Abstract
Background
Serotonin (5-HT3) receptor antagonists are commonly used to decrease nausea and vomiting for surgery patients, but these agents may be harmful. We conducted a systematic review on the comparative safety of 5-HT3 receptor antagonists.
Methods
Searches were done in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials to identify studies comparing 5-HT3 receptor antagonists with each other, placebo, and/or other antiemetic agents for patients undergoing surgical procedures. Screening search results, data abstraction, and risk of bias assessment were conducted by two reviewers independently. Random-effects pairwise meta-analysis and network meta-analysis (NMA) were conducted. PROSPERO registry number: CRD42013003564.
Results
Overall, 120 studies and 27,787 patients were included after screening of 7,608 citations and 1,014 full-text articles. Significantly more patients receiving granisetron plus dexamethasone experienced an arrhythmia relative to placebo (odds ratio (OR) 2.96, 95 % confidence interval (CI) 1.11–7.94), ondansetron (OR 3.23, 95 % CI 1.17–8.95), dolasetron (OR 4.37, 95 % CI 1.51–12.62), tropisetron (OR 3.27, 95 % CI 1.02–10.43), and ondansetron plus dexamethasone (OR 5.75, 95 % CI 1.71–19.34) in a NMA including 31 randomized clinical trials (RCTs) and 6,623 patients of all ages. No statistically significant differences in delirium frequency were observed across all treatment comparisons in a NMA including 18 RCTs and 3,652 patients.
Conclusion
Granisetron plus dexamethasone increases the risk of arrhythmia.
Electronic supplementary material
The online version of this article (doi:10.1186/s12916-015-0379-3) contains supplementary material, which is available to authorized users.
Keywords: Systematic review, Network meta-analysis, Serotonin receptor antagonists, Postoperative nausea, Postoperative vomiting
Background
Serotonin (5-HT3) receptor antagonists are a class of antiemetics recommended for patients undergoing surgery who are at risk for nausea and vomiting [1, 2]. Serotonin (5-HT3) receptor antagonists reduce nausea and vomiting by inhibiting vagal nerves in the central nervous system and intestinal mucosa [3]. However, some evidence suggests that 5-HT3 receptor antagonists can increase the risk of cardiac harm in children undergoing chemotherapy [4, 5]. Adverse events associated with these medications include a decrease in heart rate and prolongation of the QT interval. We were commissioned by Health Canada, a department of the federal government, to determine the comparative safety of 5-HT3 receptor antagonists for patients of all ages undergoing surgery due to safety concerns regarding the 5-HT3 receptor antagonists.
Methods
We used an integrated knowledge translation approach [6], entailing collaboration between researchers and research users throughout the conduct of this study. The research users involved in this study who posed the original study question were from Health Canada.
Protocol
A protocol was developed and revised using feedback from the research team and the research users. We registered our protocol with PROSPERO (CRD42013003564) and published it in an open-access journal [7]. Our methods are described briefly here; additional details can be found in the protocol publication. We originally intended to evaluate both safety and efficacy outcomes for patients undergoing surgery or chemotherapy; however, due to the enormous number of studies that met the inclusion criteria, we made slight changes to our protocol and subdivided the analyses. The current paper focuses on the safety of 5-HT3 antagonists in patients undergoing surgery. Subsequent papers will examine the efficacy of 5-HT3 antagonists for patients undergoing surgery [8], and the efficacy and safety of 5-HT3 antagonists for patients undergoing chemotherapy.
Eligibility criteria
We included experimental (randomized clinical trials (RCTs), quasi-RCTs, non-RCTs), quasi-experimental (interrupted time series, controlled before–after studies), and observational (cohort) studies involving patients of any age undergoing any type of surgery who were given a 5-HT3 receptor antagonist for nausea and/or vomiting. A list of the agents and relevant comparators that were investigated in the included studies can be found in Additional file 1: Appendix 1. The primary outcome was the number of patients experiencing arrhythmia, and secondary outcomes were QT prolongation, PR prolongation, delirium, and mortality (overall and sudden cardiac death). Given the large number of included studies we limited the review to those published in English. Studies suspected or identified as fraudulent were excluded [9].
Information sources
An experienced librarian executed searches of MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception until 11 January 2013. Unpublished studies were sought by searching trial protocol registries and conference proceedings.
Study selection and data collection
After a calibration exercise, the literature search results were screened by pairs of reviewers, working independently. The same approach was used to abstract data and appraise the quality of included studies. Conflicts at both the screening and the abstraction steps were resolved through discussion. When data was missing or clarification of published data was needed we contacted the authors.
Appraisal of methodological quality and risk of bias
To assess methodological quality and risk of bias of the included studies, we used the Cochrane Effective Practice and Organisation of Care risk of bias tool for experimental and quasi-experimental studies [10], the Newcastle-Ottawa Scale [11] for cohort studies, and the McMaster Quality Assessment Scale of Harms (known as the McHarm tool) [12] for studies reporting harms.
Synthesis of included studies
A pooled estimate of effect was derived on the odds ratio (OR) scale using random-effects pairwise meta-analysis for each outcome and comparison, if at least two studies were available. When studies reported zero events in one treatment arm, 0.5 was added to the numerator and 1 was added to the denominator. Studies with zero events in both arms were excluded from the analyses. Between-study heterogeneity for direct-comparison meta-analysis was estimated using the restricted maximum likelihood (REML) [13] and measured using the I2 statistic [14]. Each pairwise meta-analysis estimate is presented along with the corresponding 95% confidence interval (CI). These analyses were conducted using the metafor package [15] in R 3.1.2 [16].
Before embarking on network meta-analysis (NMA), we evaluated the transitivity assumption by examining the comparability of the distributions of age (children versus adults), timing of administration (all time points versus during surgery), and risk of bias (all versus removing high risk of bias for randomization, allocation concealment, and blinding of outcome assessor) as potential treatment-effect modifiers across comparisons [17]. For each outcome, we visually inspected the potential effect modifiers by using colored edges in the network according to the level of the effect modifier and the majority of trials included in each comparison [18]. We evaluated the consistency assumption for the entire network using the design-by-treatment interaction model [19]. In case we found statistically significant inconsistency, we planned to assess certain paths of the network using the loop-specific method [20, 21] to identify which piece of evidence was responsible for the inconsistency (i.e., local inconsistency). We also planned to apply network meta-regression to adjust for potential effect modifiers if local inconsistency was identified. NMAs were performed within a frequentist framework, assuming a common within-network estimate for the heterogeneity parameter across all comparisons and estimated with the REML [13, 19]. We used the surface under the cumulative ranking (SUCRA) curve to rank the safety of the various 5-HT3 receptor antagonists [22].
The treatment nodes were selected with input from clinicians, pharmacists, and statisticians on the team. Due to the complexity of the analysis, we did not account for differences in doses and durations assuming that all impact the treatment effect equally. Specifically, when a study compared different doses of an intervention against another intervention, we included only the recommended dose in the analysis [1, 23–33].
The summary treatment effect generated by each NMA is presented along with its 95 % CI and 95 % predictive interval (PrI). The PrI, representing the interval within which the estimated treatment effect of a future study is expected to lie, captures the uncertainty of the NMA estimate and the magnitude of heterogeneity within the network overall [34, 35]. To assess the presence of reporting bias (including publication bias and small-study effects), we applied the comparison-adjusted funnel plot for each outcome separately [18]. We ordered the treatments from oldest to newest and then plotted the difference between each study-specific treatment effect and the corresponding comparison-specific summary effect under the fixed-effect model, against the study-specific standard error. We carried out subgroup analyses for all outcomes according to the timing of administration of 5-HT3 receptor antagonist therapy (all time periods versus during surgery) and age (all ages versus children). To establish the robustness of our results, we performed a sensitivity analysis in which we excluded studies with high risk of bias because of incomplete outcome data. Given that our primary analysis was a network meta-analysis restricted to RCTs, we conducted a second sensitivity analysis in which non-randomized studies were added to the network, to observe the contribution of different study designs to the treatment effects. Network meta-analyses were conducted using the mvmeta command in Stata 13.0 [36, 37].
Results
Literature search
After screening 7,608 citations, we reviewed 1,014 potentially relevant full-text articles and identified 115 primary publications [10, 33, 38–150] and five companion reports [151–154] (reporting on six studies) providing data on 27,787 patients that met our inclusion criteria (Fig. 1). Overall, 77 studies were excluded because they reported results suspected or confirmed to be fraudulent [9]. One of the included studies was an unpublished conference abstract [84].
Fig. 1.
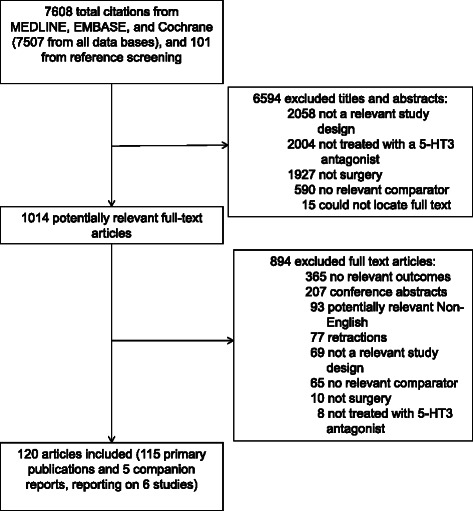
Study flow. Details the flow of information through the different phases of the review; maps out the number of records identified, included and excluded, and the reasons for their exclusion
Study and patient characteristics
The majority of the included studies were RCTs (97 %), conducted in Europe (37 %), North America (26 %), or Asia (24 %) and published between 1990 and 2013 (Table 1, Additional file 1: Appendix 2). The duration of follow-up was very short, ranging from ≤6 h to more than a week. The most frequent follow-up time observed was 12 to 24 h (69 %). The setting was not reported in the majority of trials (62 %) (Table 1).
Table 1.
