Abstract
Background
Follow-up care after radical cystectomy is poorly defined with extensive variation in practice patterns. We sought to determine sources of these variations in care as well as examine the economic impact of standardization of care to guideline recommended care.
Methods
Using linked SEER-Medicare data from 1992 to 2007; we determined follow-up care expenditures (time and geography standardized) for 24 months after surgery. Accounted expenditures included office visits, imaging studies, urine tests and blood work. A multilevel model was implemented to determine the impact of region, surgeon, and patient factors on care delivery. We then compared the actual expenditures on care in the Medicare system (interquartile range) to the expenditures if patients received care recommended by current clinical guidelines.
Results
Expenditures over 24 months of follow-up were calculated per month and per patient. The mean and median total expenditures per patient were $1108 and $805 respectively (minimum $0, maximum $9,805, 25th to 75th percentile $344 to $1503). Variations in expenditures were most explained at the patient level. After accounting for surgeon and patient levels, we found no regional variations in care. Adherence to guidelines would be associated with expenditures from 0.80–10.6 times the expenditures in current practice.
Conclusion
While some regional and surgeon-level variations in care were found, most variation in expenditure on follow-up care was at the patient-level, largely based on node positivity, chemotherapy status, final cancer stage. Standardization of care to current established guidelines would create higher expenditures on follow-up care than current practice patterns.
Keywords: Bladder cancer, Cystectomy, Follow-up, Cost Analysis, Expenditure
INTRODUCTION
In 2012, an estimated 73,510 new cases of bladder cancer were diagnosed in the United States, with an estimated 14,880 deaths.1 For patients with disease not appropriate for conservative forms of intervention, definitive surgery with radical cystectomy remains the standard of care. For the approximately 8500 patients who receive this surgery each year,2 recurrence and adverse events from urinary tract reconstruction are major concerns,3, 4 with most bladder cancer recurrences occurring within the first two years after definitive surgery.4, 5
Despite this high risk of recurrence in the first two years following invasive therapy, strong evidence to help guide clinicians in the follow-up care of their patients is lacking. In the absence of such evidence, competing guidelines have been proposed. For example, the International Consultation on Urological Diseases (ICUD) offers very broad guidelines for follow-up. They suggest a risk-stratified approach for follow-up without any specific recommendations.6 In contrast, both the National Comprehensive Cancer Network (NCCN) and the European Association of Urology have published guidelines for follow-up; though both organizations cite a dearth of strong evidence and acknowledge that many of the recommendations are based on expert opinion.7, 8 These guidelines have quite different recommendations on appropriate follow-up after definitive surgery. The lack of agreement in the guidelines, in addition to the weak evidence base, can lead to considerable variation in the follow-up care performed in clinical practice.
To determine mechanisms leading to variation in follow-up care, we performed a population-based study utilizing Surveillance, Epidemiology, and End Results (SEER)-Medicare data. We sought to elucidate whether variations in care were the result of patient, provider, or regional factors. We then determined the consequences on expenditures at the payer level if care were standardized to published guidelines.
MATERIALS AND METHODS
Study Population
Using linked SEER-Medicare data from 1992 to 2007, we identified all bladder cancer patients based on International Classification of Diseases—Oncology 3 diagnosis codes. Using the National Claims History (NCH) file (data on physician/supplier Part B bills for fee for service Medicare claims from non-institutional providers), we defined a cohort of patients who underwent surgical excision of their tumor based on the Current Procedural Terminology (CPT) codes for radical cystectomy. Patients aged 66 to 90 were included in the study to allow one full year of claims for assessment of comorbidity status. Only patients with continuous enrollment in Medicare parts A and B, no HMO enrollment prior to surgery, and who survived or were not censored by one month after surgery were included in the final study cohort.
Description of Expenditures
We determined follow-up care expenditures for up to 24 months after surgery. This was performed by tallying the expenditures related to office visits, imaging studies, urine tests and blood work as indexed from Healthcare Common Procedure Coding System (HSPCS) codes (Appendix 1). Ascertainment of care was stopped at death, loss of coverage, HMO enrollment, or the end of the follow-up in the data.
