Abstract
Objective
To test the association between hospital type and performance of candidate quality measures for treatment of muscle invasive bladder cancer (MIBC) using a large national tumor registry. Proposed quality measures include receipt of neoadjuvant chemotherapy, timely treatment, adequate lymph node dissection, and continent urinary diversion.
Methods
Using the National Cancer Database (NCDB), patients with stage ≥II urothelial carcinoma treated with radical cystectomy (RC) from 2003–2010 were identified. Hospitals were grouped by type and annual RC volume: community, comprehensive low volume (CLV), comprehensive high volume (CHV), academic low volume (ALV), and academic high volume (AHV) groups. Logistic regression models were used to test the association between hospital group and performance of quality measures, adjusting for year, demographic, and clinical/pathologic characteristics; generalized estimating equations were fitted to the models to adjust for clustering at the hospital level.
Results
23,279 patients underwent RC at community (12.4%), comprehensive (CLV: 38%; CHV: 5%), and academic (ALV: 17%; AHV: 28%) hospitals. While only 0.8% (n=175) of patients met all 4 quality criteria, 61% of patients treated at AHV hospitals met ≥2 quality metric indicators compared to ALV (45%), CHV (44%), CLV (38%), and community (37%) hospitals (p<0.001). Following adjustment, patients were more likely to receive ≥2 quality measures when treated at AHV (OR 2.4 [CI 2.0–2.9]), ALV (OR 1.3 [CI 1.1–1.6]), and CHV (OR 1.3 [CI 1.03–1.7]) hospitals compared to community hospitals.
Conclusions
Patients undergoing RC at AHV hospitals were more likely to meet quality criteria. However, performance remains low across hospital types, highlighting the opportunity to improve quality of care for MIBC.
Keywords: bladder cancer, hospital type, quality measures, radical cystectomy, surgical volume
Introduction
Emerging pay for performance reimbursement mechanisms are directed at improving delivery of high quality, valued-based care while reducing unnecessary utilization and expenditures.[1] Performance reporting has been proposed as an essential component of healthcare reform, with the aim of improving quality by increasing transparency and accountability.[2] Although a clear benefit has not been demonstrated, public disclosure of provider performance at the individual and aggregate level has increased over the past two decades[3] to empower patients as informed decision makers and reduce undesirable variation. Further, while evidence-based guidelines exist to standardize treatment practices for most medical conditions, guidelines are often underutilized, particularly for highly specialized and resource intensive services such as oncologic care.[4]
Bladder cancer remains one of the most costly malignancies from diagnosis to death per patient, and with only a 5% relative reduction in mortality rate over the past 15 years, progress in preventing bladder cancer-related mortality lags behind other malignancies such as colon, breast, and prostate cancers.[5] While small improvements in hospital length of stay and inpatient mortality have been demonstrated with the regionalization of complex surgical procedures including radical cystectomy (RC),[6] there is still considerable variation in evidence based practice for patients with bladder cancer.[7] Further, lack of level one evidence has limited development of rigorous metrics to reliably measure quality for patients undergoing surgical treatment of muscle invasive bladder cancer (MIBC).[8]
Several process measures with varying levels of supporting evidence have been proposed as candidate quality of care metrics, including receipt of neoadjuvant chemotherapy, timely treatment, adequate lymph node yield, and use of continent urinary diversion.[9] To improve quality at the national level, experts and advocacy groups have emphasized regionalization of complex procedures from low volume community hospitals to tertiary high volume centers.[10] In this study, our aim was to assess trends in performance of candidate quality measures in patients undergoing RC for MIBC by hospital type using a large national tumor registry.
Patients and Methods
Cohort Definition
A program of the American College of Surgeons, Commission on Cancer, and American Cancer Society, the National Cancer Data Base (NCDB) is a national cancer registry that was established in 1989 and serves as a comprehensive clinical surveillance resource for cancer care in the United States. The NCDB compiles data from more than 1,500 commission-accredited cancer programs in the United States and Puerto Rico and captures approximately to 70% of all newly diagnosed cancer cases.[11]
All patients with urothelial carcinoma of the bladder were identified based on International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) site codes (8120–8131). Our analytic cohort was restricted to individuals aged 18 to 90 years undergoing RC for analytic stage II-IV disease from 2003–2010. Patients with non-urothelial histologic type, stage ≤I or unknown stage, or second primary cancers were excluded. Patient socioeconomic characteristics were provided using census tract data. Co-morbidity burden was determined using the Charlson-Deyo classification and categorized as 0, 1, or ≥2. Vital status to determine trends in overall survival was only available for patients identified prior to 2006.
Based on case volume and access to cancer-related services and specialists, the NCDB classifies hospitals as unknown, community (100–500 new cancer cases per year), comprehensive community (>500 cases per year), and teaching/research (academic) centers defined by either National Cancer Institute designation or medical school affiliation. Using previously described methods[6, 12, 13], annual RC hospital volume status was defined by tercile (with high volume defined as ≥ 4 RC/year). As previous studies have demonstrated overlap in outcomes by facility type and volume status,[14] composite hospital type/volume (high versus intermediate/low) categories were created: unknown and community, comprehensive low volume (CLV), comprehensive high volume (CHV), academic low volume (ALV), and academic high volume (AHV).
