presence of pro-inflammatory cytokines and growth factors within diabetic tissues may delay the onset the proliferation and differentiation of mesenchymal progenitor cells and osteoblasts in the osseointegration process53. It has been suggested that continuous application of gowth-factors, such as FGF-2 may facilitate osseointegration and bone healing in conditions of locally- and systemically-inhibited osteogenesis such as found in diabetes1. Thus, targeted controlled local delivery of biologically-active substances may stimulate bone regeneration and rescue the inhibitory effects of diabetes on bone healing. Biologically-enhanced therapies may offer significant clinical benefits by enhancing the endogenous healing capacity of bone defects, increasing the rate and total amounts of bone formation and ultimately resulting in significantly improved bone regeneration, specially in locally or systemically-inhibited healing sites, such as observed in diabetes mellitus.
The role of BMP-2 in normal tissue repair has been investigated, and it has been demonstrated that BMP-2 is a critical endogenous mediator of the signaling cascade that governs bone repair54. Previous studies have proposed that suppressed osteoblastogenesis may be a cause and reduced expression of the osteoblast-specific genes, such as bone morphogenetic protein-2, a mechanism for low bone mass, lower mineralized bone volume/tissue volume and impaired bone regeneration in rodent models of diabetes mellitus55–57. Despite the fact that the anabolic effect of rhBMP-2 in standardized bone defects in experimental normal animal models has been well documented58–63, to our knowledge, however, no studies have investigated the degree to which rhBMP-2 can mitigate against the effects of diabetic osteopenia or whether rhBMP-2 can reverse inhibited bone formation in diabetes. We reasoned that rhBMP-2 would be highly effective in reversing the effects of diabetes on bone formation because it is a stimulator of osteoblast differentiation rather than a direct stimulator of osteoblast function and extracellular matrix production47,64.
Our results show that rhBMP-2 is quite effective in reversing diabetes-induced inhibition of bone healing, but that new bone formed is qualitatively different from that found in non-diabetic animals in two respects. First, the area of bone formed in diabetic animals is less than normal; and second a greater amount of non-mineralized tissues is found in healing diabetic lesions, thus resulting in diminished bone density in diabetic animals. These results demonstrate that bone regeneration modulated by rhBMP-2 in diabetic animals may replicate and up-regulate the biologic events of bone metabolism and repair in these animals55.
A previous study12 as proposed a role for AGE/RAGE interactions as a possible mechanism for impaired bone healing in vivo. It is of interest that AGE/RAGE interactions cause increased apoptosis of mesenchymal cells, notably fibroblasts40 and primary rat calvaria and murine MC3T3-E1 osteoblasts41. AGEs accumulated in the bone matrix have the potential to affect the growth and function of osteoblasts and impaired matrix mineralization39 by activation the AGE - RAGE pathway39 and to suppress the osteogenic properties of osteoblasts in vivo42. The decreased osteoblastogenesis seen in diabetes may be associated with the regulation of intra-cellular signaling molecules resulting in decreased expression of genes relevant to osteoblastic phenotypic development43,44. Knowledge of multiple mechanisms by which AGE/RAGE interactions alters osteoblast differentiation, survival, and function seem likely to permit development of novel multifaceted therapeutic strategies to address osteopenia that is seen as a complication of type I diabetes.
Taken together the findings of the present study may suggest important clinical considerations. Uncontrolled diabetes results in significantly reduced natural and rhBMP-2 induced regenerative bone formation, which is manifested as inhibited osseointegration due to reduced area and calcification of formed bone, as well as a reduced surface of contact between bone and implant16,17,20,21. Targeted controlled local delivery of biologically-active substances such as rhBMP-2 may stimulate bone regeneration and rescue the inhibitory effects of diabetes on bone healing and osseointegration. Biologically-enhanced procedures may offer significant clinical benefits by enhancing the endogenous healing capacity of bone defects, increasing the rate and total amounts of bone formation and ultimately resulting in significantly improved bone regeneration.