Appendix 1.
Medicare Allowable Costs Associated with follow up parameter
| Test Type | Cost | Cpt code |
|---|---|---|
|
| ||
| Renal function panel | 14.97 | 80069 |
| Blood gas any combination | 27.4 | 82805 |
| ESR, nonautomated | 5.02 | 85651 |
| Urine culture | 11.43 | 87086 |
| Urine cytology | 72.84 | 88106 |
| CT abdomen and pelvis w w/o contrast | 409.64 | 74178 |
| 3D reconstruction | 82.68 | 76377 |
| CT chest w contrast | 197.01 | 71260 |
| Chest X-ray - 2 views | 30.97 | 71020 |
| Renal Ultrasound (retroperitoneal) | 132.41 | 76770 |
| office visit, follow up - level 3 | 70.46 | 99213 |
We calculated the total expenditures on care for each patient, price-adjusted for time and geography using the Medicare-Economic Index.9 The distribution of expenditures over a 24 month follow-up period was skewed to the right with one mode; therefore we applied log transformation to each of these variables to better meet the normality assumption of the linear mixed model.
Statistical Analysis
A series of three-level mixed models with random effects were fit designed to examine the variability of patient expenditures at different levels and how much of the variability could be explained by including patient and surgeon specific factors. Patients with a surgeon only appearing once in the data were removed for the multilevel model. There were 11 regions and 384 surgeons with at least two patients in the data. Case wise deletion was used for the missing data based on the complete model. 1384 patients fit these criteria. The multilevel models were allowed to have unique (random) intercepts by region and surgeon in order to examine the variability at the region, surgeon, and patient level. The models differed in the type of factors (fixed effects) that were included. The null model included no factors. The patient model and surgeon model included only patient or surgeon specific factors respectively. The complete model included both patient [age, race, gender, marital status, neighborhood education level, zip code level median income, comorbidity, final stage of the disease (from SEER extent of disease coding), nodal status, whether chemotherapy was administered (neoadjuvant or adjuvant), and hospital readmissions (within 24 months of surgery)] and surgeon level factors [presence of a medical school at the hospital where cystectomy was performed, presence of a residency program at the hospital, National Cancer Institute designation of the hospital, employment type of provider (solo, hospital-based, group practice), and the decade of completion of residency of the provider]. Deviance tests were performed comparing each model back to its parent.
We then performed an additional analysis to assess the contribution of the individual patient level factors to the variability in follow up expenditures. A multilevel modeling framework was implemented with patients nested within surgeons who were subsequently nested into SEER-based geographical regions. We fitted 3-level linear mixed models with random intercepts. Our initial model is a random intercepts model with SEER regions and surgeons nested within SEER regions as random effects and no exploratory variables. We then added in the patient-level factors. We calculated the percentage of total variance in average expenditure explained by each of the individual patient-level factors.
Medicare Expenditures Associated with Guideline Recommended Follow-up Care
Table 2 demonstrates the follow-up regimens proposed by the NCCN and EAU. Of note, the NCCN guidelines provide ranges of follow-up intensity in their guidelines but to facilitate comparison, two follow-up routines at the high and low end of intensity were considered. When upper tract imaging was recommended, we elected a CT urogram as the most likely study to be performed as a reflection of current practice patterns. In order to determine the Medicare expenditures associated with follow-up, the Medicare allowed payment was determined for each follow-up care category in the guidelines (Appendix 2).10
Table 2.