Quality Measure Selection
Four candidate performance measures were identified: receipt of neoadjuvant chemotherapy, treatment within 3 months of diagnosis, lymph node yield ≥10, and performance of continent diversion.[9] Neoadjuvant chemotherapy was defined as systemic treatment received prior to RC using initiation of therapy date. Time to treatment was determined using time from diagnosis to surgery or initiation of neoadjuvant chemotherapy to avoid penalizing hospitals in which pre-operative chemotherapy is preferentially administered. Lymph node yield was derived from the regional lymph nodes examined field, and receipt of continent urinary diversion was determined by primary site codes.
Statistical Analyses
Trends in performance of candidate quality measures were assessed from 2003–2010 using Chi-square tests. Categorizing patients by the number of measures received, we performed exploratory analyses to assess for differences in overall survival using Kaplan Meier methods and proportional hazard regressions adjusted for all covariates. Patient demographic and clinical characteristics were compared by hospital category group using Chi-square tests. Adjusting for year, age, gender, race (white, African American, other), Hispanic ethnicity, payer group, Charlson-Deyo score, income, education, tumor grade, analytic stage, urban/rural status, and facility location, we examined the association between hospital category and receipt of individual quality measures using multivariable logistic regression. To account for clustering within hospitals, we calculated robust standard errors using Generalized Estimating Equations. As a composite metric may be more effective to reliably assess quality,[15] we performed similar regressions using receipt of ≥2 quality measures as a secondary endpoint. While limited, the association observed between survival and receipt of ≥2 measures was used to support our use of ≥2 measures as a secondary endpoint. All statistical analyses were performed using SAS software (version 9.3), with p values of <0.05 meeting statistical significance.
Results
We identified 23,279 patients (mean age 67.2 ± 10.8 years, 74% male) with stage ≥II urothelial carcinoma undergoing RC from 2003–2010. The majority were treated at academic (45%) and comprehensive community (43%) hospitals compared to community (12%) hospitals. No community hospitals met high volume criteria. Combining hospital facility type and volume tercile, the majority of patients were treated at CLV (38%) and AHV centers (27.9%), compared to ALV (16.7%), CHV (5%), and community centers (12.4%) respectively. Comparing composite category groups, subjects differed by demographic, clinical, and pathologic characteristics (Table 1).
Table 1.
Patient characteristics by composite hospital category.
| Overall | C | CLV | CHV | ALV | AHV | P Value | |
|---|---|---|---|---|---|---|---|
| N | 23,279 | 2,880 | 8,849 | 1,168 | 3,895 | 6,487 | |
| N (%) | Proportion | ||||||
| Age (years) | <0.0001 | ||||||
| <50 | 1,423 (6.1) | 5.0 | 5.1 | 6.3 | 7.8 | 7.0 | |
| ≥50 to <60 | 4,200 (18.1) | 16.3 | 16.7 | 17.3 | 21.0 | 19.1 | |
| ≥60 to <70 | 7,163 (30.8) | 30.5 | 30.0 | 30.8 | 30.5 | 32.1 | |
| ≥70 | 10,493 (45.1) | 48.2 | 48.3 | 45.6 | 40.8 | 41.9 | |
| Charlson Deyo Score | <0.0001 | ||||||
| 0 | 16,719 (71.8) | 71.1 | 69.9 | 71.2 | 72.3 | 74.7 | |
| 1 | 5,064 (21.8) | 22.0 | 23.1 | 22.5 | 21.3 | 19.9 | |
| ≥2 | 1,496 (6.4) | 6.9 | 7.0 | 6.3 | 6.4 | 5.4 | |
| Gender | <0.0001 | ||||||
| Male | 17,211 (73.9) | 72.6 | 73.6 | 75.7 | 71.8 | 75.7 | |
| Female | 6,068 (26.1) | 27.4 | 26.2 | 24.3 | 28.2 | 24.3 | |
| Race | <0.0001 | ||||||
| White | 21,240 (91.2) | 93.4 | 93.3 | 90.6 | 86.2 | 90.7 | |
| AA | 1,328 (5.7) | 4.6 | 4.4 | 6.8 | 10.9 | 4.7 | |
| Other | 711 (3.1) | 2.0 | 2.3 | 2.7 | 2.9 | 4.7 | |
| Hispanic | <0.0001 | ||||||
| No | 20,672 (88.8) | 87.7 | 90.1 | 80.2 | 85.5 | 91.1 | |
