Abstract
Rationale
Acid-Sensing Ion Channels (ASICs) are Na+ channels that are activated by acidic pH. Their expression in cardiac afferents and remarkable sensitivity to small pH changes has made them leading candidates to sense cardiac ischemia.
Objective
Four genes encode six different ASIC subunits, however it is not yet clear which of the ASIC subunits contribute to the composition of ASICs in cardiac afferents.
Methods and Results
Here we labeled cardiac afferents using a retrograde tracer dye in mice, which allowed for patch-clamp studies of murine cardiac afferents. We found that a higher percentage of cardiac sensory neurons from the dorsal root ganglia (DRG) respond to acidic pH and generated larger currents compared to those from the nodose ganglia (NG). The ASIC-like current properties of the cardiac DRG neurons from wild-type mice most closely matched the properties of ASIC2a/3 heteromeric channels. This was supported by studies in ASIC null mice: acid-evoked currents from ASIC3 −/− cardiac afferents matched the properties of ASIC2a channels, and currents from ASIC2 −/− cardiac afferents matched the properties of ASIC3 channels.
Conclusions
We conclude that ASIC2a and -3 are the major ASIC subunits in cardiac DRG neurons, and provide potential molecular targets to attenuate chest pain and deleterious reflexes associated with cardiac disease.
Keywords: ASICs, H+-gated channel, cardiac afferent
INTRODUCTION
Cardiac sensory neurons (afferents) continuously sense hemodynamic conditions within the heart and contribute to reflex control of the cardiovascular system. Additionally, they sense pathological conditions within the heart, most notably during myocardial ischemia or infarction. During such conditions, humans often experience pain or discomfort within the chest, termed angina pectoris. Rather than just acting as passive sensors, there is increasing evidence that cardiac afferent activation initiates detrimental reflexes during cardiac disease processes.1, 2 A number of chemical mediators activate cardiac afferents,3 and within the past decade studies have begun to determine the molecular identity of their sensory receptors. One such compound is lactic acid,4, 5 which is released from ischemic cardiac myocytes, and receptors of lactic acid are the pH-sensitive Acid-Sensing Ion Channels (ASICs).
ASICs belong to the DEG/ENaC family of ion channels and include four genes (ASIC1, -2, -3, and -4) that encode for six subunits (ASIC1 and -2 both have alternative splice transcripts: ASIC1a, -1b, -2a, and -2b).6, 7 Functional ASIC channels consist of a complex of three subunits;8 individually expressed subunits form homomultimeric channels, while coexpression of two or more subunits allows for the formation of heteromultimeric channels. ASIC2b does not form H+ -gated channels when expressed alone, but alters current properties when coexpressed with other ASICs,9, 10 and mammalian ASIC4 does not contribute to channel gating properties.11 Each of the other various ASIC homomeric and heteromeric channels have unique biophysical and/or pharmacological properties, and their selective expression in various populations of neurons may contribute to a diversity of functions.6, 7 As with other visceral organs, the mammalian heart is innervated by two populations of sensory neurons; those that follow the sympathetic nerve tracts to their cell bodies located in the upper thoracic dorsal root ganglia (DRG), and those that follow the vagal nerve tracts to their cell bodies located in the nodose ganglia (NG). Using a retrograde tracer dye to identify cardiac afferents in rat, we previously found very large ASIC-like pH-activated currents in isolated cardiac DRG neurons compared to those from the NG.12 This data correlates with studies showing that cardiac afferents in the DRG are primarily responsible for pain transmission during ischemia.3 Moreover, these ASIC channels are activated in the narrow range of extracellular pH (7.0 – 6.8) that occurs during myocardial ischemia,4, 13, 14 and are more sensitive to lactic acid than to other forms of acid.15
The biophysical properties of ASIC-like currents in rat cardiac DRG neurons suggested a contribution by ASIC3 subunits.13 However, it is not clear if other ASIC subunits contribute to the channel complex. To address this question, we took advantage of genetically altered mice. Given their purported role as sensors of ischemia in the heart, we tested the hypothesis that the composition of ASIC channels in cardiac afferents is unique compared to other neurons. By studying labeled cardiac sensory neurons in wild-type and ASIC null mice, and comparing their current properties to those of heterologously expressed ASICs, we were able to define the ASIC subunit composition in cardiac afferents.
MATERIALS AND METHODS
For details see Online Data Supplements.
Labeling and culture of mouse cardiac afferents
Mouse cardiac sensory neurons were labeled for identification in primary dissociated culture by injecting DiI (Molecular Probes) –coated polystyrene microbeads into the pericardial space in a modification of a previous protocol in rat.12 2 to 3 weeks after the injection of DiI-beads suspension, the DRG (C8-T3) and/or NG were collected, and dissociated as previously discribed.16 Cells were studied 18–48 h after plating. See Online Data Supplements for details and figure.
Generation of ASIC knockout mice
The generation of mice missing individual ASIC1, -2, and -3 subunits have been reported. Subsequently, these mice were produced on a C57BL/6J congenic background, and crossed to generate mice with the simultaneous disruption of multiple ASIC subunits.
Heterologous Expression of cDNA in CHO Cells
Mouse ASIC1a, ASIC2a, and ASIC3 were previously cloned into the pMT3 plasmid vector, and Chinese hamster ovarian (CHO) cells were transfected as previously described.17 Cells were studied 48–72 hours after transfection.
Electrophysiology
Whole-cell patch-clamp recordings from sensory neurons and CHO cells were performed as previously described.16, 17 Data are means ± SEM. Statistical significance was assessed using unpaired Student’s t-test.
Quantitative RT-PCR
Total RNA was isolated using the RNeasy mini kit (Qiagen), according to the manufacturer’s protocol. cDNA synthesis was performed using the AffinityScript qPCR cDNA Synthesis kit (Stratagene) followed by qPCR using the Brilliant II SYBR Green qPCR Master Mix (Stratagene). Primer sequences are listed in Online Data Supplement.
