Abstract
Background
Accurate identification of nonverbal emotional cues is essential to successful social interactions, yet most research is limited to emotional face expression labeling. Little research focuses on the processing of emotional prosody, or tone of verbal speech, in clinical populations.
Methods
Using the Diagnostic Analysis of Nonverbal Accuracy, the current study examined whether youths with pediatric-onset bipolar disorder (BD) and/or those with chronic and severe irritability (i.e. the severe mood dysregulation phenotype) are impaired in their ability to identify the emotional prosody of a spoken sentence with neutral content.
Results
Youths with severe mood dysregulation (n = 67) performed more poorly than healthy comparison children (n = 57), even when the sample was limited to unmedicated patients. Medicated BD youths (n = 52) exhibited impairment relative to healthy comparison children. No interactions between group and emotion were observed, suggesting that emotional prosody labeling problems may represent a general deficit in chronically irritable youths and in medicated youths with BD.
Conclusion
In concert with previously documented facial emotion labeling deficits, difficulties ascertaining the correct emotional tone of a spoken sentence may contribute to emotion dysregulation in chronically irritable children, and possibly also in youths with BD.
Keywords: Bipolar disorder, emotion recognition
Introduction
The diagnostic boundary of bipolar disorder (BD) in youths is the subject of considerable debate. Specifically, whether youths with chronic and impairing irritability and hyperarousal symptoms exhibit a developmental form of BD is unclear. To facilitate research into the diagnostic status of this group, Leibenluft, Charney, Towbin, Bhangoo, and Pine (2003) operationalized symptom criteria for severe mood dysregulation syndrome (SMD) so that youths with SMD could be compared with those with ‘classic’ forms of BD. However, SMD is not a currently recognized disorder in the DSM. Symptom profiles of BD and SMD are distinguished primarily by the episodicity/chronic nature of mood symptoms. For example, the symptoms of BD include distinct manic and depressive episodes, whereas those of SMD are characterized by severe, chronic irritability (Baroni, Lunsford, Luckenbaugh, Towbin, & Leibenluft, 2009). Studies contrasting BD and SMD on clinical, behavioral and pathophysiological measures could shed light on this controversy. A recent review concluded that familial, longitudinal, behavioral and neuroimaging data indicate differences between BD and SMD (Leibenluft, 2011). Notably, longitudinal studies of youths with SMD or irritability indicate that these clinical presentations in childhood are associated with increased risk of unipolar depression and anxiety disorders, but not BD, in adulthood (Brotman et al., 2006; Stringaris, Cohen, Pine, & Leibenluft, 2009; Stringaris et al., 2010). One pilot family history study found that, compared to youths with SMD, youths with BD are at increased risk to have a parent with BD (Brotman et al., 2007). Finally, while SMD and BD share some behavioral deficits (e.g. on face emotion processing tasks; Guyer et al., 2007), suggesting the presence of similar pathophysiological mechanisms, two studies indicate that the neural responses underlying these shared behavioral deficits may differ between groups (Brotman et al., 2010; Rich et al., 2007). However, the existing literature is small, so further studies comparing BD and SMD are needed to elucidate the pathophysiology of these syndromes and inform the development of novel treatments.
An important domain of comparison for SMD and BD is the ability to process social cues, because these deficits may contribute to interpersonal difficulties. Such difficulties have been documented in pediatric BD (Geller et al., 2000; Goldstein et al., 2009). The SMD inclusion criteria specify that the patient’s irritability must be impairing in two of three contexts (home, school and peers); such impairment invariably manifests in conflictual interpersonal relationships in each of these domains. Evidence suggests that BD youths exhibit deficits on behavioral tasks assessing the ability to identify emotional facial expressions (Brotman, Guyer, et al., 2008; Guyer et al., 2007; McClure et al., 2005; Schenkel, Pavuluri, Herbener, Harral, & Sweeney, 2007). Specifically, they require higher levels of emotional intensity to identify emotional expressions accurately (Brotman, Skup, et al., 2008; Rich et al., 2008) and have difficulty differentiating between subtle variations in facial expression intensity (Schenkel et al., 2007). They also display other social deficits, including impairments in taking another person’s perspective in emotional, but not neutral, contexts (Schenkel, Marlow-O’Connor, Moss, Sweeney, & Pavuluri, 2008), and reduced awareness of the appropriate language to use in social situations (McClure et al., 2005). SMD populations are less frequently studied. However, evidence indicates that they have similar deficits. Indeed, in one study SMD youths made an equal number of errors when labeling facial emotions as youths with BD, but more errors than healthy children and youths with mood, anxiety, conduct or attention deficit disorders (Guyer et al., 2007). In a second study using morphed facial expressions, SMD youths required more emotional intensity to identify a facial expression than healthy children, and were equally as impaired as BD youths (Rich et al., 2008). On a final face processing task, youths with SMD exhibited amygdala hypoactivation relative to BD and healthy comparison controls (Brotman et al., 2010). Such deficits in SMD youths might result in disrupted social cue processing, including difficulties identifying the emotional tone of a spoken word or phrase, given the role that the amygdala plays in assigning salience to stimuli.
