Introduction
Late age-related macular degeneration (AMD), specifically central geographic atrophy (GA) and choroidal neovascularization (CVN),1 is the leading cause of irreversible vision loss in the elderly in the developed world.2 AMD is categorized as either i) non-neovascular, or dry, when CNV is not present, or ii) neovascular, or wet, when CNV is present. Increasing age is the most significant risk factor for AMD development.3 In the United States, AMD is more common in Caucasians, and smoking is strongly associated. The hallmark of AMD clinically is the presence of drusen situated under the retinal pigment epithelium (RPE). Drusen size is categorized as small (≤62μm), medium (63–124μm), or large (≥125μm), with presence of large drusen being a significant predictor of progression to late AMD and associated vision loss.4 The Age-Related Eye Disease Studies (AREDS and AREDS2) have shown that high-dose antioxidant and zinc supplementation delay the progression of AMD in moderate- and high-risk patients.5, 6 Anti-vascular endothelial growth factor (VEGF) treatment is the mainstay of therapy for wet AMD.7 Currently, there is no treatment for GA.
While AMD pathogenesis is undoubtedly multifactorial, including the effects of aging and oxidative stress as well as genetic and environmental factors, significant evidence has emerged implicating inflammation and the immune system. The major role of the immune system is to identify and respond to physiologic insults, such as infection, malignancy, and tissue damage. Often this takes the form of robust inflammatory responses, such as those seen in various forms of uveitis, despite the potent down-regulatory immune environment within the eye.8 In AMD, immune dysregulation in the form of overt intraocular inflammation is not clinically apparent. It has been proposed that the role of inflammation is not always negative. The concept of parainflammation has been suggested to describe inflammatory responses to tissue stress that are intermediate between basal and robust inflammatory states and function in a reparative manner.9, 10 As AMD is not accompanied by an intense inflammatory reaction, it is possible that dysregulation of reparative parainflammatory mechanisms, in the context of the aging eye, lead to a low grade chronic inflammatory response and subsequent AMD pathology. This review will focus on the potential inflammatory mechanisms of AMD pathogenesis based on evidence from human studies.
Immunogenetics of AMD
Over the past 10 years, AMD has been associated with several genetic single nucleotide polymorphisms (SNP), many of which encode proteins involved in inflammatory cascades and the immune system (Table 1). Variants of several genes encoding proteins in the complement system, including complement factor H (CFH),11–14 CFB, complement component 2 (C2),15, 16 C3,17–19 and C5,20 have been associated with AMD suggesting that dysregulation of the complement cascade may be involved in AMD pathogenesis. CFH functions to down regulate the alternative complement pathway.21 The CFH polymorphism resulting in tyrosine to histidine at position 402 (Y402H) may be associated with up to 50% of all AMD cases22 and has also been associated with sarcoidosis-related and other forms of posterior uveitis,23, 24 possibly suggesting a larger role for this polymorphism in ocular inflammation. CFB and C2 are activators of the alternative and classical complement pathways, respectively, and variants of these genes were found to be protective against development of AMD.15, 16, 25
Table 1.
Immune-related gene variants associated with AMD
| Gene | Chromosomal Location | Function | Variant Effect | References |
|---|---|---|---|---|
| CFH | 1q32 | Alternative pathway inhibitor | Harmful | 11–14 |
|
|
||||
| CFB | 6p21 | Alternative pathway activator | Protective | 15, 16, 25 |
|
|
||||
| C2 | 6p21 | Classical pathway activator | Protective | 14, 15, 22 |
|
|
||||
| C3 | 19p13 | Complement component; multiple inflammatory functions | Harmful | 17–19 |
|
|
||||
| C5 | 9q33-q34 | Complement component; multiple inflammatory functions | Depends on variant | 20 |
|
|
||||
| CX3CR1 | 3p21 | Chemokine receptor found on retinal microglia | Conflicting/Harmful | 27–31 |
|
|
||||
| CCR3 | 3p21 | Chemokine receptor | Harmful | 32 |
|
|
||||
| IL-8/CXCL8 | 4q13-q21 | Chemokine for neutrophils; pro-angiogenic | Harmful | 33, 34 |
CFH: Complement factor H; CFB: Complement factor B; C2: Complement component 2; C3: Complement component 3; C5: Complement component 5; IL: Interleukin
In addition to the complement system, polymorphisms in genes encoding chemokines and their receptors have been associated with AMD. Chemokine receptors are expressed on immune cells as well as other cell types, such as endothelial cells, and function to direct cells to sites of inflammation in response to ligation by their cognate chemokine. Genetic variants of the chemokine receptor CX3CR1, which is expressed on retinal microglia,26 have been associated with AMD.27, 28 However, not all studies have confirmed this association29, 30 and different studies have identified distinct polymorphisms that may be associated with AMD.31 Polymorphisms in the genes encoding the chemokine receptor CCR332 and chemokine CXCL8 (also known as IL-8),33, 34 which have been implicated in angiogenesis,35, 36 have also been associated with AMD. Therefore, AMD has been associated with genetic variants of various inflammatory molecules perhaps suggesting that several inflammatory pathways can lead to the same clinical disease.