RESULTS
Cardiac afferent responses to protons, capsaicin or ATP
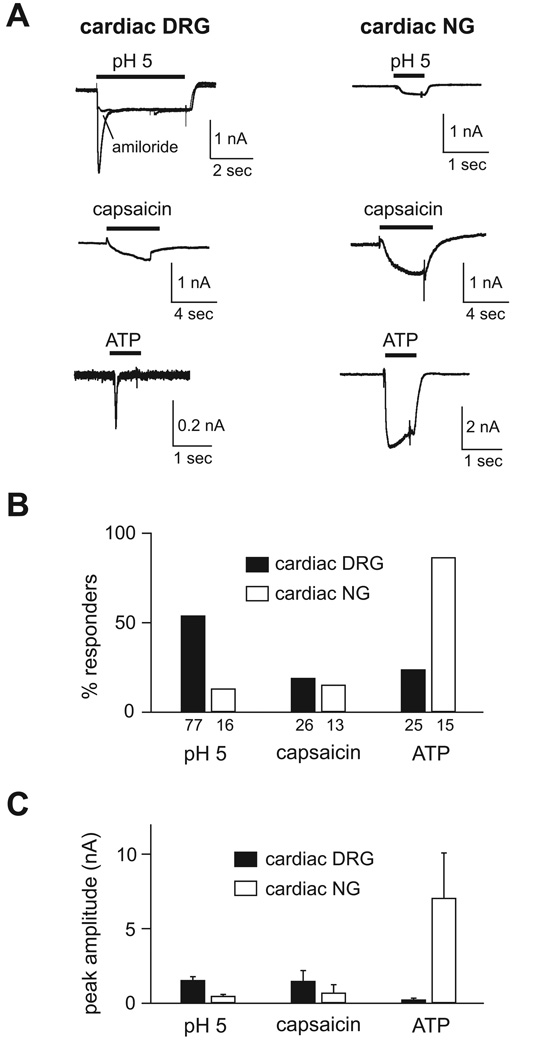
Using a modification of a protocol from rat,12 we injected a retrograde tracer dye into the pericardial space of mice, which allowed us to identify and study labeled cardiac afferents in primary culture by patch-clamp technique (supplementary Figure 1). In wild-type mice, we first recorded currents in cardiac sensory neurons from both the DRG (diameter range 20–40 µm, mean = 30.4 µm) and NG (diameter range 25–35 µm, mean = 30.3 µm) evoked by protons (pH 5), capsaicin (1 µmol/L), or ATP (30 µmol/L), all of which have been reported to activate cardiac afferents.13, 18, 19 Figure 1A shows typical currents recorded from cardiac DRG and NG neurons. Switching from pH 7.4 to pH 5 solution induced rapidly activating and desensitizing (transient) currents that were followed by sustained currents of smaller amplitude in slightly over half (40 of 74) of cardiac DRG neurons. The transient phase of the proton-activated current was blocked by the ASIC channel blocker, amiloride, whereas the sustained phase was not (Figure 1A). The fast kinetics of the transient current and amiloride block are signature characteristics of ASIC channels (sustained currents generated by ASICs are insensitive to amiloride).14, 20, 21 In contrast, very few cardiac NG neurons (2 of 16) had acid-evoked currents, and only one cell generated a transient current (the current in Figure 1A is from a NG neuron with a sustained pH-evoked response). Capsaicin, an activator of transient receptor potential vanilloid 1 (TRPV1) channels, induced sustained or slowly desensitizing currents in a small percentage of both populations of cells (Figure 1A). In contrast, ATP evoked larger currents and in a greater percentage of NG compared to DRG neurons (Figure 1A). Additionally, the ATP-evoked currents observed in NG neurons displayed markedly slower activation and desensitization kinetics than those from DRG neurons (Figure 1A), a finding that has previously been described and most likely represents different P2X channel subunits.22
Figure 1.
Cardiac afferent activation by acidic solution, capsaicin and ATP. A, Representative currents evoked by application of a pH 5 solution, capsaicin (1 µmol/L), or ATP (30 µmol/L) in cardiac DRG (left) and NG (right) neurons. Note the different scale bars and application times. Amiloride (1 mmol/L) blocked the transient pH-evoked current (Iamiloride/IpH5 = 0.27 ± 0.02, n = 4), but not the sustained phase (Iamiloride/IpH5 = 1.09 ± 0.14, n = 4). B, Percentages of cardiac DRG and NG neurons that responded to pH 5, capsaicin and ATP. A positive response was defined as an evoked current > 60 pA. The number of neurons studied for each group is listed below each bar. C, Mean peak amplitude of the responding neurons.
Comparing the percentage of responders to a given agonist (Figure 1B), and their peak current amplitudes (Figure 1C), revealed interesting differences between the populations of cells (also see Table 1). A higher percentage of cardiac DRG neurons responded to protons than to capsaicin or ATP, and these proton-evoked currents were more prevalent and larger than those from cardiac NG neurons. In contrast, ATP generated the most consistent and largest responses in cardiac NG neurons. These data suggest that cardiac DRG neurons are enriched in ASIC channels compared to P2X or TRPV1 channels, whereas cardiac NG neurons express high levels of P2X channels.
Table 1.
Properties of ASIC currents in cardiac and non-cardiac DRG neurons from wild-type and ASIC null mice, and heterologously expressed ASIC subunits.