An understudied aspect of nonverbal communication, particularly in pediatric psychopathology, is an individual’s ability to identify the emotional prosody or tone of a statement. Like visual cues, deficits in recognizing the emotional prosody of spoken words (e.g. interpreting playful sarcasm as hurtful criticism) can lead to inappropriate responses (e.g. angry outbursts) which can strain interpersonal relationships, reduce social support, and increase risk for mood episodes and/or exacerbate symptoms. Also, increased understanding of emotional prosody identification deficits may facilitate the development of more effective psychosocial treatments. Many psychosocial treatments for youths with BD focus on higher order social skills (e.g. initiating conversation and resolving conflicts; for review, see West & Pavuluri, 2009) rather than on emotional cue perception. Some promising findings have emerged from preliminary studies of nonverbal emotion identification training in other psychiatric populations (e.g. Horan et al., 2011), and these might be promising adjunctive treatments in youths with BD and SMD.
Evidence of impaired emotional prosody identification exists among adults with BD or unipolar depression (Kan, Mimura, Kamijima, & Kawamura, 2004; Murphy & Cutting, 1990; Peron et al., 2011; Uekermann, Abdel-Hamid, Lehmkamper, Vollmoeller, & Daum, 2008), even during euthymia (Bozikas et al., 2007). Specifically, individuals with major depressive disorder (MDD) and BD are less likely to correctly identify the emotional prosody of sentences or pseudowords with semantically neutral content relative to healthy adults. In one study, the impairment in MDD adults was specific to the surprise emotion (Kan et al., 2004); however, other studies noted more general deficits across emotion categories in both MDD and BD populations (Murphy & Cutting, 1990; Peron et al., 2011; Uekermann et al., 2008). One study in adult BD indicates aberrant patterns of neural activation, including the amygdala, uncus, superior temporal gyrus and inferior frontal gyrus, when attending to the emotional prosody of sentences with no semantic content (Mitchell, Elliott, Barry, Cruttenden, & Woodruff, 2004). In the one study of prosody identification in children with mood problems, school-age boys scoring high on self-report measures of depression display impaired prosody identification of pseudowords spoken in angry, happy, sad and neutral tones relative to nondepressed comparisons (Emerson, Harrison, & Everhart, 1999). Youths with high self-reported depression scores were impaired on all emotions relative to healthy children. However, these subjects were recruited from a school setting and did not receive diagnostic assessments. Thus, it remains unknown whether emotional prosody deficits exist in children with clinically significant mood problems.
Here, we investigate whether BD and SMD youths have deficits identifying emotional prosody. Such deficits would indicate that these patients have impairments in identifying emotions from both visual and auditory nonverbal cues. By contrast, intact emotional prosody identification abilities would suggest that BD and SMD youths have specific deficits labeling facial emotion expressions. We used the Diagnostic Analysis of Nonverbal Accuracy (DANVA–2; Baum & Nowicki, 1998; Rothbaum & Nowicki, 2004) –a standardized measure of participants’ ability to identify the emotional nonverbal communication of a neutral phrase spoken in one of four emotional prosodies (anger, happiness, fear or sadness). Given prior work with facial recognition deficits, we hypothesized that both BD and SMD youths would exhibit impairments relative to healthy children when labeling emotional prosody. As prior studies on related nonverbal cue processing in BD and mood disordered youths found deficits across emotion categories (e.g. Emerson et al., 1999; Guyer et al., 2007), we expected deficits to manifest across emotions.
Method
Participants
Participants and parents gave written informed assent and consent in this National Institute of Mental Health (NIMH) Institutional Review Board-approved study. Participants were 8–17 years old and included BD (n = 76), SMD (n = 67) and healthy control youths (HC; n = 57). Participants were assessed using the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS–PL; Kaufman et al., 1997), with a supplemental module to ascertain SMD. To measure mood symptoms, clinicians administered the Children’s Depression Rating Scale (CDRS; Poznanski et al., 1984) and the Mood Symptom Questionnaire (designed to assess the severity of irritability; A. Stringaris, unpublished data) to patients with BD and SMD and the Young Mania Rating Scale (YMRS; Young et al., 1978) to BD youths. Euthymia was defined as: CDRS < 40 and YMRS ≤ 12 for BD and CDRS < 40 for SMD. Depression was defined as CDRS ≥ 40 in SMD and CDRS ≥ 40 and YMRS ≤ 12 in BD; hypomania/mania as: CDRS < 40 and YMRS > 12; and mixed state: CDRS ≥ 40 and YMRS > 12; see Table 1). Interviews and mood ratings were conducted with children and their parents separately by masters- or doctoral level clinicians who have all achieved high interrater reliability (κ ≥ .9) on each measure following a training procedure that involves observing and conducting ratings under the supervision of a clinician who has obtained reliability. Training clinicians achieved reliability after reliability statistical analyses for individual item responses for achieved κ ≥ .9 on each measure.