24 Month Follow-up Recommendations by Guideline
| Months after Cystectomy
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | |||
| NCCN | High Intensity | Labs, Urine Cytology | X | X | X | X | X | X | X | X |
| CXR, CT Urogram | X | X | X | X | X | X | X | X | ||
| Conservative | Labs, Urine Cytology | - | X | - | X | - | X | - | X | |
| CXR, CT Urogram | - | - | - | X | - | - | - | X | ||
|
| ||||||||||
| EAU | < pT1 | Renal Ultrasound | X | - | - | - | - | - | - | - |
| CT Thor/Urogram | - | - | - | X | - | - | - | X | ||
| Labs, Culture, Cytology | X | X | X | X | - | - | - | X | ||
| = pT2 | Renal Ultrasound | X | - | - | - | - | - | - | - | |
| CT Thor/Urogram | - | X | - | X | - | X | - | X | ||
| Labs, Culture, Cytology | X | X | - | X | - | - | - | X | ||
| ≥ pT3 | Renal Ultrasound | X | - | - | - | - | - | - | - | |
| CT Thor/Urogram | X | X | - | X | - | X | - | X | ||
| Labs, Culture, Cytology | X | X | - | X | - | - | - | X | ||
Comparison of Expenditures on Follow-up from SEER-Medicare Patient Data to the Guideline Recommended Care
We wished to understand how the follow-up care expenditure among SEER-Medicare patients related to the expenditure of adhering to the reviewed guidelines. For the SEER- Medicare data, we looked only at patients who survived at least 24 months during the follow-up period and the total expenditures on follow-up care over the 24 month follow-up period were assessed for these patients. For consistency with the prior analysis, we excluded expenditures within one month of surgery as these were more likely related to initial surgery instead of follow-up care. The Medicare expenditures associated with the NCCN and EAU guidelines were calculated over a 24 month follow-up period. To evaluate the dispersion of the variance of expenditures in the population, we compared the 25th, 50th, and 75th percentile of 24 month expenditures within the SEER-Medicare data to the calculated expenditures for 24 months of follow-up for both high and low intensity follow-up recommended by NCCN. For the comparison to the expenditures on recommended follow-up by the EAU, we calculated the actual 24 month follow-up expenditures at the 25th, 50th, and 75th percentile for patients in our cohort by stage (Stage ≤ T1, Stage = T2, Stage > T2). These calculated expenditures were compared to the calculated follow-up expenditures per stage category from the EAU recommendations.
All statistical tests were conducted with the use of SAS 9.2. All tests were two-sided at a significance level of 0.05. The Institutional Review Board of Washington University approved this study.
RESULTS
The demographic information of our cohort is shown in Table 1. The mean and median total expenditures per patient were $1108 and $805 respectively (minimum $0, maximum $9,805, 25th to 75th percentile $344 to $1503). The highest expenditures on follow-up care were in patients treated with chemotherapy or who were node positive. Patients with lower stage disease, no hospital readmissions within 24 months of cystectomy, or who were of Black race had lower expenditures on follow-up care.
Table 1.
Patient Characteristics by Time Period and Expenditure Groups
| N | % | Average Monthly Expenditure Per Patient | |
|---|---|---|---|
|
| |||
| Total Patients | 1807 | - | $88.33 |
|
| |||
| Age Category | |||
|
| |||
| 66 to 69 | 366 | 20.3% | $100.79 |
| 70 to 74 | 526 | 29.1% | $86.83 |
