Abstract
The multiple effects of opiate alkaloids, important therapeutic drugs used for pain control, are mediated by the neuronal μ-opioid receptor. Among the side effects of these drugs is a profound impairment of gastrointestinal transit. Endomorphins are opioid peptides recently isolated from the nervous system, which have high affinity and selectivity for μ-opioid receptors. Since the μ-opioid receptor undergoes ligand-induced receptor endocytosis in an agonist-dependent manner, we compared the ability of endomorphin-1, endomorphin-2 and the μ-opioid receptor peptide agonist, [D-Ala2,MePhe4,Gly-ol5]-enkephalin (DAMGO), to induce receptor endocytosis in cells transfected with epitope-tagged μ-opioid receptor complementary DNA, and in myenteric neurons of the guinea-pig ileum, which naturally express this receptor. Immunohistochemistry with antibodies to the FLAG epitope or to the native receptor showed that the μ-opioid receptor was mainly located at the plasma membrane of unstimulated cells. Endomorphins and DAMGO induced μ-opioid receptor endocytosis into early endosomes, a process that was inhibited by naloxone. Quantification of surface receptors by flow cytometry indicated that endomorphins’ and DAMGO stimulated endocytosis with similar time-course and potency. They inhibited with similar potency electrically induced cholinergic contractions in the longitudinal muscle–myenteric plexus preparation through an action antagonized by naloxone. The apparent affinity estimate of naloxone (pA2 ~ 8.4) is consistent with antagonism at the μ-opioid receptor in myenteric neurons.
These results indicate that endomorphins directly activate the μ-opioid receptor in neurons, thus supporting the hypothesis that they are ligands mediating opioid actions in the nervous system. Endomorphin-induced μ-opioid receptor activation can be visualized by receptor endocytosis.
Keywords: myenteric neurons, receptor endocytosis, opioid peptides, opiate alkaloids, neurogenic cholinergic contractions, excitatory and inhibitory enteric neurons
The three opioid receptors, designated δ, κ and μ, mediate the biological effects of opioid peptides in the central and peripheral nervous system, and can be distinguished by their affinity for peptide and alkaloid agonists.24,29,31 For instance, dynorphins are selective for κ-opioid receptor, enkephalins are the preferred ligands for δ, whereas endorphins bind to μ and δ with similar affinity.6 However, enkephalins, dynorphins and endorphins bind all subtypes with moderate selectivity. The μ-opioid receptor (MOR) is also the target of potent analgesics with high potential for abuse, such as morphine and fentanyl, and it is considered the main mediator in the development of tolerance and drug addiction.29 In view of the important biological actions of alkaloids, which include analgesia, respiratory depression, inhibition of intestinal transit and secretion, and drug dependence,7,16,18,31 the recent identification of endogenous peptides, named endomorphin-1 and endomorphin-2, with high affinity and selectivity for the MOR,38 may have important implications for the physiological regulation of μ receptor by endogenous ligands. Indeed, endomorphin-1 has a 4000- and 15 000-fold preference for the MOR over δ and κ receptors, respectively. Endomorphin-1 and endomorphin-2 are related peptides isolated from the brain, which have powerful μ-selective biological activity, including analgesia. They have similar affinity for the MOR as [D-Ala2,MePhe4,Gly-ol5]enkephalin (DAMGO), one of the most potent MOR-selective enkephalin analogs, but far greater selectivity. However, direct activation of neuronal MOR by endomorphin-1 and endomorphin-2 has not been shown.