Table 1.
Demographic and clinical characteristics of youths with BD, SMD or no psychiatric history: age, rating scales, number of diagnoses and medications for the three study groups
| Characteristic | Children with BD (n = 76)
|
Children with SMD (n = 67)
|
Healthy children (n = 57)
|
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 12.84 | 2.67 | 12.47 | 1.85 | 13.20 | 2.13 |
| FSIQa | 106.07 | 15.23 | 103.88 | 13.08 | 112.56 | 13.90 |
| YMRSb | 48.99 | 7.57 | – | – | – | – |
| CDRS | 28.84 | 10.11 | 28.73 | 10.27 | – | – |
| MSQb | 22.69 | 9.00 | 26.07 | 7.84 | – | – |
| Number of comorbid/co-occurring diagnosesb | 1.99 | 1.65 | 2.62 | 1.22 | – | – |
| Number of medicationsb | 2.38 | 1.76 | 1.67 | 1.67 | – | – |
BD = bipolar disorder; SMD = severe mood dysregulation; FSIQ = full-scale IQ measured using Wechsler Abbreviated Scale of Intelligence; YMRS = Young Mania Rating Scale; CDRS = Children’s Depression Rating Scale; MSQ = Mood Symptoms Questionnaire.
HC > BD = SMD.
Data were not available for all participants for these variables. The following subsample of data was included in each variable for BD participants: YMRS (n = 75); MSQ (n = 68); medication status (n = 68). For SMD participants, co-occurring diagnoses (n = 66).
Bipolar disorder participants met criteria for ‘narrow-phenotype’ BD, that is, lifetime history of at least one hypomanic or manic episode meeting DSM–IV–TR duration criteria with elevated/expansive mood and at least three ‘B’ mania symptoms (Leibenluft et al., 2003). Most were BD I (n = 58; 77.3%; data unavailable for 1 youth), euthymic (n = 44; 57.9%); and medicated (n = 52; 76.5%; data unavailable for 8 youths) when tested (see Table 2).
Table 2.
Demographic and clinical characteristics of youths with BD, SMD or no psychiatric history: breakdown by gender, BD and comorbid diagnoses, mood states and types of medication for the three study groups
| Characteristic | Children with BD (n = 76)
|
Children with SMD (n = 67)
|
Healthy children (n = 57)
|
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Male | 47 | 61.8 | 47 | 70.2 | 30 | 52.6 |
| BD Ia | 58 | 77.3 | – | – | – | – |
| BD IIa | 16 | 21.3 | – | – | – | – |
| Comorbid diagnosesa | ||||||
| ADHD | 44 | 57.9 | 58 | 87.9 | – | – |
| ODD or CD | 23 | 30.3 | 51 | 77.3 | – | – |
| MDD | – | – | 11 | 16.7 | – | – |
| Anxiety | 42 | 55.3 | 32 | 48.5 | – | – |
| Mood state | ||||||
| Euthymic | 44 | 57.9 | 61 | 91.0 | – | – |
| Depressed | 5 | 6.6 | 6 | 9.0 | – | – |
| Manic or hypomanic | 21 | 27.6 | – | – | – | – |
| Mixed | 6 | 7.9 | – | – | – | – |
| Medicationa | ||||||
| Unmedicated | 16 | 23.5 | 25 | 37.3 | – | – |
| Atypical antipsychotic | 40 | 58.8 | 23 | 34.3 | – | – |
| Lithium | 21 | 30.9 | 6 | 9.0 | – | – |
| Antiepileptic | 34 | 50.0 | 16 | 23.9 | – | – |
| Antidepressant | 22 | 32.4 | 15 | 22.4 | – | – |
| Stimulants | 14 | 20.6 | 26 | 38.8 | – | – |
Percentages indicate percentage of available data. BD = bipolar disorder; SMD = severe mood dysregulation; ADHD = attention deficit hyperactivity disorder; ODD = oppositional defiant disorder; CD = conduct disorder; MDD = major depressive disorder; anxiety = any anxiety disorder.
Data were not available for all participants for these variables. The following subsample of data was included in each variable for BD participants: BD I or BD II status (n = 75); medication status (n = 68). For SMD participants, co-occurring diagnoses (n = 66).
Severe mood dysregulation participants met criteria for SMD, that is, nonepisodic irritability, excessive reactivity and hyperarousal (Leibenluft et al., 2003). The syndrome must begin prior to age 12, and last for at least 1 year without a period of remission exceeding 2 months. Symptoms must cause severe impairment in at least one setting (home, school and peers), and at least mild impairment in another. Children with a history of hypomanic episodes of longer than 1 day were excluded. Most SMD youths were not depressed (n = 61; 91.0%) and were medicated (n = 42; 62.7%) when tested (Table 2).