Study characteristics
| Characteristic | Number of studiesa (n = 115) | Percentage of studies (%) |
|---|---|---|
| Year of publication | ||
| 1990–1994 | 7 | 6.1 |
| 1995–1999 | 37 | 32.2 |
| 2000–2004 | 19 | 16.5 |
| 2005–2009 | 36 | 31.3 |
| 2010–2013 | 16 | 13.9 |
| Geographic region | ||
| Europe | 42 | 36.5 |
| North America | 30 | 26.1 |
| Asia | 28 | 24.3 |
| Multi-continent | 8 | 7.0 |
| Australasia | 3 | 2.6 |
| Africa | 2 | 1.7 |
| Not reported | 1 | 0.9 |
| South America | 1 | 0.9 |
| Study design | ||
| Randomized clinical trial | 112 | 97.4 |
| Non-randomized clinical trial | 2 | 1.7 |
| Controlled before–after study | 1 | 0.9 |
| Study conduct period | ||
| 1990–1999 | 1 | 0.9 |
| 2000–2009 | 15 | 13.0 |
| 2010–2013 | 1 | 0.9 |
| Not reported | 98 | 85.2 |
| Duration of follow-up b | ||
| 0 to ≤6 | 9 | 7.8 |
| >6 to ≤12 | 2 | 1.7 |
| >12 to ≤24 | 79 | 68.7 |
| >24 to ≤48 | 17 | 14.8 |
| >48 to ≤72 | 2 | 1.7 |
| >72 to ≤1 week | 3 | 2.6 |
| Not reported | 3 | 2.6 |
| Interventions examined: frequency c | ||
| Serotonin antagonists: Reported as administered alone (administered with dexamethasone) | ||
| Ondansetron | 79 (7) | 68.70 (6.1) |
| Granisetron | 14 (4) | 12.2 (3.5) |
| Tropisetron | 15 (0) | 13.0 (0.0) |
| Dolasetron | 15 (1) | 13.0 (0.9) |
| Palonosetron | 4 (0) | 3.5 (0.0) |
| Ramosetron | 3 (1) | 2.6 (0.9) |
| Comparator antiemetics: | ||
| Butyrophenone | 26 | 22.61 |
| Benzamide | 14 | 12.17 |
| Dexamethasone | 6 | 5.2 |
| Phenothiazine | 2 | 1.7 |
| Antihistamine | 3 | 2.61 |
| NK-1 | 4 | 3.5 |
| Anticholinergic | 0 | 0 |
| Serotonin antagonists given with other antiemetic: | ||
| Serotonin antagonist + dexamethasone | 13 | 11.3 |
| Serotonin antagonist + butyrophenone | 5 | 4.4 |
| Serotonin antagonist + benzamide | 0 | 0 |
| Serotonin antagonist + antihistamine | 1 | 0.9 |
| Serotonin antagonist + NK-1 | 1 | 0.9 |
| Serotonin antagonist + phenothiazine | 0 | 0 |
| Placebo or no treatment | 86 | 74.78 |
| Outcomes examined: frequency d | ||
| Arrhythmia | 53 | 46.1 |
| Delirium | 34 | 29.6 |
| Mortality | 28 | 24.3 |
| QT prolongation | 18 | 15.7 |
| Setting | ||
| Not reported | 71 | 61.7 |
| Hospital | 25 | 21.7 |
| Medical center | 16 | 13.9 |
| Multi-center | 3 | 2.6 |
aIncludes unpublished data [84]; bduration is in hours unless otherwise noted; cmultiple interventions and comparators examined across the studies; dmultiple interventions and outcomes reported per study. NK-1: Neurokinin 1 receptor antagonist
The interventions examined were ondansetron (0.1−48 mg/day) (69 %), granisetron (0.1−3 mg/day) (12 %), tropisetron (0.3−5 mg/day) (13 %), dolasetron (12.5−200 mg/day) (13 %), palonosetron (0.025−0.07 mg/day) (4 %), and ramosetron (0.1−0.6 mg/day) (3 %). Some studies examined 5-HT3 receptor antagonists administered concomitantly with other antiemetics, dexamethasone (2–16 mg/day) (11 %) and droperidol (2.5 mg/day) (4 %), being the most common (Table 1, Additional file 1: Appendix 3).
Arrhythmia was the most frequently reported outcome (46 %). Only five studies reported QT prolongation, and 13 reported on the QT interval. None of the studies reported the number of patients experiencing PR prolongation or sudden cardiac death. We abstracted data from all of the included studies, and included 51 studies in our analyses. Reasons for excluding studies from the analyses included the manner in which the outcome was reported (e.g., mean versus number of patients), reporting zero events for all treatment arms, and investigating a single 5-HT3 receptor antagonist (with a different dosage in each treatment arm).
The average sample size was 242 participants ranging from 28 to 1,044, and 64% of participants were women (Table 2, Additional file 1: Appendix 4). Most of the studies involved only adult patients (63 %), patients with American Society of Anesthesiologists physical status I or II (58 %), and patients who were undergoing obstetrical and gynecological (32 %) surgery. Patients’ history of postoperative nausea and vomiting was reported in 58% of the studies, and history of motion sickness was reported in 43 % of the studies. Comorbidities were rarely reported (6 %) (Table 2).
Table 2.
Patient characteristics
| Total number of patients | 27,787 | |
|---|---|---|
| Mean sample size | 242 | |
| Mean percentage female (%) | 64 | |
| Number of studies (n = 115)a | Percentage of studies (%) | |
| Age category | ||
| Children only (aged <18 years) | 22 | 19.1 |
| Adults only (aged ≥18 years to ≤65 years) | 72 | 62.6 |
| Children and adults (aged ≤65 years) | 2 | 1.7 |
| Adults and elderly (aged ≥18 years) | 16 | 13.9 |
| All ages | 2 | 1.7 |
| Not reported | 1 | 0.9 |
| American Society of Anesthesiologists (ASA) physical status | ||
| I | 4 | 3.5 |
| I or II | 62 | 53.9 |
| I or II or III | 32 | 27.8 |
| Not reported | 17 | 14.8 |
| Surgery type | ||
| Obstetric and gynecological | 37 | 32.2 |
| Eye | 12 | 10.4 |
| Gastrointestinal | 9 | 7.8 |
| General dentistry, oral and maxillofacial surgery, and orthodontics | 5 | 4.3 |
| Orthopedic | 5 | 4.3 |
| Neurological | 3 | 2.6 |
| Otolaryngological | 2 | 1.7 |
| Breast | 1 | 0.9 |
| Cardiovascular | 1 | 0.9 |
| Urological | 1 | 0.9 |
| Miscellaneous (includes multiple surgery types, abdominal surgery, and plastic surgery unspecified) | 39 | 33.9 |
| History of motion sickness | ||
| Yes | 49 | 42.6 |
| No or not reported | 66 | 57.4 |
| History of postoperative nausea and vomiting | ||
| Yes | 67 | 58.3 |
| No or not reported | 48 | 41.7 |
| Comorbidities b | ||
| Not reported | 109 | 94.8 |
| Diabetes mellitus | 3 | 2.6 |
| Cardiovascular | 2 | 1.7 |
| Obesity | 1 | 0.9 |
| Urological | 1 | 0.9 |
| Migraines | 1 | 0.9 |
| Liver disease | 1 | 0.9 |
aIncludes unpublished data; bsome studies considered more than one comorbidity
Methodological quality and risk of bias
The majority of the included experimental and quasi-experimental studies had unclear or high risk of bias on the following items: allocation concealment (57 %), similarity of baseline outcome characteristics (88 %), incomplete outcome data (51 %), selective outcome reporting bias (97 %), and other types of bias, including the potential for funding bias because the study was funded by private industry and an author on the publication was employed by the company sponsoring the study (88 %) (Additional file 1: Appendix 5, 6). None of the 115 studies reporting harms outcomes fully reported all items on the McHarm tool (Additional file 1: Appendix 7, 8). The visual inspection of the comparison adjusted funnel plots showed that there was no evidence for small-study effects and publication bias (Additional file 1: Appendix 9).
Statistical analysis
Arrhythmia
The network meta-analysis for arrhythmia included 31 RCTs with 6,623 patients [40, 43, 45, 53, 59, 74, 78, 79, 83, 86–89, 97, 102, 108, 112–115, 117, 119, 123, 125, 128, 130, 132, 138, 141, 142, 150]. The network geometry and included drugs can be found in Fig. 2a. Twenty-one studies were excluded from the analysis because they reported zero events in all arms [39, 43, 57, 60, 61, 71, 73, 81, 82, 86, 90, 92–94, 98, 110, 121, 127, 145, 155, 156]. Although the definitions of arrhythmia varied across the studies (Additional file 1: Appendix 10), there was no evidence of network inconsistency (χ2 = 3.49, degrees of freedom = 10, P = 0.968, heterogeneity variance = 0.01), and the within-network heterogeneity variance was estimated to be 0.00. Among patients of all ages receiving granisetron plus dexamethasone, significantly more experienced arrhythmia compared with placebo (OR 2.96, 95 % CI 1.11–7.94), ondansetron (OR 3.23, 95 % CI 1.17–8.95), dolasetron (OR 4.37, 95 % CI 1.51–12.62), tropisetron (OR 3.27, 95 % CI 1.02–10.43), and ondansetron plus dexamethasone (OR 5.75, 95 % CI 1.71–19.34) (Fig. 3, Table 3, Additional file 1: Appendix 11). According to the SUCRA curves (Additional file 1: Appendix 12), the safest agents for arrhythmia were ondansetron plus dexamethasone (83 % probability) and dolasetron (82 % probability).
Fig. 2.
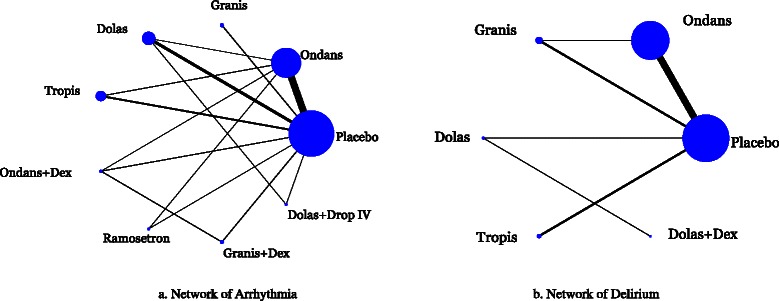
Network meta-analysis diagrams for (a) arrhythmia and (b) delirium. Nodes are proportional to the number of patients included in the corresponding treatments, and edges are weighted according to the number of studies included in the respective comparisons. Dex: Dexamethasone; Dolas: Dolasetron; Drop: Droperidol; Granis: Granisetron; Ondans: Ondansetron; Tropis: Tropisetron
Fig. 3.