The complement system in AMD
The complement system encompasses over 30 proteins that function in an inflammatory cascade resulting in enhanced chemotaxis, phagocytosis, agglutination, and target cell lysis via formation of the membrane attack complex (MAC). As discussed above, several complement gene variants have been associated with AMD suggesting a role for the complement system in AMD pathogenesis. Complement activation products, including C3a, C5a, as well as C5b-9 that comprise the MAC, have been identified in drusen from human eyes with AMD37–42 and were found to be elevated in serum of AMD patients compared with age-matched controls.43 Levels of these complement activation markers correlated with the presence of at least one copy of the CFH Y402H risk allele. In addition, dry AMD patients homozygous for the CFH variant had higher serum levels of interleukin (IL)-6, IL-18, and tumor necrosis factor (TNF)-α compared with those either heterozygous for or lacking the variant.44 Given the function of CFH as an inhibitor of the complement cascade, these results suggest that the Y402H CFH variant may reduce its inhibitory function allowing deregulated activation of the complement system and subsequent inflammatory and cytolytic responses. In accord with this notion, a rare genetic variant of C3, which reduces its ability to bind CHF, has also been associated with AMD.45, 46
The C5a receptor has been identified in human choroid47 as well as on ARPE-19 cells.48 Exposure of choriocapillaris endothelial cells to C5a in vitro lead to upregulation of ICAM-1, an adhesion molecule that binds activated leukocytes.47 Exposure of ARPE-19 cells to C5a in vitro suppressed RPE-derived transforming growth factor (TGF)-β2, an important immunosuppressive molecule in the eye, and reduced RPE viability.48 Furthermore, C5a increased the proliferative capacity of peripheral blood mononuclear cells (PBMC) and decreased the ability of RPE cells to suppress PBMC proliferation. In a separate study, C5a was shown to promote interleukin (IL)-22 and IL-17A secretion from human CD4 T cells isolated from patients with exudative AMD in a monocyte-dependent mechanism.49 IL-17A has been shown to induce angiogenesis in human choroidal endothelial cells in vitro.50 IL-17A can also induce pyroptosis and apoptosis in ARPE-19 cells.51 These results suggest that RPE: i) choroidal endothelial cell, and circulating immune cells are responsive to complement activation products, ii) RPE/choroid exposed to complement activation products can modulate interactions between monocytes and lymphocytes, and that iii) cytokines expressed by T cells exposed to complement activation products may promote neovascularization and cell death.
Recently, there have been a number of reports implicating exosomes in the pathogenesis of AMD. Exosomes are small membrane-bound vesicles of endocytic origin secreted by many cell types and thought to play a role in intercellular communication.52 Abundant oxidative stress within the RPE is a well-accepted component of AMD pathogenesis.53 Oxidative stress results in production of reactive oxygen species (ROS), which can damage mitochondrial DNA (mtDNA) and other macromolecules. Mitochondrial damage has been illustrated in human AMD maculae.53, 54 In an in vitro model where mtDNA damage was induced in ARPE-19 cells by treatment with rotenone, a mitochondrial complex I inhibitor, increased autophagy as well as increased exosome release and chemokine secretion were observed.55 Markers of autophagy and exosomes were also demonstrated in drusen of human AMD patients. In the in vitro model, secreted exosomes were coated with complement factor C3, which was able to bind CFH. The authors speculated that mutation of CFH might decrease the ability of CFH to bind and clear C3-coated exosomes, leading to enhanced drusen formation and AMD.
Inflammatory cytokines in AMD
A number of inflammatory cytokines have been found to be elevated either systemically in the serum or locally in the ocular tissue or fluids of patients with AMD. As mentioned above, systemic levels IL-6, IL-18, and TNF-α correlated with CFH haplotypes in AMD patients.44 Furthermore, systemic IL-6 levels have been shown to correlate with the incidence56 and progression57 of AMD. IL-18 has recently been reported to play a protective role against development of CNV.58, 59 However, results are conflicting as to whether IL-18 functions in anti-angiogenic manner.60 As discussed below, IL-18 may also be involved in RPE atrophy.61
IL-22 and IL-17A, cytokines produced by the Th17 subset of helper CD4 T cells, were found to be elevated in the serum of patients with exudative AMD.49 Two SNPs within the IL-17A gene were recently reported to be associated with AMD as well as higher levels of IL-17A secretion from PBMC stimulated ex vivo.62 Elevated IL-17A and IL-17RC levels were also detected in AMD lesions compared with normal macular tissues.51 In vitro, IL-22 induced apoptosis of primary human RPE cells,63 and IL-17A was shown to enhance production of inflammatory cytokines, including IL-6, IL-8 and CCL2, from ARPE-19 cells64 as well as induce angiogenesis in human choroidal endothelial cells.50
Several studies have employed multiplex protein screens to identify cytokines in ocular fluids of patient with neovascular AMD.65–70 Table 2 lists select cytokines with direct involvement in inflammatory cascades. Consistent with the systemic findings discussed above, IL-6, which has pro-angiogenic properties, has been found in higher concentrations in aqueous fluid of eye with neovascular AMD compared to controls.68, 69 However, as with many observations from human samples, whether these cytokines are involved in the primary pathogenesis of AMD or are a result of the disease process remains to be determined.