| % Response to pH 5 |
Amplitude at pH 4 (nA) |
pH50 | τ desensitization at pH 6 (sec) |
τ desensitization at pH 4 (sec) |
|
|---|---|---|---|---|---|
| DRG neurons | |||||
| wild-type | |||||
| cardiac | 54 (40/74) | 1.9 ± 0.36 (18) | 5.6 (11) | 0.13 ± 0.01 (15) | 1.94 ± 0.63*(9) |
| non-cardiac | 53 (41/77) | 3.4 ± 0.71 (14) | 6.2 (8) | 0.15 ± 0.03 (21) | 0.38 ± 0.05 (7) |
| ASIC2 −/− | |||||
| cardiac | 29 (11/38) | 1.5 ± 0.28*(9) | 6.6 (8) | 0.20 ± 0.02**††(7) | 0.43 ± 0.07†(7) |
| non-cardiac | 44 (23/52) | 3.9 ± 0.93 (15) | 6.6 (14) | 0.16 ± 0.01 (16) | 0.38 ± 0.02 (7) |
| ASIC3 −/− | |||||
| cardiac | 67 (10/15) | 1.5 ± 0.26*(12) | 3.8 (8) | - | 1.03 ± 0.23*(7) |
| non-cardiac | 42 (14/33) | 3.8 ± 0.78 (10) | 6.1 (4) | 1.65 ± 0.28††(5) | 0.35 ± 0.13 (6) |
| ASIC2/3 −/− | |||||
| cardiac | 0 (0/14) | - | - | - | - |
| non-cardiac | 20 (4/20) | 2.3 ± 0.80 (4) | - | 0.92 ± 0.32 (3) | - |
| ASIC subunits expressed in CHO cells | |||||
| ASIC1a | 6.6 (9) | 1.60 ± 0.46 (4) | 0.45 ± 0.08 (9) | ||
| ASIC2a | 3.8 (9) | - | 1.15 ± 0.12 (5) | ||
| ASIC3 | 6.8 (12) | 0.29 ± 0.01 (18) | 0.47 ± 0.07 (7) | ||
| ASIC1a + ASIC2a | 5.4 (7) | 0.60 ± 0.06 (6) | 0.86 ± 0.10 (6) | ||
| ASIC1a + ASIC3 | 6.7 (7) | 0.10 ± 0.01 (7) | 0.32 ± 0.08 (6) | ||
| ASIC2a + ASIC3 | 5.6 (16) | 0.14 ± 0.01 (16) | 1.76 ± 0.45 (11) | ||
| ASIC1a + ASIC2a + ASIC3 | 6.0 (12) | 0.13 ± 0.03 (9) | 0.31 ± 0.04 (10) |
Values for half-maximal pH activation (pH50) are derived from curve fits of the means. N’s are denoted in parentheses after each value.
(*P < 0.05, and **P < 0.01 cardiac vs. non-cardiac DRG neurons of the same genotype; † P < 0.05, and †† P < 0.01 ASIC null vs. wild-type DRG neurons)
Biophysical properties of ASIC-like currents in cardiac DRG neurons from wild-type mice
To delineate the ASIC subunit composition underlying the pH-evoked currents in cardiac DRG neurons, we measured three properties that have proven useful to distinguish ASIC channels composed of different subunits: 1) pH sensitivity of the transient current, 2) sustained current amplitude normalized to peak transient current, and 3) desensitization kinetics.9, 14, 16
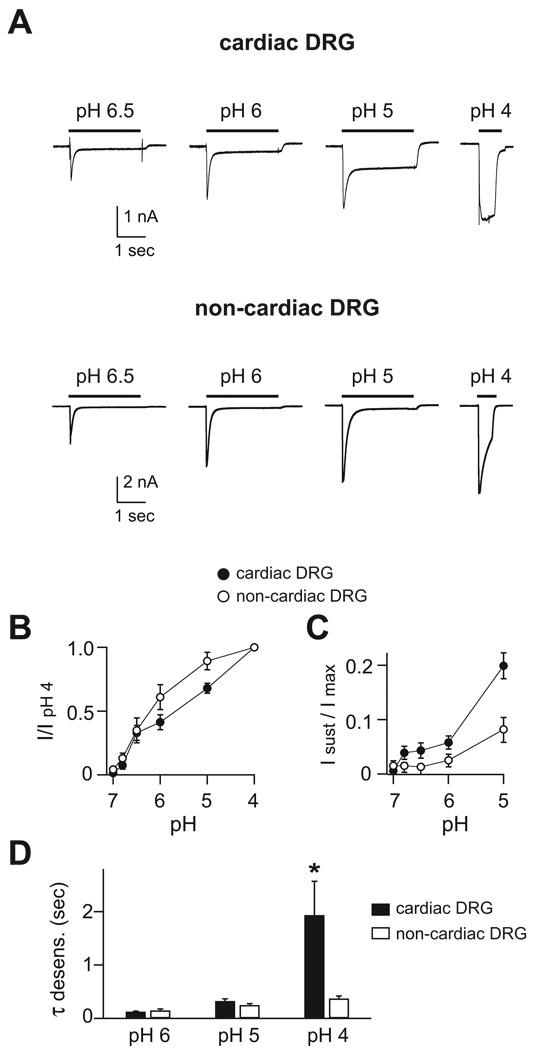
For comparison, we also studied unlabeled DRG neurons in the same cultures (n = 83; mean diameter = 31.4 µm). Presumably, the majority of these unlabeled DRG neurons represented sensory neurons that innervate somatic tissues (skin, muscle, etc.) and we termed them “non-cardiac” neurons. Application of acidic pH solutions evoked transient and sustained currents in both cardiac and non-cardiac DRG cells (Figure 2A). However, closer inspection of their biophysical properties revealed significant differences. First, although the pH sensitivity as measured by half-maximal activation was similar between cardiac and non-cardiac DRG neurons (Table 1 and Figure 2B), the pH activation curve of cardiac neurons appears to have more than one component. This suggests that cardiac neurons might have more than one population of ASIC channels, each with different pH sensitivities. Second, the sustained currents were significantly larger in cardiac compared to non-cardiac DRG neurons (Figure 2C). Third, the rate of desensitization at pH 4 was markedly slower in cardiac DRG neurons (Figure 2D).
Figure 2.
Biophysical properties of ASIC-like currents in cardiac and non-cardiac DRG neurons. A, Representative currents evoked by indicated pH solutions from a control solution of pH 7.4 in cardiac and non-cardiac DRG neurons. pH-dose response data of B, transient and C, sustained currents in cardiac and non-cardiac DRG neurons. Both sets of data were normalized to the peak transient currents evoked by pH 4. D, Mean time constants of desensitization as measured from single exponential fits to the falling phase of the transient currents evoked by the indicated pH solutions in cardiac and non-cardiac DRG neurons. Rates were calculated from pH applications of three second duration, except those from pH 4 (0.6 sec) (neurons did not tolerate longer applications of pH 4, and for this same reason we did not calculate sustained currents at pH 4. While these calculations are not as precise as those make from longer applications, they still delineate qualitative differences). N = 5–21; *P < 0.05 vs. non-cardiac DRG neurons.
