Abstract
The alpha isoform of the phosphatidylinositol-3-kinases (PI3Kα) is often mutated, amplified and overex-pressed in human tumors. In an effort to develop new inhibitors targeting this enzyme, we carried out a pharmacophore model study based on six PI3Kα-selective compounds. The pharmacophore searching identified three structurally novel inhibitors of PI3Kα and its H1047R mutant. Our biological studies show that two of our hit molecules suppressed the formation of pAKT, a downstream effector of PI3Kα, and induced apoptosis in the HCT116 colon cancer cell line. QPLD-based docking showed that residues Asp933, Glu849, Val851, and Gln859 appeared to be key binding residues for active inhibitors.
Keywords: PI3K, Docking, Pharmacophore modeling, Apoptosis, LY294002, GSK2126458
Phosphatidylinositol 3-kinases (PI3Ks) are a group of lipid kinases that phosphorylate phosphatidylinositol at the 3-OH position of the inositol ring, generating phosphatidylinositol 3,4,5-triphosphate (PIP3).1,2 PIP3 regulates a wide variety of cellular functions including cell growth, differentiation and proliferation.3 The alpha isoform of PI3Ks (PI3Kα) is encoded by the gene PIK3CA, which is mutated, amplified and overexpressed in numerous human tumors including breast, urinary tract, cervical, gastric, squamous cell lung, non-small-cell lung, and colon cancers.4 PI3Kα somatic heterozygous point mutations can be grouped in frequent ‘hotspots’ located in the helical (E542K or E545K) or kinase (H1047R) domains, thereby conferring a gain in lipid kinase activity.4 The gain-of-function in the H1047R mutation is dependent upon allosteric changes mediated by p85α.5 The prevalence of PI3Kα and its H1047R mutation in various cancers suggests that inhibitors targeting the wild-type or the H1047R mutant of PI3Kα would be beneficial.
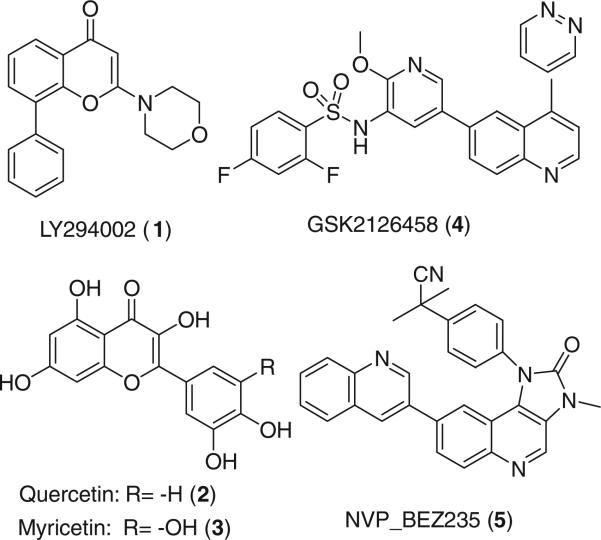
The efforts to develop PI3Kα inhibitors have led to the discovery of LY294002 (1), quercetin (2), myricetin (3), GSK2126458 (4) and NVP_BEZ235 (5) (Fig. 1) as antitumor agents. Compound 1 is a potent, reversible and selective inhibitor of PI3K kinase and is a common reference compound in many PI3K inhibition studies.6 In addition, flavonols 2 and 3 are associated with reduced pancreatic cancer risk7 and both are considered to be broad spectrum inhibitors of many protein kinases, including PI3Ks. 48 and 59 are currently evaluated in clinical trials. Compounds 1–5 non-selectively inhibit both PI3Kα and PI3Kγ; the latter is a structural homolog to the a isoform and mediates inflammatory pathways in rheumatoid arthritis, psoriasis, allergic diseases and cardiovascular disorders.10
Figure 1.
Chemical structures of PI3K inhibitors.