Control subjects were psychiatrically healthy, based on the K-SADS–PL. Also, having a first degree relative with a mood disorder was exclusionary for this group. Exclusion criteria for all groups included full-scale IQ (FSIQ) < 70, unstable and/or chronic medical illness, or substance abuse within the past 2 months. We also excluded individuals with suspected pervasive developmental disorder (PDD). A best estimate procedure was used, which relied on information gathered from medical records, conversations with treating clinicians, observations during evaluation and scores on three PDD measures – Social Communication Questionnaire (Berument, Rutter, Lord, Pickles, & Bailey, 1999), Children’s Communication Checklist (Bishop, 1998) and Social Responsiveness Scale (Constantino et al., 2003). These latter scales were useful in characterizing the child’s function in terms of PDD characteristics but were not used as exclusionary based on specific scores, as prior work has shown that they yield inconsistent results in our study populations in terms of screening children into the ‘PDD-likely’ range (Towbin, Pradella, Gorrindo, Pine, & Leibenluft, 2005). Instead, the best estimate procedure consisted of our clinical staff, including a child psychiatrist with expertise in the assessment of PDD, reviewing all of the available information and, as a group, making a consensus decision as to whether a participant’s level of PDD symptoms was sufficient to warrant concern about a possible diagnosis and therefore ineligibility for the study. Only children with no more than mild social and language impairment were eligible for the study. Children with moderate to severe impairment in the social reciprocity or communicative domains were excluded.
Measures
The Child and Adult Paralanguage subtests of the Diagnostic Analysis of Nonverbal Accuracy (DANVA–2; Baum & Nowicki, 1998; Rothbaum & Nowicki, 2004) were used to assess emotional prosody labeling. This standardized computerized instrument was designed for use with pediatric populations and has good internal consistency and reliability. Assessments of construct validity indicate associations between performance on the adult and child paralanguage subtests and measures of social competence across a wide range of ages (for review see Baum & Nowicki, 1998; Rothbaum & Nowicki, 2004). This test has also been used to probe nonverbal identification abilities in children with disruptive behavior disorders (Cadesky, Mota, & Schachar, 2000; McClure & Nowicki, 1998; Stevens, Charman, & Blair, 2001); the face emotion identification subtests have been used with youths with BD, SMD, MDD, anxiety and conduct/attention problems (e.g. Brotman, Guyer, et al., 2008; Guyer et al., 2007; McClure et al., 2005). For additional information about the psychometric qualities of this test, please see: http://psychology.emory.edu/clinical/interpersonal/DANVAmanual03.doc.
Each subtest features a standardized set of recordings of child (n = 24) or adult (n = 24) actors repeating a neutral phrase (I’m going out of the room now, but I’ll be back later) in happy, sad, angry or fearful emotional tones. Using a button press, participants indicated which emotion the actor expressed. The primary outcome variable was the total number of errors made in each emotion and actor category.
Data analysis
Separate analyses of variance (ANOVAs) assessed group differences in age and FSIQ, while chi-square tested gender differences. Because FSIQ differed between groups, it served as a covariate. A Group (BD, SMD, HC) × Emotion (happy, sad, angry, fearful) × Actor (child, adult) repeated measures analysis of covariance (ANCOVA), with FSIQ as a covariate, examined the number of errors per emotion category. Post hoc ANCOVAs were used for post hoc analyses and partial η2 effect sizes (η2p) are reported.
A secondary Group (BD, SMD, HC) × Emotion (happy, sad, angry, fearful) × Intensity (low, high) repeated measures ANCOVA, with FSIQ as a covariate, was used to examine whether the number of errors differed depending on emotional intensity.
To examine medication effects, BD and SMD groups were stratified by medication status (medicated or unmedicated). ANOVAs comparing total voice errors between each subgroup and HC were conducted, and FSIQ was covaried when appropriate. Pearson correlations between number of errors made (both total errors and the number of errors made in each emotion category) and CDRS, YMRS (n = 75) and MSQ (n = 68) scores were completed for BD and between total voice errors and CDRS and MSQ scores for SMD.
Results
Demographics
Groups differed in FSIQ, but not age or gender (see Tables 1 and 2). IQ scores of SMD and BD did not differ, but both were lower than HC [Group: F(2, 197) = 6.17, p < .005, η2p = .06]. Consequently, FSIQ was included as a covariate in the primary analyses.