| Yes | 662 (2.8) | 2.1 | 2.1 | 4.5 | 4.3 | 3.0 | |
| Unknown | 1,945 (9.4) | 10.2 | 7.8 | 15.3 | 10.2 | 60.0 | |
| Median income | <0.0001 | ||||||
| <$30K | 2,810 (12.1) | 11.8 | 11.7 | 7.9 | 14.7 | 11.9 | |
| $30–34.9K | 4,353 (18.7) | 20.6 | 19.4 | 16.4 | 16.7 | 18.5 | |
| $35–44.9K | 6,587 (28.3) | 31.9 | 29.2 | 31.3 | 24.6 | 27.1 | |
| >$45K | 8,288 (35.6) | 30.5 | 34.2 | 39.6 | 38.9 | 37.1 | |
| Unknown | 1,241 (5.3) | 5.1 | 5.5 | 4.9 | 5.1 | 5.4 | |
| Percent less than high school education | <0.0001 | ||||||
| >29% | 3,428 (14.7) | 15.8 | 13.5 | 11.8 | 17.4 | 14.8 | |
| 20–29% | 5,273 (22.7) | 23.1 | 22.5 | 20.3 | 23.6 | 22.6 | |
| 14–20% | 5,738 (24.7) | 29.3 | 24.9 | 23.6 | 22.6 | 23.6 | |
| <14% | 7,599 (32.6) | 26.7 | 33.5 | 39.5 | 31.3 | 33.7 | |
| Unknown | 1,241 (5.3) | 5.1 | 5.5 | 4.9 | 5.1 | 5.4 | |
| Payor Group | <0.0001 | ||||||
| Private | 2,004 (8.6) | 10.3 | 8.9 | 8.2 | 8.6 | 7.6 | |
| Managed Care | 5,879 (25.3) | 20.3 | 23.9 | 27.6 | 25.9 | 28.5 | |
| Medicaid | 976 (4.2) | 3.9 | 3.1 | 3.8 | 7.6 | 3.9 | |
| Medicare | 12,949 (55.6) | 60.6 | 59.1 | 56.3 | 49.1 | 52.5 | |
| None/other | 1,471 (6.3) | 4.9 | 5.1 | 4.1 | 8.9 | 7.5 | |
| Urban/Rural | <0.0001 | ||||||
| Rural | 1,890 (8.1) | 13.8 | 7.8 | 2.8 | 4.4 | 9.3 | |
| Suburban | 2,865 (12.3) | 15.5 | 12.2 | 5.9 | 9.2 | 14.1 | |
| Small metropolitan | 6,955 (29.9) | 28.1 | 37.5 | 13.4 | 29.2 | 23.7 | |
| Large metropolitan | 10,186 (43.8) | 36.9 | 36.6 | 74.5 | 50.6 | 46.9 | |
| Unknown | 1,383 (5.9) | 5.8 | 6.0 | 3.4 | 6.6 | 6.0 | |
| Analytic Stage | <0.0001 | ||||||
| II | 8735 (37.5) | 42.0 | 38.8 | 36.5 | 37.3 | 34.2 | |
| III | 7411 (31.8) | 30.6 | 33.2 | 32.6 | 30.5 | 31.3 | |
| IV | 7133 (30.6) | 27.5 | 28.1 | 30.9 | 32.2 | 34.6 | |
| Tumor Grade | <0.0001 | ||||||
| Low Grade (1/2) | 1040 (4.5) | 6.5 | 4.7 | 4.2 | 4.5 | 3.3 | |
| High Grade (3/4) | 21010 (90.3) | 88.2 | 89.8 | 90.2 | 89.7 | 92.1 | |
| Unknown | 1229 (5.3) | 5.3 | 5.5 | 5.7 | 5.8 | 4.6 | |
| Facility Location | <0.0001 | ||||||
| Northeast | 1425 (6.1) | 7.9 | 5.2 | 0 | 11.2 | 4.6 | |
| Atlantic | 3360 (14.4) | 9.1 | 11.0 | 0 | 18.5 | 21.6 | |
| Southeast | 4513 (19.4) | 18 | 19.6 | 40 | 18.2 | 16.8 | |
| Great Lakes | 4453 (19.1) | 25.9 | 18.1 | 22 | 23.3 | 14.6 | |
| South | 1600 (6.9) | 6.1 | 7.3 | 4.5 | 3.7 | 9.0 | |
| Midwest | 2393 (10.3) | 9.8 | 11.9 | 5.7 | 5.9 | 11.7 | |
| West | 1725 (7.4) | 8.0 | 7.7 | 11.5 | 8.9 | 5.2 | |
| Mountain | 1099 (4.7) | 2.7 | 7.5 | 5.1 | 3.7 | 2.4 | |
| Pacific | 2711 (11.7) | 12.7 | 11.7 | 11.3 | 6.6 | 14.2 | |
| Year of Diagnosis | <0.0001 | ||||||
| 2003 | 2869 (12.3) | 13.2 | 12.6 | 11.5 | 13.5 | 11.1 | |
| 2004 | 2788 (12.0) | 14.7 | 12.5 | 11.2 | 12.1 | 10.1 | |
| 2005 | 2911 (12.5) | 13.1 | 13.0 | 13.4 | 11.9 | 11.8 | |
| 2006 | 2861 (12.3) | 12.1 | 12.1 | 12.0 | 12.1 | 12.9 | |
| 2007 | 2902 (12.5) | 12.5 | 12.0 | 11.8 | 12.3 | 13.4 | |
| 2008 | 2995 (12.9) | 12.7 | 12.5 | 13.4 | 12.6 | 13.5 | |
| 2009 | 2920 (12.5) | 11.6 | 12.3 | 13.3 | 12.6 | 13.1 | |
| 2010 | 3033 (13.0) | 10.1 | 13.1 | 13.4 | 12.9 | 14.3 | |
C-Community, CLV – Comprehensive low volume, CHV – Comprehensive high volume, ALV – academic low volume, AHV – academic high volume, AA-African American
19,554 of patients (84%) underwent treatment within 3 months, 2,885 (12%) received neoadjuvant chemotherapy, 10,243 (44%) had lymph node dissection yielding ≥10 nodes, and 1,992 (9%) underwent continent urinary diversion. Only 175 (0.8%) patients met all proposed quality criteria, while 2,130 (9%), 8,334 (35.8%), 10,848 (46.6%), and 1,792 (7.7%) met 3, 2, 1, and 0 measures respectively. Adjusted survival analyses revealed that receipt of ≥ 2 measures was associated with increased survival (HR 0.88 [CI 0.82–0.94]), while receipt of ≤ 1 measures did not reach statistical significance (HR 0.996 [CI 0.9–1.1]) (Figure 1). Results from the proportional hazards regression for additional covariates are detailed in Supplementary Table 1.