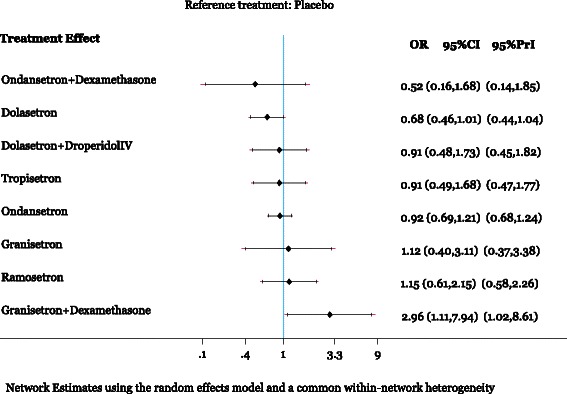
Network meta-analysis results for arrhythmia. All treatments are compared to placebo. The black horizontal lines represent the 95 % confidence intervals (CI) of the summary treatment effects and red horizontal lines the 95 % predictive intervals (PrI). Results are presented on the odds ratio (OR) scale. Among patients of all ages receiving granisetron plus dexamethasone, significantly more experienced arrhythmia compared with placebo (OR 2.96, 95 % CI 1.11–7.94), ondansetron (OR 3.23, 95 % CI 1.17–8.95), dolasetron (OR 4.37, 95 % CI 1.51–12.62), tropisetron (OR 3.27, 95 % CI 1.02–10.43), and ondansetron plus dexamethasone (OR 5.75, 95 % CI 1.71–19.34). Note: Reference treatment is placebo. CI: Confidence interval; OR: Odds ratio; PrI: Predictive interval
Table 3.
Statistically significant results of network meta-analysis for all time periods of drug administration
| All ages | Children only | |||||
|---|---|---|---|---|---|---|
| Treatment comparison | Number of studies | MA estimate: OR (95 % CI) | NMA estimate: OR (95 % CI) | Number of studies | MA estimate: OR (95 % CI) | NMA estimate: OR (95 % CI) |
| Arrhythmia | 31 RCTs and 6,623 patients | 9 RCTs and 1,572 patients | ||||
| Granisetron + DEX vs placebo | 2 | 2.63 (0.75– 9.29) | 2.96 (1.11–7.94) | 1 | 4.89 (1.15–20.79) | 5.15 (1.33–19.91) |
| Granisetron + DEX vs ondansetron | NA | NA | 3.23 (1.17–8.95) | NA | NA | 4.71 (1.08–20.46) |
| Granisetron + DEX vs dolasetron | NA | NA | 4.37 (1.51–12.62) | NA | NA | NA |
| Granisetron + DEX vs tropisetron | NA | NA | 3.27 (1.02–10.43) | NA | NA | NA |
| Granisetron + DEX vs ondansetron + DEX | 2 | 8.10 (1.92–34.13) | 5.75 (1.71–19.34) | 1 | 7.67 (1.47–40.00) | 7.12 (1.66–30.63) |
CI: Confidence interval; DEX: Dexamethasone; MA: Meta-analysis; NA: Not applicable; NMA: Network meta-analysis; OR: Odds ratio
A subgroup analysis was conducted for 26 RCTs involving 4,878 patients in which the agents were administered during surgery [40, 43, 45, 53, 59, 74, 78, 79, 83, 86–89, 97, 102, 112, 113, 115, 117, 119, 123, 125, 132, 138, 142, 150]. The results were the same as for the primary analysis, except that significantly fewer patients of all ages receiving dolasetron experienced arrhythmia compared with placebo (OR 0.58, 95 % CI 0.36–0.93) and ramosetron (OR 0.38, 95 % CI 0.17–0.92) (Additional file 1: Appendix 13). According to the SUCRA curves for this subgroup analysis, the safest agents were dolasetron (86 % probability) and ondansetron plus dexamethasone (83 %).
Another subgroup analysis was conducted for nine RCTs involving a total of 1,572 patients to examine the intra-operative administration of ondansetron, ondansetron plus dexamethasone, and granisetron plus dexamethasone to children (Table 3, Additional file 1: Appendix 13) [53, 79, 86, 89, 97, 113, 117, 123, 138]. Significantly more children receiving granisetron plus dexamethasone during surgery experienced arrhythmia compared with placebo (OR 5.15, 95 % CI 1.33–19.91), ondansetron (OR 4.71, 95 % CI 1.08–20.46), and ondansetron plus dexamethasone (OR 7.12, 95 % CI 1.66–30.63). According to the SUCRA curves, the safest agent in terms of arrhythmia was ondansetron plus dexamethasone (80 % probability). Finally, a sensitivity analysis was conducted in which one RCT was removed because of high risk of incomplete outcome data [128], and the same results were observed (Additional file 1: Appendix 13).
Delirium
The network meta-analysis for delirium included 18 studies involving 3,652 patients in which ondansetron, granisetron, dolasetron, tropisetron, and dolasetron plus dexamethasone were administered during surgery [52, 60, 68, 69, 76, 79, 96, 100, 105, 106, 118, 124, 128, 133, 137, 139, 144, 146]. The network geometry and included drugs can be found in Fig. 2b. Ten studies were excluded from the analysis because they reported zero events in all arms [49, 69, 75, 90, 99, 103, 129, 135, 140, 143]. No statistically significant results were observed and the within-network heterogeneity variance in the network meta-analysis model was estimated to be 0.00 (Additional file 1: Appendix 14). Although the definitions of delirium varied across the studies (Additional file 1: Appendix 15, 16), there was no evidence of network inconsistency (χ2 = 0.32, degrees of freedom = 2, P = 0.851, heterogeneity variance = 0.00).
Mortality
A meta-analysis was conducted for three studies including 1,255 patients that reported mortality for comparisons of ondansetron with placebo [10, 111, 142]. No statistically significant effects were observed (OR 1.92, 95 % CI 0.30–12.21). Twenty-five studies were excluded from this analysis because they reported zero events in both arms [38, 41, 44, 55, 56, 58, 62, 67, 70, 72, 77, 78, 80, 107, 109, 115, 120, 126, 128, 130, 131, 134, 149, 157, 158].
QT prolongation
Two RCTs reported the number of patients experiencing QT prolongation [55, 116]. In one of these studies, there was no statistically significant difference between ondansetron and placebo (OR 0.75, 95 % CI 0.47–1.20) [55], and in the other there was no statistically significant difference between granisetron and placebo (OR 0.32, 95 % CI 0.01–8.02) [116]. Three studies did not inform the analysis and were excluded, as they reported zero events in both arms [58, 115, 159].
Discussion
More patients receiving granisetron plus dexamethasone experienced arrhythmia compared to other agents. The safest 5-HT3 receptor antagonists with respect to arrhythmia were ondansetron plus dexamethasone and dolasetron for patients of all ages and ondansetron plus dexamethasone for children (none of the included studies examined dolasetron in children). These results were consistent across subgroup and sensitivity analyses. None of the agents caused significantly more patients to experience delirium. Few studies reported QT prolongation, and no statistically significant results for this outcome were reported in the two studies reporting at least one event. As well, no statistically significant differences in mortality were observed between ondansetron and placebo in a meta-analysis of three studies that reported this outcome. None of the studies included in this analysis reported the number of patients experiencing PR prolongation or sudden cardiac death.
Our finding of no increased risk of cardiac arrhythmia in association with ondansetron therapy supports the results of a previous systematic review [160]. Although we are aware of other systematic reviews and meta-analyses of 5-HT3 receptor antagonists [9, 161], the previous researchers did not conduct network meta-analysis, so we cannot compare our results with theirs. Notably, because of our comprehensive literature search and broad eligibility criteria, we included 62 studies involving a total of 14,705 patients that were not included in any of the previous reviews (Additional file 1: Appendix 17).
We found no increased risk of arrhythmia with dolasetron for patients of any age. This does not mean that a cardiac risk does not exist; we found no studies examining other cardiac harms, such as PR prolongation and sudden cardiac death. We identified no studies examining dolasetron administered to children. We found other data gaps through the conduct of this review. In particular, most of the studies focused on effectiveness outcomes, and relatively few reported harms. Our network meta-analysis results for the effectiveness outcomes have been reported in another publication [8].
The studies included in our analysis had some methodological limitations. Most of the studies were small (average sample size 242 patients) and larger sample sizes are required to assess harms, in particular harms that occur only rarely, such as arrhythmia and delirium. Indeed, the need for larger sample sizes is the reason we included non-randomized studies in our review. Although these non-randomized studies involved more patients than the RCTs, their inclusion did not change the network meta-analysis results obtained for arrhythmia or delirium. As well, many of the studies failed to report baseline characteristics or all items assessed by the McHarm tool, and many of the included trials had an unclear or high risk of bias on important items for the conduct of trials, including allocation concealment, selective outcome reporting bias, and potential for funding bias.
Our systematic review process also had some limitations. Slight changes to our original protocol [7] were necessary, because of the enormous number of studies that met our inclusion criteria. For example, we were unable to report data on patients undergoing chemotherapy in this paper (but these will be disseminated in an upcoming paper), we did not include studies written in languages other than English, and we focused inclusion to unpublished conference abstracts from the past 10 years that included relevant data. However, we were able to include unpublished data from one study [84], and our funnel plots showed no evidence of small-study effects or publication bias. Furthermore, we assumed that the effects of the different doses and durations were identical across the treatments, and that they defined the same node they belong to. We are currently exploring these assumptions in another paper [162]. Finally, we had to exclude 77 studies because they contained data known or suspected to be fraudulent, as identified by editors and authors in the field and presented in a paper [9]; we did not conduct a sensitivity analysis including these articles to examine the effect of excluding these studies on our results.
Conclusion
We conclude that most 5-HT3 receptor antagonists that do not cause delirium. Granisetron plus dexamethasone increased the risk of cardiac harm (arrhythmia), with the number needed to harm ranging from five to eight. We are unable to comment on the relationship between 5-HT3 receptor antagonists and other cardiac harms, such as for PR prolongation and sudden cardiac death, as no studies reported these important outcomes.
Acknowledgements
This systematic review was funded by the Canadian Institutes for Health Research/Drug Safety and Effectiveness Network (CIHR/DSEN). ACT and BH are funded by a CIHR/DSEN New Investigator Award in Knowledge Synthesis. BRH receives funding from the Alberta Heritage Foundation for Medical Research. AAV is funded by the Banting Postdoctoral Fellowship Program from the CIHR. DM is funded by a University of Ottawa Research Chair. SES is funded by a Tier 1 Canada Research Chair in Knowledge Translation. We thank Laure Perrier for conducting the literature searches, Becky Skidmore for peer-reviewing the literature search, and Drs Maggie Chen and Joseph Beyene for providing feedback on conceptualization of the review. We also thank Vladi Struchkov, Vera Nincic, Roberta Cardoso, Christy Johnson, and Derek Roberts for screening some of the citations, and/or abstracting some of the data, and/or appraising quality for a few of the included studies. Finally, we thank Wing Hui for abstracting some of the data, Ana Guzman and Inthuja Selvaratnam for formatting the manuscript, and Peggy Robinson for copyediting the manuscript.