Emotion labeling errors
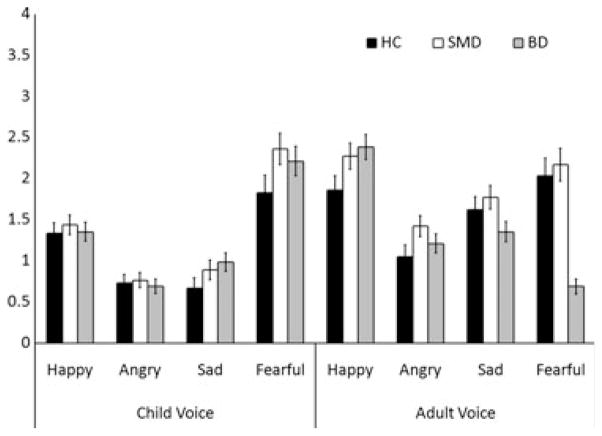
There were no two-way or three-way interactions; therefore, we focused on main effects. Groups differed in total number of emotion labeling errors, Group: F(2, 196) = 3.22, p < .05, η2p = .03 (Figure 1, Table 3). Post hoc ANCOVAs exploring this main effect demonstrated that SMD (M = 13.28, SD = 4.68) made more errors than HC (M = 10.77, SD = 3.89; p < .02, η2p = .05). A trend for greater errors among BD (M = 12.51, SD = 4.24) relative to HC was also observed (p < .09; η2p = .02). The two clinical groups did not differ (p > .35, η2p = .006). A trend for a main effect of Actor (p < .06, η2p = .02) emerged, suggesting all participants made more errors for adult than child voices. There was no main effect of Emotion.
Figure 1.
Mean number of errors made in each emotion and actor category per diagnostic group. A main effect of diagnostic group [F(2, 196) = 3.22, p < .05] revealed significantly more errors among youths with SMD as compared with healthy comparison children (p < .02). Youths with BD exhibited a trend toward greater errors than healthy children (p < .09). No interactions among diagnostic group, emotion, or actor were observed. SMD = youths with severe mood dysregulation; BD = youths with bipolar disorder
Table 3.
Analysis of covariance results comparing errors made by youths with BD, SMD and HC children
| Effect | F | df | p-Value | Post hoc results |
|---|---|---|---|---|
| Group | 3.22 | 2,196 | .04 | SMD < HC*; BD < HC † |
| Actor | 3.63 | 1,196 | .06 | Adult > child † |
| Emotion | 2.20 | 3,588 | .10 | |
| Actor × Emotion | 1.08 | 3,588 | .35 | |
| Group × Actor | 0.10 | 2,196 | .90 | |
| Group × Emotion | 0.23 | 6,588 | .95 | |
| Group × Actor × Emotion | 1.72 | 6,588 | .12 |
Analyses were conducted after covarying full-scale IQ. BD = bipolar disorder (n = 76); SMD = severe mood dysregulation (n = 67); HC = healthy children (n = 57).
p < .10;
p < .05.
The secondary analysis of emotion intensity revealed that all participants made more errors identifying the prosody of low intensity voices relative to high intensity voices [Intensity: F(1, 196) = 9.25, p < .005]. However, there were no interactions between Intensity and any of the other factors, including Group.
Impact of medication and mood state
Medicated BD youths (n = 52) made more errors than HC children, F(1, 107) = 4.22, p < .05, η2p = .04, but no differences emerged between unmedicated BD (n = 16) and HC youths (p > .60, η2p = .02). Unmedicated SMD youths (n = 25) made more errors than HC children Group, F(1, 79) = 6.04, p < .02, η2p = .07, but medicated SMD (n = 42) did not differ from HC children (p > .09, η2p = .03).
The potential confounding role of mood state was examined. No significant correlations between clinical variables (CDRS, YMRS and MSQ) and either total error scores or the number of errors in each emotional category were observed for either BD or SMD (all rs < |.18| all ps > .10).
These results indicate that error rates are related to medication status. Namely, elevated error rates, relative to healthy subjects, are present only in medicated BD and unmedicated SMD. In each group, error rates are unrelated to current symptoms of depression, mania or irritability.
Discussion
Our findings suggest that the facial emotion recognition deficits that have been observed previously in BD and SMD (Guyer et al., 2007; McClure et al., 2005; Schenkel et al., 2007) may reflect more general deficits in identifying emotional information from nonverbal social cues. Specifically, SMD youths exhibited greater errors labeling the emotional prosody of sentences with neutral semantic content relative to healthy comparisons. The BD sample as a whole exhibited a trend in this direction; however, medicated BD youths differed significantly from HC. Errors were unrelated to current symptoms of depression, irritability and mania.
The existence of emotional prosody identification deficits among SMD youths and medicated BD youths adds to the literature in several ways. Although emotional prosody is not widely studied, the ability to identify the emotional tone of spoken information is an important element of social interactions. Hence, the deficits we observed in SMD and medicated BD may contribute to the social impairments documented in youths with BD (Geller et al., 2000; Goldstein et al., 2009) and the difficulties observed in SMD because of the impact of their extreme irritability on interpersonal relationships. Interestingly, there was no Group × Emotion interaction, suggesting a generalized difficulty with emotional prosody identification similar to the global face emotion identification deficits observed in these populations. Studies with morphed facial stimuli suggest that both SMD and BD youths require more emotional information than HC to classify facial expressions correctly (Brotman, Skup, et al., 2008; Rich et al., 2008) and have difficulty discriminating between varying face emotion intensity levels (Schenkel et al., 2007). Similar studies might be conducted with emotional prosody to assess impairments distinguishing subtleties in vocal tone. Such deficits might have important implications for the patients’ ability to establish and maintain reciprocal relationships. This may influence patient’s support networks, leaving them more vulnerable to life stressors and subsequent risk for future mood episodes. In addition, existing psychosocial treatments may benefit from including emotional cue recognition training modules to remedy these deficits, as in schizophrenia (Horan et al., 2011).