Figure 1.
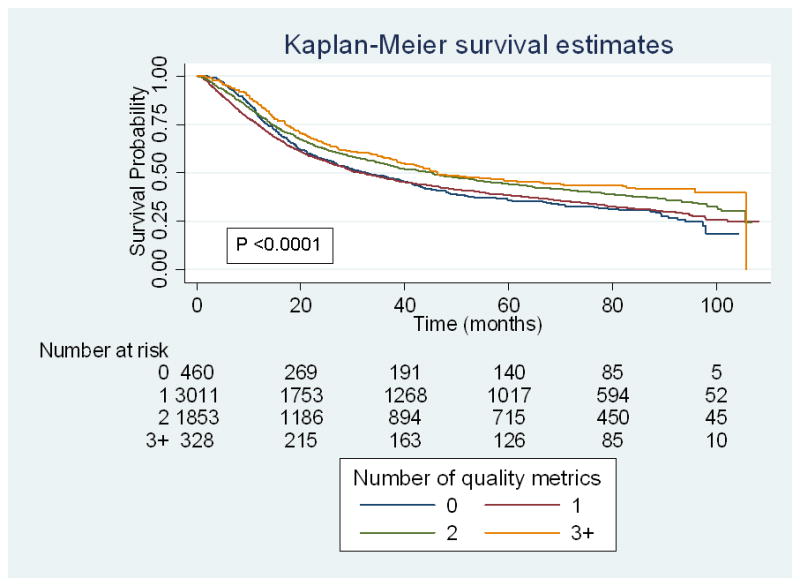
Unadjusted overall survival by number of quality measures for patients treated from 2003–2005
Evaluating unadjusted trends over time (2003 to 2010), receipt of neoadjuvant chemotherapy increased from 6 to 23% (p<0.0001) and lymph node yield ≥ 10 nodes increased from 35 to 55% (p <0.0001), while the proportion of patients undergoing timely treatment and continent urinary diversion decreased from 86 to 84% (p<0.0001) and 9 to 7% (p=0.02) respectively. Following adjustment, patients at AHV centers were more likely to receive neoadjuvant chemotherapy (OR 1.6 [CI 1.3–2.0]), adequate lymph node dissection (OR 3.14 [CI 2.5–4.0]), and continent diversion (OR 2.4 [CI 1.8–3.3]), but were less likely to have timely treatment (OR 0.72 [CI 0.6–0.9]), compared with Community hospitals (Table 2).
Table 2.
Association between hospital type and individual quality measures
| Treatment within 3 months | Neoadjuvant chemotherapy | ≥10 Lymph nodes | Continent diversion | |||||
|---|---|---|---|---|---|---|---|---|
| Hospital Type | OR [CI] | P Value | OR [CI] | P Value | OR [CI] | P Value | OR [CI] | P Value |
| Community | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Comprehensive low volume | 1.07 [0.9–1.2] | 0.47 | 1.00 [0.8–1.2] | 0.99 | 1.00 [0.9–1.1] | 0.99 | 1.03 [0.8–1.3] | 0.82 |
| Comprehensive high volume | 1.17 [0.9–1.5] | 0.23 | 1.15 [0.7–1.8] | 0.54 | 1.36 [1.04–1.8] | 0.03 | 1.19 [0.7–1.9] | 0.49 |
| Academic low volume | 0.86 [0.7–1.01] | 0.07 | 0.96 [0.8–1.2] | 0.71 | 1.53 [1.3–1.8] | <0.001 | 1.10 [0.8–1.5] | 0.53 |
| Academic high volume | 0.72 [0.6–0.9] | 0.001 | 1.60 [1.3–2.0] | <0.0001 | 3.14 [2.5–4.0] | <0.001 | 2.40 [1.8–3.3] | <0.0001 |
Adjusting for year, age, gender, race, Hispanic ethnicity, payor group, Charlson-Deyo score, income, education, tumor grade, analytic stage, urban/rural status, and facility location
The proportion of patients meeting ≥2 quality criteria increased from 2003 to 2010 (39 to 58%, p<0.0001). Comparing the proportion of patients undergoing RC by hospital type, patients were most likely to meet ≥2 quality criteria if treated at an AHV center (61%) and least likely if treated at a community center (37%) (Figure 2). Following adjustment, patients were more likely to receive ≥2 quality measures when treated at AHV (OR 2.4 [CI 2.0–2.9]), ALV (OR 1.3 [CI 1.1–1.6]), and CHV (OR 1.3 [CI 1.1–1.7]) hospitals compared to community hospitals. Notably, African Americans (OR 0.84 [CI 0.7–0.9]) and those insured with Medicaid (OR 0.76 [CI 0.6–0.9]), Medicare (OR 0.86 [CI 0.8–0.96]), or uninsured (OR 0.86 [CI 0.8–0.99]) were less likely to receive ≥2 quality measures, and decreasing associations between receipt of ≥2 quality measures were observed with increasing age and Charlson-Deyo score categories (Table 3).