In summary, the present study demonstrates that rhBMP-2 significantly stimulates bone regeneration in vivo and partially rescued impaired bone healing in diabetic animals to levels similar to normal untreated animals. These properties of rhBMP-2 bone defect therapy may have potential clinical use as a biologically enhanced procedure for stimulation of bone regeneration in normal as well as in diabetic patients. Qualitative differences in the healing potential of bone defects from normal and diabetic animals likely reflect a condition of multi-factorial etiology that deserves additional investigation focused on mechanistic aspects of these phenomena. Local delivery of rhBMP-2 in combination with other therapeutic factors that address additional mechanistic features of diabetic osteopenia may be developed as novel therapeutic approaches to address impaired bone healing in diabetic patients in need of bone reconstruction.
ACKNOLEDGEMENTS
This study was supported by NIH/NIDCR grant R01 DE011004 for PCT.
REFERENCES
- 1.Santana RB, Trackman PC. Controlled release of fibroblast growth factor 2 stimulates bone healing in an animal model of diabetes mellitus. Int J Oral Maxillofac Implants. 2006;21:711–718. [PubMed] [Google Scholar]
- 2.Buser D, von Arx T, ten Bruggenkate C, Weingart D. Basic surgical principles with ITI implants. Clin Oral Implants Res. 2000;11:59–68. doi: 10.1034/j.1600-0501.2000.011s1059.x. [DOI] [PubMed] [Google Scholar]
- 3.Neukam FW, Esser E. Implantology. Mund Kiefer Gesichtschir. 2000;4:249–256. doi: 10.1007/PL00014546. [DOI] [PubMed] [Google Scholar]
- 4.von Wilmowsky C, Stockmann P, Metzler P, Harsch IA, Amann K, Schlegel KA. Establishment of a streptozotocin-induced diabetic domestic pig model and a systematic evaluation of pathological changes in the hard and soft tissue over a 12-month period. Clin Oral Impl Res. 2010;21:709–717. doi: 10.1111/j.1600-0501.2010.01914.x. [DOI] [PubMed] [Google Scholar]
- 5.Retzepi M, Donos N. The effect of diabetes mellitus on osseous healing. Clin Oral Implants Res. 2010;21:673–681. doi: 10.1111/j.1600-0501.2010.01923.x. [DOI] [PubMed] [Google Scholar]
- 6.Bouillon R. Diabetic bone disease. Calcif Tissue Int. 1991;49:155–160. doi: 10.1007/BF02556109. [DOI] [PubMed] [Google Scholar]
- 7.Seino Y, Ishida H. Diabetic osteopenia: pathophysiology and clinical aspects. Diabetes Metab Rev. 1995;11:21–35. doi: 10.1002/dmr.5610110103. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes – a meta-analysis. Osteoporos Int. 2007;18:427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 9.Levin ME, Boisseau VC, Avioli LV. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N Engl J Med. 1976;294:241–245. doi: 10.1056/NEJM197601292940502. [DOI] [PubMed] [Google Scholar]
- 10.Santiago JV, McAlister WH, Ratzan SK, Bussman Y, Haymond MW, Shackelford G, et al. Decreased cortical thickness & osteopenia in children with diabetes mellitus. J Clin Endocrinol Metab. 1977;45:845–848. doi: 10.1210/jcem-45-4-845. [DOI] [PubMed] [Google Scholar]
- 11.Cozen L. Does diabetes delay fracture healing? Clin Orthoped. 1972;82:134–140. [PubMed] [Google Scholar]
- 12.Santana RB, Xu L, Babakhanlou-Chase H, Amar S, Graves DT, Trackman PC. A role for advanced glycation end products in diminished bone healing in type I diabetes. Diabetes. 2003;52:1502–1510. doi: 10.2337/diabetes.52.6.1502. [DOI] [PubMed] [Google Scholar]
- 13.Follak N, Kloting I, Wolf E, Merk H. Histomorphometric evaluation of the influence of the diabetic metabolic state on bone defect healing depending on the defect size in spontaneously diabetic BB/OK rats. Bone. 2004;35:144–152. doi: 10.1016/j.bone.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Devlin H, Garland H, Sloan P. Healing of tooth extraction sockets in experimental diabetes mellitus. Oral Maxillofac Surg. 1996;54:1087–1091. doi: 10.1016/s0278-2391(96)90166-4. [DOI] [PubMed] [Google Scholar]