Abbreviations
- 5-HT3
Serotonin
- CI
Confidence interval
- IV
Intravenous
- NK-1
Neurokinin 1 receptor antagonist
- NMA
Network meta-analysis
- OR
Odds ratio
- PrI
Predictive interval
- RCT
Randomized control trial
- REML
Restricted maximum likelihood
- SUCRA
Surface under the cumulative ranking
Additional file
Appendix 1–17. Includes 17 appendices with supplementary data.
Footnotes
Competing interests
RR owns stock in a company (GlaxoSmithKline Inc) that manufactures one of the interventions included in our study. All other authors declare that they have no competing interests.
Authors’ contributions
ACT conceived the study, designed the study, helped obtain funding for the study, guided the analysis, interpreted the results, and drafted the manuscript. CS coordinated the review, screened citations and full-text articles, abstracted data, appraised quality, cleaned the data, and edited the manuscript. EB, LS, and HA screened citations and full-text articles, abstracted data, appraised quality, cleaned the data, and edited the manuscript. AAV analyzed the data, interpreted the data, wrote the synthesis section, and edited the manuscript. PAK, AV, JI, HM, ER, RR, JH, CN, and KM screened citations and full-text articles, appraised quality, and edited the manuscript. JA provided methodological support and screened citations and full-text articles. BH, BRH, and DM helped conceive the study and edited the manuscript. SES conceived and designed the study, obtained the funding, interpreted the results, and edited the manuscript. All authors read and approved the final paper and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Contributor Information
Andrea C Tricco, Email: TriccoA@smh.ca.
Charlene Soobiah, Email: SoobiahC@smh.ca.
Erik Blondal, Email: BlondalE@smh.ca.
Areti A Veroniki, Email: VeronikiA@smh.ca.
Paul A Khan, Email: pakahn@hotmail.com.
Afshin Vafaei, Email: VafaeiA@smh.ca.
John Ivory, Email: john.d.ivory@gmail.com.
Lisa Strifler, Email: StriflerL@smh.ca.
Huda Ashoor, Email: AshoorH@smh.ca.
Heather MacDonald, Email: hrmacdonald@gmail.com.
Emily Reynen, Email: ereynen@gmail.com.
Reid Robson, Email: reidcrobson@gmail.com.
Joanne Ho, Email: jmho2001@gmail.com.
Carmen Ng, Email: carmen_hm_ng@yahoo.ca.
Jesmin Antony, Email: AntonyJ@smh.ca.
Kelly Mrklas, Email: kmrklas@gmail.com.
Brian Hutton, Email: bhutton@ohri.ca.
Brenda R Hemmelgarn, Email: Brenda.Hemmelgarn@albertahealthservices.ca.
David Moher, Email: dmoher@ohri.ca.
Sharon E Straus, Email: sharon.straus@utoronto.ca.
References
- 1.Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71. doi: 10.1213/01.ANE.0000068580.00245.95. [DOI] [PubMed] [Google Scholar]
- 2.McCracken G, Houston P, Lefebvre G. Guideline for the management of postoperative nausea and vomiting. J Obstet Gynaecol Can. 2008;30:600–7. doi: 10.1016/S1701-2163(16)32895-X. [DOI] [PubMed] [Google Scholar]
- 3.Constenla M. 5-HT3 receptor antagonists for prevention of late acute-onset emesis. Ann Pharmacother. 2004;38:1683–91. doi: 10.1345/aph.1D191. [DOI] [PubMed] [Google Scholar]
- 4.Buyukavci M, Olgun H, Ceviz N. The effects of ondansetron and granisetron on electrocardiography in children receiving chemotherapy for acute leukemia. Am J Clin Oncol. 2005;28:201–4. doi: 10.1097/01.coc.0000144849.41300.0a. [DOI] [PubMed] [Google Scholar]
- 5.Pinarli FG, Elli M, Dagdemir A, Baysal K, Acar S. Electrocardiographic findings after 5-HT3 receptor antagonists and chemotherapy in children with cancer. Pediatr Blood Cancer. 2006;47:567–71. doi: 10.1002/pbc.20639. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon ML. Moving knowledge to action through dissemination and exchange. J Clin Epidemiol. 2011;64:25–31. doi: 10.1016/j.jclinepi.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Tricco AC, Soobiah C, Antony J, Hemmelgarn B, Moher D, Hutton B, et al. Safety of serotonin (5-HT3) receptor antagonists in patients undergoing surgery and chemotherapy: protocol for a systematic review and network meta-analysis. Syst Rev. 2013;2:46. doi: 10.1186/2046-4053-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tricco AC, Soobiah C, Blondal E, Veroniki AA, Khan PA, Vafaei A et al. Comparative efficacy of serotonin (5-HT3) receptor antagonists in patients undergoing surgery: a systematic review and network meta-analysis. BMC Med. 2015; Ahead of print. [DOI] [PMC free article] [PubMed]
- 9.Carlisle JB. The analysis of 168 randomised controlled trials to test data integrity. Anaesthesia. 2012;67:521–37. doi: 10.1111/j.1365-2044.2012.07128.x. [DOI] [PubMed] [Google Scholar]
- 10.Gan TJ, Gu J, Singla N, Chung F, Pearman MH, Bergese SD, et al. Rolapitant for the prevention of postoperative nausea and vomiting: a prospective, double-blinded, placebo-controlled randomized trial. Anesth Analg. 2011;112:804–12. doi: 10.1213/ANE.0b013e31820886c3. [DOI] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: University of Ottawa; 2014. [Google Scholar]
- 12.Glacy J, Putnam K, Godfrey S, Falzon L, Mauger B, Samson D, et al. Treatments for seasonal allergic rhinitis [Internet]. Rockville: Agency for Healthcare Research and Quality; 2013. (Comparative Effectiveness Reviews, No. 120.) Appendix F, McMaster Quality Assessment Scale of Harms (McHarm). http://www.ncbi.nlm.nih.gov/books/NBK153703/. Accessed Jan 2015.
- 13.Raudenbush SW. Analyzing effect sizes: Random-effects models. In: Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2. New York: The Russell Sage Foundation; 2009. pp. 295–316. [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 16.R Core Team. R: A language and environment for statistical computing. http://www.R-project.org/. Accessed: Jan 2015.
- 17.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Meth. 2012;3:80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 18.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White IR, Barrett JK, Jackson D, Higgins J. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Res Synth Meth. 2012;3:111–25. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42:332–45. doi: 10.1093/ije/dys222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–71. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Chen CC, Lin CS, Ko YP, Hung YC, Lao HC, Hsu YW. Premedication with mirtazapine reduces preoperative anxiety and postoperative nausea and vomiting. Anesth Analg. 2008;106:109–13. doi: 10.1213/01.ane.0000289636.09841.bc. [DOI] [PubMed] [Google Scholar]
- 24.Omran HASA, Nasr DAM. Effect of premedication with mirtazapine versus ondansetron on postoperative nausea and vomiting in breast surgery. Egypt J Anaesth. 2011;27:135–9. doi: 10.1016/j.egja.2011.06.003. [DOI] [Google Scholar]
- 25.Homesley HD, Hahne WF, McLees B, Heck K, Barrett RJ, Lentz SS, et al. Randomized comparison of the antiemetic efficacy of a serotonin type 3 receptor antagonist (MDL 72,222) with a high-dose metoclopramide regimen. Am J Clin Oncol. 1993;16:175–9. doi: 10.1097/00000421-199304000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Gan TJ, Meyer TA, Apfel CC, Chung F, Davis PJ, Habib AS, et al. Society for Ambulatory Anesthesia guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2007;105:1615–28. doi: 10.1213/01.ane.0000295230.55439.f4. [DOI] [PubMed] [Google Scholar]
- 27.Golembiewski J, Chernin E, Chopra T. Prevention and treatment of postoperative nausea and vomiting. Am J Health Syst Pharm. 2005;62:1247–60. doi: 10.2146/ajhp050374. [DOI] [PubMed] [Google Scholar]
- 28.Kovac AL. Prevention and treatment of postoperative nausea and vomiting. Drugs. 2000;59:213–43. doi: 10.2165/00003495-200059020-00005. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm SM, Dehoorne-Smith ML, Kale-Pradhan PB. Prevention of postoperative nausea and vomiting. Ann Pharmacother. 2007;41(1):68–78. doi: 10.1345/aph.1H398. [DOI] [PubMed] [Google Scholar]
- 30.Yun MJ, Kim YH, Kim AR. Comparison of azasetron and ondansetron for preventing postoperative nausea and vomiting in patients undergoing gynecological laparoscopic surgery. Yonsei Med J. 2010;51:88–92. doi: 10.3349/ymj.2010.51.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunter JB, McAuliffe JJ, Beckman EC, Wittkugel EP, Spaeth JP, Varughese AM. A factorial study of ondansetron, metoclopramide, and dexamethasone for emesis prophylaxis after adenotonsillectomy in children. Paediatr Anaesth. 2006;16:1153–65. doi: 10.1111/j.1460-9592.2006.01952.x. [DOI] [PubMed] [Google Scholar]
- 32.Gupta P, Khanna J, Mitramustafi AK, Bhartia VK. Role of pre-operative dexamethasone as prophylaxis for postoperative nausea and vomiting in laparoscopic surgery. J Minim Access Surg. 2006;2:12–5. doi: 10.4103/0972-9941.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta SD, Pal R, Sarkar A, Mukherjee S, Mitra K, Roy S, et al. Evaluation of Ondansetron-induced QT interval prolongation in the prophylaxis of postoperative emesis. J Nat Sci Biol Med. 2011;2:119–24. doi: 10.4103/0976-9668.82313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:964–7. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White IR. Multivariate random-effects meta-regression: updates to mvmeta. Stata J. 2011;11:255–70. [Google Scholar]
- 37.StataCorp. Stata Statistical Software: Release 13. College Station: StataCorp LP; 2013.