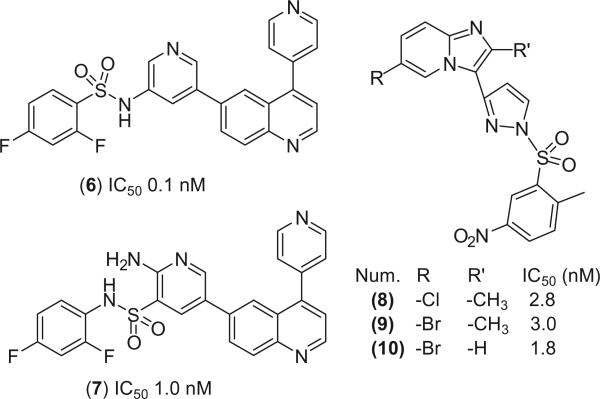
The aim of this study was to find novel lead compounds that are active against both the overexpressed wild-type PI3Kα (wt PI3Kα) and the frequently observed H1047R mutant PI3Kα. To achieve this goal, we used 4, its structural homologs 6 and 7 and the PI3Kα-selective imidazopyridine derivatives 8–10 (Fig. 2)11,12 to build a pharmacophore model in MOE13 to search against the National Cancer Institute (NCI) compound database.14 The identified hit molecules were tested for their ability to suppress tumor growth in cells harboring both the wt and H1047R mutant PI3Kα. The suppressive activity of these compounds on the downstream AKT and their apoptosis effect were also examined in colon cancer cell lines. In this work, we discovered three novel PI3Kα inhibitors with potencies comparable to that of 1.
Figure 2.
PI3Kα-selective inhibitors used in pharmacophore modeling.
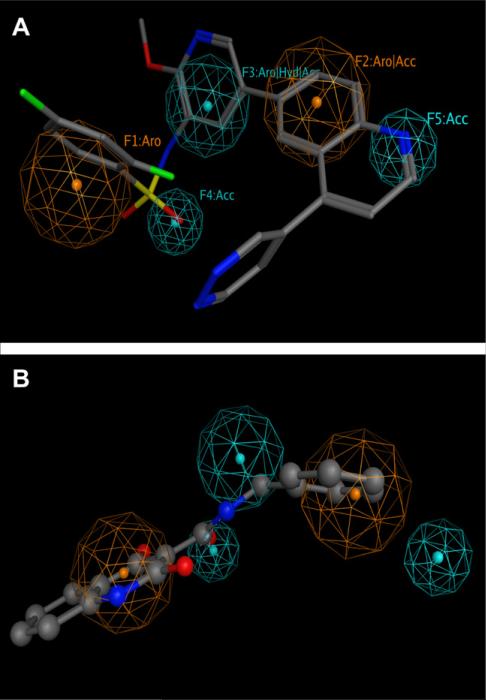
The selection of pyridylsulfonamide analogs (4, 6, 7) and imidazopyridine derivatives (8–10) to build a pharmacophore for potential PI3Kα inhibitors was based on their high potency. The superposition of 6–10 to 4 generated a pharmacophore with two H-bond acceptors (F4 and F5), one aromatic ring (F1), one aromatic or H-bond acceptor (F2) and one aromatic or hydrophobic or H-bond acceptor (F3) (Fig. 3).
Figure 3.
Pharmacophore model for PI3Kα with 4 (A, stick model), and 13 (B, ball-and-stick model). Aro stands for aromatic rings; Acc for H-bond acceptor; and Hyd for hydrophobic groups. F2 (Aro|Acc) is due to the imidazopridine in 8–10 which are both aromatic and contain a nitrogen atom (H-bond acceptor).
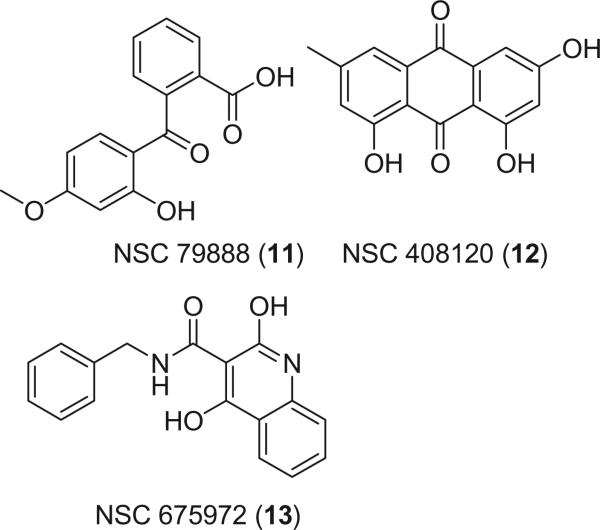
This model was used to search against the NCI database with 260,071 compounds, which were subject to the filtration with Lipinski's rule of five.15 3,716 entries satisfied the pharmacophore and were reported as hits. Further refinement with ranges of logP and logS of active inhibitors yielded a list of 49 structures as our final hit list. From these 49 compounds, NSC 79888 (11), NSC 408120 (12), and NSC 675972 (13) were chosen as potential PI3Kα inhibitors (Fig. 4) based on their availability from commercial sources. Efforts to obtain other hit molecules from various sources failed. Figure 3B showed that 13 satisfied four out of five pharmacophores. Similarly, 11 and 12 matched four pharmacophoric points.