- 15.Iyama S, Takeshita F, Ayukawa Y, Kido MA, Suetsugu T, Tanaka T. A study of the regional distribution of bone formed around hydroxyapatite implants in the tibiae of streptozotocin-induced diabetic rats using multiple fluorescent labeling and confocal laser scanning microscopy. J Periodontol. 1997;68:1169–1175. doi: 10.1902/jop.1997.68.12.1169. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita F, Murai K, Iyama S, Ayukawa Y, Suetsugu T. Uncontrolled diabetes hinders bone formation around titanium implants in rat tibiae. A light and fluorescence microscopy, and image processing study. J Periodontol. 1998;69:314–320. doi: 10.1902/jop.1998.69.3.314. [DOI] [PubMed] [Google Scholar]
- 17.Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants. 1998;13:620–629. [PubMed] [Google Scholar]
- 18.Fiorellini JP, Nevins ML, Norkin A, Weber HP, Karimbux NY. The effect of insulin therapy on osseointegration in a diabetic rat model. Clin Oral Implants Res. 1999;10:362–328. doi: 10.1111/j.1600-0501.1999.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 19.Giglio MJ, Giannunzio G, Olmedo D, Guglielmotti MB. Histomorphometric study of bone healing around laminar implants in experimental diabetes. Implant Dent. 2000;9:143–149. doi: 10.1097/00008505-200009020-00006. [DOI] [PubMed] [Google Scholar]
- 20.McCracken M, Lemons JE, Rahemtulla F, Prince CW, Feldman D. Bone response to titanium alloy implants placed in diabetic rats. Int J Oral Maxillofac Implants. 2000;15:345–354. [PubMed] [Google Scholar]
- 21.Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiya P. Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent. 2003;12:242–251. doi: 10.1097/01.id.0000074440.04609.4f. [DOI] [PubMed] [Google Scholar]
- 22.Ottoni CE, Chopard RP. Histomorphometric evaluation of new bone formation in diabetic rats submitted to insertion of temporary implants. Braz Dent J. 2004;15:87–92. doi: 10.1590/s0103-64402004000200001. [DOI] [PubMed] [Google Scholar]
- 23.Gerritsen M, Lutterman JA, Jansen JA. Wound healing around bone-anchored percutaneous devices in experimental diabetes mellitus. J Biomed Mater Res. 2000;53:702–709. doi: 10.1002/1097-4636(2000)53:6<702::aid-jbm13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Margonar R, Sakakura CE, Holzhausen M, Pepato MT, Alba RC, Marcantonio E. The influence of diabetes mellitus and insulin therapy on biomechanical retention around dental implants: a study in rabbits. Implant Dent. 2003;12:333–339. doi: 10.1097/01.id.0000086482.65273.b7. [DOI] [PubMed] [Google Scholar]
- 25.Shore RM, Chesney RW, Mazess RB, Rose PG, Bargman GJ. Osteopenia in juvenile diabetes. Calcif Tissue Intern. 1981;33:455–457. doi: 10.1007/BF02409473. [DOI] [PubMed] [Google Scholar]
- 26.Aubia J, Serrano LI, Marinoso L, Hojman L, Diez A, Lloveras J, Masramon J. Osteodystrophy of diabetics in chronic dialysis: a histomorphometric study. Calc Tiss Intern. 1988;42:297–301. doi: 10.1007/BF02556363. [DOI] [PubMed] [Google Scholar]
- 27.Rico H, Hernandez ER, Cabranes JA, Gomez-Castresana F. Suggestion of a deficient osteoblastic function in diabetes mellitus: the possible cause of osteopenia in diabetics. Calcif Tissue Int. 1989;45:71–73. doi: 10.1007/BF02561404. [DOI] [PubMed] [Google Scholar]
- 28.Bouillon R. Diabetic bone disease. Low turnover osteoporosis related to decreased IGF-I production. Verh K Acad Geneeskd Belg. 1992;54:365–391. [PubMed] [Google Scholar]
- 29.Bouillon R, Bex M, Van Herck E, Laureys J, Dooms L, Lesaffre E, Ravussin E. Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab. 1995;80:1194–1202. doi: 10.1210/jcem.80.4.7714089. [DOI] [PubMed] [Google Scholar]
- 30.Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1994;44:775–782. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 31.Verhaeghe J, Van Herck E, van Bree R, Moermans K, Bouillon R. Decreased osteoblast activity in spontaneously diabetic rats. In vivo studies on the pathogenesis. Endocrine. 1997;7:165–175. doi: 10.1007/BF02778138. [DOI] [PubMed] [Google Scholar]