Table 2.
Select inflammatory cytokines elevated in intraocular fluids of neovascular AMD patients
| Cytokine | Function | References |
|---|---|---|
| IL-6 | Acute phase reactant; pyrogenic; pro-angiogenic | 68, 69 |
|
|
||
| IL-8/CXCL8 | Chemokine for neutrophils; pro-angiogenic | 68, 69 |
|
|
||
| MCP-1/CCL2 | Chemokine for monocytes, memory T cells, and dendritic cells | 67, 69, 70 |
|
|
||
| ICAM-1 | Adhesion molecule that facilitates transmigration of leukocytes | 69, 70 |
IL: Interleukin; MCP: Monocyte chemotactic protein; ICAM: Intercellular adhesion molecule
Inflammasome activation in AMD
Recently, inflammatory signaling cascades within RPE cells have been implicated in AMD pathogenesis. The term inflammasome refers to multi-protein intracellular complexes formed in response to stimulation of pattern recognition receptors, such as NOD-like receptors (NLR), by microbes, toxins, or products of cellular stress.71 Inflammasome activation leads to production of active caspase-1, an enzyme capable of cleaving and activating several pro-peptides, including IL-18. Drusen isolated from human eyes with AMD were shown to activate the NLRP3 inflammasome in vitro.58 In addition, RPE from human eyes with GA was shown to have increased mRNA levels of NLRP3 and IL-18 and increased protein levels of NLRP3 and caspase-1 compared to age-matched normal controls.72 The same group also reported increased levels of a non-coding RNA sequence known as Alu RNA as well as decreased levels of the RNase DICER1 in RPE from human eyes with GA.73 DICER1 cleaves and inactivates Alu RNA; therefore, deficiency in DICER1 could result in increased levels of Alu RNA, which was shown to activate the NLRP3 inflammasome with subsequent elaboration of active IL-18.72 Furthermore, RPE from human eyes with GA were shown to have increased protein levels of the phosphorylated IRAK1 and IRAK4,72 which are involved in MyD88 signaling, as well as active caspase 874 compared to controls. Inhibiting caspase 8 in vitro prevented RPE cell death.74 Taken together, these findings suggest that DICER1 deficiency may result in increased levels of Alu RNA, which activates the NLRP3 inflammasome resulting in production of active IL-18, which signals via MyD88 to elicit active caspase 8, which causes apoptosis of RPE cells. Why DICER levels are decreased in eyes with GA remains to be determined. Nonetheless, targeting this pathway may lead to novel therapeutic strategies for GA, which currently has no treatment.
Microglia and Macrophages in AMD
Innate immune cells, such as microglia and macrophages, are sentinels in the body’s response to tissue insult and function to initiate inflammatory responses, clear debris, and remodel tissue to a state of homeostasis. Microglia are specialized myeloid-derived cells that reside in the retina and central nervous system (CNS). In rodent models, microglia enter the CNS prior to closure of the blood-brain barrier, and their population is maintained through self-renewal in the absence of known stem cells.75 Microglia cell bodies are typically situated in the inner retina in the normal human retina.26 These cells can migrate to the subretinal space in response to inflammatory stimuli.10 Activated microglia have been found in the outer retina and subretinal space in eyes with AMD.26, 76 Retinal microglia express the chemokine receptor CX3CR1.26 Genetic polymorphisms in the CX3CR1 gene have been associated with AMD and reduce the chemotactic ability of monocytes.26 In murine models, deficiency of CXCR3 lead to subretinal accumulation of microglia with normal aging and exacerbated laser-induced CNV.
Several studies have identified the presence of macrophages in or around drusen, areas of geographic atrophy, and choroidal neovascular membranes on pathologic analysis of eyes from AMD patients.53, 77–82 However, it is unclear from these studies whether macrophages are involved in the development of these pathologic findings or accumulate as a consequence of the pathology. In support of a causative role for macrophages in wet AMD, macrophages from human CNV tissue were found to express the pro-angiogenic protein VEGF, suggesting that macrophages are involved in the development of CNV lesions.83 Alternatively, macrophages from human CNV tissue have also been shown to express scavenger receptors for oxidized proteins, such as lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), suggesting that these cells may migrate to sites of oxidative damage to clear debris.84, 85 However, these conclusions are not mutually exclusive, and animal models of laser-induced CNV have also provided conflicting results with some studies suggesting macrophages contribute to CNV formation while others show a protective role for macrophages in preventing CNV.86–88 Macrophages are a heterogeneous and can adopt a spectrum of phenotypes. For instance, M1 macrophages are proinflammatory, while M2 macrophages are relatively anti-inflammatory and may be pro-angiogenic in certain microenvironments.89 Interestingly, M1 macrophages were more common in the eyes with GA AMD and M2 macrophages were more common in the neovascular AMD eyes.90 Therefore, the polarization of macrophages within AMD lesions may in part determine the course of pathology. Further research is required to determine the precise role of microglia and macrophages in human AMD pathogenesis.