Figure 4.
Chemical structures of the hit molecules.
In order to examine the antiproliferative activities of 11–13 against the wt or H1047R mutant PI3Kα, or both, we evaluated the growth inhibition of 1–3 (as references) and 11–13 in the colon cancer cell lines HCT116, HCT116-WT and HCT116-MUT (H1047R). HCT116 is a highly malignant colon carcinoma cell line with both wt and mutant PI3K (H1047R) that was established in tissue culture from a primary tumor.16 HCT116-WT and HCT116-MUT are colon cancer cell lines with the wt only or H1047R mutant only (MUT) PI3Kα, respectively. These cell lines were obtained via asymmetrical knockout of the MUT or wt PIK3CA allele.17
All three hit molecules (11–13) inhibited both the wt and H1047R mutant of PI3Kα, with IC50 values comparable to those of LY294002 (1). Compound 13 appeared to be the most potent. In most cases, the IC50 values of HCT116-MUT are lower than those expressing WT cells (HCT116-WT only, or HCT116, which contains both wt and H1047R mutant) (Table 1). This result is in good agreement with the gain-of-function observation that cells expressing mutant PI3KCA are hypersensitive to PI3K inhibition.
Table 1.
Growth inhibition of HCT116, HCT116-WT, and HCT116-MUT (H1047R) by compounds 1-3 and 11-13
| Compounds (IC50, μM) | HCT116 | HCT116-WT | HCT116-MUT (H1047R) |
|---|---|---|---|
| 1 | 5.8 | 6.7 | 5.3 |
| 2 | 6.7 | 23 | 5.7 |
| 3 | 24 | 23 | 4.1 |
| 11 | 13 | 31 | 18 |
| 12 | 6.2 | 5.3 | 3.8 |
| 13 | 1.8 | 1.1 | 0.73 |
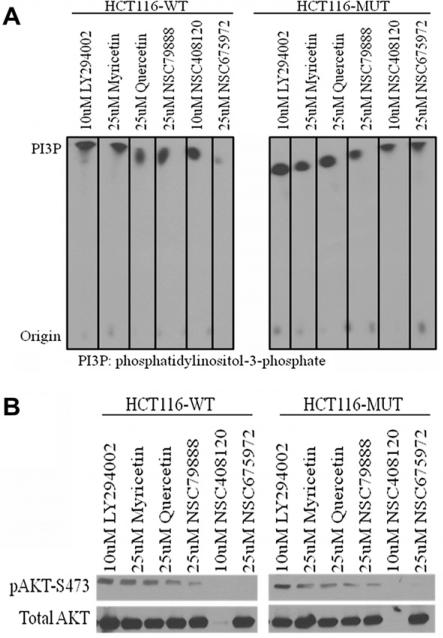
Next, we tried to determine whether the cell growth inhibition of the tested compounds was mediated by PI3K pathway. We carried out kinase assays on PI3Kα and immunoblot analysis on AKT (a downstream effector of PI3K signaling) using HCT116-WT and HCT116-MUT cells.
For the HCT116-WT cells, 13 showed the best inhibition of p110α-PI3K whereas 11 and 12 exhibited similar activities as 3. Compounds 3, 11–13 all inhibited PI3K more potently than 1 (Fig. 5A). These results show that the tested compounds can directly inhibit PI3K kinase activity. The immunoblot analysis of phospho-AKT (pAKT) at Ser473 and total AKT revealed that other than 12, all other tested compounds effectively blocked AKT phosphorylation without altering total AKT protein in both HCT116-WT and HCT116-MUT cells, with 12 and 13 exhibiting the best inhibitory activity in preventing the formation of pAKT-S473. Compound 12 appeared to exert its PI3K inhibitory effect by not only suppressing the formation of pAKT but also by inhibiting the expression of AKT (total AKT level) (Fig. 5B). Taken together with the PI3Kα kinase assay data, our results confirm that 11–13 directly inhibit PI3Kα and indirectly block downstream signaling pathways.