Emotional prosody identification deficits may have implications beyond immediate interpersonal interactions. For example, studies link accurate emotion perception (including emotional prosody identification) with poor occupational outcomes among adults with schizophrenia (Kee, Green, Mintz, & Brekke, 2003). Also, emotional prosody may influence how semantic information is encoded and recalled (Schirmer, 2010). Thus, nonverbal emotion labeling deficits may reflect general abnormalities in emotion processing and contribute to poor emotion regulation. This preliminary study used a small number of experimentally controlled emotional prosody variables to investigate potential deficits in SMD and BD youths. However, research using more ecologically valid stimuli, measures of later recall, and emotion regulation measures may elucidate areas of dysfunction in these populations and provide a basis for future psychosocial treatments.
Evidence for emotional prosody deficits in SMD and medicated BD also helps to constrain hypotheses regarding the neural circuitry dysfunction mediating social cognition deficits in these populations. Specifically, our data suggest that emotion processing deficits in SMD and BD are mediated by dysfunction in areas central to emotional processing or attention, including the amygdala, orbitofrontal cortex and prefrontal cortex, rather than in areas associated with visual or prosody-specific processing. As we only measured behavioral responses, such hypotheses are speculative and should be tested using fMRI (functional magnetic resonance imaging). However, they are consistent with the neural dysfunction observed in BD adults during emotional prosody (Mitchell et al., 2004).
Consistent with data on face emotion identification (Guyer et al., 2007; Rich et al., 2008), BD and SMD did not differ in ability to label emotional prosody, although both were impaired (at least at a trend level) relative to HC. The SMD syndrome was developed to define a phenotype that had been postulated to be a developmental manifestation of BD (Leibenluft et al., 2003); the two populations are differentiated mainly by the presence (in BD) or absence (in SMD) of episodic mania. In earlier work, both groups exhibited impairment on a face emotion labeling task relative to healthy controls and to patients with unipolar depressed/anxious or conduct/attention deficit hyperactivity disorders (Guyer et al., 2007). However, early examinations of the neural underpinnings of such face emotion identification deficits point to important brain-based distinctions between BD and SMD, despite similar performance on behavioral tasks. Specifically, during emotional ratings of neutral faces, SMD youths exhibited less amygdala activation than controls or BD (Brotman et al., 2010). Whether similar neural distinctions would emerge for emotional prosody recognition in these populations is unknown. Several brain regions integral to the identification of emotional prosody and face emotions have been implicated in the dysfunctional neural circuitry of both BD and SMD. Dysfunction in this circuitry may act as a ‘final common pathway’ for nonverbal emotion identification deficits in BD and SMD; future research could clarify whether the mediating circuitry differs between groups and/or sensory modalities. In this way, fMRI research into nonverbal emotional processing could elucidate similarities and differences between the two disorders, with implications for diagnosis and treatment.
Future research should also investigate the specificity of our findings to medicated BD youths. Possibly, emotional prosody deficits occur only in BD youths with severe illness who cannot be taken off medication. Alternatively, medications may cause the deficit. The impact of medication on nonverbal emotion processing is unclear. Studies document face emotion labeling deficits regardless of medication status (Brotman, Guyer, et al., 2008; Guyer et al., 2007). Other studies describe nonverbal processing impairment only among medicated BD youths (Schenkel et al., 2007), and research suggests that particular medications (e.g. lithium; Holmes et al., 2008) can impair performance on emotional tasks in adults with BD. We cannot investigate the impact of specific medications on emotional prosody deficits due to ethical concerns that preclude discontinuing severely ill children from medication for research, and because most children were taking medications from several categories. Research is necessary to investigate the role of medications on nonverbal emotion identification processes.
Limitations
Without an additional psychiatric comparison group lacking emotional prosody deficits, deficits in SMD and medicated BD youths may represent nonspecific abnormalities associated with childhood psychopathology. Data indicate face emotion labeling deficits in BD and SMD but not in other mood, anxiety and conduct disorders (Guyer et al., 2007); however, emotion prosody deficits have been observed in youths with depression symptoms (Emerson et al., 1999) or ADHD (Uekermann et al., 2008), and adults with schizophrenia and autism spectrum disorders (Hoekert, Kahn, Pijnenborg, & Aleman, 2007; Philip et al., 2010). Correlations between social anxiety symptoms and emotional prosody deficits have been documented as well (McClure & Nowicki, 2001). However, we are unaware of any studies directly comparing emotional prosody identification abilities between youths with mood disorders and those with other psychiatric disorders. Therefore, the specificity of emotion prosody labeling deficits is unknown and studies directly comparing different psychiatric groups in this domain would contribute to the literature.