Biological responses to agonists of the MOR are terminated by receptor desensitization and down-regulation, which are mediated by receptor uncoupling from G-proteins and receptor endocytosis, intracellular sorting and recycling.15,19,20,23,30 These processes limit the duration of excitability and may contribute to opiate tolerance and dependence. However, there are remarkable differences in the ability of peptide and alkaloid agonists to activate these mechanisms. Whereas enkephalins and the alkaloids, morphine and etorphine, activate and desensitize the MOR through the same signaling pathway, only enkephalins and etorphine induce rapid endocytosis.17,33 In human embryonic kidney, 293 cells stably transfected with murine MOR cDNA containing the signal FLAG epitope at the amino terminus, the MOR internalizes rapidly to early endosomes through a mechanism that is likely to be clathrin dependent. The MOR is the first example of a G-protein-coupled receptor for which intracellular trafficking appears to be highly dependent on individual agonists. This agonist selectivity in MOR internalization suggests the existence of different mechanisms regulating opioid system functions, and it may play an important role in the development of tolerance.
In this study, we examined the activation of the MOR by endomorphin-1 and endomorphin-2, since their effects on the subcellular distribution of the MOR are unknown. The aims were to: (i) determine if endomorphins induce endocytosis of the MOR in transfected cell lines with similar potency to DAMGO, a high-affinity MOR agonist; (ii) examine whether endomorphins activate the MOR in enteric neurons that naturally express the MOR; and (iii) determine the effects of this interaction on neurogenic (cholinergic) contractions to electrical field stimulation in the longitudinal muscle–myenteric plexus preparation from the guinea-pig ileum.
EXPERIMENTAL PROCEDURES
Materials
Endomorphin-1 (Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (Tyr-Pro-Phe-Phe-NH2) were purchased from Anaspec. Inc. (San Jose, CA). DAMGO, tetrodotoxin and atropine sulfate were from Sigma Chemical Co. (St Louis, MO). Naloxone was from the UCLA Pharmacy. Mouse monoclonal antibodies (M1, M2) to the FLAG epitope were from Eastman Kodak Co. (New Haven, CT). A rabbit antibody to a C-terminal fragment of the rat MOR384–398 was from Incstar Science, Technology and Research (Stillwater, MN). Staining by this antibody was comparable to that obtained with a previously characterized affinity-purified antibody (MOR387–398)33 and was abolished by preincubation with C-terminal receptor fragments, confirming its specificity. A mouse monoclonal antibody to the transferrin receptor was from Dr Ian Trowbridge (Salk Institute, San Diego, CA). Affinity-purified goat or donkey anti-rabbit or anti-mouse immunoglobulin G coupled to fluorescein isothiocyanate or Texas Red was from Cappel Research Products (Durham, NC) or Jackson Immunoresearch Laboratories (West Grove, PA).
Cell line
Complementary DNA encoding mouse MOR with an N-terminal FLAG epitope (Met-DYKDDDDK) subcloned into pcDNA3 was provided by Dr M. von Zastrow (UCSF). Kristen murine sarcoma virus transformed rat kidney epithelial cells (KNRK) were transfected with this vector by lipofection. Cells were grown in Dulbecco’s modified Eagle’s medium with 4.5 g/l of glucose containing 10% (v/v) fetal calf serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 400 μg/ml G418. Clones were selected by immunofluorescence using the FLAG M1 antibody. Clone KNRK-MOR No. 6 showed uniformly high MOR immunoreactivity and was used for all experiments. Cells were plated for 48 h before use on glass coverslips coated with poly-L-lysine for immunofluorescence, or on plastic for flow cytometry. For immunohistochemistry, KNRK-MOR cells were washed in 100 mM phosphate-buffered saline (PBS; pH 7.4) and placed in Dulbecco’s modified Eagle’s medium containing 0.1% bovine serum albumin (BSA), 10 μM amastatin, 1 μM phosphoramidon and 1 μM captopril at 37°C. Cells were incubated with 1 nM–10 μM endomorphin-1, endomorphin-2 or DAMGO for 0–120 min at 37°C. To determine specificity of agonist interaction with the MOR, cells were preincubated with 1 μM naloxone for 10 min before addition of agonists. They were fixed in 4% paraformaldehyde in PBS for 20 min at 4°C.