Figure 5.
(A) Direct inhibition of PI3K kinase activity by tested compounds; (B) inhibition of AKT or phosphorylation of AKT (pAKT).
Having shown that our hit molecules 11–13, especially 12 and 13, caused growth inhibition and blocked AKT phosphorylation in colon cancer HCT116-WT and HCT116-MUT cell lines, we next questioned whether the cell growth inhibition was due to cell death caused by apoptosis. To address this issue, we performed DNA fragmentation and MTT assays in the three cell lines described above.
The DNA fragmentation data (Table 2, and Supplementary Fig. 1S) showed that 1 led to more than an eightfold increase in DNA fragments than the control in the HCT116-WT and HCT116-MUT cells. This is consistent with the observation that inhibition of the PI3K/AKT pathway by 1 significantly increased ursolic acid-induced apoptosis in human HepG2 cells.18 Compound 3 produced a similar degree of apoptosis as 1 in HCT116-MUT cells, whereas the cell death caused by 2 in the mutant only cells was much weaker—only half of that of 1. In addition, the cell cycle arrest and apoptosis induced by 2 may be mediated through caspases-3, -8, and -9 as observed in MDA-MB-231 human breast cancer cells.19
Table 2.
Quantification of DNA fragmentation of 1-3 and 11-13 in HCT116, HCT116-WT, and HCT116-MUT cells (48 h) at 25 μM
| Compound | HCT116 | HCT116-WT | HCT116-MUT |
|---|---|---|---|
| 1 | 3.8 | 8.5 | 8.6 |
| 2 | 1.0 | 2.5 | 4.0 |
| 3 | 1.5 | 2.6 | 8.5 |
| 11 | 1.8 | 1.3 | 3.0 |
| 12 | 8.6 | 12 | 13 |
| 13 | 2.9 | 3.2 | 11 |
The DNA fragmentation assays showed that 12 and 13 had stronger apoptosis effects than did the established PI3K inhibitors 1–3. 12 appeared to induce the most apoptosis in all three cell lines. The potent apoptotic effect of 12 correlated with its ability to block AKT expression (total AKT) and to suppress the formation of pAKT-S473 (Fig. 5B). Previously, the antiproliferative effect of 12 was considered via the activation of caspase-3 induced apoptosis.20 Our data provides an additional mechanism of the tumoricidal effect of 12 via the inhibition of the PI3K/AKT pathway by blocking the formation of AKT and pAKT-S473 and by inducing apoptosis. It is known that AKT suppresses apoptosis either by stimulating NF-κB by upregulating the transactivation potential of p65,21 or by phosphorylating BCL-2 family member BAD, thereby suppressing apoptosis and promoting cell survival.22 The suppression of pAKT and the blockage of AKT expression by 12 led to apoptosis in three HCT116 cell lines, especially in H1047R mutant cells.
Similarly, 13 produced more apoptosis than 1 in the H1047R mutant only cells (HCT116-MUT). The apoptotic effect induced by 13 was not as strong as that of 1 in cells expressing wt PI3Kα (HCT116 or HCT116-WT cells). The apoptosis-inducing effect of 11 is not as strong as that caused by 12 or 13, but it exhibited similar activity toward apoptosis as 2, a proven chemical known to cause cell cycle arrest and apoptosis.19 To our knowledge, our study is the first one to reveal the antitumor properties of 11–13 in colon cancer cells expressing H1047R mutant of PI3Kα. Compounds with the benzophenone core structure of 11 were reported to induce apoptosis in Ehrlich ascites tumor (EAT) cells through activation of caspase-3.23
In order to gain detailed insight into the binding interactions of our experimentally verified inhibitors with PI3K wt and H1047R mutant, we carried out docking studies of our tested compounds to proteins PI3K wt (model 2RD0) and H1047R mutant (model 3HHM) using the QPLD-based docking approach. Cho et al. showed that incorporating the charges that were derived from the quantum mechanical/molecular mechanical (QM/MM) approach into molecular docking significantly enhanced the predictive outcome of a docking program.24 This novel protein–ligand docking method is called Quantum–Polarized Ligand Docking (QPLD)-based docking, as described by Cho et al.24 and Zhong et al.25 In the QPLD docking, the Qsite program was used to generate a new set of atomic partial charges for ligand poses which were derived from the Glide dock program. The ligand pose with QM-generated partial charges were redocked to the protein using Glide program with the XP-scoring function. With such a treatment, the polarization effects of the protein binding pocket were taken into account. The QPLD docking method has shown its superiority compared to other docking programs in the study case of 40 protein–ligand complexes with root-mean-square deviations (RMSDs) of docked pose to native pose in most cases less than 1.00 Å.24
The validation of the QPLD-based docking method was performed by comparing the docked pose with the cocrystal structure of wortmannin in H1047R PI3Kα/wortmannin complex (PDB id: 3HHM).26 The RMSD for heavy atoms of wortmannin between the QPLD-generated docked pose and the native cocrystal conformation was 0.98 Å. This indicates that the QPLD-based docking can successfully predict ligand binding conformations.