| 75 to 79 | 528 | 29.2% | $86.28 |
| >=80 | 387 | 21.4% | $81.36 |
| Race | |||
|
| |||
| White | 1667 | 92.3% | $88.58 |
| Black | 56 | 3.1% | $77.32 |
| Other | 84 | 4.6% | $90.65 |
| Sex | |||
|
| |||
| Male | 1176 | 65.1% | $84.66 |
| Female | 631 | 34.9% | $95.15 |
| Marital Status | |||
|
| |||
| Married | 585 | 32.4% | $90.04 |
| Unmarried/Unknown | 1222 | 67.6% | $87.50 |
| Education Level* | |||
|
| |||
| <10% Did not Graduate HS | 538 | 29.8% | $92.48 |
| 10–19% Did not Graduate HS | 773 | 42.8% | $89.11 |
| 20–29% Did not Graduate HS | 263 | 14.6% | $84.55 |
| >=30% Did not Graduate HS | 182 | 10.1% | $82.13 |
| Unknown | 51 | 2.8% | $74.31 |
| Zip Code Income | |||
|
| |||
| < $38,657 | 432 | 23.9% | $85.49 |
| $38,658–$47,921 | 436 | 24.1% | $84.38 |
| $47,922–$62,354 | 450 | 24.9% | $85.44 |
| >$62,354 | 438 | 24.2% | $99.65 |
| Unknown | 51 | 2.8% | $74.31 |
| Comorbidity | |||
|
| |||
| 0 | 1018 | 56.3% | $88.94 |
| 1 | 503 | 27.8% | $87.92 |
| 2 | 209 | 11.6% | $79.82 |
| 3 | 77 | 4.3% | $105.89 |
| Final Stage of Disease | |||
|
| |||
| Stage 1 | 390 | 21.6% | $74.09 |
| Stage 2 | 317 | 17.5% | $71.69 |
| Stage 3 | 416 | 23.0% | $91.89 |
| Stage 4 | 395 | 21.9% | $115.74 |
| Unknown/No Cancer | 289 | 16.0% | $83.20 |
| Nodal Status | |||
|
| |||
| All nodes negative | 690 | 38.2% | $74.81 |
| Nodes Positive | 248 | 13.7% | $139.35 |
| No Nodes Examined | 869 | 48.1% | $84.49 |
| Chemotherapy Administered | |||
|
| |||
| No Chemotherapy | 1173 | 64.9% | $64.12 |
| Neoadjuvant Only | 98 | 5.4% | $88.15 |
| Adjuvant Only | 226 | 12.5% | $154.25 |
| Neoadjuvant/Adjuvant | 19 | 1.1% | $168.62 |
| Treatment Only | 180 | 10.0% | $105.54 |
| Neoadjuvant/Adjuvant/Treatment | 87 | 4.8% | $147.22 |
| Continuous | 24 | 1.3% | $245.36 |
| Admission to Hospital after Initial Discharge | |||
|
| |||
| None | 505 | 27.9% | $62.79 |
| 1 Admission | 435 | 24.1% | $82.26 |
| 2 Admissions | 328 | 18.2% | $111.03 |
| 3 Admissions | 539 | 29.8% | $103.33 |
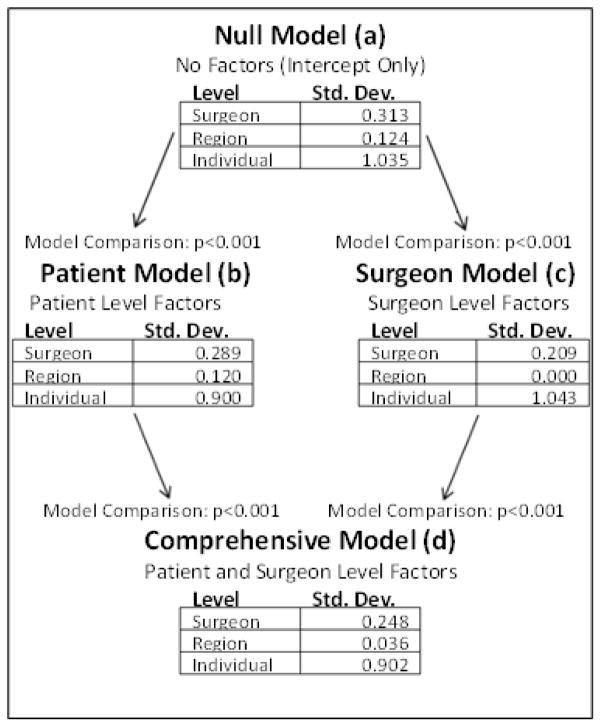
The multilevel null model (figure 1a) reveals that a majority of the variability in expenditures lies at the individual level, and there is also a substantial amount of variability at the surgeon level. The region level results reveal that there is very little difference occurring in expenditures by region. Both patient (1b) and surgeon (1c) models were fit. Adding patient specific factors lowered the variability of expenditures in all three levels with the largest decrease at the patient level. The surgeon model explains a substantial amount of the surgeon level expenditure variability but had no effect on the patient level variability. It also essentially removes all of the variability present at the region level. The complete model (1d) puts all the patient information and surgeon information into one final model. Addition of patient factors to surgeon factors explained some of the surgeon level expenditure variability. Each of these models is a significantly better fit than its predecessor(s) (p<0.001). Table 3 illustrates the percentage of total variance explained by each of the individual patient-level factors for average expenditure over 24 months. The major contributors accounting for greater than 1% of the variation in expenditure were nodal status, administration of chemotherapy, hospital readmissions, and final stage of the disease.