Adaptive immunity in AMD
Adaptive immunity refers to antigen-specific responses generated by B and T lymphocytes against peptides the body detects as foreign. Direct evidence implicating the adaptive immune system in AMD is sparse in humans. Several studies have reported the presence of anti-retinal antibodies in the serum of AMD patients.91–93 Expression of major histocompatibility (MHC) class II molecules, which are required for presentation of antigen to CD4 T cells, is seen on RPE in human eyes with AMD (Figure 1). Furthermore, T cells from AMD patients have been shown to proliferate in response to retinal antigens (unpublished data from RBN). Therefore, the adaptive immune system is able to sense and respond to retinal antigens in patients with AMD. However, whether these responses are the cause or result of retinal damage is unknown.
Figure 1.
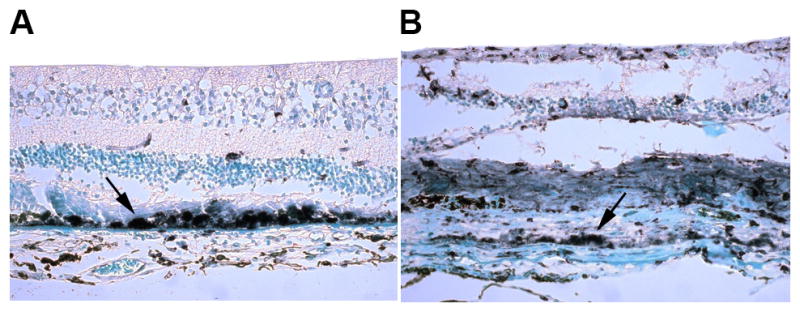
HLA-DR expression on RPE in eyes with (A) geographic atrophic and (B) neovascular AMD. Arrows indicate areas of strongly positive staining.
As people age, the immune system undergoes changes referred to as immunosenescence, a phenomenon perhaps most apparent in the T cell compartment where the ratio of memory to naïve T cells drastically increases, in part due to reduced thymic output of naïve T cells with age.94 In addition, T cells undergo phenotypic changes with age, including down-regulation of CD28, a co-stimulatory molecule involved in the generation of antigen-specific responses, and upregulation of markers typically seen on natural killer (NK) cells, such as CD56, that may allow antigen non-specific reactivity. A case-control study of over one hundred AMD patients and controls reported an increased frequency of CD28−CD56+ T cells in the peripheral blood of AMD patients compared with controls.95 Most of this difference appeared to be in the CD8 T cell compartment. These results suggest that heightened immunosenescent responses may play a role in AMD pathogenesis. Unfortunately, the control group was significantly younger than the AMD group confounding interpretation of these results.
As discussed above, CD4 T cells from AMD patients secreted IL-22 and IL-17A in a monocyte dependent manner in response to the complement component C5a,49 which has been found in drusen and is also elevated in the serum of AMD patients. In addition, IL-17 induced angiogenesis in human choroidal endothelial cells and cell death in ARPE-19 cells in vitro.50, 51 Therefore, interplay between the innate (complement system and monocytes) and adaptive (T cells and their cytokines) may be involved in the complex pathogenesis of AMD.
Immunotherapy for AMD
There are several recently completed and ongoing clinical trials evaluating immune-based interventions for both dry and wet AMD. As discussed above, T cells may play a role in AMD pathogenesis. In patients with GA, subconjunctival sirolimus, an mTOR inhibitor that suppresses T cell responses, did not reduce GA area compared to fellow non-treated eyes.96 In a randomized non-masked trial in patients with CNV, however, systemic sirolimus treatment was shown to significantly reduce the number of anti-VEGF intravitreal injections required to control CNV.97 In the same study, systemic daclizumab, a monoclonal antibody directed against the alpha subunit (CD25) of the IL-2 receptor expressed on T cells, also reduced the number of anti-VEGF injections in patients with CNV. These results suggest that systemic inhibition of T cell responses may alter the clinical course of wet AMD.
TNF-α has been localized to human CNV lesions with the suggestion that it may promote angiogenesis.98 Systemic treatment of neovascular AMD patients with the anti-TNF-α monoclonal antibody, infliximab, had no effect on the number of anti-VEGF injections required or visual acuity, but the power of the study was low.97 A number a case reports describe the use of intravitreal anti-TNF-α agents in neovascular AMD patients refractory to anti-VEGF therapy; however, all reports describe significant intraocular inflammation as a complication.99–101
Broad spectrum immunosuppressive medications have also been tested in neovascular AMD. Topical bromfenac, a non-steroidal anti-inflammatory drug (NSAID),102 and intravitreal triamcinolone,103 have been tested in conjunction with anti-VEGF intravitreal injections in the treatment of neovascular AMD. While having no effect on visual acuity, both agents reduced the number of required anti-VEGF injections. Therefore, therapies directed against certain inflammatory molecules and pathways may alter the clinical course of AMD.