In the present study, participants were recruited from a wide age range, spanning a number of significant developmental changes. Because no group differences in age were observed, we believe that the emotional prosody deficits seen in the SMD and medicated BD groups cannot be attributable to age. However, future investigations of emotional prosody would benefit from a closer examination of developmental stages.
To maximize clinical differences between BD and SMD, BD was limited to the ‘narrow-phenotype’, requiring at least one manic or hypomanic episode with euphoria or grandiosity. It is unknown whether these findings generalize to youths with manic episodes characterized by irritability rather than elation.
Future research might include a neutral prosody condition. Some data indicate that BD and SMD youths perceive neutral facial stimuli as more threatening than do HC (Brotman et al., 2010; Rich et al., 2006), while other social cognition deficits may be specific to emotional versus neutral conditions (Schenkel et al., 2008). In addition, nonverbal language and social skills assessments would be helpful to correlate with performance on standardized behavioral measures.
Conclusions
This study of emotional prosody recognition suggests that emotional labeling deficits in SMD, and possibly BD, extend to emotional prosody as well as face emotion labeling. A diffuse pattern of nonverbal emotional labeling deficits may contribute to social deficits in these samples, with potential implications for psychosocial interventions. Research into this understudied topic, including the role of psychotropic medications and the neural underpinnings of emotional prosody identification processes, could provide important information about psychosocial function in BD and SMD, including similarities and distinctions between them. Finally, information about nonverbal emotion processing differences could facilitate the development of psychotherapeutic interventions to address systematic errors and improve social skills in these youths.
Key points.
Youths with severe mood dysregulation and a medicated subsample of youths with bipolar disorder are impaired in their ability to identify the emotional prosody of a spoken sentence with neutral content.
These findings extend our understanding of nonverbal emotional processing abnormalities in these populations.
These findings may inform treatment emphasizing focus on social skills including the accurate identification of nonverbal emotional cues.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA. We thank the children and families for their participation, which made this research possible. We also thank the staff of the Emotion and Development Branch at NIMH.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Baroni A, Lunsford JR, Luckenbaugh DA, Towbin KE, Leibenluft E. Practitioner review: The assessment of bipolar disorder in children and adolescents. Journal of Child Clinical Psychology and Psychiatry. 2009;50:203–215. doi: 10.1111/j.1469-7610.2008.01953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum KM, Nowicki SN., Jr Perception of emotion: Measuring decoding accuracy of adult prosodic cues varying in intensity. Journal of Nonverbal Behavior. 1998;22:89–107. [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bishop DV. Development of the Children’s Communication Checklist (CCC): A method for assessing qualitative aspects of communicative impairment in children. Journal of Child Clinical Psychology and Psychiatry. 1998;39:879–891. [PubMed] [Google Scholar]
- Bozikas VP, Kosmidis MH, Tonia T, Andreou C, Focas K, Karavatos A. Impaired perception of affective prosody in remitted patients with bipolar disorder. Journal of Neuropsychiatry and Clinical Neuroscience. 2007;19:436–440. doi: 10.1176/jnp.2007.19.4.436. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Guyer AE, Lawson ES, Horsey SE, Rich BA, Dickstein DP, Leibenluft E. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. American Journal of Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Kassem L, Reising MM, Guyer AE, Dickstein DP, Rich BA, Leibenluft E. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. American Journal of Psychiatry. 2007;164:1238–1241. doi: 10.1176/appi.ajp.2007.06101619. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, Towbin K. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA, Schmajuk M, Rich BA, Dickstein DP, Guyer AE, Costello EJ, Leibenluft E. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biological Psychiatry. 2006;60:991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Skup M, Rich BA, Blair KS, Pine DS, Blair JR, Leibenluft E. Risk for bipolar disorder is associated with face-processing deficits across emotions. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1455–1461. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadesky EB, Mota VL, Schachar RJ. Beyond words: How to children with ADHD and/or conduct problems process nonverbal information about affect. Journal of the Academy of Child and Adolescent Psychiatry. 2000;39:1160–1167. doi: 10.1097/00004583-200009000-00016. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Reich W. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Emerson CS, Harrison DW, Everhart DE. Investigation of receptive affective prosodic ability in school-aged boys with and without depression. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1999;12:102–109. [PubMed] [Google Scholar]