Organotypic cultures of the ileum
Male albino, Harlan Porcellus guinea-pigs (Hartley; 200–300 g) were deeply anesthetized with sodium pentobarbital (50 mg/kg) and killed by exsanguination. Animal protocols were in line with the NIH recommendations for the humane use of animals. Because in vitro preparations were used throughout the study, discomfort was reduced to a minimum. The distal ileum was removed, opened along the longitudinal axis and washed with Kreb’s solution (mM: 5.9 KCl, 118 NaCl, 2.5 CaCl2·2H2O, 1.2 MgSO4·7H2O, 1.4 NaH2PO4, 22.7 NaHCO3; 1 g/l D-glucose; pH 7.4), containing 100 μg/ml streptomycin, 100 IU/ml penicillin and 2.5 μg/ml fungi-zone, for three 10-min periods at 4°C.3 The full thickness of the ileum was incubated in Dulbecco’s Modified Medium Nutrient Mixture F-12 HAM containing 10% fetal bovine serum (FBS), streptomycin, penicillin and fungizone, in 95% O2/5% CO2 for 30 min at 37°C. The intestine was pinned flat in Kreb’s solution containing 100 μM nicardipine to relax the muscle. Ileum specimens (full thickness) were incubated in Dulbecco’s Modified Medium Nutrient Mixture F-12 HAM containing 10% FBS, 10 μM amastatin, 1 μM phosphoramidon and 1 μM captopril with 100 nM–10 μM endomorphin-1, endomorphin-2 or DAMGO for 0–15 min at 37°C. In control experiments, 10 μM naloxone was added to the agonists. Organotypic cultures were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) overnight, and stored in PB containing 0.01% sodium azide. Whole mounts of longitudinal muscle with attached myenteric plexus were prepared.33,35
Immunohistochemistry
Fixed KRNK-MOR cells were incubated in PBS containing 1% normal goat serum and 0.1% saponin for three 10-min periods, and then incubated in the same solution with primary antibodies to the FLAG epitope (M1; 5 μg/ml), MOR384–398 (1:4000) or transferrin receptor (1:4000) over-night at 4°C.13,14 Cells were washed and incubated with secondary antibodies (1:200) for 2 h at room temperature. Whole mount preparations from organotypic cultures of the ileum were incubated in PB containing 0.5% Triton X-100 for three 30-min periods, incubated in 5% normal goat serum in 0.5% Triton X-100/PB for 60 min, and then incubated in the same solution with primary antibody for 48 h at 4°C. Whole mounts were washed and incubated with secondary antibodies (1:100) for 1 h at room temperature. Cells and whole mounts were examined by confocal microscopy using Bio-Rad (MRC 1000) and Zeiss (410) Laser Scanning Microscopes.12,32,33
Flow cytometry
KNRK-MOR cells were dissociated with enzyme-free cell dissociation buffer (Life Technologies/BRL, Gaithersburg, MD), and adjusted to a density of 1.5 × 106 cells/ml in Iscove’s medium containing 1% BSA. Cells were resuspended in the same medium containing 10 μM amastatin, 1 μM phosphoramidon and 1 μM captopril at 37°C. Cells were incubated with 100 nM–10 μM endomorphin-1, endomorphin-2 or DAMGO for 0–120 min at 37°C. In control experiments, cells were preincubated with 1 μM naloxone for 10 min before addition of agonists. They were washed, incubated in 200 μl medium containing 0.5% BSA, 5% FBS and 30 μg/ml FLAG M2 antibody for 60 min at 4°C, washed again, then incubated with fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (1:200) for 60 min at 4°C. Cells were washed and resuspended in cell dissociation buffer containing 0.3% FBS and 2 μg/ml propidium iodide. Cells were analysed by flow cytometry, as described.4,14 A minimum of 10 000 events was analysed per sample. Viability of cells, as determined by exclusion of propidium iodide, exceeded 75%. Non-specific fluorescence was determined in non-transfected cells and in transfected cells without primary or secondary antibodies. Changes in specific surface fluorescence were determined from the geometric mean of all gated events.