Conclusions
Several lines of evidence implicate various components of the immune system in AMD pathogenesis. However, the exact inflammatory mechanisms involved remain elusive. While vision loss attributable to AMD-associated CNV has been drastically reduced over the past decade with the introduction of intravitreal anti-VEGF therapies, there remains no effective treatment for the more common atrophic form of late AMD. Therapies targeting several components of the complement cascade are currently being tested.104 Clinical trials are also underway investigating transplantation of stem cell-derived RPE into areas of GA in human patients.105 This raises additional issues regarding inflammatory responses and their potential effect on photoreceptors and RPE. Beyond the inflammatory reaction induced by surgery, RPE derived from fetal embryonic stem cells will likely be seen as foreign by the host requiring systemic immunosuppression to prevent rejection.
Oral tolerance is another potential therapeutic avenue that may prove beneficial in the treatment of AMD. Induction of immunologic tolerance, meaning a specific dampening of immune responses toward specific antigens, has been accomplished by oral administration of antigens to humans with several inflammatory conditions,106–108 including uveitis where patients fed S antigen were able to stably discontinue systemic immunosuppressive medications.109 It is clear that further research is needed to elucidate the inflammatory mechanisms involved in AMD pathogenesis if novel targeted therapeutics are to be effective.
Acknowledgments
Funding: Supported by Intramural funds from the National Eye Institute, National Institutes of Health
Footnotes
Disclosures: The authors declare that they have no conflicts of interest to disclose
References
- 1.Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. American journal of ophthalmology. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 3.Coleman HR, Chan CC, Ferris FL, 3rd, Chew EY. Age-related macular degeneration. Lancet. 2008;372:1835–45. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Archives of ophthalmology. 2005;123:1570–4. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of ophthalmology. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Age-Related Eye Disease Study 2 Research G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. Jama. 2013;309:2005–15. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. The New England journal of medicine. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 8.Nussenblatt RB, Ferris F., 3rd Age-related macular degeneration and the immune response: implications for therapy. American journal of ophthalmology. 2007;144:618–26. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Progress in retinal and eye research. 2009;28:348–68. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 14.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 15.Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nature genetics. 2006;38:458–62. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maller J, George S, Purcell S, et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nature genetics. 2006;38:1055–9. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 17.Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. The New England journal of medicine. 2007;357:553–61. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 18.Despriet DD, van Duijn CM, Oostra BA, et al. Complement component C3 and risk of age-related macular degeneration. Ophthalmology. 2009;116:474–480. e2. doi: 10.1016/j.ophtha.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 19.Spencer KL, Olson LM, Anderson BM, et al. C3 R102G polymorphism increases risk of age-related macular degeneration. Human molecular genetics. 2008;17:1821–4. doi: 10.1093/hmg/ddn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baas DC, Ho L, Ennis S, et al. The complement component 5 gene and age-related macular degeneration. Ophthalmology. 2010;117:500–11. doi: 10.1016/j.ophtha.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Molecular immunology. 2004;41:355–67. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Thakkinstian A, Han P, McEvoy M, et al. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Human molecular genetics. 2006;15:2784–90. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- 23.Thompson IA, Liu B, Sen HN, et al. Association of complement factor H tyrosine 402 histidine genotype with posterior involvement in sarcoid-related uveitis. American journal of ophthalmology. 2013;155:1068–1074. e1. doi: 10.1016/j.ajo.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara DC, Merriam JE, Freund KB, et al. Analysis of major alleles associated with age-related macular degeneration in patients with multifocal choroiditis: strong association with complement factor H. Archives of ophthalmology. 2008;126:1562–6. doi: 10.1001/archopht.126.11.1562. [DOI] [PubMed] [Google Scholar]
- 25.Spencer KL, Hauser MA, Olson LM, et al. Protective effect of complement factor B and complement component 2 variants in age-related macular degeneration. Human molecular genetics. 2007;16:1986–92. doi: 10.1093/hmg/ddm146. [DOI] [PubMed] [Google Scholar]
- 26.Combadiere C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. The Journal of clinical investigation. 2007;117:2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuo J, Smith BC, Bojanowski CM, et al. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:1297–9. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Hu J, Zhang J, Guan H. Polymorphisms in CFH, HTRA1 and CX3CR1 confer risk to exudative age-related macular degeneration in Han Chinese. The British journal of ophthalmology. 2010;94:1211–4. doi: 10.1136/bjo.2009.165811. [DOI] [PubMed] [Google Scholar]