- 38.Choi YS, Shim JK, Ahn SH, Kwak YL. Efficacy comparison of ramosetron with ondansetron on preventing nausea and vomiting in high-risk patients following spine surgery with a single bolus of dexamethasone as an adjunct. Korean J Anesthesiol. 2012;62:543–7. doi: 10.4097/kjae.2012.62.6.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang PK, Tsay PJ, Huang CC, Lai HY, Lin PC, Huang SJ, et al. Comparison of dexamethasone with ondansetron or haloperidol for prevention of patient-controlled analgesia-related postoperative nausea and vomiting: a randomized clinical trial. World J Surg. 2012;36:775–81. doi: 10.1007/s00268-012-1446-y. [DOI] [PubMed] [Google Scholar]
- 40.Ekinci O, Malat I, Isitmangil G, Aydin N. A randomized comparison of droperidol, metoclopramide, tropisetron, and ondansetron for the prevention of postoperative nausea and vomiting. Gynecol Obstet Invest. 2011;71:59–65. doi: 10.1159/000320747. [DOI] [PubMed] [Google Scholar]
- 41.Park SK, Cho EJ. A randomized, double-blind trial of palonosetron compared with ondansetron in preventing postoperative nausea and vomiting after gynaecological laparoscopic surgery. J Int Med Res. 2011;39:399–407. doi: 10.1177/147323001103900207. [DOI] [PubMed] [Google Scholar]
- 42.Ryu JH, Jeon YT, Hwang JW, Oh AY, Moon JY, Ro YJ, et al. Intravenous, oral, and the combination of intravenous and oral ramosetron for the prevention of nausea and vomiting after laparoscopic cholecystectomy: a randomized, double-blind, controlled trial. Clin Ther. 2011;33:1162–72. doi: 10.1016/j.clinthera.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 43.Sahoo T, SenDasgupta C, Goswami A, Hazra A. Reduction in spinal-induced hypotension with ondansetron in parturients undergoing caesarean section: a double-blind randomised, placebo-controlled study. Int J Obstet Anesth. 2012;21:24–8. doi: 10.1016/j.ijoa.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Bilgin TE, Birbicer H, Ozer Z, Doruk N, Tok E, Oral U. A comparative study of the antiemetic efficacy of dexamethasone, ondansetron, and metoclopramide in patients undergoing gynecological surgery. Med Sci Monit. 2010;16:CR336–341. [PubMed] [Google Scholar]
- 45.Choi DK, Chin JH, Lee EH, Lim OB, Chung CH, Ro YJ, et al. Prophylactic control of post-operative nausea and vomiting using ondansetron and ramosetron after cardiac surgery. Acta Anaesthesiol Scand. 2010;54:962–9. doi: 10.1111/j.1399-6576.2010.02275.x. [DOI] [PubMed] [Google Scholar]
- 46.Jee YS, Yoon HJ, Jang CH. Prophylactic antiemetic effects in gynecologic patients receiving fentanyl IV-patient controlled analgesia: comparison of combined treatment with ondansetron and dexamethasone with metoclopramide and dexamethasone. Korean J Anesthesiol. 2010;59:335–9. doi: 10.4097/kjae.2010.59.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jokela R, Ahonen J, Seitsonen E, Marjakangas P, Korttila K. The influence of ondansetron on the analgesic effect of acetaminophen after laparoscopic hysterectomy. Clin Pharmacol Ther. 2010;87:672–8. doi: 10.1038/clpt.2009.281. [DOI] [PubMed] [Google Scholar]
- 48.Mehta D, Sanatani S, Whyte SD. The effects of droperidol and ondansetron on dispersion of myocardial repolarization in children. Paediatr Anaesth. 2010;20:905–12. doi: 10.1111/j.1460-9592.2010.03408.x. [DOI] [PubMed] [Google Scholar]
- 49.Shakya S, Chaturvedi A, Sah BP. Prophylactic low dose ketamine and ondansetron for prevention of shivering during spinal anaesthesia. J Anaesthesiol Clin Pharmacol. 2010;26:465–9. [PMC free article] [PubMed] [Google Scholar]
- 50.Singla NK, Singla SK, Chung F, Kutsogiannis DJ, Blackburn L, Lane SR, et al. Phase II study to evaluate the safety and efficacy of the oral neurokinin-1 receptor antagonist casopitant (GW679769) administered with ondansetron for the prevention of postoperative and postdischarge nausea and vomiting in high-risk patients. Anesthesiology. 2010;113:74–82. doi: 10.1097/ALN.0b013e3181d7b13a. [DOI] [PubMed] [Google Scholar]
- 51.Feng PH, Chu KS, Lu IC, Shieh JP, Tzeng JI, Ho ST, et al. Haloperidol plus ondansetron prevents postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiol Taiwan. 2009;47:3–9. doi: 10.1016/S1875-4597(09)60013-8. [DOI] [PubMed] [Google Scholar]
- 52.Jain V, Mitra JK, Rath GP, Prabhakar H, Bithal PK, Dash HH. A randomized, double-blinded comparison of ondansetron, granisetron, and placebo for prevention of postoperative nausea and vomiting after supratentorial craniotomy. J Neurosurg Anesthesiol. 2009;21:226–30. doi: 10.1097/ANA.0b013e3181a7beaa. [DOI] [PubMed] [Google Scholar]
- 53.Riad W, Marouf H. Combination therapy in the prevention of PONV after strabismus surgery in children: granisetron, ondansetron, midazolam with dexamethasone. Middle East J Anesthesiol. 2009;20:431–6. [PubMed] [Google Scholar]
- 54.Rosow CE, Haspel KL, Smith SE, Grecu L, Bittner EA. Haloperidol versus ondansetron for prophylaxis of postoperative nausea and vomiting. Anesth Analg. 2008;106:1407–9. doi: 10.1213/ane.0b013e3181609022. [DOI] [PubMed] [Google Scholar]
- 55.Candiotti KA, Kovac AL, Melson TI, Clerici G, Joo Gan T. Palonosetron 04–06 Study G. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo for preventing postoperative nausea and vomiting. Anesth Analg. 2008;107:445–51. doi: 10.1213/ane.0b013e31817b5ebb. [DOI] [PubMed] [Google Scholar]
- 56.Choi YS, Shim JK, Yoon do H, Jeon DH, Lee JY, Kwak YL. Effect of ramosetron on patient-controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. Spine (Phila Pa 1976) 2008;33:E602–6. doi: 10.1097/BRS.0b013e31817c6bde. [DOI] [PubMed] [Google Scholar]
- 57.Contreras-Dominguez V, Carbonell-Bellolio P. Prophylactic antiemetic therapy for acute abdominal surgery. A comparative study of droperidol, metoclopramide, tropisetron, granisetron and dexamethasone. Rev Bras Anestesiol. 2008;58:35–44. doi: 10.1590/S0034-70942008000100005. [DOI] [PubMed] [Google Scholar]
- 58.Kovac AL, Eberhart L, Kotarski J, Clerici G, Apfel C. Palonosetron 04–07 Study G. A randomized, double-blind study to evaluate the efficacy and safety of three different doses of palonosetron versus placebo in preventing postoperative nausea and vomiting over a 72-hour period. Anesth Analg. 2008;107:439–44. doi: 10.1213/ane.0b013e31817abcd3. [DOI] [PubMed] [Google Scholar]
- 59.Owczuk R, Wenski W, Polak-Krzeminska A, Twardowski P, Arszulowicz R, Dylczyk-Sommer A, et al. Ondansetron given intravenously attenuates arterial blood pressure drop due to spinal anesthesia: a double-blind, placebo-controlled study. Reg Anesth Pain Med. 2008;33:332–9. doi: 10.1016/j.rapm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 60.Piper SN, Rohm K, Boldt J, Kranke P, Maleck W, Seifert R, et al. Postoperative nausea and vomiting after surgery for prognathism: not only a question of patients’ comfort. A placebo-controlled comparison of dolasetron and droperidol. J Craniomaxillofac Surg. 2008;36:173–9. doi: 10.1016/j.jcms.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Said-Ahmed HAF. Does granisetron affect the analgesic efficacy of tramadol in patients undergoing abdominal hysterectomy? Acta Anaesthesiologica Italica. 2008;59:256–66. [Google Scholar]
- 62.Bestas A, Önal SA, Bayar MK, Yildirim A, Aygen E. Effects of ondansetron and granisetron on postoperative nausea and vomiting in adult patients undergoing laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled clinical trial. Curr Ther Res Clin Exp. 2007;68:303–12. doi: 10.1016/j.curtheres.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diemunsch P, Gan TJ, Philip BK, Girao MJ, Eberhart L, Irwin MG, et al. Single-dose aprepitant vs ondansetron for the prevention of postoperative nausea and vomiting: a randomized, double-blind phase III trial in patients undergoing open abdominal surgery. Br J Anaesth. 2007;99:202–11. doi: 10.1093/bja/aem133. [DOI] [PubMed] [Google Scholar]
- 64.Gan TJ, Apfel CC, Kovac A, Philip BK, Singla N, Minkowitz H, et al. A randomized, double-blind comparison of the NK1 antagonist, aprepitant, versus ondansetron for the prevention of postoperative nausea and vomiting. Anesth Analg. 2007;104:1082–9. doi: 10.1213/01.ane.0000263277.35140.a3. [DOI] [PubMed] [Google Scholar]
- 65.Han DW, Hong SW, Kwon JY, Lee JW, Kim KJ. Epidural ondansetron is more effective to prevent postoperative pruritus and nausea than intravenous ondansetron in elective cesarean delivery. Acta Obstet Gynecol Scand. 2007;86:683–7. doi: 10.1080/00016340701302616. [DOI] [PubMed] [Google Scholar]
- 66.Lee Y, Wang PK, Lai HY, Yang YL, Chu CC, Wang JJ. Haloperidol is as effective as ondansetron for preventing postoperative nausea and vomiting. Can J Anaesth. 2007;54:349–54. doi: 10.1007/BF03022656. [DOI] [PubMed] [Google Scholar]
- 67.Oksuz H, Zencirci B, Ezberci M. Comparison of the effectiveness of metoclopramide, ondansetron, and granisetron on the prevention of nausea and vomiting after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2007;17:803–8. doi: 10.1089/lap.2006.0243. [DOI] [PubMed] [Google Scholar]