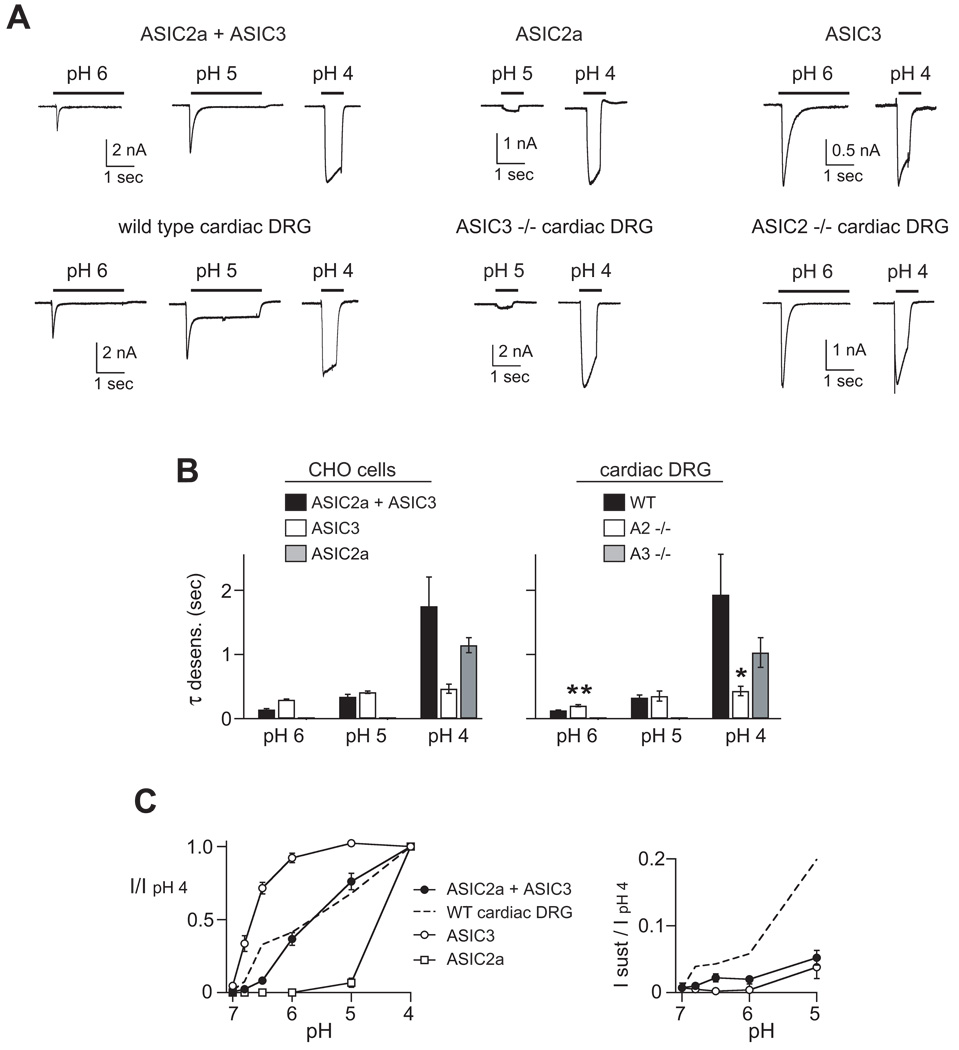
From these data, we make two general conclusions. First, the pH-evoked currents in cardiac DRG neurons have unique properties, indicating that the subunit composition of the ASIC channels in these cells is different than those of the greater population of sensory neurons. These differences also confirmed that our labeling technique had successfully identified a distinct population of sensory neurons. Second, the data allows us to begin to make predictions about the ASIC subunit composition in these neurons. The rate of desensitization at pH 6 was faster in both cardiac (128 ± 6 msec) and non-cardiac DRG neurons (151 ± 26 msec) than homomeric channels of any of the individual ASIC subunits.9, 16, 23 In fact, pH 6-evoked currents with a rate constant less than 200 msec only occur when ASIC3 is present in a heteromeric complex with ASIC2a and/or ASIC1 subunits (refs9, 16, and Table 1). These data suggest that most ASIC channels are heteromeric channels comprised of multiple different subunits, and ASIC3 is a necessary component. Moreover, the large sustained currents in cardiac DRG neurons suggested that the channel complexes in these cells might be heteromeric channels comprised of ASIC2a and -3 subunits, since this ASIC heteromeric complex generates the largest sustained currents.9, 14
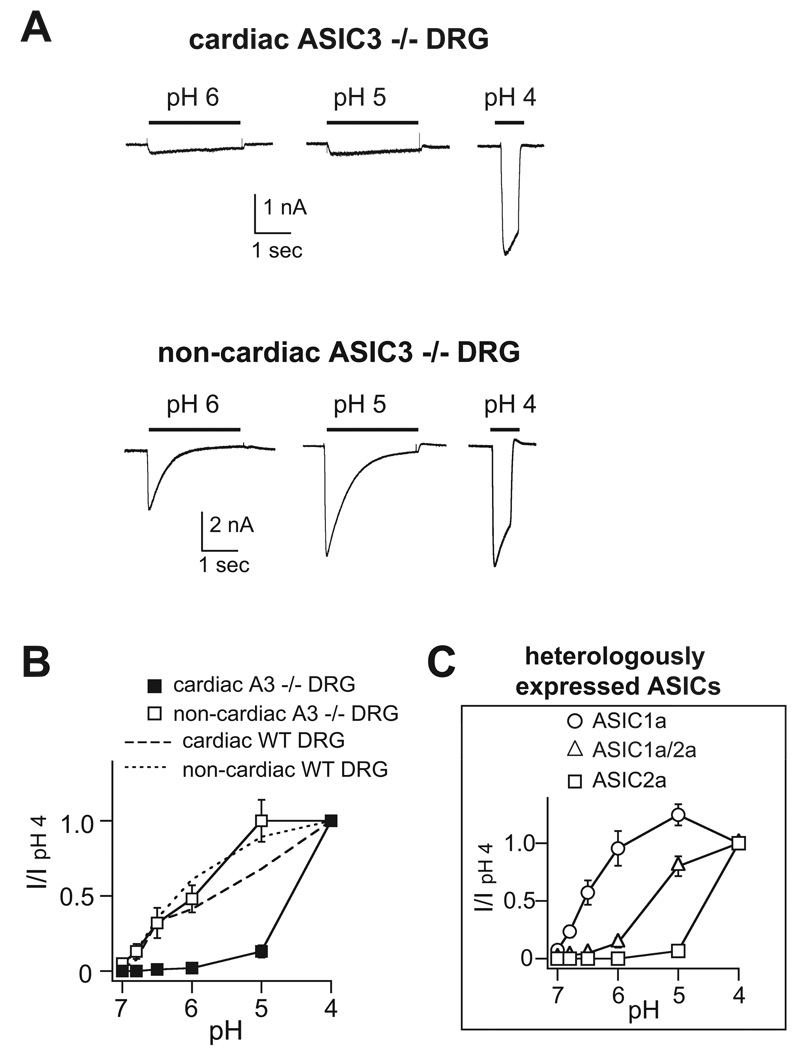
ASIC-like currents in cardiac DRG neurons from ASIC3 −/− mice
To further test the prediction that ASIC3 contributes to the channel complex in cardiac DRG neurons, we studied cells from ASIC3 null mice. The first thing to note is that we found transient pH-evoked currents in a similar percentage of ASIC3 −/− cardiac neurons and the mean pH 4-current amplitude was similar to those from wild-type cardiac neurons (Table 1). This indicates that ASIC3 is not the only subunit that generates acid-evoked currents in cardiac DRG neurons. However, loss of ASIC3 dramatically altered the current properties. By comparing the pH-evoked currents from wild-type (Figure 2A) to those from ASIC3 −/− cardiac DRG neurons (Figure 3A), it is obvious that loss of ASIC3 produced a significant decrease in the pH sensitivity of the cells (Figure 3B). In fact, the low pH sensitivity of the currents in ASIC3 −/− cardiac DRG neurons could only be generated by one ASIC channel – ASIC2a homomeric channels (refs 9, 16, and Table 1). To confirm this notion, we heterologously expressed candidate combinations of ASIC subunits that might compose the ASIC channels in ASIC3 −/− neurons. Indeed, only ASIC2a homomers reproduced the pH insensitivity (Figure 3C), as well as other properties of the currents in ASIC3 −/− cardiac DRG neurons (Table 1).