- 29.Zerbib J, Puche N, Richard F, et al. No association between the T280M polymorphism of the CX3CR1 gene and exudative AMD. Experimental eye research. 2011;93:382–6. doi: 10.1016/j.exer.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Ryu E, Fridley BL, Tosakulwong N, Bailey KR, Edwards AO. Genome-wide association analyses of genetic, phenotypic, and environmental risks in the age-related eye disease study. Molecular vision. 2010;16:2811–21. [PMC free article] [PubMed] [Google Scholar]
- 31.Schaumberg DA, Rose L, DeAngelis MM, Semba RD, Hageman GS, Chasman DI. Prospective study of common variants in CX3CR1 and risk of macular degeneration: pooled analysis from 5 long-term studies. JAMA ophthalmology. 2014;132:84–95. doi: 10.1001/jamaophthalmol.2013.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma NK, Gupta A, Prabhakar S, Singh R, Bhatt AK, Anand A. CC chemokine receptor-3 as new target for age-related macular degeneration. Gene. 2013;523:106–11. doi: 10.1016/j.gene.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Tsai YY, Lin JM, Wan L, et al. Interleukin gene polymorphisms in age-related macular degeneration. Investigative ophthalmology & visual science. 2008;49:693–8. doi: 10.1167/iovs.07-0125. [DOI] [PubMed] [Google Scholar]
- 34.Goverdhan SV, Ennis S, Hannan SR, et al. Interleukin-8 promoter polymorphism −251A/T is a risk factor for age-related macular degeneration. The British journal of ophthalmology. 2008;92:537–40. doi: 10.1136/bjo.2007.123190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghasemi H, Ghazanfari T, Yaraee R, Faghihzadeh S, Hassan ZM. Roles of IL-8 in ocular inflammations: a review. Ocular immunology and inflammation. 2011;19:401–12. doi: 10.3109/09273948.2011.618902. [DOI] [PubMed] [Google Scholar]
- 36.Takeda A, Baffi JZ, Kleinman ME, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460:225–30. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14:835–46. [PubMed] [Google Scholar]
- 38.Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2328–33. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Experimental eye research. 2000;70:441–9. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 40.Hageman GS, Mullins RF, Russell SR, Johnson LV, Anderson DH. Vitronectin is a constituent of ocular drusen and the vitronectin gene is expressed in human retinal pigmented epithelial cells. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1999;13:477–84. doi: 10.1096/fasebj.13.3.477. [DOI] [PubMed] [Google Scholar]
- 41.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Experimental eye research. 2001;73:887–96. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 42.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholl HP, Charbel Issa P, Walier M, et al. Systemic complement activation in age-related macular degeneration. PloS one. 2008;3:e2593. doi: 10.1371/journal.pone.0002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao S, Ko A, Partanen M, et al. Relationship between systemic cytokines and complement factor H Y402H polymorphism in patients with dry age-related macular degeneration. American journal of ophthalmology. 2013;156:1176–83. doi: 10.1016/j.ajo.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan X, Larson DE, Wang C, et al. Identification of a rare coding variant in complement 3 associated with age-related macular degeneration. Nature genetics. 2013;45:1375–9. doi: 10.1038/ng.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helgason H, Sulem P, Duvvari MR, et al. A rare nonsynonymous sequence variant in C3 is associated with high risk of age-related macular degeneration. Nature genetics. 2013;45:1371–4. doi: 10.1038/ng.2740. [DOI] [PubMed] [Google Scholar]
- 47.Skeie JM, Fingert JH, Russell SR, Stone EM, Mullins RF. Complement component C5a activates ICAM-1 expression on human choroidal endothelial cells. Investigative ophthalmology & visual science. 2010;51:5336–42. doi: 10.1167/iovs.10-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu M, Liu B, Jawad S, et al. C5a contributes to intraocular inflammation by affecting retinal pigment epithelial cells and immune cells. The British journal of ophthalmology. 2011;95:1738–44. doi: 10.1136/bjophthalmol-2011-300235. [DOI] [PubMed] [Google Scholar]
- 49.Liu B, Wei L, Meyerle C, et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. Journal of translational medicine. 2011;9:1–12. doi: 10.1186/1479-5876-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Zhong M, Liang L, Gu F, Peng H. Interleukin-17 induces angiogenesis in human choroidal endothelial cells in vitro. Investigative ophthalmology & visual science. 2014;55:6968–75. doi: 10.1167/iovs.14-15029. [DOI] [PubMed] [Google Scholar]
- 51.Ardeljan D, Wang Y, Park S, et al. Interleukin-17 retinotoxicity is prevented by gene transfer of a soluble interleukin-17 receptor acting as a cytokine blocker: implications for age-related macular degeneration. PloS one. 2014;9:e95900. doi: 10.1371/journal.pone.0095900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC. Extracellular vesicles: potential roles in regenerative medicine. Frontiers in immunology. 2014;5:608. doi: 10.3389/fimmu.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Progress in retinal and eye research. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ardeljan CP, Ardeljan D, Abu-Asab M, Chan CC. Inflammation and Cell Death in Age-Related Macular Degeneration: An Immunopathological and Ultrastructural Model. Journal of clinical medicine. 2014;3:1542–1560. doi: 10.3390/jcm3041542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PloS one. 2009;4:e4160. doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein R, Myers CE, Cruickshanks KJ, et al. Markers of inflammation, oxidative stress, and endothelial dysfunction and the 20-year cumulative incidence of early age-related macular degeneration: the Beaver Dam Eye Study. JAMA ophthalmology. 2014;132:446–55. doi: 10.1001/jamaophthalmol.2013.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seddon JM, George S, Rosner B, Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Archives of ophthalmology. 2005;123:774–82. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 58.Doyle SL, Campbell M, Ozaki E, et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nature medicine. 2012;18:791–8. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Doyle SL, Ozaki E, Brennan K, et al. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Science translational medicine. 2014;6:230ra44. doi: 10.1126/scitranslmed.3007616. [DOI] [PubMed] [Google Scholar]