Figure 2.
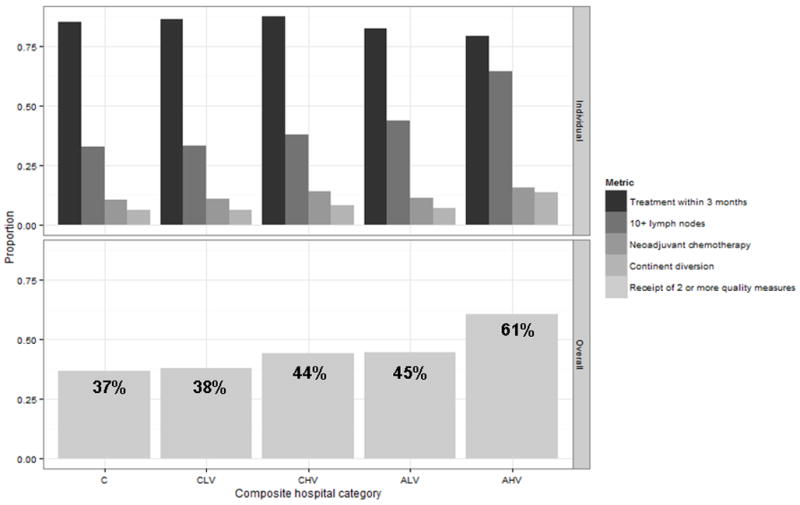
Performance of individual and summary quality measures by composite hospital category (C - community, CLV - comprehensive low volume, CHV – comprehensive high volume, ALV - academic low volume, AHV – academic high volume).
Table 3.
Characteristics associated with receipt of ≥2 candidate quality measures.
| Characteristic | OR [CI] | P Value |
|---|---|---|
| Age (years) | ||
| <50 | 1.0 | |
| ≥50 to <60 | 0.82 [0.7–0.9] | 0.0007 |
| ≥60 to <70 | 0.70 [0.6–0.8] | <0.0001 |
| ≥70 | 0.50 [0.4–0.6] | <0.0001 |
| Gender | ||
| Male | 1.1 [1.02–1.09] | <0.0001 |
| Race | ||
| White | 1.0 | |
| AA | 0.84 [0.7–0.9] | 0.003 |
| Other | 0.99 [0.8–1.2] | 0.949 |
| Charlson-Deyo Score | ||
| 0 | 1.0 | |
| 1 | 0.90 [0.8–0.96] | 0.003 |
| ≥2 | 0.71 [0.6–0.8] | <0.0001 |
| Analytic Stage | ||
| II | 1.0 | |
| III | 0.93 [0.9–0.99] | 0.03 |
| IV | 1.09 [1.03–1.2] | 0.005 |
| Tumor Grade | ||
| Low Grade (1/2) | 1.0 | |
| High Grade (3/4) | 1.03 [0.9–1.2] | 0.678 |
| Unknown | 0.97 [0.8–1.2] | 0.698 |
| Median Income | ||
| <$30K | 1.0 | |
| $30–34.9K | 1.09 [0.98–1.2] | 0.111 |
| $35–44.9K | 1.00 [0.9–1.1] | 0.950 |
| >$45K | 1.05 [0.9–1.2] | 0.488 |
| Unknown | 1.13 [0.9–1.4] | 0.241 |
| Percent less than high school education | ||
| >29% | 1.0 | |
| 20–29% | 1.02 [0.9–1.1] | 0.655 |
| 14–20% | 1.11 [1.0–1.2] | 0.054 |
| <14% | 1.14 [1.01–1.3] | 0.03 |
| Hospital Category | ||
| Community | 1.0 | |
| CLV | 1.02 [0.9–1.2] | 0.709 |
| CHV | 1.32 [1.03–1.7] | 0.03 |
| ALV | 1.33 [1.1–1.6] | 0.0003 |
| AHV | 2.37 [2.0–2.9] | <0.0001 |
| Payor Group | ||
| Private | 1.0 | |
| Managed Care | 1.1 [0.9–1.2] | 0.403 |
| Medicaid | 0.76 [0.6–0.9] | 0.0007 |
| Medicare | 0.86 [0.8–0.96] | 0.006 |
| None/unknown | 0.86 [0.8–0.99] | 0.04 |
| Geographic Location | ||
| Rural | 1.0 | |
| Suburban | 1.0 [0.9–1.1] | 0.992 |
| Small metropolitan | 0.93 [0.8–1.1] | 0.223 |
| Large metropolitan | 0.88 [0.8–1.01] | 0.063 |
| Unknown | 0.95 [0.8–1.2] | 0.634 |
| Facility Location | ||
| Northeast | 0.92 [0.8–1.1] | 0.308 |
| Atlantic | 0.79 [0.7–0.9] | 0.0006 |
| Southeast | 0.96 [0.9–1.1] | 0.429 |
| Great Lakes | 1.16 [1.04–1.3] | 0.009 |
| South | 0.89 [0.7–1.1] | 0.248 |
| Midwest | 1.08 [0.9–1.3] | 0.295 |
| West | 1.20 [1.01–1.4] | 0.041 |
| Mountain | 1.01 [0.8–1.2] | 0.949 |
| Pacific | 1.0 | |
| Year of Diagnosis | ||
| 2003 | 0.77 [0.7–0.8] | <0.0001 |
| 2004 | 0.79 [0.7–0.9] | <0.0001 |
| 2005 | 0.76 [0.7–0.8] | <0.0001 |
| 2006 | 0.91 [0.8–0.98] | 0.02 |
| 2007 | 0.97 [0.9–1.1] | 0.521 |
| 2008 | 1.16 [1.1–1.2] | 0.0001 |
| 2009 | 1.31 [1.2–1.4] | <0.0001 |
| 2010 | 1.0 | |
Controlling for age, gender, race, Charlson-Deyo score, analytic stage, tumor grade, hospital category, payor group, geographic location, median income, proportion with less than high school education, year, region
Performance of sensitivity analyses excluding receipt of continent diversion as a quality metric did not substantially impact the proportion of patients receiving ≥ 2 quality criteria treated at AHV versus community hospitals (57 vs. 34% patients; p<0.0001) or the main findings of our multivariable models (AHV OR 2.3, p<0.0001). Similarly, use of alternative definitions of high volume status, including 25 RC/year (AHV OR 3.2, p<0.001) and 50 RC/year (AHV OR 1.9, p<0.0001) minimally impacted our main findings. As such these data are not fully presented.
Discussion
Using the NCDB, we evaluated performance of four candidate quality measures for patients undergoing RC for MIBC and found that performance of these measures varied widely by hospital type. Using a composite measure of facility type and procedural volume, patients treated at AHV centers were more likely to meet proposed quality criteria compared to those treated at community centers. Further, while performance of these measures increased over time, only 46% of patients met at least two quality criteria, and surprisingly less than 1% (n=175) met all four criteria.