Longitudinal muscle–myenteric plexus preparation26
Guinea-pigs were killed by CO2 inhalation. The distal ileum was removed and placed in Tyrode’s solution (mM: 136.9 NaCl, 2.7 KCl, 1.8 CaCl2, 1.04 MgCl2, 0.4 NaH2PO4, 11.9 NaHCO3, 5.5 glucose; 95% O2/5% CO2) at 37°C. Segments were threaded onto a glass rod, and longitudinal muscle with the intact myenteric plexus was teased from the underlying circular muscle using cotton balls soaked in Tyrode’s solution. Strips 4 cm long were folded in half and mounted in organ baths containing 4.5 ml of Tyrode’s solution (95% O2/5% CO2) at 37°C, under tension of 5 mN. Isometric contractions were recorded with a force–displacement transducer (Harvard Instruments, South Natick, MA). After 45 min equilibration, strips were maximally stimulated (60 V, 0.1 Hz, 0.5 ms) using two platinum electrodes with an S44 stimulator (Grass Instrument Co., Quincy, MA). Concentration–response curves for the inhibitory effects of endomorphin-1, endomorphin-2 and DAMGO (each at 30 pM–1 μM) on electrically induced contractions were constructed in non-cumulative fashion, in the absence or presence of 30 nM naloxone (incubation time 20 min). After each agonist administration, strips were washed for three 5-min periods, and contractions were allowed to return to baseline before any subsequent pharmacological treatment. Apparent affinity estimates (pA2) from single antagonist concentrations were calculated using the Gaddum10 equation.
RESULTS
μ-Opioid receptor localization in cell lines
We examined agonist-induced endocytosis of the MOR with an extracellular FLAG epitope expressed in KNRK cells. These types of cells have been proven useful for the delineation of intracellular pathways of agonist-induced trafficking of other peptide receptors.13,14 The FLAG epitope permitted localization of the MOR by immunofluorescence and quantification of surface receptors by flow cytometry. The N-terminal FLAG does not influence signaling or endocytosis of other peptide receptors.
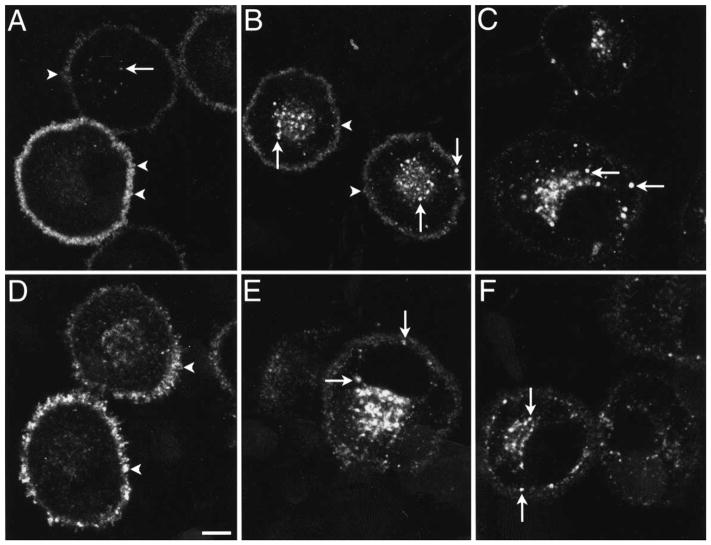
The MOR was localized in KNRK-MOR cells using an antibody to the N-terminal FLAG epitope or the C-terminus of the native receptor, with identical results. In untreated cells, the MOR was principally localized at the plasma membrane, and also detected in a few superficial and perinuclear vesicles (Fig. 1A). After incubation with 1 μM endomorphin-1 for 5–10 min at 37°C, the MOR was detected initially in numerous superficial endosomes and then in perinuclear endosomes, with little change in the intensity of surface labeling (Fig. 1B). After 30–120 min, the MOR was localized to a prominent perinuclear pool of endosomes and there was diminished labeling of the plasma membrane (Fig. 1C). Preincubation with the opioid receptor antagonist, naloxone, markedly inhibited endomorphin-1-induced endocytosis (Fig. 1D). Incubation with 1 μM endomorphin-2 or DAMGO caused similar changes in the subcellular localization of the MOR (Fig. 1E, F). Endomorphin-2- and DAMGO-induced MOR endocytosis was also inhibited by naloxone (not shown). Incubation of cells with 10 or 100 nM endomorphin-1, endomorphin-2 or DAMGO also caused detectable endocytosis, but lower concentrations did not affect the subcellular distribution of the MOR. Endosomes containing the MOR at 5–10 min after exposure to 1 μM endomorphin-1, endomorphin-2 or DAMGO also contained the transferrin receptor, as determined by superimposition of confocal images (not shown), which indicates that these vesicles are early endosomes. Together, these results show that endomorphins specifically bind to and activate the MOR in transfected cells and induce endocytosis in a manner similar to DAMGO.