Figure 3.
Properties of acid-evoked currents in cardiac and non-cardiac DRG neurons from ASIC3 −/− mice. A, Currents evoked by the indicated pH solutions from cardiac and non-cardiac ASIC3 −/− DRG neurons. B, pH dose-response of transient currents in cardiac and non-cardiac ASIC3 −/− DRG neurons (n = 3–9). For comparison, the respective data from wild-type neurons is superimposed. C, pH dose-response data from currents recorded from CHO cells expressing ASIC1a, ASIC2a, or coexpressing ASIC1a and ASIC2a (n = 6–9).
Whereas loss of ASIC3 produced a large shift in the pH sensitivity of cardiac DRG neurons, it did not affect the pH sensitivity of non-cardiac DRG neurons (Figure 3B). This suggests that ASIC1 subunits are components of the complexes in these cells. However, consistent with previous studies, we found ASIC3 is a major component of the ASIC channels in non-cardiac DRG neurons as evident from the significant slowing of the desensitization kinetics of pH 6-evoked currents in ASIC3 −/− neurons (refs16, 24, and Table 1). We conclude from these data that ASIC3 is indeed a major contributor to ASIC channels in most DRG neurons that expressed ASICs. In addition, currents in ASIC3 −/− cardiac DRG neurons matched those of ASIC2a homomers, further suggesting that the native channels in these cells might be ASIC2a/3 heteromers.
ASIC-like currents in cardiac DRG neurons from ASIC2 −/− mice
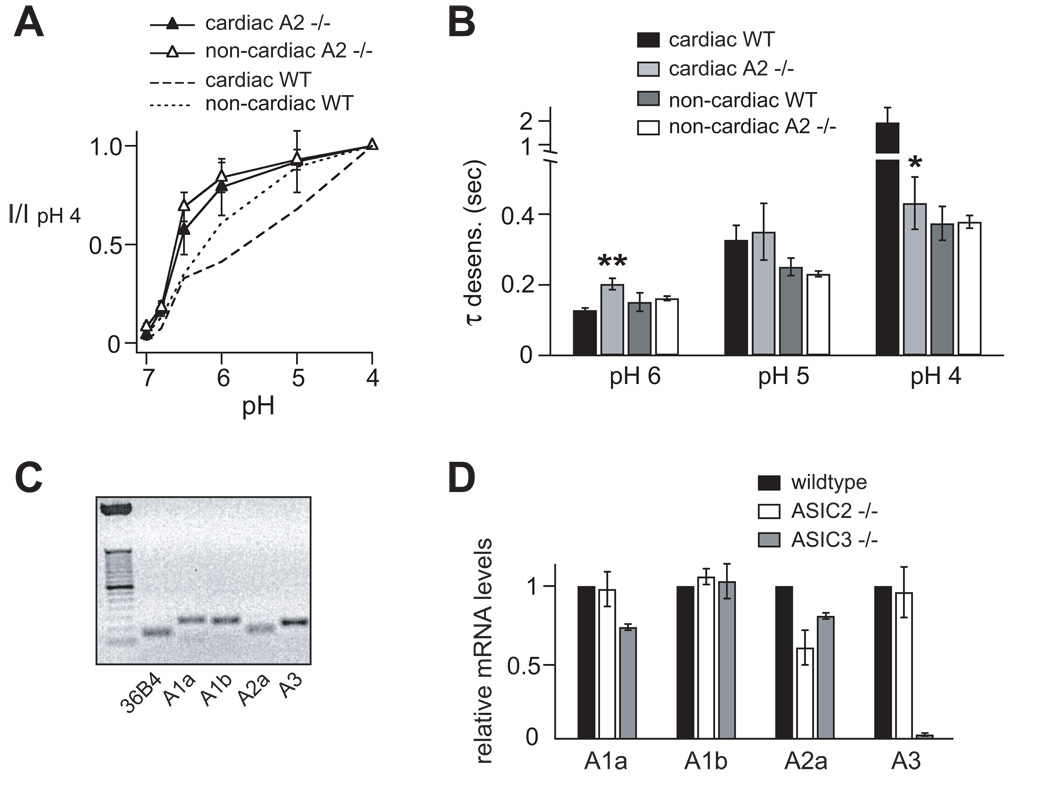
To directly test the contribution of ASIC2 subunits, we next studied pH-evoked currents from ASIC2 null mice. Similar to ASIC3, loss of ASIC2 did not abolish currents, suggesting that ASIC2 subunits are not solely responsible for acid-evoked currents in cardiac DRG neurons. However, both cardiac and non-cardiac DRG neurons were more pH sensitive in ASIC2 −/− than wild-type mice (Figure 4A). Although both ASIC2a and -2b are deleted in this mouse strain, the shift in pH sensitivity was most certainly due to loss of ASIC2a subunits, since ASIC2b does not cause significant changes in pH sensitivity or kinetics when coexpressed with other ASIC subunits.9, 10 The kinetics of desensitization where also altered in cardiac neurons by deletion of ASIC2 (Figure 4B, also see Figure 6A). The kinetics did not change in ASIC2 −/− non-cardiac neurons, remaining fast at pH 6, which indicates that the residual ASIC channel in these cells was primarily still a heteromer.