Defining “high quality care” is complex, and efforts to define surgical quality have lagged behind those of medical conditions such as diabetes, cardiovascular and chronic pulmonary disease due to the lack of rigorous data to support evidence based practice[16]. In an elegant review, Hollenbeck et al. detail how the Donabedian conceptual framework of structure, process, and outcome[17] can be applied to the evaluation of quality of care among RC patients. Identifying 29 structural, 22 process, and 10 outcome measures, this hypothetical framework illustrates both how challenging quality of care assessment can be as well as the granularity of the data needed to adequately measure it.[18]
In the absence of rigorously validated quality indicators, variation in performance rates between regions, hospitals, and providers likely reflects differing interpretations of the evidence base for each respective candidate measure.[19] For our study, we chose to assess four metrics captured by registry data that are supported either by randomized controlled trials, such as neoadjuvant chemotherapy,[20] institutional or observational study evidence, such as timely treatment[21] and adequate lymph node dissection,[22] or expert consensus, such as receipt of continent urinary diversion.[23]
When attempting to measure adherence to recommended care using utilization rates, it is important to consider that some subjects in the analytic sample may, in fact, not be eligible for the specified metric being assessed.[24] Illustrating this point, not all patients with MIBC are candidates for neoadjuvant chemotherapy for reasons such as poor performance status or pre-existing renal dysfunction. However, it is concerning that despite strong evidence of a survival benefit,[20] utilization of neoadjuvant chemotherapy could only be documented in 23% of patients undergoing RC in 2010.
In our sample, patients were less likely to undergo treatment within 3 months if treated at AHV centers. Reasons for delayed care are likely multifactorial, but may include increased travel distance as complex surgical care has become increasingly regionalized over time[25] as well as the increased utilization of neoadjuvant chemotherapy at more experienced centers. We attempted to account for this by using initiation of systemic therapy ≤90 days to define time to treatment for those undergoing neoadjuvant chemotherapy, but to date this metric has not been validated as a quality measure. For lymph node yield, we chose a cutoff of 10 nodes as our quality benchmark which is consistent with prior reports[22], but a clear nodal count threshold has yet to be identified that most clearly defines a survival benefit.[26] Further, ongoing investigations evaluating the prognostic impact of lymph node density and extended lymph node dissection on survival will influence the utility of lymph node threshold as a process measure.[27]
Patient selection and individual preferences are critical to the decision to perform a continent diversion, and it is not possible using existing registry data sources to identify the proper denominator of appropriate candidates to determine an accurate utilization rate. Without an established health related quality of life benefit,[28] and low performance rates in our own and other population based studies,[29] receipt of continent diversion as a quality indicator is controversial and will unlikely reach consensus among experts. One could argue that, if possible to ascertain, a more appropriate process measure would be documentation of an informed discussion regarding the risks and benefits of continent diversion for a patient without contraindication.[9] For these reasons, we performed sensitivity analyses excluding receipt of continent urinary diversion as a quality metric which did not significantly impact our main findings.