Figure 1.
Results from the multilevel model. With no patient or surgeon factors included, most variation in care was explained at the surgeon and patient levels, with little variation in care resulting from region of treatment (a). Addition of patient factors (b) explained some of the variation at the region and surgeon levels. Addition of surgeon factors (c) did not change the variation explained by patient factors, but removed the influence of region completely from the model. The final model including both patient and surgeon factors shows the largest variations in care resided at the patient level, with some variation at the surgeon level. Region of treatment did not account for any variations in expenditures on follow up care after radical cystectomy.
Table 3.
Percentage of total variance explained by each of the individual patient-level factors for average expenditure at 24 months
| Race | 0.12% |
| Gender | 0.15% |
| Martial Status | <0.00% |
| Neighborhood Education Level | <0.00% |
| Age Category | <0.00% |
| Median Income | 0.73% |
| Charlson Comorbidity | 0.24% |
| Node Status | 4.80% |
| Chemotherapy Administered | 17.77% |
| Readmission to Hospital | 7.27% |
| Final Stage of Disease | 3.49% |
Table 4 demonstrates the expenditures related to follow-up care by type of follow-up per the guidelines in comparison to the interquartile range of expenditure from the SEER-Medicare data. Expenditures on care for each comparison are higher for guideline recommended care than for the actual care received in the cohort with the exception of the NCCN conservative follow-up category. Follow-up care would be 20% less costly if NCCN conservative follow-up recommendations were followed compared to the 75th percentile of patients in the cohort. The largest difference in expenditure was found between the NCCN high intensity follow-up and the care actually received in the 25th percentile of expenditure in the cohort. Here, switching to the NCCN guideline recommended follow-up creates an over ten times increase in expenditures to the Medicare program. Furthermore, in both guideline recommended care and actual clinical practice, imaging accounts for the majority of expenditures on follow up care.
Table 4.
Cost of 24 Months of Follow Up Per Guideline Recommendations in Comparison to Observed Cost of Follow Up from SEER-Medicare Data
| Total cost after 24 Months of Follow Up | Total cost over24 months ($) | Total cost of imaging over24 months ($) | Factor greater than SEER-Medicare follow up (25th) | Factor greater than SEER-Medicare follow up (50th) | Factor greater than SEER-Medicare follow up (75th) | |
|---|---|---|---|---|---|---|
|
| ||||||
| NCCN Guideline Recommendations | High Intensity follow up | 4832.96 | 3525 | 10.6 | 5.13 | 2.78 |
| Conservative follow up | 1383.86 | 881 | 3.04 | 1.47 | 0.80 | |
|
| ||||||
| SEER-Medicare 25th/50th/75th (N=915) | 456/942/1737 | 236/563/1229 | n/a | n/a | n/a | |
|
| ||||||
| EAU Guideline Recommendations | Stage ≤ T1 | 2023.75 | 1346 | 4.74 | 2.43 | 1.36 |
|
| ||||||
| SEER-Medicare 25th/50th/75th Stage 1 (N=262) | 427/834/1485 | 220/511/1009 | n/a | n/a | n/a | |
|
| ||||||
| Stage = T2 | 3388.45 | 2559 | 7.60 | 3.80 | 2.02 | |
|
| ||||||
| SEER-Medicare 25th/50th/75th Stage 2 (N=209) | 446/892/1674 | 235/590/1157 | n/a | n/a | n/a | |
|
| ||||||
| Stage > T2 | 4070.8 | 3166 | 7.48 | 3.55 | 1.95 | |
|
| ||||||
| SEER-Medicare 25th/50th/75th Stage 3,4 (N=303) | 544/1148/2084 | 282/677/1486 | n/a | n/a | n/a | |
DISCUSSION
Our results demonstrate variation at regional, surgeon, and patient levels, with most of the variation in expenditure on follow-up care situated at the patient level. Most of these patient factors, including nodal status, receipt of chemotherapy, and tumor stage, are directly related to the need to monitor for treatment response and recurrence of disease. However, most of the variation in expenditures on follow-up care could not be accounted for in the models. This variation in practice could be tempered by closer adherence to published guidelines, but at increased expense for patients and payers.