Figure 4.
Properties of acid-evoked currents in cardiac and non-cardiac DRG neurons from ASIC2 −/− mice. A, pH dose-response of transient currents in cardiac and non-cardiac ASIC2 −/− DRG neurons, and the respective data from wild-type neurons superimposed (n = 8–15). B, Comparison of the time constants of desensitization of cardiac and non-cardiac DRG neurons from wild-type and ASIC2 −/− mice. N = 7–21; *P < 0.05 and **P < 0.01 vs. cardiac wild-type DRG neurons. C, ASICs subunit mRNA from thoracic DRG from wildtype mice were collected and analyzed by quantitative real time RT-PCR (qPCR), and the products were run on a 2% agarose gel. See table in Online Supplemental Data for product lengths. D, DRG mRNA levels from wildtype, ASIC2 −/−, or ASIC3 −/− mice were measured by qPCR, and transcript levels were normalized to the levels in wildtype DRG. Data from ASIC null mice represent means from 3 mice each.
Figure 6.
Comparison of the properties of ASIC-like currents in cardiac DRG neurons with currents generated by heterologously expressed ASIC subunits. A, Currents evoked by the indicated pH solutions from wild-type, ASIC3 −/−, or ASIC2−/− cardiac DRG neurons compared to currents from CHO cells coexpressing ASIC2a, ASIC3, or ASIC2a+ASIC3 subunits. B, Comparison of the time constants of desensitization of currents from heterologously expressed ASICs (left), and those from cardiac DRG neurons (right). The data from heterologously expressed ASIC2a and ASIC3 −/− mice at pH 6 and 5 were not included because the currents were too small (see their pH activation range in Figure 3B and 3C) to accurately fit (n = 6–18; *P < 0.05 and **P < 0.01 vs. cardiac wild-type DRG neurons. pH-dose response data of C, transient and D, sustained currents in cardiac wild-type DRG neurons and heterologously expressed ASICs. ASIC2a does not generate sustained currents. Both sets of data were normalized to the peak transient currents evoked by pH 4 (n = 3–16).
To test whether there was compensatory upregulation of ASIC subunits in ASIC −/− mice, which could confound our results, we measured levels of ASIC mRNAs in thoracic DRG by quantitative real time RT-PCR. As expected, we found consistent mRNA expression of all tested ASIC subunits (Figure 4C), and none of the residual subunits were upregulated in ASIC2 −/− or ASIC3 −/− mice (Figure 4D). In ASIC2 −/− mice, the level of ASIC2a mRNA was decreased, but not abolished. Since ASIC2 −/− mice were generated by deletion of two exons encoding the second membrane spanning domain, we suspect that our upstream primers detected a truncated residual transcript.25
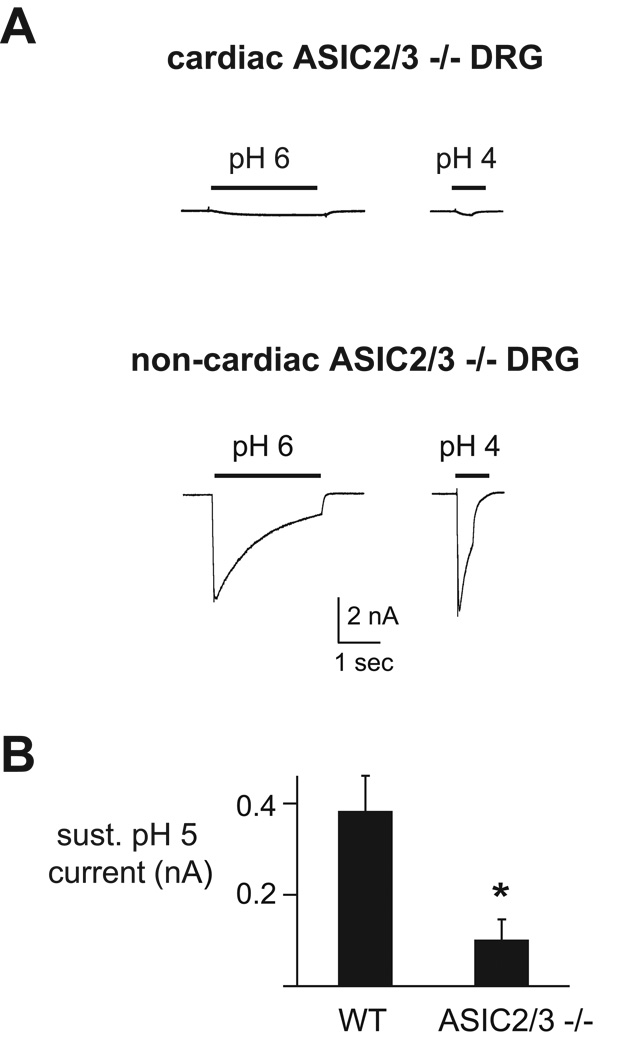
ASIC-like currents are absent in cardiac DRG neurons from ASIC2/3 double −/− mice
Our data thus far suggested that ASIC3 and ASIC2a subunits contribute to ASIC channels in cardiac DRG neurons. To further test this possibility, we studied mice lacking both ASIC2 and ASIC3 genes. As illustrated in Figure 5A, no ASIC2/3 −/− cardiac DRG neurons had ASIC-like transient pH-evoked currents. On the other hand, 4 out of 20 ASIC2/3 −/− non-cardiac DRG neurons displayed transient acid-evoked currents. Their slow rates of desensitization (Table 1) and recovery from desensitization (see Figure 5 legend) were consistent with ASIC1 channels (ref 16 and Table 1). We suspect that the relatively low percentage of cells that displayed ASIC1-like currents was perhaps due to the fact that these mice were heterozygous for the ASIC1 gene. These data confirm that ASIC1 subunits contribute to ASIC channels in some DRG neurons, but are not significant contributors in cardiac DRG neurons.