With increased performance of individual quality metrics as well as our composite quality measure documented at AHV centers, our findings lend some support to volume-outcomes studies promoting the further regionalization of high-risk surgical care[10]. Interestingly, performance at CHV centers was comparable to ALV hospitals, which illustrates that quality improvement may be a modifiable target that is not dependent on academic or cancer center designation. Furthermore, our study adds to the growing body of evidence suggesting decreased access to quality care for African Americans, the uninsured, and those insured with Medicaid or Medicare.[30] In fact, regionalization may exacerbate existing access disparities by increasing delays in evaluation and therapy for the underserved, and by overwhelming the resources of the recipient hospitals through increased referrals.[25] The majority of our sample was treated at CLV centers, and increasing efforts to promote adherence to quality metrics via transparent public reporting of outcomes, integration of health information technology, rigorous re-certification efforts, and evolving reimbursement schema may be more effective means of improving quality at the local or community level.[31]
Our study is limited by use of ≥2 measures as a composite quality metric, the inability to rigorously adjust for disease severity or selection biases, and concerns regarding the face validity of the selected quality measures. Given the constraints of data availability, we were unable to evaluate individual surgeon performance, and relied on hospital self-report for quality assurance. Further, limited mortality data prohibits the ability to rigorously evaluate the association between adherence to candidate measures and cancer specific or overall survival. While the procedural volume threshold of 4 cystectomies per year derived in our study may be questioned, our chosen methods have been rigorously applied in secondary data analyses examining the volume-outcome relationship.[6, 12, 13] A recent meta-analysis examining the association between high-volume hospitals and post-operative mortality following cystectomy[32] demonstrated that while considerable variation in high volume threshold (3.8–24 procedures/year) exists between studies, a meta-regression failed to identify a relationship between the cutoff point used and the strength of the relationship to postoperative mortality. Further highlighting that derived volume thresholds from secondary data are dependent on the data in question and are not meant to be universally applied, we performed sensitivity analyses using 25 and 50 annual procedures to determine high volume status that did not impact our main findings. Despite adjustment for comorbidity, patient preference in selection of care providers and type of urinary diversion can impart residual bias. Finally, the reliability of data for patients who received some of their treatment at a hospital that does not report to the NCDB may be limited. Nevertheless, to our knowledge, our study is the first to examine evidence-based practice for patients with MIBC undergoing RC using a contemporary, all payer dataset. This provides a unique opportunity to assess current trends in national practice and gain new insights into gaps in quality of care.
Conclusions
In the NCDB, patients undergoing RC at AHV hospitals were more likely to meet candidate quality of care criteria compared to non-academic and low volume centers. However, adherence remains low across hospital types, highlighting the opportunity to improve quality of care for MIBC. Future efforts should prioritize the identification and assessment of processes of care that are most likely to impact quality, are easily measurable, and translate into significant improvement in cancer outcomes at the population level.
Supplementary Material
Acknowledgments
Source of Funding: This publication was supported by the National Cancer Institute at the National Institutes of Health (grant number: P30 CA006927, RU) and the Department of Defense, Physician Research Training Award (AK).
Footnotes
No financial disclosures or conflicts of interest.
References
- 1.McWilliams JM, Landon BE, Chernew ME. Changes in health care spending and quality for Medicare beneficiaries associated with a commercial ACO contract. JAMA : the journal of the American Medical Association. 2013 Aug 28;310:829–36. doi: 10.1001/jama.2013.276302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renzi C, Sorge C, Fusco D, Agabiti N, Davoli M, Perucci CA. Reporting of quality indicators and improvement in hospital performance: the P.Re.Val.E. Regional Outcome Evaluation Program. Health services research. 2012 Oct;47:1880–901. doi: 10.1111/j.1475-6773.2012.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketelaar NA, Faber MJ, Flottorp S, Rygh LH, Deane KH, Eccles MP. Public release of performance data in changing the behaviour of healthcare consumers, professionals or organisations. Cochrane Database Syst Rev. 2011:CD004538. doi: 10.1002/14651858.CD004538.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsen B, Glenton C, Pope C. Thou shalt versus thou shalt not: a meta–synthesis of GPs’ attitudes to clinical practice guidelines. Br J Gen Pract. 2007 Dec;57:971–8. doi: 10.3399/096016407782604820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013 Jan;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 6.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007 May;69:871–5. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamie K, Saigal CS, Lai J, et al. Compliance with guidelines for patients with bladder cancer: variation in the delivery of care. Cancer. 2011 Dec 1;117:5392–401. doi: 10.1002/cncr.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran AT, Smaldone MC, Kutikov A, Uzzo RG. Addressing the Evidence Gap – Randomized Controlled Trials for the Surgical Management of Localized Genitourinary Malignancies. Urologic Oncology–Seminars and Original Investigations. 2013 In Press. [Google Scholar]