- 68.Rusch D, Arndt C, Martin H, Kranke P. The addition of dexamethasone to dolasetron or haloperidol for treatment of established postoperative nausea and vomiting. Anaesthesia. 2007;62:810–7. doi: 10.1111/j.1365-2044.2007.05136.x. [DOI] [PubMed] [Google Scholar]
- 69.Sagir O, Gulhas N, Toprak H, Yucel A, Begec Z, Ersoy O. Control of shivering during regional anaesthesia: prophylactic ketamine and granisetron. Acta Anaesthesiol Scand. 2007;51:44–9. doi: 10.1111/j.1399-6576.2006.01196.x. [DOI] [PubMed] [Google Scholar]
- 70.Sandhu T, Tanvatcharaphan P, Cheunjongkolkul V. Ondansetron versus metoclopramide in prophylaxis of nausea and vomiting for laparoscopic cholecystectomy: a prospective double-blind randomized study. Asian J Surg. 2008;31:50–4. doi: 10.1016/S1015-9584(08)60057-3. [DOI] [PubMed] [Google Scholar]
- 71.Siddik-Sayyid SM, Aouad MT, Taha SK, Azar MS, Hakki MA, Kaddoum RN, et al. Does ondansetron or granisetron prevent subarachnoid morphine-induced pruritus after cesarean delivery? Anesth Analg. 2007;104:421–4. doi: 10.1213/01.ane.0000253668.10453.de. [DOI] [PubMed] [Google Scholar]
- 72.Bridges JD, Nettle CB, Dugirrala VJ, Suda KJ, Garey KW. Low-dose granisetron for the prevention of postoperative nausea and vomiting. J Appl Res. 2006;6:223–9. [Google Scholar]
- 73.Chan MT, Choi KC, Gin T, Chui PT, Short TG, Yuen PM, et al. The additive interactions between ondansetron and droperidol for preventing postoperative nausea and vomiting. Anesth Analg. 2006;103:1155–62. doi: 10.1213/01.ane.0000239223.74552.0a. [DOI] [PubMed] [Google Scholar]
- 74.Kelsaka E, Baris S, Karakaya D, Sarihasan B. Comparison of ondansetron and meperidine for prevention of shivering in patients undergoing spinal anesthesia. Reg Anesth Pain Med. 2006;31:40–5. doi: 10.1097/00115550-200601000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Sarvela PJ, Halonen PM, Soikkeli AI, Kainu JP, Korttila KT. Ondansetron and tropisetron do not prevent intraspinal morphine- and fentanyl-induced pruritus in elective cesarean delivery. Acta Anaesthesiol Scand. 2006;50:239–44. doi: 10.1111/j.1399-6576.2006.00934.x. [DOI] [PubMed] [Google Scholar]
- 76.Tosun Z, Akin A, Dogan H, Boyaci A. A randomized, placebo-controlled trial of a single dose of tropisetron for the prevention of vomiting after strabismus surgery in children. Mt Sinai J Med. 2006;73:1106–11. [PubMed] [Google Scholar]
- 77.D’Angelo R, Philip B, Gan TJ, Kovac A, Hantler C, Doblar D, et al. A randomized, double-blind, close-ranging, pilot study of intravenous granisetron in the prevention of postoperative nausea and vomiting in patients abdominal hysterectomy. Eur J Anaesthesiol. 2005;22:774–9. doi: 10.1017/S0265021505001286. [DOI] [PubMed] [Google Scholar]
- 78.Gan TJ, Coop A, Philip BK. A randomized, double-blind study of granisetron plus dexamethasone versus ondansetron plus dexamethasone to prevent postoperative nausea and vomiting in patients undergoing abdominal hysterectomy. Anesth Analg. 2005;101:1323–9. doi: 10.1213/01.ANE.0000180366.65267.F6. [DOI] [PubMed] [Google Scholar]
- 79.Khalil SN, Roth AG, Cohen IT, Simhi E, Ansermino JM, Bolos ME, et al. A double-blind comparison of intravenous ondansetron and placebo for preventing postoperative emesis in 1- to 24-month-old pediatric patients after surgery under general anesthesia. Anesth Analg. 2005;101:356–61. doi: 10.1213/01.ANE.0000155261.27335.29. [DOI] [PubMed] [Google Scholar]
- 80.Kocamanoglu IS, Baris S, Karakaya D, Sener B, Tur A, Cetinkaya M. Effects of granisetron with droperidol or dexamethasone on prevention of postoperative nausea and vomiting after general anesthesia for cesarean section. Methods Find Exp Clin Pharmacol. 2005;27:489–93. doi: 10.1358/mf.2005.27.7.920930. [DOI] [PubMed] [Google Scholar]
- 81.Kontrimaviciute E, Baublys A, Ivaskevicius J. Postoperative nausea and vomiting in patients undergoing total abdominal hysterectomy under spinal anaesthesia: a randomized study of ondansetron prophylaxis. Eur J Anaesthesiol. 2005;22:504–9. doi: 10.1017/S0265021505000864. [DOI] [PubMed] [Google Scholar]
- 82.Pirat A, Tuncay SF, Torgay A, Candan S, Arslan G. Ondansetron, orally disintegrating tablets versus intravenous injection for prevention of intrathecal morphine-induced nausea, vomiting, and pruritus in young males. Anesth Analg. 2005;101:1330–6. doi: 10.1213/01.ANE.0000180830.12355.D9. [DOI] [PubMed] [Google Scholar]
- 83.Treschan TA, Zimmer C, Nass C, Stegen B, Esser J, Peters J. Inspired oxygen fraction of 0.8 does not attenuate postoperative nausea and vomiting after strabismus surgery. Anesthesiology. 2005;103:6–10. doi: 10.1097/00000542-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 84.White PF, Scuderi PE. Prevention of postoperative nausea and vomiting (PONV): A dose-ranging study involving palonosetron, a potent 5-HT3 receptor antagonist. Anesthesiology. 2005;103:A703. [Google Scholar]
- 85.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–51. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Binstock W, Rubin R, Bachman C, Kahana M, McDade W, Lynch JP. The effect of premedication with OTFC, with or without ondansetron, on postoperative agitation, and nausea and vomiting in pediatric ambulatory patients. Paediatr Anaesth. 2004;14:759–67. doi: 10.1111/j.1460-9592.2004.01296.x. [DOI] [PubMed] [Google Scholar]
- 87.Eberhart LH, Morin AM, Hoerle S, Wulf H, Geldner G. Droperidol and dolasetron alone or in combination for prevention of postoperative nausea and vomiting after vitrectomy. Ophthalmology. 2004;111:1569–75. doi: 10.1016/j.ophtha.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 88.Hanaoka K, Toyooka H, Kugimiya T, Ohashi Y. Granisetron Study Group of Japan. Efficacy of prophylactic intravenous granisetron in postoperative emesis in adults. J Anesth. 2004;18:158–65. doi: 10.1007/s00540-004-0236-6. [DOI] [PubMed] [Google Scholar]
- 89.Samarkandi AH, Riad W, Altaf R, Fatani R. Dexamethasone-ondansetron combination in prevention of nausea and vomiting after strabismus surgery in children. Egypt J Anaesth. 2004;20:399–403. [Google Scholar]
- 90.Charuluxananan S, Kyokong O, Somboonviboon W, Narasethakamol A, Promlok P. Nalbuphine versus ondansetron for prevention of intrathecal morphine-induced pruritus after cesarean delivery. Anesth Analg. 2003;96:1789–93. doi: 10.1213/01.ANE.0000066015.21364.7D. [DOI] [PubMed] [Google Scholar]
- 91.Loewen P, Lamb S, Clugston P. Randomized, double-blind trial of dolasetron versus droperidol for prophylaxis of postoperative nausea and vomiting in patients undergoing TRAM flap breast reconstruction surgery. Ann Plast Surg. 2003;51:472–7. doi: 10.1097/01.SAP.0000070650.60249.2F. [DOI] [PubMed] [Google Scholar]
- 92.O’Brien CM, Titley G, Whitehurst P. A comparison of cyclizine, ondansetron and placebo as prophylaxis against postoperative nausea and vomiting in children. Anaesthesia. 2003;58:707–11. doi: 10.1046/j.1365-2044.2003.03189_6.x. [DOI] [PubMed] [Google Scholar]
- 93.Argiriadou H, Papaziogas B, Pavlidis T, Parlapani A, Georgiou M, Papagiannopoulou P, et al. Tropisetron vs ondansetron for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy: a randomized double-blind, placebo-controlled study. Surg Endosc. 2002;16:1087–90. doi: 10.1007/s00464-001-9191-6. [DOI] [PubMed] [Google Scholar]
- 94.Gurkan Y, Toker K. Prophylactic ondansetron reduces the incidence of intrathecal fentanyl-induced pruritus. Anesth Analg. 2002;95:1763–6. doi: 10.1097/00000539-200212000-00054. [DOI] [PubMed] [Google Scholar]
- 95.Dabbous A, Khoury SJ, Chehab IR, Bartelmaos T, Khoury G. Ondansetron versus dehydrobenzoperidol and metoclopramide for management of postoperative nausea in laparoscopic surgery patients. JSLS. 2001;5:139–42. [PMC free article] [PubMed] [Google Scholar]
- 96.Kathirvel S, Dash HH, Bhatia A, Subramaniam B, Prakash A, Shenoy S. Effect of prophylactic ondansetron on postoperative nausea and vomiting after elective craniotomy. J Neurosurg Anesthesiol. 2001;13:207–12. doi: 10.1097/00008506-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Subramaniam B, Madan R, Sadhasivam S, Sennaraj B, Tamilselvan P, Rajeshwari S, et al. Dexamethasone is a cost-effective alternative to ondansetron in preventing PONV after paediatric strabismus repair. Br J Anaesth. 2001;86:84–9. doi: 10.1093/bja/86.1.84. [DOI] [PubMed] [Google Scholar]
- 98.Ahmed AB, Hobbs GJ, Curran JP. Randomized, placebo-controlled trial of combination antiemetic prophylaxis for day-case gynaecological laparoscopic surgery. Br J Anaesth. 2000;85:678–82. doi: 10.1093/bja/85.5.678. [DOI] [PubMed] [Google Scholar]
- 99.Charuluxananan S, Somboonviboon W, Kyokong O, Nimcharoendee K. Ondansetron for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Reg Anesth Pain Med. 2000;25:535–9. doi: 10.1097/00115550-200009000-00017. [DOI] [PubMed] [Google Scholar]
- 100.Jensen AB, Christiansen DB, Coulthard K, Wilkins A, Roberts G, Walt JH, et al. Tropisetron reduces postoperative vomiting in children undergoing tonsillectomy. Paediatr Anaesth. 2000;10:69–75. doi: 10.1046/j.1460-9592.2000.00401.x. [DOI] [PubMed] [Google Scholar]