Figure 5.
ASIC-like currents are absent in cardiac DRG neurons from ASIC2/3 double −/− mice. A, Currents evoked by the indicated pH solutions from cardiac and non-cardiac ASIC2/3 −/− DRG neurons. In three non-cardiac ASIC2/3 −/− DRG neurons that displayed ASIC-like currents, we measured the time course for the channels to recover from desensitization (not shown). Current was completely desensitized by a prolonged 10 sec pH 6 application. Cells were then bathed in pH 7.4 for two seconds before again exposed to a second pH 6 application. Recovery is the percentage of current evoked by the second pH 6 application compared to the first. In all three cells, the percent recovery at 2 seconds was < 50 %. B, Mean sustained current amplitudes evoked by pH 5 in wildtype and ASIC2/3 −/− cardiac DRG neurons. N = 8–12; *P = 0.01.
To determine if ASICs contribute to the sustained pH-activated currents (since the sustained component of ASIC currents is insensitive to amiloride), we measured the amplitudes of the sustained currents in ASIC2/3 −/− cardiac DRG neurons and found they were significantly smaller compared to those from wildtype mice (Figure 5B). This suggests that ASIC2 and/or ASIC3 subunits contribute to the sustained currents in cardiac DRG neurons.
Properties of ASIC 2a/3 heteromultimers closely match those of cardiac DRG neurons
We conclude that ASIC2a and-3 are necessary components of ASIC channels in cardiac DRG neurons. Next, we asked if coexpression of ASIC2a and -3 was sufficient to reproduce the properties of native ASIC channels in cardiac afferents. Coexpression of the two subunits generated pH 6-evoked currents that desensitized faster, and pH 4-evoked currents that desensitized slower than currents generated by either subunit expressed alone (Figure 6A,B), confirming that ASIC2a and -3 formed heteromultimers. Across a range of pH values, the desensitization kinetics of ASIC2a/3 heteromers matched almost exactly those of wild-type cardiac DRG neurons. Moreover, the desensitization rates of ASIC3 homomers matched the data from ASIC2 −/− mice, and the desensitization rates of ASIC2a homomers matched those from ASIC3 −/− mice (Figure 6A, B). The pH-sensitivity of ASIC2a/3 also matched that of wild-type cardiac DRG neurons (Figure 6C, left). However, at the foot of pH activation (pH 6 to 7 range) the curves diverged slightly; between pH 7.0 and 6.0, the activation of cardiac neurons could be fitted by a Hill curve with a pH50 of 6.6. This value is intermediate between those of heterologously expressed ASIC3 (pH50 = 6.8) and ASIC2a+ASIC3 (pH50 = 5.6), suggesting that cardiac DRG neurons express a mixture of different channels. We also tested if coexpression of ASIC2a and -3 could reproduce the large sustained currents in cardiac DRG neurons. While ASIC2a/3 generate the largest sustained currents of the ASIC channels,9, 14 they were still relatively smaller than those in observed in cardiac afferents (Figure 6C, right).
DISCUSSION
ASICs are purported pH sensor in sensory neurons innervating the heart. To delineate their molecular composition, we labeled and isolated mouse cardiac sensory neurons, which allowed for detailed electrophysiological characterization of the native ASIC channels by the patch-clamp technique. We then studied and compared data from mice that had undergone targeted deletion of specific ASIC subunits. The properties of ASIC channels in cardiac DRG neurons from wild-type mice suggested that they were heteromeric channels composed of ASIC2a and -3. This was confirmed by studying cardiac afferents from ASIC null mice: currents from ASIC3 −/− mice matched the properties of ASIC2a homomeric channels, currents from ASIC2 −/− mice matched the properties of ASIC3 homomeric channels, and ASIC-like currents were absent from ASIC2/3 double −/− mice.
ASICs in cardiac afferents are unique
Most ASIC channels in mouse DRG neurons are heteromeric channels consisting of more than one ASIC subunit type. This is best appreciated by measuring the kinetics of desensitization; ASIC pH 6-evoked currents in DRG neurons desensitize faster than those from all ASIC homomeric channels, however when ASIC3 is coexpressed with other ASIC subunits, heteromeric channels are formed that match the fast kinetics of the native channels (refs9, 16, and Table 1). This was true for both cardiac and non-cardiac DRG neurons, suggesting that ASIC3 is a major contributor in the majority of DRG neurons.16, 24
However, in other respects cardiac afferents were unique. pH-evoked currents from cardiac afferents were slightly less pH sensitive at more extreme pH changes, had larger sustained currents across a broad pH activation range, and had markedly different desensitization kinetics at pH 4 compared to non-cardiac DRG neurons. Our studies of ASIC null mice confirmed that all of these differences were due to an increased contribution of ASIC2a, and a lack of ASIC1 subunits to the currents properties in cardiac afferents. We previously studied ASIC1a −/− mice and found that ASIC1 subunits are indeed major contributors to ASIC channels in most non-cardiac DRG neurons,16 and our data here supports this conclusion. Why are ASIC1 subunits major contributors to ASIC channels in sensory neurons that innervate skin and other somatic tissue (these are the majority of neurons in DRG) whereas they are absent from sensory neurons that innervate the heart? We speculate below that ASIC2a/3 heteromeric channel properties make them ideal sensors of myocardial ischemia.
Previous work from rat cardiac afferents suggested that ASIC3 is a major contributor,13 and our data here is in agreement. However, our data also reveal some differences between ASIC channels in rat and mouse cardiac afferents. First, greater than 90% of rat cardiac DRG neurons expressed ASIC-like currents, and the mean amplitude of pH 5-evoked currents was ~10 nA.12, 14 In contrast, we found that ~50% of mouse cardiac DRG neurons expressed ASIC-like currents, and the mean amplitude of pH 5-evoked currents was smaller (1.57 nA). A second difference is that cardiac DRG neurons from rat were more pH sensitive (pH50 = 6.6)12 than those from mice (pH50 = 5.6). A previous study directly comparing the ASIC-like currents in unlabeled rat and mouse DRG suggested there are differences in the expression of ASICs between the two species.26 The functional significance of these species differences is unclear.