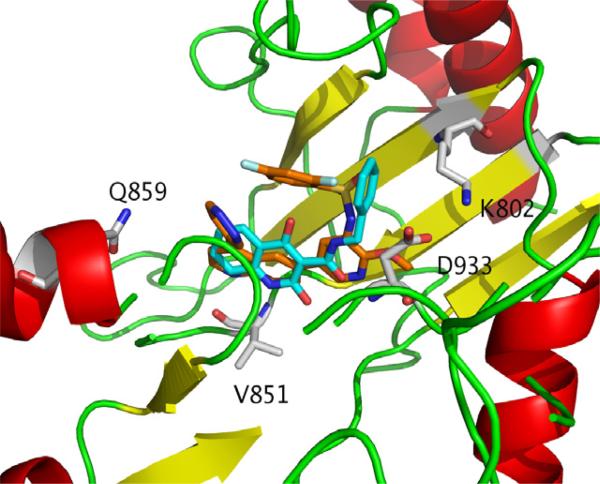
Our QPLD-based docking results of 1–4, and 11–13 against the wt and H1047R mutant PI3Kα reveal that first all compounds bind to the kinase catalytic domain of PI3K, and second, most tested compounds tend to form H-bonds with the wt and mutant PI3Kα via Asp933, Gln859, Val851 (via backbone NH), Glu849, and Lys802 (Fig. 6 and Table 3). This suggested that these residues are important for ligand binding to the wt and H1047R mutant. The importance of these residues in ligand binding to the wt and H1047R mutant of PI3Kα has been confirmed by other experimental26 and computational data.27,28 Among these residues, Asp933 was considered to be the most important.29 Our docking studies appeared to confirm this observation. The corresponding position (Gln859 of PI3Kα) in PI3Kc is Lys890. Our previously docking results suggested that the difference between these two residues might be used to develop PI3Kα-selective inhibitors.28 To evaluate the QPLD-based docking, we also carried out the regular Glide Dock of our compounds to the 2RD0 native protein. Supplementary Figure 2S shows that there is a positive linear relationship (r2 = 0.65) between the docking scores from the Glide versus QPLD-methods. The morpholino moiety in 1 and the catechol-/pyrogallol-like structure in 2 and 3 may have contributed to their differences in activities, docking scores and H-bond behaviors. The importance of a morpholino moiety has been illustrated in many PI3Kα active compounds.30
Figure 6.
Protein/ligand interactions of the complexes of PI3Kα with 4 (carbon atoms in orange) and 13 (carbon atoms in cyan).
Table 3.
Docking scores (kcal/mol) and H-bond interactions between compounds and proteins PI3Kα (the wt and the mutant).