- 60.Hirano Y, Yasuma T, Mizutani T, et al. IL-18 is not therapeutic for neovascular age-related macular degeneration. Nature medicine. 2014;20:1372–5. doi: 10.1038/nm.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ijima R, Kaneko H, Ye F, et al. Interleukin-18 induces retinal pigment epithelium degeneration in mice. Investigative ophthalmology & visual science. 2014;55:6673–8. doi: 10.1167/iovs.14-15367. [DOI] [PubMed] [Google Scholar]
- 62.Zhang S, Liu Y, Lu S, Cai X. Genetic Variants of Interleukin 17A Are Functionally Associated with Increased Risk of Age-Related Macular Degeneration. Inflammation. 2014 doi: 10.1007/s10753-014-9973-3. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Liu B, Maminishkis A, et al. Gene expression profiling in autoimmune noninfectious uveitis disease. Journal of immunology. 2008;181:5147–57. doi: 10.4049/jimmunol.181.7.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PloS one. 2011;6:e18139. doi: 10.1371/journal.pone.0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cha DM, Woo SJ, Kim HJ, Lee C, Park KH. Comparative analysis of aqueous humor cytokine levels between patients with exudative age-related macular degeneration and normal controls. Investigative ophthalmology & visual science. 2013;54:7038–44. doi: 10.1167/iovs.13-12730. [DOI] [PubMed] [Google Scholar]
- 66.Jonas JB, Tao Y, Neumaier M, Findeisen P. Cytokine concentration in aqueous humour of eyes with exudative age-related macular degeneration. Acta ophthalmologica. 2012;90:e381–8. doi: 10.1111/j.1755-3768.2012.02414.x. [DOI] [PubMed] [Google Scholar]
- 67.Kramer M, Hasanreisoglu M, Feldman A, et al. Monocyte chemoattractant protein-1 in the aqueous humour of patients with age-related macular degeneration. Clinical & experimental ophthalmology. 2012;40:617–25. doi: 10.1111/j.1442-9071.2011.02747.x. [DOI] [PubMed] [Google Scholar]
- 68.Miao H, Tao Y, Li XX. Inflammatory cytokines in aqueous humor of patients with choroidal neovascularization. Molecular vision. 2012;18:574–80. [PMC free article] [PubMed] [Google Scholar]
- 69.Jonas JB, Jonas RA, Neumaier M, Findeisen P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina. 2012;32:2150–7. doi: 10.1097/IAE.0b013e3182576d07. [DOI] [PubMed] [Google Scholar]
- 70.Jonas JB, Tao Y, Neumaier M, Findeisen P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Archives of ophthalmology. 2010;128:1281–6. doi: 10.1001/archophthalmol.2010.227. [DOI] [PubMed] [Google Scholar]
- 71.Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–41. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tarallo V, Hirano Y, Gelfand BD, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–59. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–30. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y, Tarallo V, Kerur N, et al. DICER1/Alu RNA dysmetabolism induces Caspase-8-mediated cell death in age-related macular degeneration. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16082–7. doi: 10.1073/pnas.1403814111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Experimental eye research. 2003;76:463–71. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 77.Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye. 1990;4 ( Pt 4):613–21. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- 78.Grossniklaus HE, Miskala PH, Green WR, et al. Histopathologic and ultrastructural features of surgically excised subfoveal choroidal neovascular lesions: submacular surgery trials report no. 7. Archives of ophthalmology. 2005;123:914–21. doi: 10.1001/archopht.123.7.914. [DOI] [PubMed] [Google Scholar]
- 79.Dastgheib K, Green WR. Granulomatous reaction to Bruch’s membrane in age-related macular degeneration. Archives of ophthalmology. 1994;112:813–8. doi: 10.1001/archopht.1994.01090180111045. [DOI] [PubMed] [Google Scholar]
- 80.Lopez PF, Grossniklaus HE, Lambert HM, et al. Pathologic features of surgically excised subretinal neovascular membranes in age-related macular degeneration. American journal of ophthalmology. 1991;112:647–56. doi: 10.1016/s0002-9394(14)77270-8. [DOI] [PubMed] [Google Scholar]
- 81.Csaky K, Baffi J, Chan CC, Byrnes GA. Clinicopathologic correlation of progressive fibrovascular proliferation associated with occult choroidal neovascularization in age-related macular degeneration. Archives of ophthalmology. 2004;122:650–2. doi: 10.1001/archopht.122.4.650. [DOI] [PubMed] [Google Scholar]
- 82.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 83.Grossniklaus HE, Ling JX, Wallace TM, et al. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Molecular vision. 2002;8:119–26. [PubMed] [Google Scholar]
- 84.Kamei M, Yoneda K, Kume N, et al. Scavenger receptors for oxidized lipoprotein in age-related macular degeneration. Investigative ophthalmology & visual science. 2007;48:1801–7. doi: 10.1167/iovs.06-0699. [DOI] [PubMed] [Google Scholar]
- 85.Suzuki M, Kamei M, Itabe H, et al. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Molecular vision. 2007;13:772–8. [PMC free article] [PubMed] [Google Scholar]