Significance of ASIC2a/3 heteromers as cardiac pH sensors
We found that mouse cardiac afferents generated relatively large sustained currents at pH values ≤ 6.8 compared to non-cardiac DRG neurons. ASIC2a/3 heteromers generate the largest sustained currents of the ASIC channels in this pH range,9, 14 and our data suggests that these channels underlie, in part, the large sustained currents in cardiac afferents. Sustained current through ASIC channels triggers persistent firing of action potentials in cardiac afferents,14 which might contribute to the typical persistent nature of angina during myocardial ischemia.
Although we did not extensively study currents at modest pH changes here, ASIC channels that contain ASIC3 are exquisitely sensitive to small changes in pH.13, 14 The threshold of activation for both ASIC3 homomers and ASIC2b/3 heteromers occurs with a pH change from 7.4 to 7.2.14 Furthermore, when the proton carrier is lactate, as in myocardial ischemia, the threshold of activation is further shifted because ASIC channels are more sensitive to lactate than other acids.15 We speculate the biphasic shape of the pH activation curve of mouse cardiac DRG neurons suggests they possess a population of ASIC channels that is poised to sense slight pH changes within the myocardium (ASIC3 homomers or ASIC2b/3 heteromers), and another population that would increase sensory activation at more extreme pH changes (ASIC2a/3 heteromers).
Contribution of other receptors to cardiac sensation
Of the ASIC channels, heterologous expression of ASIC2a/3 heteromers generates the largest sustained acid-evoked currents at pH values less than 6.8. Still, these currents were not as large as the sustained currents recorded from cardiac DRG neurons. This suggests that channels other than ASICs, such as TRPV119 or 2 pore K+ channels,27 might contribute to pH sensing in cardiac afferents at these pH ranges. Our data also suggests that cardiac NG neurons express high levels of P2X channels and thus ATP might be an important mediator in these sensory neurons. Cardiac afferent activation during myocardial ischemia, and during normal physiological conditions, most certainly involves multiple mediators and receptors.
Physiological significance
On the whole, our work has demonstrated that ASIC channels are highly expressed in cardiac DRG neurons, which are the major sensory pathway for cardiac pain in patients with myocardial ischemia or infarction. As remarkably sensitive pH and lactate sensors, we also speculate that ASICs function as metabolic sensors within the heart, particularly during stress and exercise, when the metabolic demands of the heart can increase five-fold. In cardiac disease states, ASIC activation might contribute to the initiation of deleterious neuro-hormonal reflexes that are pervasive during such states. Understanding the molecular composition of ASIC channels within cardiac afferents is critical; most of our current pharmacological tools to modulate ASICs are specific to individual ASIC subunits. Here, we have defined the ASIC2a/3 heteromeric channel as a potential molecular target to treat chest pain and/or cardiac diseases.
Supplementary Material
ACKNOWLEDGMENT
We thank Justin Yesis, Kathryn Kaufman, and the DNA core facility (NIH Grant DK25295) for technical assistance. Present address for T.Hattori: Division of Emergency Medicine, Nagoya City University Hospital, Nagoya, Japan.
SOURCES OF FUNDING
This work was supported by the NIH/NHLBI grant HL076419.
Non-standard Abbreviations and Acronyms
- ASIC
acid-sensing ion channel
- DRG
dorsal root ganglia
- NG
nodose ganglia
- CHO
Chinese hamster ovarian
- TRPV1
transient receptor potential vanilloid 1
Footnotes
Subject codes: Ion channels/membrane transport, Ischemic biology - basic studies
DISCLOSURES
None.
REFERENCES
- 1.Wang W, Ma R. Cardiac Sympathetic Afferent Reflexes in Heart Failure. Heart Failure Reviews. 2000;5:57–71. doi: 10.1023/A:1009898107964. [DOI] [PubMed] [Google Scholar]
- 2.Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138:9–23. doi: 10.1016/j.autneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meller ST, Gebhart GF. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience. 1992;48:501–524. doi: 10.1016/0306-4522(92)90398-l. [DOI] [PubMed] [Google Scholar]
- 4.Pan HL, Longhurst JC, Eisenach JC, Chen SR. Role of protons in activation of cardiac sympathetic C-fibre afferents during ischaemia in cats. J. Physiol. (Lond) 1999;518:857–866. doi: 10.1111/j.1469-7793.1999.0857p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchida Y, Murao S. Excitation of afferent cardiac sympathetic nerve fibers during coronary occlusion. Am J Physiol. 1974;226:1094–1099. doi: 10.1152/ajplegacy.1974.226.5.1094. [DOI] [PubMed] [Google Scholar]
- 6.Lingueglia E. Acid-sensing ion channels in sensory perception. J Biol Chem. 2007;282:17325–17329. doi: 10.1074/jbc.R700011200. [DOI] [PubMed] [Google Scholar]
- 7.Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 9.Hesselager M, Timmermann DB, Ahring PK. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- 10.Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J. Biol. Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 11.Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- 12.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ. Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc. Natl. Acad. Sci. U. S. A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]
- 15.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat. Neurosci. 2001;4:869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 16.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshcol JO, Harding AM, Hattori T, Costa V, Welsh MJ, Benson CJ. Acid-sensing ion channel 3 (ASIC3) cell surface expression is modulated by PSD-95 within lipid rafts. Am J Physiol Cell Physiol. 2008;295:C732–C739. doi: 10.1152/ajpcell.00514.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katchanov G, Xu J, Clay A, Pelleg A. Electrophysiological-anatomic correlates of ATP-triggered vagal reflex in the dog. IV. Role of LV vagal afferents. Am J Physiol. 1997;272:H1898–H1903. doi: 10.1152/ajpheart.1997.272.4.H1898. [DOI] [PubMed] [Google Scholar]
- 19.Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol. 2003;551:515–523. doi: 10.1113/jphysiol.2003.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J. Neurochem. 1999;72:51–57. doi: 10.1046/j.1471-4159.1999.0720051.x. [DOI] [PubMed] [Google Scholar]
- 21.Waldmann R, Bassilana F, de Weille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J. Biol. Chem. 1997;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- 22.Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP- gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- 23.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- 24.Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J Neurophysiol. 2002;87:2835–2843. doi: 10.1152/jn.2002.87.6.2835. [DOI] [PubMed] [Google Scholar]
- 25.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 26.Leffler A, Monter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience. 2006;139:699–709. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. Embo. J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.