| Compound | wt (2RD0) |
H1047R mutant (3HHM) |
||
|---|---|---|---|---|
| Docking score (kcal/mol) | Binding residues | Docking score (kcal/mol) | Binding residues | |
| 1 | –9.0 | Asp933 | –7.4 | Val851, Gln859 |
| 2 | –11 | Tyr836, Val851 | –8.6 | Val851, Asp933 |
| 3 | –8.3 | Glu849, Val851, Asp933 | –10 | Glu849,Val851,Asp933 |
| 4 | –13 | Lys776, Tyr836, Val851 | –11 | Met872, Val851, Asn853, Gln859 |
| 11 | –11 | Glu849, Val851, Gln859 | –9.1 | Val851 |
| 12 | –9.8 | Glu849 | –10 | Val851 |
| 13 | –9.9 | Lys802, Asp810, Glu849, Asp933 | –8.7 | Val851,Asp933 |
The docking of 4 to the wt and H1047R mutant of PI3Kα showed that 4 is the tightest binder (the best docking scores in Table 3) to the wt and mutant proteins. Compound 4 formed H-bonds with wt PI3Kα via Lys776, Tyr836, and Val851, and with the H1047R mutant via Met872, Val851, Asn853, and Gln859. Our hit molecules 11 and 12 formed only one H-bond with the main chain amide nitrogen of Val851, and 13 with Val851 and Asp933 in the H1047R mutant (Table 3). This suggests that other than Asp933 and Val851, Asn853 and/or Met872 may be important for ligand binding as well.
In summary, our pharmacophore modeling and database searching led to the identification of two novel PI3Kα inhibitors (12 and 13) with growth inhibitory activities similar to that of the known PI3Kα inhibitor 1. Both 12 and 13 suppressed the formation of pAKT, a downstream effector of PI3Kα and induced apoptosis in colon cancer cells, generating more DNA fragments than that induced by 1. Our docking studies suggests that the ability to form H-bonds with Asp933, Gln859, Val851, Glu849, and Lys802 may be critical for ligand binding to PI3Kα. The HCT116 colon cancer cell line data show that 12 and 13 may be good candidates for further optimization. Future studies may include an examination of 12 and 13 against a panel of kinases to investigate their selectivity. The ready availability of 12 and 13 from commercial sources may make them invaluable pharmacological tools to study PI3Kα/AKT pathway, especially those affected by H1047R mutants. We are synthesizing a series of compounds based on 13 and the preliminary data will be reported elsewhere. Whether 11 and 12 are promiscuous remains to be determined. Future hit identification could involve fingerprint-based similarity searching.
Supplementary Material
Acknowledgments
This work is partially supported by the Research Corporation for Science Advancement. D.A.S. acknowledges Al-Zaytoonah Private University of Jordan for financial support, and the Bukey Fellowship from University of Nebraska Medical Center. N.A.S. and M.G.B. wanted to thank NIH for support (Grant numbers CA72001 and CA38173).
Abbreviations
- PI3Ks
phosphatidylinositol 3-kinases
- PIP3
phosphatidylinositol 3,4,5-triphosphate
- QPLD
Quantum–Polarized Ligand Docking
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bmcl.2011.12.044.
References and notes
- 1.Huang C-H, Mandelker D, Schmidt-Kittler O, Samuels Y, Velculescu VE, Kinzler KW, Vogelstein B, Gabelli SB, Amzel LM. Science. 2007;318:1744. doi: 10.1126/science.1150799. [DOI] [PubMed] [Google Scholar]
- 2.Cantrell DA. J. Cell Sci. 2001;114:1439. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 3.Liu P, Cheng H, Roberts TM, Zhao JJ. Nat. Rev. Drug Disc. 2009;8:627. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikenoue T, Kanai F, Hikiba Y, Obata T, Tanaka Y, Imamura J, Ohta M, Jazag A, Guleng B, Tateishi K, Asaoka Y, Matsumura M, Kawabe T, Omata M. Cancer Res. 2005;65:4562. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 5.Zhao L, Vogt PK. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2652. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlahos CJ, Matter WF, Hui KY, Brown RF. J. Biol. Chem. 1994;269:5241. [PubMed] [Google Scholar]
- 7.Nöthlings U, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN. Am. J. Epidemiol. 2007;166:924. doi: 10.1093/aje/kwm172. [DOI] [PubMed] [Google Scholar]