It is important to weigh the pros and cons of strict adherence to a follow-up protocol after cancer treatment. Most patients who develop a recurrence after definitive therapy for urothelial cancer of the bladder, will do so within 6–18 months after surgery. As reviewed, the established guidelines recommend increased follow-up intensity at least during the first 24 months after definitive therapy. Close adherence to such guidelines could ameliorate problems with disparities, as we found for neighborhood income levels, in receipt of follow-up care. For most patients, adherence to guidelines would increase the receipt of follow-up care.
Improved adherence to guidelines could also help improve patient outcomes; however, the utility of follow up care after cystectomy has not been clearly defined. Contrasting studies have shown an improvement in patient survival when recurrences are found in an asymptomatic state, and no improvement in survival in this same setting.11, 12 Additionally, aspects of follow-up care, including urine testing and doctor visits, are associated with improvements in survival in both population based13 and institutional studies.11, 14 If guidelines focused on care most associated with improved survival, and the guidelines were followed, patient outcomes would be substantially improved.
Unfortunately, guideline adherence also could create negative consequences. In a survey of urologic oncologists, there was no uniformity in the type of follow-up care provided after cystectomy,15 and there was lack of compliance with a predetermined follow-up schedule after cystectomy. With such variability in the follow-up being performed, there is a real concern for unnecessary quantity of follow-up leading to excess expenditures on care; yet, this was not reflected in our analysis. Despite the two prevailing recommendations for relatively intensive surveillance following extirpative surgery for bladder cancer, our analysis of current practice patterns suggests that providers are conducting a more conservative approach that is, for the most part, less costly than published guidelines. Greater compliance with current guidelines would actually increase expenditures for payers and patients. Additionally, strict adherence to follow-up protocols may restrict patients’ choice, potentially negatively affecting patient’s quality of life.16 Without any prospective studies demonstrating a survival benefit with follow-up, it appears that current providers are not putting unnecessary economic strain on the medical system.
There are some limitations to address for this study. First, though our SEER-Medicare linked data only included patients aged ≥ 65, it still remains relevant to the bladder cancer population as approximately 72% of bladder cancer diagnoses are among patients over 65 years of age.17 Second, only the direct expenditures on care were addressed. Thus true economic costs from a societal perspective were not assessed. These additional inputs, including lost time for patients, expenditures on transportation to testing and visits, and loss of productivity for caregivers would only increase the economic burden of closer adherence to current guidelines. Third, the frequency of physician office visits was assessed for all providers which may have increased the overall cost of follow-up in the cohort when compared with the established guidelines. However, the incremental cost of a physician office visit is low and we have previously established that imaging is the primary driving factor for cost of follow-up care.13 Finally, we did not limit our assessment of care to diagnosis codes indicating bladder cancer. By including all follow up testing after cystectomy, we may have increased the quantity and expenditures on follow up care above the amounts truly performed for bladder cancer follow up. This inflation, however, would only increase the discrepancy between current guideline recommendations and current practice.
CONCLUSION
We demonstrated that patient level factors were primarily responsible for the variance in expenditures related to follow-up care after cystectomy for bladder cancer. Accounting for final pathologic stage, we found greater adherence to published guidelines would substantially increase expenditures on follow-up care for these patients. Adherence to a more conservative guideline, like the NCCN follow up pattern, would allow standardization of care without substantial increases in expenditures.
Highlights.