- 101.Kreisler NS, Spiekermann BF, Ascari CM, Rhyne HA, Kloth RL, Sullivan LM, et al. Small-dose droperidol effectively reduces nausea in a general surgical adult patient population. Anesth Analg. 2000;91:1256–61. doi: 10.1097/00000539-200011000-00038. [DOI] [PubMed] [Google Scholar]
- 102.Philip BK, Pearman MH, Kovac AL, Chelly JE, Wetchler BV, McKenzie R, et al. Dolasetron for the prevention of postoperative nausea and vomiting following outpatient surgery with general anaesthesia: a randomized, placebo-controlled study. The Dolasetron PONV Prevention Study Group. Eur J Anaesthesiol. 2000;17:23–32. doi: 10.1097/00003643-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 103.Zarate E, Watcha MF, White PF, Klein KW, Sa Rego M, Stewart DG. A comparison of the costs and efficacy of ondansetron versus dolasetron for antiemetic prophylaxis. Anesth Analg. 2000;90:1352–8. doi: 10.1097/00000539-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 104.Koivuranta M, Ala-Kokko TI, Jokela R, Ranta P. Comparison of ondansetron and tropisetron combined with droperidol for the prevention of emesis in women with a history of post-operative nausea and vomiting. Eur J Anaesthesiol. 1999;16:390–5. doi: 10.1097/00003643-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 105.McCall JE, Stubbs K, Saylors S, Pohlman S, Ivers B, Smith S, et al. The search for cost-effective prevention of postoperative nausea and vomiting in the child undergoing reconstructive burn surgery: ondansetron versus dimenhydrinate. J Burn Care Rehabil. 1999;20:309–15. doi: 10.1097/00004630-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 106.Sinha PK, Tripathi M, Ambesh SP. Efficacy of ondansetron in prophylaxis of postoperative nausea and vomiting in patients following infratentorial surgery: a placebo-controlled prospective double-blind study. J Neurosurg Anesthesiol. 1999;11:6–10. doi: 10.1097/00008506-199901000-00002. [DOI] [PubMed] [Google Scholar]
- 107.Tsui SL, Ng KF, Wong LC, Tang GW, Pun TC, Yang JC. Prevention of postoperative nausea and vomiting in gynaecological laparotomies: a comparison of tropisetron and ondansetron. Anaesth Intensive Care. 1999;27:471–6. doi: 10.1177/0310057X9902700506. [DOI] [PubMed] [Google Scholar]
- 108.Diemunsch P, Korttila K, Leeser J, Helmers JH, Wilkey B, Nave S, et al. Oral dolasetron mesylate for prevention of postoperative nausea and vomiting: a multicenter, double-blind, placebo-controlled study. The Oral Dolasetron PONV Prevention Study Group. J Clin Anesth. 1998;10:145–52. doi: 10.1016/S0952-8180(97)00259-6. [DOI] [PubMed] [Google Scholar]
- 109.Goodarzi M. A double blind comparison of droperidol and ondansetron for prevention of emesis in children undergoing orthopaedic surgery. Paediatr Anaesth. 1998;8:325–9. doi: 10.1046/j.1460-9592.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- 110.Hamid SK, Selby IR, Sikich N, Lerman J. Vomiting after adenotonsillectomy in children: a comparison of ondansetron, dimenhydrinate, and placebo. Anesth Analg. 1998;86:496–500. doi: 10.1213/00000539-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 111.Morris RW, Aune H, Feiss P, Hanson A, Hasselstrom L, Maltby JR, et al. International, multicentre, placebo-controlled study to evaluate the effectiveness of ondansetron vs. metoclopramide in the prevention of post-operative nausea and vomiting. Eur J Anaesthesiol. 1998;15:69–79. doi: 10.1097/00003643-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 112.Scholz J, Hennes HJ, Steinfath M, Farber L, Schweiger C, Dick W, et al. Tropisetron or ondansetron compared with placebo for prevention of postoperative nausea and vomiting. Eur J Anaesthesiol. 1998;15:676–85. doi: 10.1097/00003643-199811000-00009. [DOI] [PubMed] [Google Scholar]
- 113.Tramer MR, Sansonetti A, Fuchs-Buder T, Rifat K. Oculocardiac reflex and postoperative vomiting in paediatric strabismus surgery. A randomised controlled trial comparing four anaesthetic techniques. Acta Anaesthesiol Scand. 1998;42:117–23. doi: 10.1111/j.1399-6576.1998.tb05091.x. [DOI] [PubMed] [Google Scholar]
- 114.Diemunsch P, D’Hollander A, Paxton L, Schoeffler P, Wessel P, Nave S, et al. Intravenous dolasetron mesilate in the prevention of postoperative nausea and vomiting in females undergoing gynecological surgery. J Clin Anesth. 1997;9:365–73. doi: 10.1016/S0952-8180(97)00063-9. [DOI] [PubMed] [Google Scholar]
- 115.Diemunsch P, Leeser J, Feiss P, D’Hollander A, Bradburn BG, Paxton D, et al. Intravenous dolasetron mesilate ameliorates postoperative nausea and vomiting. Can J Anaesth. 1997;44:173–81. doi: 10.1007/BF03013007. [DOI] [PubMed] [Google Scholar]
- 116.Graczyk SG, McKenzie R, Kallar S, Hickok CB, Melson T, Morrill B, et al. Intravenous dolasetron for the prevention of postoperative nausea and vomiting after outpatient laparoscopic gynecologic surgery. Anesth Analg. 1997;84:325–30. doi: 10.1213/00000539-199702000-00015. [DOI] [PubMed] [Google Scholar]
- 117.Klockgether-Radke A, Neumann S, Neumann P, Braun U, Muhlendyck H. Ondansetron, droperidol and their combination for the prevention of post-operative vomiting in children. Eur J Anaesthesiol. 1997;14:362–7. doi: 10.1097/00003643-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 118.Koivuranta M, Laara E, Ranta P, Ravaska P, Alahuhta S. Comparison of ondansetron and droperidol in the prevention of postoperative nausea and vomiting after laparoscopic surgery in women. A randomised, double-blind, placebo-controlled trial. Acta Anaesthesiol Scand. 1997;41:1273–9. doi: 10.1111/j.1399-6576.1997.tb04644.x. [DOI] [PubMed] [Google Scholar]
- 119.Korttila K, Clergue F, Leeser J, Feiss P, Olthoff D, Payeur-Michel C, et al. Intravenous dolasetron and ondansetron in prevention of postoperative nausea and vomiting: a multicenter, double-blind, placebo-controlled study. Acta Anaesthesiol Scand. 1997;41:914–22. doi: 10.1111/j.1399-6576.1997.tb04809.x. [DOI] [PubMed] [Google Scholar]
- 120.Kovac AL, Scuderi PE, Boerner TF, Chelly JE, Goldberg ME, Hantler CB, et al. Treatment of postoperative nausea and vomiting with single intravenous doses of dolasetron mesylate: a multicenter trial. Dolasetron Mesylate PONV Treatment Study Group. Anesth Analg. 1997;85:546–52. doi: 10.1213/00000539-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 121.Mikawa K, Takao Y, Nishina K, Shiga M, Maekawa N, Obara H. Optimal dose of granisetron for prophylaxis against postoperative emesis after gynecological surgery. Anesth Analg. 1997;85:652–6. doi: 10.1213/00000539-199709000-00030. [DOI] [PubMed] [Google Scholar]
- 122.Monagle J, Barnes R, Goodchild C, Hewitt M. Ondansetron is not superior to moderate dose metoclopramide in the prevention of post-operative nausea and vomiting after minor gynaecological surgery. Eur J Anaesthesiol. 1997;14:604–9. doi: 10.1097/00003643-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 123.Morton NS, Camu F, Dorman T, Knudsen KE, Kvalsvik O, Nellgard P, et al. Ondansetron reduces nausea and vomiting after paediatric adenotonsillectomy. Paediatr Anaesth. 1997;7:37–45. doi: 10.1046/j.1460-9592.1997.d01-39.x. [DOI] [PubMed] [Google Scholar]
- 124.Patel RI, Davis PJ, Orr RJ, Ferrari LR, Rimar S, Hannallah RS, et al. Single-dose ondansetron prevents postoperative vomiting in pediatric outpatients. Anesth Analg. 1997;85:538–45. doi: 10.1213/00000539-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 125.Purhonen S, Kauko M, Koski EM, Nuutinen L. Comparison of tropisetron, droperidol, and saline in the prevention of postoperative nausea and vomiting after gynecologic surgery. Anesth Analg. 1997;84:662–7. doi: 10.1097/00000539-199703000-00036. [DOI] [PubMed] [Google Scholar]
- 126.Rung GW, Claybon L, Hord A, Patel C, Kallgren M, Koppel J, et al. Intravenous ondansetron for postsurgical opioid-induced nausea and vomiting. S3A-255 Study Group. Anesth Analg. 1997;84:832–8. doi: 10.1213/00000539-199704000-00025. [DOI] [PubMed] [Google Scholar]
- 127.Scuderi PE, Weaver RG, Jr, James RL, Mims G, Elliott WG, Weeks DB. A randomized, double-blind, placebo controlled comparison of droperidol, ondansetron, and metoclopramide for the prevention of vomiting following outpatient strabismus surgery in children. J Clin Anesth. 1997;9:551–8. doi: 10.1016/S0952-8180(97)00143-8. [DOI] [PubMed] [Google Scholar]
- 128.Taylor AM, Rosen M, Diemunsch PA, Thorin D, Houweling PL. A double-blind, parallel-group, placebo-controlled, dose-ranging, multicenter study of intravenous granisetron in the treatment of postoperative nausea and vomiting in patients undergoing surgery with general anesthesia. J Clin Anesth. 1997;9:658–63. doi: 10.1016/S0952-8180(97)00190-6. [DOI] [PubMed] [Google Scholar]
- 129.Ulusoy HO, Akturk G, Luleci N, Kalac N, Albayrak D. Prophylactic administration of ondansetron in emergency intraabdominal operations. Middle East J Anesthesiol. 1997;14:45–58. [PubMed] [Google Scholar]