- 8.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, García-Echeverría C. Mol. Cancer Ther. 2008;7:1851. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 9.Knight SD, Adams ND, Burgess JL, Chaudhari AM, Darcy MG, Donatelli CA, Luengo JI, Newlander KA, Parrish CA, Ridgers LH, Sarpong MA, Schmidt SJ, Van Aller GS, Carson JD, Diamond MA, Elkins PA, Gardiner CM, Garver E, Gilbert SA, Gontarek RR, Jackson JR, Kershner KL, Luo L, Raha K, Sherk CS, Sung C-M, Sutton D, Tummino PJ, Wegrzyn RJ, Auger KR, Dhanak D. ACS Med. Chem. Lett. 2010;1:39. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rückle T, Schwarz MK, Rommel C. Nat. Rev. Drug Disc. 2006;5:903. doi: 10.1038/nrd2145. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa M, Kaizawa H, Kawaguchi K-I, Ishikawa N, Koizumi T, Ohishi T, Yamano M, Okada M, Ohta M, Tsukamoto S-I, Reynaud FI, Waterfield MD, Parker P, Workman P. Bioorg. Med. Chem. 2007;15:403. doi: 10.1016/j.bmc.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa M, Kawaguchi K-I, Kaizawa H, Koizumi T, Ohishi T, Yamano M, Okada M, Ohta M, Tsukamoto S-I, Reynaud FIR, Parker P, Workman P, Waterfield MD. Bioorg. Med. Chem. 2007;15:5837. doi: 10.1016/j.bmc.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 13.MOE software . Chemical Computing Group Inc.; Montreal, Canada: 2009. Web address: http://www.chemcomp.com. [Google Scholar]
- 14.NCI Open Database Compounds, release 3. National Cancer Institute, National Institutes of Health; Bethseda, MD: Sep, 2003. [18.08.2008]. Available online at: http://cactus.nci.nih.gov/download/nci. [Google Scholar]
- 15.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv. Drug Delivery Rev. 1997;23:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 16.Brattain MG, Levine AE, Chakrabarty S, Yeoman LC, Willson JK, Long B. Cancer Metastasis Rev. 1984;3:177. doi: 10.1007/BF00048384. [DOI] [PubMed] [Google Scholar]
- 17.Samuels Y, Diaz LA, Jr., Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Cancer Cell. 2005;7:561. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Tang C, Lu YH, Xie JH, Wang F, Zou JN, Yang JS, Xing YY, Xi T. Anticancer Drugs. 2009;20:249. doi: 10.1097/CAD.0b013e328327d476. [DOI] [PubMed] [Google Scholar]
- 19.Chien SY, Wu YC, Chung JG, Yang JS, Lu HF, Tsou MF, Wood WG, Kuo SJ, Chen DR. Hum. Exp. Toxicol. 2009;28:493. doi: 10.1177/0960327109107002. [DOI] [PubMed] [Google Scholar]
- 20.Wang CG, Yang JQ, Liu BZ, Jin DT, Wang C, Zhong L, Zhu D, Wu Y. Eur. J. Pharmacol. 2010;627:33. [Google Scholar]
- 21.Madrid LV, Wang C-Y, Guttridge DC, Schottelius AJG, Baldwin AS, Jr., Mayo MW. Mol. Cell. Biol. 2000;20:1626. doi: 10.1128/mcb.20.5.1626-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Cell. 1997;91:231. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakar BT, Khanum SA, Jayashree K, Salimath BP, Shashikanth S. Bioorg. Med. Chem. 2006;14:435. doi: 10.1016/j.bmc.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Cho AE, Guallar V, Berne BJ, Friesner RJ. Comput. Chem. 2005;26:915. doi: 10.1002/jcc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong H, Kirschner KN, Lee M, Bowen JP. Bioorg. Med. Chem. Lett. 2008;18:542. doi: 10.1016/j.bmcl.2007.11.090. [DOI] [PubMed] [Google Scholar]
- 26.Mandelker D, Gabelli S, Schmidt-kittler O, Zhu J, Cheong I, Huang CH, Kinzler K, Vogelstein B, Amzel M. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16996. doi: 10.1073/pnas.0908444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Wang Y, Zhang FJ. Mol. Model. 2010;9:1449. doi: 10.1007/s00894-010-0659-y. [DOI] [PubMed] [Google Scholar]
- 28.Sabbah DA, Vennerstrom JL, Zhong HJ. Chem. Inf. Model. 2010;50:1887. doi: 10.1021/ci1002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinson J-A, Schmidt-Kittler O, Zhu J, Jennings IG, Kinzler KW, Vogelstein B, Chalmers DK, Thompson PE. ChemMedChem. 2011;6:514. doi: 10.1002/cmdc.201000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabbah DA, Brattain MG, Zhong H. Curr. Med. Chem. 2011;18:5528. doi: 10.2174/092986711798347298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.