Patient factors explain most variation in follow-up expenditures after cystectomy
Nodal status, chemotherapy, readmissions, and disease stage drive expenditures
Actual expenditures are lower than the costs of testing in guidelines
Adherence to conservative NCCN guidelines standardize care without increasing costs
Acknowledgments
This publication was supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ), and Grant Number KM1CA156708 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moyer VA, Force USPST. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 2.Hollenbeck BK, Taub DA, Dunn RL, Wei JT. Quality of care: partial cystectomy for bladder cancer--a case of inappropriate use? J Urol. 2005;174:1050–1054. doi: 10.1097/01.ju.0000169477.30477.3d. discussion 1054. [DOI] [PubMed] [Google Scholar]
- 3.Nieuwenhuijzen JA, de Vries RR, Bex A, et al. Urinary diversions after cystectomy: the association of clinical factors, complications and functional results of four different diversions. Eur Urol. 2008;53:834–842. doi: 10.1016/j.eururo.2007.09.008. discussion 842–834. [DOI] [PubMed] [Google Scholar]
- 4.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 5.Malkowicz SB, van Poppel H, Mickisch G, et al. Muscle-invasive urothelial carcinoma of the bladder. Urology. 2007;69:3–16. doi: 10.1016/j.urology.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Soloway M, Khoury S, editors. Bladder Cancer. Paris, France: International Consultation of Urological Diseases-European Association of Urology; 2012. [Google Scholar]
- 7. [accessed 09/30/2013];NCCN Clinical Practice Guidelines in Oncology (NCCN Guielines): Bladder Cancer. 2013 Available from http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 8.Stenzl A, Cowan NC, De Santis M, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2009;55:815–825. doi: 10.1016/j.eururo.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40:IV-104–117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 10.Sanderson KM, Cai J, Miranda G, Skinner DG, Stein JP. Upper tract urothelial recurrence following radical cystectomy for transitional cell carcinoma of the bladder: an analysis of 1,069 patients with 10-year followup. J Urol. 2007;177:2088–2094. doi: 10.1016/j.juro.2007.01.133. [DOI] [PubMed] [Google Scholar]
- 11.Giannarini G, Kessler TM, Thoeny HC, Nguyen DP, Meissner C, Studer UE. Do patients benefit from routine follow-up to detect recurrences after radical cystectomy and ileal orthotopic bladder substitution? Eur Urol. 2010;58:486–494. doi: 10.1016/j.eururo.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Volkmer BG, Kuefer R, Bartsch GC, Jr, Gust K, Hautmann RE. Oncological followup after radical cystectomy for bladder cancer-is there any benefit? J Urol. 2009;181:1587–1593. doi: 10.1016/j.juro.2008.11.112. discussion 1593. [DOI] [PubMed] [Google Scholar]
- 13.Strope SA, Chang SH, Chen L, Sandhu G, Piccirillo JF, Schootman M. Survival Impact of Follow-up Care after Radical Cystectomy for Bladder Cancer. J Urol. 2013 doi: 10.1016/j.juro.2013.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boorjian SA, Tollefson MK, Cheville JC, Costello BA, Thapa P, Frank I. Detection of asymptomatic recurrence during routine oncological followup after radical cystectomy is associated with improved patient survival. J Urol. 2011;186:1796–1802. doi: 10.1016/j.juro.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Dalbagni G, Bochner BH, Cronin A, Herr HW, Donat SM. A plea for a uniform surveillance schedule after radical cystectomy. J Urol. 2011;185:2091–2096. doi: 10.1016/j.juro.2011.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hack TF, Degner LF, Watson P, Sinha L. Do patients benefit from participating in medical decision making? Longitudinal follow-up of women with breast cancer. Psychooncology. 2006;15:9–19. doi: 10.1002/pon.907. [DOI] [PubMed] [Google Scholar]
- 17.Croswell JM, Kramer BS, Kreimer AR, et al. Cumulative incidence of false-positive results in repeated, multimodal cancer screening. Ann Fam Med. 2009;7:212–222. doi: 10.1370/afm.942. [DOI] [PMC free article] [PubMed] [Google Scholar]