- 32.Morris HF, Ochi S, Winkler S. Implant survival in patients with type 2 diabetes: placement to 36 months. Ann Periodontol. 2000;5:157–165. doi: 10.1902/annals.2000.5.1.157. [DOI] [PubMed] [Google Scholar]
- 33.Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants. 2005;20:569–577. [PubMed] [Google Scholar]
- 34.Mombelli A, Cionca N. Systemic diseases affecting osseointegration therapy. Clin Oral Implants Res. 2006;17(Suppl 2):97–103. doi: 10.1111/j.1600-0501.2006.01354.x. [DOI] [PubMed] [Google Scholar]
- 35.Shernoff AF, Colwell JA, Bingham SF. Implants for type II diabetic patients: interim report. VA implants in diabetes study group. Implant Dent. 1994;3:183–185. doi: 10.1097/00008505-199409000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Fiorellini JP, Chen PK, Nevins M, Nevins ML. A retrospective study of dental implants in diabetic patients. Intern J Periodont Rest Dent. 2000;20:366–373. [PubMed] [Google Scholar]
- 37.Lalla E, Lamster IB, Schmidt AM. Enhanced interaction of advanced glycation end products with their cellular receptor RAGE: implications for the pathogenesis of accelerated periodontal disease in diabetes. Ann Periodontol. 1998;3:13–19. doi: 10.1902/annals.1998.3.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 39.Franke S, Siggelkow H, Wolf G, Hein G. Advanced glycation endproducts influence the mRNA expression of RAGE, RANKL and various osteoblastic genes in human osteoblasts. Arch Physiol Biochem. 2007;113:154–161. doi: 10.1080/13813450701602523. [DOI] [PubMed] [Google Scholar]
- 40.Alikhani Z, Alikhani M, Boyd C, Nagao K, Trackman PC, Graves DT. Advanced glycation endproducts enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem. 2005;280:12087–12095. doi: 10.1074/jbc.M406313200. [DOI] [PubMed] [Google Scholar]
- 41.Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, Pischon N, Trackman PC, Gerstenfeld L, Graves DT. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345–353. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franke S, Roster C, Pester J, Hofmann G, Oelzner P, Wolf G. Advanced glycation end products affect growth and function of osteoblasts. Clin Exp Rheumatol. 2011;29:650–660. [PubMed] [Google Scholar]
- 43.Hie M, Iitsuka N, Otsuka T, Tsukamoto I. Insulin-dependent diabetes mellitus decreases osteoblastogenesis associated with the inhibition of Wnt signaling through increased expression of Sost and Dkk1 and inhibition of Akt activation. Int J Mol Med. 2011;28:455–462. doi: 10.3892/ijmm.2011.697. [DOI] [PubMed] [Google Scholar]
- 44.Hie M, Tsukamoto I. Increased expression of the receptor for activation of NF-kappaB and decreased runt-related transcription factor 2 expression in bone of rats with streptozotocin-induced diabetes. Int J Mol Med. 2010;26:611–618. doi: 10.3892/ijmm_00000506. [DOI] [PubMed] [Google Scholar]
- 45.Khosravi R, Sodek KL, Faibish M, Trackman PC. Collagen advanced glycation inhibits its Discoidin Domain Receptor 2 (DDR2)-mediated induction of lysyl oxidase in osteoblasts. Bone. 2014;58:33–41. doi: 10.1016/j.bone.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- 47.Wozney JM. The bone morphogenetic protein family: multifunctional cellular regulators in the embryo and adult. Eur J Oral Sci. 1998;106(Suppl 1):160–166. doi: 10.1111/j.1600-0722.1998.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 48.Hay E, Lemonnier J, Modrowski D, Lomri A, Lasmoles F, Marie PJ. N- and E-cadherin mediate early human calvaria osteoblast differentiation promoted by bone morphogenetic protein-2. J Cell Physiol. 2000;183:117–128. doi: 10.1002/(SICI)1097-4652(200004)183:1<117::AID-JCP14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Villarino ME, Sánchez LM, Bozal CB, Ubios AM. Influence of short-term diabetes on osteocytic lacunae of alveolar bone. A histomorphometric study. Acta Odontol Latinoam. 2006;19:23–28. [PubMed] [Google Scholar]