Fig. 1.
Localization of the MOR in KNRK-MOR cells. Cells were unstimulated (A), incubated with 1 μM endomorphin-1 for 10 min (B) or 30 min (C), or 30 min with 1 μM naloxone (D), or incubated with 1 μM endomorphin-2 for 30 min (E) or 1 μM DAMGO for 30 min (F). The MOR was detected using FLAG M1 (A, C, E) or MOR384–398 (B, D, F) antibodies. Images are single optical sections. In unstimulated cells, the MOR was present at the plasma membrane (arrowheads) and in a few endosomes (arrow). After incubation with agonists, the MOR was present in many superficial and perinuclear endosomes (arrows), and there was diminished surface immunoreactivity. Cells illustrated in A are all stained, but with different intensity. This reflects variability in staining seen in transfected cells. Naloxone caused retention of the MOR at the plasma membrane (arrowheads).
Scale bar = 5 μm.
Flow cytometry
We used an antibody to the extracellular FLAG epitope to quantify surface MOR in non-permeabilized cells by flow cytometry. When KNRK-MOR cells were incubated with 10 μM endomorphin-1 or endomorphin-2 for 30 min at 37°C, surface FLAG immunoreactivity was reduced to 71 ± 4.8% and 72 ± 6.8% of that observed in untreated cells, respectively (Fig. 2A). When cells were treated with 10 μM DAMGO for 30 min, surface FLAG was 59 ± 4.4% of that in controls. Surface FLAG remained constant after incubation with endomorphin-1 or endomorphin-2 for up to 120 min, but increased slightly after exposure to DAMGO for 60 and 120 min. When cells were incubated with 10, 5 or 1 μM endomorphin-1, endomorphin-2 or DAMGO for 30 min, surface FLAG immunoreactivity declined in a concentration-dependent manner (Fig. 2B). However, there was no detectable loss of surface FLAG after incubation with 0.1 μM agonist for 30 min. Preincubation of cells with naloxone abolished the loss of FLAG immunoreactivity induced by 5 μM endomorphin-1, endomorphin-2 or DAMGO (Fig. 2B). Therefore, incubation of transfected cells with endomorphins induces a loss of surface MOR due to receptor endocytosis. The extent, time-course and concentration dependency of endocytosis in response to endomorphin-1 and endomorphin-2 are similar to those observed with DAMGO. However, most of the receptor remained at the cell surface even in the presence of high concentrations of any of these peptide agonists.
Fig. 2.