- 86.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Investigative ophthalmology & visual science. 2003;44:3586–92. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 87.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Investigative ophthalmology & visual science. 2003;44:3578–85. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 88.Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS medicine. 2006;3:e310. doi: 10.1371/journal.pmed.0030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 90.Cao X, Shen D, Patel MM, et al. Macrophage polarization in the maculae of age-related macular degeneration: a pilot study. Pathology international. 2011;61:528–35. doi: 10.1111/j.1440-1827.2011.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Penfold PL, Provis JM, Furby JH, Gatenby PA, Billson FA. Autoantibodies to retinal astrocytes associated with age-related macular degeneration. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1990;228:270–4. doi: 10.1007/BF00920033. [DOI] [PubMed] [Google Scholar]
- 92.Patel N, Ohbayashi M, Nugent AK, et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115:422–30. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morohoshi K, Ohbayashi M, Patel N, Chong V, Bird AC, Ono SJ. Identification of anti-retinal antibodies in patients with age-related macular degeneration. Experimental and molecular pathology. 2012;93:193–9. doi: 10.1016/j.yexmp.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 94.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–46. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Faber C, Singh A, Kruger Falk M, Juel HB, Sorensen TL, Nissen MH. Age-related macular degeneration is associated with increased proportion of CD56(+) T cells in peripheral blood. Ophthalmology. 2013;120:2310–6. doi: 10.1016/j.ophtha.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 96.Wong WT, Dresner S, Forooghian F, et al. Treatment of geographic atrophy with subconjunctival sirolimus: results of a phase I/II clinical trial. Investigative ophthalmology & visual science. 2013;54:2941–50. doi: 10.1167/iovs.13-11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nussenblatt RB, Byrnes G, Sen HN, et al. A randomized pilot study of systemic immunosuppression in the treatment of age-related macular degeneration with choroidal neovascularization. Retina. 2010;30:1579–87. doi: 10.1097/IAE.0b013e3181e7978e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oh H, Takagi H, Takagi C, et al. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Investigative ophthalmology & visual science. 1999;40:1891–8. [PubMed] [Google Scholar]
- 99.Arias L, Caminal JM, Badia MB, Rubio MJ, Catala J, Pujol O. Intravitreal infliximab in patients with macular degeneration who are nonresponders to antivascular endothelial growth factor therapy. Retina. 2010;30:1601–8. doi: 10.1097/IAE.0b013e3181e9f942. [DOI] [PubMed] [Google Scholar]
- 100.Wu L, Arevalo JF, Hernandez-Bogantes E, Regatieri CV, Roca JA, Farah ME. Intravitreal tumor necrosis factor-alpha inhibitors for neovascular age-related macular degeneration suboptimally responsive to antivascular endothelial growth factor agents: a pilot study from the Pan American Collaborative Retina Study Group. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013;29:366–71. doi: 10.1089/jop.2012.0203. [DOI] [PubMed] [Google Scholar]
- 101.Semeraro F, Romano MR, Danzi P, Angi M, Costagliola C. Intravitreal infliximab for choroidal neovascularization in patients refractory to conventional treatments. International journal of immunopathology and pharmacology. 2013;26:765–8. doi: 10.1177/039463201302600321. [DOI] [PubMed] [Google Scholar]
- 102.Gomi F, Sawa M, Tsujikawa M, Nishida K. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina. 2012;32:1804–10. doi: 10.1097/IAE.0b013e31825be87f. [DOI] [PubMed] [Google Scholar]
- 103.Ahmadieh H, Taei R, Riazi-Esfahani M, et al. Intravitreal bevacizumab versus combined intravitreal bevacizumab and triamcinolone for neovascular age-related macular degeneration: six-month results of a randomized clinical trial. Retina. 2011;31:1819–26. doi: 10.1097/IAE.0b013e31820d58f2. [DOI] [PubMed] [Google Scholar]
- 104.Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nature reviews Immunology. 2013;13:438–51. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2014 doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 106.Kapp K, Maul J, Hostmann A, et al. Modulation of systemic antigen-specific immune responses by oral antigen in humans. European journal of immunology. 2010;40:3128–37. doi: 10.1002/eji.201040701. [DOI] [PubMed] [Google Scholar]
- 107.Koffeman EC, Genovese M, Amox D, et al. Epitope-specific immunotherapy of rheumatoid arthritis: clinical responsiveness occurs with immune deviation and relies on the expression of a cluster of molecules associated with T cell tolerance in a double-blind, placebo-controlled, pilot phase II trial. Arthritis and rheumatism. 2009;60:3207–16. doi: 10.1002/art.24916. [DOI] [PubMed] [Google Scholar]
- 108.Postlethwaite AE, Wong WK, Clements P, et al. A multicenter, randomized, double-blind, placebo-controlled trial of oral type I collagen treatment in patients with diffuse cutaneous systemic sclerosis: I. oral type I collagen does not improve skin in all patients, but may improve skin in late-phase disease. Arthritis and rheumatism. 2008;58:1810–22. doi: 10.1002/art.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nussenblatt RB, Gery I, Weiner HL, et al. Treatment of uveitis by oral administration of retinal antigens: results of a phase I/II randomized masked trial. American journal of ophthalmology. 1997;123:583–92. doi: 10.1016/s0002-9394(14)71070-0. [DOI] [PubMed] [Google Scholar]