- 50.Mishima N, Sahara N, Shirakawa M, Ozawa H. Effect of streptozotocin-induced diabetes mellitus on alveolar bone deposition in the rat. Arch Oral Biol. 2002;47:843–849. doi: 10.1016/s0003-9969(02)00152-8. [DOI] [PubMed] [Google Scholar]
- 51.Devlin H, Garland H, Sloan P. Healing of tooth extraction sockets in experimental diabetes mellitus. Oral Maxillofacial Surgery. 1996;54:1087–1091. doi: 10.1016/s0278-2391(96)90166-4. [DOI] [PubMed] [Google Scholar]
- 52.Retzepi M, Lewis MP, Donos N. Effect of diabetes and metabolic control on de novo bone formation following guided bone regeneration. Clin Oral Implants Res. 2010;21:71–79. doi: 10.1111/j.1600-0501.2009.01805.x. [DOI] [PubMed] [Google Scholar]
- 53.Colombo JS, Balani D, Sloan AJ, St Crean J, Okazaki J, Waddington RJ. Delayed osteoblast differentiation and altered inflammatory response around implants placed in incisor sockets of type 2 diabetic rats. Clin Oral Implants Res. 2011;22:578–586. doi: 10.1111/j.1600-0501.2010.01992.x. [DOI] [PubMed] [Google Scholar]
- 54.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 55.Hamann C, Goettsch C, Mettelsiefen J, Henkenjohann V, Rauner M, Hempel U, et al. Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. Am J Physiol Endocrinol Metab. 2011;301:E1220–E1228. doi: 10.1152/ajpendo.00378.2011. [DOI] [PubMed] [Google Scholar]
- 56.Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, Petrov S, Alawi F, Graves DT. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012;26:1423–1430. doi: 10.1096/fj.11-196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Younis WH, Al-Rawi NH, Mohamed MA, Yaseen NY. Molecular events on tooth socket healing in diabetic rabbits. Br J Oral Maxillofac Surg. 2013;51:932–936. doi: 10.1016/j.bjoms.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 58.Marden LJ, Hollinger JO, Chaudhari A, Turek T, Schaub RG, Ron E. Recombinant human bone morphogenetic protein-2 is superior to demineralized bone matrix in repairing craniotomy defects in rats. J Biomed Mater Res. 1994;28:1127–1138. doi: 10.1002/jbm.820281003. [DOI] [PubMed] [Google Scholar]
- 59.Linde A, Hedner E. Recombinant bone morphogenetic protein-2 enhances bone healing, guided by osteopromotive e-PTFE membranes: an experimental study in rats. Calcif Tissue Int. 1995;56(6):549–553. doi: 10.1007/BF00298588. [DOI] [PubMed] [Google Scholar]
- 60.Boyne PJ. Animal studies of application of rhBMP-2 in maxillofacial reconstruction. Bone. 1996;19(1 Suppl):83S–92S. doi: 10.1016/s8756-3282(96)00144-5. [DOI] [PubMed] [Google Scholar]
- 61.Zellin G, Linde A. Importance of delivery systems for growth-stimulatory factors in combination with osteopromotive membranes. An experimental study using rhBMP-2 in rat mandibular defects. J Biomed Mater Res. 1997;35:181–190. doi: 10.1002/(sici)1097-4636(199705)35:2<181::aid-jbm6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 62.Toriumi DM, O'Grady K, Horlbeck DM, Desai D, Turek TJ, Wozney J. Mandibular reconstruction using bone morphogenetic protein 2: long-term follow-up in a canine model. Laryngoscope. 1999;109:1481–1489. doi: 10.1097/00005537-199909000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Wikesjö UM, Qahash M, Huang YH, Xiropaidis A, Polimeni G, Susin C. Bone morphogenetic proteins for periodontal and alveolar indications; biological observations - clinical implications. Orthod Craniofac Res. 2009;12:263–270. doi: 10.1111/j.1601-6343.2009.01461.x. [DOI] [PubMed] [Google Scholar]
- 64.Suzawa M, Takeuchi Y, Fukumoto S, Kato S, Ueno N, Miyazono K, Matsumoto T, Fujita T. Extracellular matrix-associated bone morphogenetic proteins are essential for differentiation of murine osteoblastic cells in vitro. Endocrinology. 1999;140:2125–2133. doi: 10.1210/endo.140.5.6704. [DOI] [PubMed] [Google Scholar]