- 130.Warriner CB, Knox D, Belo S, Cole C, Finegan BA, Perreault L. Prophylactic oral dolasetron mesylate reduces nausea and vomiting after abdominal hysterectomy. The Canadian Dolasetron Study Group. Can J Anaesth. 1997;44:1167–73. doi: 10.1007/BF03013339. [DOI] [PubMed] [Google Scholar]
- 131.Ali-Melkkila T, Kanto J, Katevuo R. Tropisetron and metoclopramide in the prevention of postoperative nausea and vomiting. A comparative, placebo controlled study in patients undergoing ophthalmic surgery. Anaesthesia. 1996;51:232–5. doi: 10.1111/j.1365-2044.1996.tb13639.x. [DOI] [PubMed] [Google Scholar]
- 132.Capouet V, De Pauw C, Vernet B, Ivens D, Derijcke V, Versichelen L, et al. Single dose i.v. tropisetron in the prevention of postoperative nausea and vomiting after gynaecological surgery. Br J Anaesth. 1996;76:54–60. doi: 10.1093/bja/76.1.54. [DOI] [PubMed] [Google Scholar]
- 133.Kovac AL, Pearman MH, Khalil SN, Scuderi PE, Joslyn AF, Prillaman BA, et al. Ondansetron prevents postoperative emesis in male outpatients. S3A-379 Study Group. J Clin Anesth. 1996;8:644–51. doi: 10.1016/S0952-8180(96)00173-0. [DOI] [PubMed] [Google Scholar]
- 134.Naguib M, el Bakry AK, Khoshim MH, Channa AB, el Gammal M, el Gammal K, et al. Prophylactic antiemetic therapy with ondansetron, tropisetron, granisetron and metoclopramide in patients undergoing laparoscopic cholecystectomy: a randomized, double-blind comparison with placebo. Can J Anaesth. 1996;43:226–31. doi: 10.1007/BF03011739. [DOI] [PubMed] [Google Scholar]
- 135.Desilva PH, Darvish AH, McDonald SM, Cronin MK, Clark K. The efficacy of prophylactic ondansetron, droperidol, perphenazine, and metoclopramide in the prevention of nausea and vomiting after major gynecologic surgery. Anesth Analg. 1995;81:139–43. doi: 10.1097/00000539-199507000-00028. [DOI] [PubMed] [Google Scholar]
- 136.Litman RS, Wu CL, Lee A, Griswold JD, Voisine R, Marshall C. Prevention of emesis after strabismus repair in children: a prospective, double-blinded, randomized comparison of droperidol versus ondansetron. J Clin Anesth. 1995;7:58–62. doi: 10.1016/0952-8180(94)00008-R. [DOI] [PubMed] [Google Scholar]
- 137.Paech MJ, Pavy TJ, Evans SF. Single-dose prophylaxis for postoperative nausea and vomiting after major abdominal surgery: ondansetron versus droperidol. Anaesth Intensive Care. 1995;23:548–54. doi: 10.1177/0310057X9502300503. [DOI] [PubMed] [Google Scholar]
- 138.Paxton D, Taylor RH, Gallagher TM, Crean PM. Postoperative emesis following otoplasty in children. Anaesthesia. 1995;50:1083–5. doi: 10.1111/j.1365-2044.1995.tb05957.x. [DOI] [PubMed] [Google Scholar]
- 139.Kaufmann MA, Rosow C, Schnieper P, Schneider M. Prophylactic antiemetic therapy with patient-controlled analgesia: a double-blind, placebo-controlled comparison of droperidol, metoclopramide, and tropisetron. Anesth Analg. 1994;78:988–94. doi: 10.1213/00000539-199405000-00027. [DOI] [PubMed] [Google Scholar]
- 140.Ummenhofer W, Frei FJ, Urwyler A, Kern C, Drewe J. Effects of ondansetron in the prevention of postoperative nausea and vomiting in children. Anesthesiology. 1994;81:804–10. doi: 10.1097/00000542-199410000-00006. [DOI] [PubMed] [Google Scholar]
- 141.Dupeyron JP, Conseiller C, Levarlet M, Hemmingsen C, Schoeffler P, Pedersen FM, et al. The effect of oral ondansetron in the prevention of postoperative nausea and vomiting after major gynaecological surgery performed under general anaesthesia. Anaesthesia. 1993;48:214–8. doi: 10.1111/j.1365-2044.1993.tb06904.x. [DOI] [PubMed] [Google Scholar]
- 142.Helmers JH, Briggs L, Abrahamsson J, Soni J, Moodley J, Forrler M, et al. A single i.v. dose of ondansetron 8 mg prior to induction of anaesthesia reduces postoperative nausea and vomiting in gynaecological patients. Can J Anaesth. 1993;40:1155–61. doi: 10.1007/BF03009605. [DOI] [PubMed] [Google Scholar]
- 143.Raphael JH, Norton AC. Antiemetic efficacy of prophylactic ondansetron in laparoscopic surgery: randomized, double-blind comparison with metoclopramide. Br J Anaesth. 1993;71:845–8. doi: 10.1093/bja/71.6.845. [DOI] [PubMed] [Google Scholar]
- 144.Du Pen S, Scuderi P, Wetchler B, Sung YF, Mingus M, Clayborn L, et al. Ondansetron in the treatment of postoperative nausea and vomiting in ambulatory outpatients: a dose-comparative, stratified, multicentre study. Eur J Anaesthesiol Suppl. 1992;6:55–62. [PubMed] [Google Scholar]
- 145.Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102:1094–100. doi: 10.1097/00000542-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 146.Kovac A, McKenzie R, O’Connor T, Duncalf D, Angel J, Gratz I, et al. Prophylactic intravenous ondansetron in female outpatients undergoing gynaecological surgery: a multicentre dose-comparison study. Eur J Anaesthesiol Suppl. 1992;6:37–47. [PubMed] [Google Scholar]
- 147.Lerman J, Sims C, Sikich N, Gow R, Chin C, Dempsey E, et al. Pharmacokinetics of the active metabolite (MDL 74,156) of dolasetron mesylate after oral or intravenous administration to anesthetized children. Clin Pharmacol Ther. 1996;60:485–92. doi: 10.1016/S0009-9236(96)90144-7. [DOI] [PubMed] [Google Scholar]
- 148.Wagner DS, Gauger V, Chiravuri D, Faust K. Ondansetron oral disintegrating tablets for the prevention of postoperative vomiting in children undergoing strabismus surgery. Ther Clin Risk Manag. 2007;3:691–4. [PMC free article] [PubMed] [Google Scholar]
- 149.Rose JB, Brenn BR, Corddry DH, Thomas PC. Preoperative oral ondansetron for pediatric tonsillectomy. Anesth Analg. 1996;82:558–62. doi: 10.1097/00000539-199603000-00023. [DOI] [PubMed] [Google Scholar]
- 150.El-Deeb A, Abd el motlb E. Prophylactic multimodal antiemetic in women undergoing cesarean section under spinal anesthesia. Egypt J Anaesth. 2011;27:107–11. doi: 10.1016/j.egja.2011.04.003. [DOI] [Google Scholar]
- 151.Jokela RM, Cakmakkaya OS, Danzeisen O, Korttila KT, Kranke P, Malhotra A, et al. Ondansetron has similar clinical efficacy against both nausea and vomiting. Anaesthesia. 2009;64:147–51. doi: 10.1111/j.1365-2044.2008.05732.x. [DOI] [PubMed] [Google Scholar]
- 152.Pearman MH. Single dose intravenous ondansetron in the prevention of postoperative nausea and vomiting. Anaesthesia. 1994;49:11–5. doi: 10.1111/j.1365-2044.1994.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 153.Scuderi P, Wetchler B, Sung YF, Mingus M, DuPen S, Claybon L, et al. Treatment of postoperative nausea and vomiting after outpatient surgery with the 5-HT3 antagonist ondansetron. Anesthesiology. 1993;78:15–20. doi: 10.1097/00000542-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 154.McKenzie R, Kovac A, O’Connor T, Duncalf D, Angel J, Gratz I, et al. Comparison of ondansetron versus placebo to prevent postoperative nausea and vomiting in women undergoing ambulatory gynecologic surgery. Anesthesiology. 1993;78:21–8. doi: 10.1097/00000542-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 155.Bagameri A, Bosze P. Ondansetron (Zofran) treatment of chemotherapy induced nausea and vomiting in patients with gynecological malignancies. Magy Noorv Lapja. 1994;57:291–3. [Google Scholar]
- 156.Chen LK, Fan SZ, Huang CH, Chao A, Cherng YG, Chen CL, et al. Effects of ondansetron on postoperative emesis in Chinese children. Acta Anaesthesiol Sin. 1998;36:87–91. [PubMed] [Google Scholar]
- 157.Ebrahim Soltani AR, Mohammadinasab H, Goudarzi M, Arbabi S, Mohammadinasab A, Mohammadinasab F, et al. Comparing the efficacy of prophylactic p6 acupressure, ondansetron, metoclopramide and placebo in the prevention of vomiting and nausea after strabismus surgery. Acta Med Iran. 2011;49:208–12. [PubMed] [Google Scholar]
- 158.Ng WW, Li AC, Lee DW, Leung TL, Ko CW, Leung KF, et al. Effectiveness of Ondansetron to prevent postoperative nausea and vomiting in ambulatory two port needlescopic cholecystectomy: a randomised controlled trial. Ambul Surg. 2008;14:1–14. [Google Scholar]
- 159.Aouad MT, Siddik-Sayyid SM, Taha SK, Azar MS, Nasr VG, Hakki MA, et al. Haloperidol vs. ondansetron for the prevention of postoperative nausea and vomiting following gynaecological surgery. Eur J Anaesthesiol. 2007;24:171–8. doi: 10.1017/S0265021506001323. [DOI] [PubMed] [Google Scholar]
- 160.Freedman SB, Uleryk E, Rumantir M, Finkelstein Y. Ondansetron and the risk of cardiac arrhythmias: a systematic review and postmarketing analysis. Ann Emerg Med. 2014;64:19–25. doi: 10.1016/j.annemergmed.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 161.Rawlinson A, Kitchingham N, Hart C, McMahon G, Ong SL, Khanna A. Mechanisms of reducing postoperative pain, nausea and vomiting: a systematic review of current techniques. Evid Based Med. 2012;17:75–80. doi: 10.1136/ebmed-2011-100265. [DOI] [PubMed] [Google Scholar]
- 162.Tricco A, Veroniki A, Blondal E, Hamid J, Straus S. Incorporating dosages increases the relevance of network meta-analysis for decision-makers: A case example using data from a systematic review of serotonin (5-HT3) receptor antagonists in patients undergoing surgery. Toronto: Canadian Association of Pharmacy Technicians (CAPT) Conference; 2014. [Google Scholar]