- Geller B, Bolhofner K, Craney JL, Williams M, DelBello MP, Gundersen K. Psychosocial functioning in a prepubertal and early adolescent bipolar disorder phenotype. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1543–1548. doi: 10.1097/00004583-200012000-00018. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Birmaher B, Axelson D, Goldstein BI, Gill MK, Esposito-Smythers C, Keller M. Psychosocial functioning among bipolar youth. Journal of Affective Disorders. 2009;114:174–183. doi: 10.1016/j.jad.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure EB, Adler AD, Brotman MA, Rich BA, Kimes AS, Leibenluft E. Specificity of facial expression labeling deficits in childhood psychopathology. Journal of Child Clinical Psychology and Psychiatry. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: Review and meta-analysis. Schizophrenia Research. 2007;96:135–145. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Holmes MK, Erickson K, Luckenbaugh DA, Drevets WC, Bain EE, Cannon DM, Zarate CA. A comparison of cognitive functioning in medicated and unmedicated subjects with bipolar depression. Bipolar Disorders. 2008;10:806–815. doi: 10.1111/j.1399-5618.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Tripp C, Hellemann G, Wynn JK, Bell M, Green MF. Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. Journal of Psychiatric Research. 2011;45:1113–1122. doi: 10.1016/j.jpsychires.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y, Mimura M, Kamijima K, Kawamura M. Recognition of emotion from moving facial and prosodic stimuli in depressed patients. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75:1667–1671. doi: 10.1136/jnnp.2004.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kee KS, Green MF, Mintz J, Brekke JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophrenia Bulletin. 2003;29:487–497. doi: 10.1093/oxfordjournals.schbul.a007021. [DOI] [PubMed] [Google Scholar]
- Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. American Journal of Psychiatry. 2011;168:129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. American Journal of Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- McClure EB, Nowicki S., Jr Emotion processing ability and severe psychological problems of children; Paper presented to the Psychoeducational Network of Georgia meetings; Brunswick, GA. 1998. Aug, [Google Scholar]
- McClure EB, Nowicki S., Jr Associations between social anxiety and nonverbal processing skill in preadolescent boys and girls. Journal of Nonverbal Behavior. 2001;25:3–19. [Google Scholar]
- McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Leibenluft E. Deficits in social cognition and response flexibility in pediatric bipolar disorder. American Journal of Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- Mitchell RL, Elliott R, Barry M, Cruttenden A, Woodruff PW. Neural response to emotional prosody in schizophrenia and in bipolar affective disorder. British Journal of Psychiatry. 2004;184:223–230. doi: 10.1192/bjp.184.3.223. [DOI] [PubMed] [Google Scholar]
- Murphy D, Cutting J. Prosodic comprehension and expression in schizophrenia. Journal of Neurology, Neurosurgery, and Psychiatry. 1990;53:727–730. doi: 10.1136/jnnp.53.9.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron J, El Tamer S, Grandjean D, Leray E, Travers D, Drapier D, Millet B. Major depressive disorder skews the recognition of emotional prosody. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:987–996. doi: 10.1016/j.pnpbp.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Philip RC, Whalley HC, Stanfield AC, Sprengelmeyer R, Santos IM, Young AW, Hall J. Deficits in facial, body movement and vocal emotional processing in autism spectrum disorders. Psychological Medicine. 2010;40:1919–1929. doi: 10.1017/S0033291709992364. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Developmental Psychopathology. 2008;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. American Journal of Psychiatry. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, McClure EB, Leibenluft E. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proceedings of the National Academy of Sciences USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum AD, Nowicki S., Jr A measure of the ability to identify emotion in children’s tone of voice. Journal of Nonverbal Behavior. 2004;28:67–92. [Google Scholar]
- Schenkel LS, Marlow-O’Connor M, Moss M, Sweeney JA, Pavuluri MN. Theory of mind and social inference in children and adolescents with bipolar disorder. Psychological Medicine. 2008;38:791–800. doi: 10.1017/S0033291707002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel LS, Pavuluri MN, Herbener ES, Harral EM, Sweeney JA. Facial emotion processing in acutely ill and euthymic patients with pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1070–1079. doi: 10.1097/chi.0b013e3180600fd6. [DOI] [PubMed] [Google Scholar]
- Schirmer A. Mark my words: Tone of voice changes affective word representations in memory. PLoS ONE. 2010;5:e9080. doi: 10.1371/journal.pone.0009080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens D, Charman T, Blair RJR. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. The Journal of Genetic Psychology. 2001;162:201–211. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- Stringaris A, Baroni A, Haimm C, Brotman M, Lowe CH, Myers F, Leibenluft E. Pediatric bipolar disorder versus severe mood dysregulation: Risk for manic episodes on follow-up. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:397–405. [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: A 20-year prospective community-based study. American Journal of Psychiatry. 2009;166:1048–1054. doi: 10.1176/appi.ajp.2009.08121849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin KE, Pradella A, Gorrindo T, Pine DS, Leibenluft E. Autism spectrum traits in children with mood and anxiety disorders. Journal of Child and Adolescent Psychopharmacology. 2005;15:452–464. doi: 10.1089/cap.2005.15.452. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Abdel-Hamid M, Lehmkamper C, Vollmoeller W, Daum I. Perception of affective prosody in major depression: A link to executive functions? Journal of the International Neuropsychological Society. 2008;14:552–561. doi: 10.1017/S1355617708080740. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British journal of psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- West AE, Pavuluri MN. Psychosocial treatments for childhood and adolescent bipolar disorder. Child and Adolescent Psychiatric Clinics of North America. 2009;18:471–482. x–xi. doi: 10.1016/j.chc.2008.11.009. [DOI] [PubMed] [Google Scholar]