- 9.Cooperberg MR, Porter MP, Konety BR. Candidate quality of care indicators for localized bladder cancer. Urologic oncology. 2009 Jul-Aug;27:435–42. doi: 10.1016/j.urolonc.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Dimick JB. Potential benefits of the new Leapfrog standards: effect of process and outcomes measures. Surgery. 2004 Jun;135:569–75. doi: 10.1016/j.surg.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Winchester DP, Stewart AK, Bura C, Jones RS. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol. 2004 Jan;85:1–3. doi: 10.1002/jso.10320. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. The New England journal of medicine. 2002 Apr 11;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 13.Elting LS, Pettaway C, Bekele BN, et al. Correlation between annual volume of cystectomy, professional staffing, and outcomes: a statewide, population–based study. Cancer. 2005 Sep 1;104:975–84. doi: 10.1002/cncr.21273. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Bentrem DJ, Talamonti MS, Stewart AK, Winchester DP, Ko CY. Risk–based selective referral for cancer surgery: a potential strategy to improve perioperative outcomes. Ann Surg. 2010 Apr;251:708–16. doi: 10.1097/SLA.0b013e3181c1bea2. [DOI] [PubMed] [Google Scholar]
- 15.Chassin MR, Loeb JM. The ongoing quality improvement journey: next stop, high reliability. Health Aff (Millwood) 2011 Apr;30:559–68. doi: 10.1377/hlthaff.2011.0076. [DOI] [PubMed] [Google Scholar]
- 16.Birkmeyer NJ, Birkmeyer JD. Strategies for improving surgical quality––should payers reward excellence or effort? N Engl J Med. 2006 Feb 23;354:864–70. doi: 10.1056/NEJMsb053364. [DOI] [PubMed] [Google Scholar]
- 17.Donabedian A. Evaluating the quality of medical care. Milbank Mem Fund Q. 1966 Jul;44 (Suppl):166–206. [PubMed] [Google Scholar]
- 18.Hollenbeck BK, Montie JE, Wei JT. Radical cystectomy and surgical quality of care. J Natl Compr Canc Netw. 2005 Jan;3:37–42. doi: 10.6004/jnccn.2005.0002. [DOI] [PubMed] [Google Scholar]
- 19.Shojania KG, Grimshaw JM. Evidence–based quality improvement: the state of the science. Health Aff (Millwood) 2005 Jan-Feb;24:138–50. doi: 10.1377/hlthaff.24.1.138. [DOI] [PubMed] [Google Scholar]
- 20.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. The New England journal of medicine. 2003 Aug 28;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 21.Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS Urologic Diseases in America P. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results–Medicare analysis. Cancer. 2009 Mar 1;115:988–96. doi: 10.1002/cncr.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollenbeck BK, Ye Z, Wong SL, Montie JE, Birkmeyer JD. Hospital lymph node counts and survival after radical cystectomy. Cancer. 2008 Feb 15;112:806–12. doi: 10.1002/cncr.23234. [DOI] [PubMed] [Google Scholar]
- 23.Nabi G, Yong SM, Ong E, McPherson G, Grant A, N’Dow J. Is orthotopic bladder replacement the new gold standard? Evidence from a systematic review. J Urol. 2005 Jul;174:21–8. doi: 10.1097/01.ju.0000162021.24730.4f. [DOI] [PubMed] [Google Scholar]
- 24.Malin JL, Schneider EC, Epstein AM, Adams J, Emanuel EJ, Kahn KL. Results of the National Initiative for Cancer Care Quality: how can we improve the quality of cancer care in the United States? J Clin Oncol. 2006 Feb 1;24:626–34. doi: 10.1200/JCO.2005.03.3365. [DOI] [PubMed] [Google Scholar]
- 25.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Oct 1;27:4671–8. doi: 10.1200/JCO.2008.20.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppie TM, Vickers AJ, Vora K, Dalbagni G, Bochner BH. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer. 2006 Nov 15;107:2368–74. doi: 10.1002/cncr.22250. [DOI] [PubMed] [Google Scholar]
- 27.Fang AC, Ahmad AE, Whitson JM, Ferrell LD, Carroll PR, Konety BR. Effect of a minimum lymph node policy in radical cystectomy and pelvic lymphadenectomy on lymph node yields, lymph node positivity rates, lymph node density, and survivorship in patients with bladder cancer. Cancer. 2010 Apr 15;116:1901–8. doi: 10.1002/cncr.25011. [DOI] [PubMed] [Google Scholar]
- 28.Cody JD, Nabi G, Dublin N, et al. Urinary diversion and bladder reconstruction/replacement using intestinal segments for intractable incontinence or following cystectomy. Cochrane Database Syst Rev. 2012;2:CD003306. doi: 10.1002/14651858.CD003306.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gore JL, Saigal CS, Hanley JM, Schonlau M, Litwin MS. Variations in reconstruction after radical cystectomy. Cancer. 2006 Aug 15;107:729–37. doi: 10.1002/cncr.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kagawa–Singer M, Dadia AV, Yu MC, Surbone A. Cancer, culture, and health disparities: time to chart a new course? CA Cancer J Clin. 2010 Jan-Feb;60:12–39. doi: 10.3322/caac.20051. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high–income countries. Lancet Oncol. 2011 Sep;12:933–80. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 32.Goossens–Laan CA, Gooiker GA, van Gijn W, et al. A systematic review and meta–analysis of the relationship between hospital/surgeon volume and outcome for radical cystectomy: an update for the ongoing debate. European urology. 2011 May;59:775–83. doi: 10.1016/j.eururo.2011.01.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