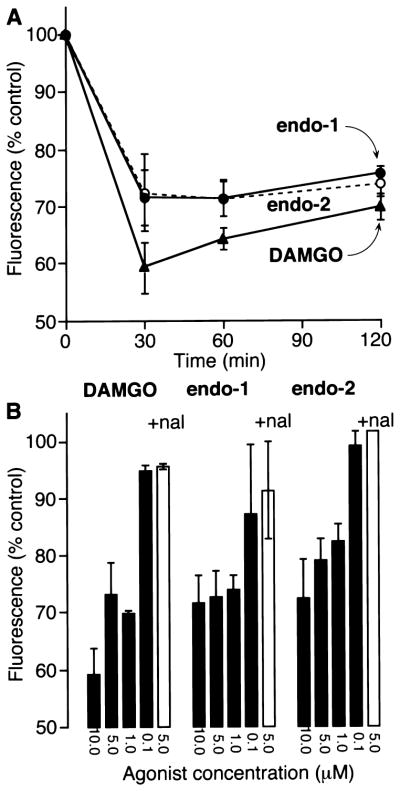
Quantification of surface MOR in KNRK-MOR cells by flow cytometry. (A) Cells were incubated with 10 μM endomorphin-1, endomorphin-2 or DAMGO for 0–120 min. (B) Cells were incubated with 0.1–10 μM endomorphin-1, endomorphin-2 or DAMGO for 30 min (filled bars) or with 5 μM agonists and 1 μM naloxone (open bars). Surface MOR was quantified by flow cytometry using an M2 antibody to the extracellular FLAG epitope. Results are expressed as percentage of surface fluorescence detected in untreated cells and are the mean ± S.E. of duplicate observations from three to five experiments.
μ-Opioid receptor localization in myenteric neurons
We used enteric neurons of the guinea-pig ileum as a model system to determine the effect of endomorphins on highly differentiated cells, since these neurons naturally express the MOR.33 MOR immunoreactivity was localized to neuronal cell bodies and fibers within the myenteric plexus of the guinea-pig ileum, to interconnecting fibers between myenteric ganglia and to fibers within the circular and longitudinal muscle layers.33 MOR-immunoreactive myenteric neurons had the morphological characteristics of Dogiel type I neurons, with an ovoid cell body, several broad dendrites and a long axon, which comprise motor neurons and perhaps interneurons.9 In unstimulated ileum, the MOR was detected at the plasma membrane and in a few vesicles of the soma, dendrites and axons (Fig. 3A). After incubation of ileal segments with 1 μM endomorphin-1 for 15 min, the MOR was no longer detectable at the plasma membrane, but was concentrated in prominent endosomes in the soma and neurites (Fig. 3B). Preincubation with naloxone abolished endomorphin-1-induced endocytosis of the MOR (Fig. 3C). Incubation of ileal segments with 1 μM endomorphin-2 or DAMGO caused similar changes in the subcellular localization of the MOR (Fig. 3D, E). Endomorphin-2- and DAMGO-induced MOR endocytosis was prevented by naloxone (not shown). Incubation of ileal tissue with 100 nM endomorphin-1, endomorphin-2 or DAMGO also caused detectable endocytosis. These results indicate that endomorphins specifically bind to and activate the MOR in a subpopulation of enteric neurons in the guinea-pig myenteric plexus to induce receptor endocytosis.
Fig. 3.
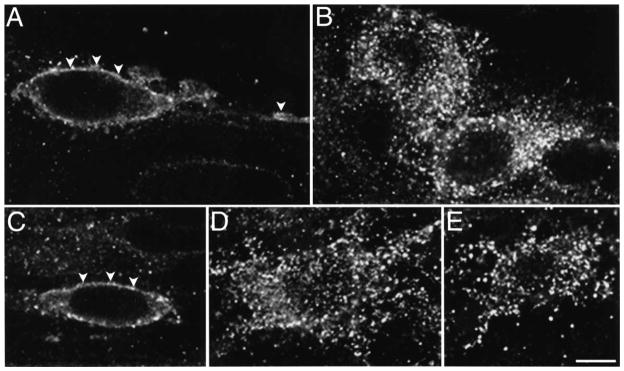
Localization of the MOR in myenteric neurons. Ileal segments were untreated (A), incubated with 1 μM endomorphin-1 for 15 min (B), or 15 min with 10 μM naloxone (C), or incubated with 1 μM endomorphin-2 for 15 min (D) or 1 μM DAMGO for 15 min (E). The MOR was detected using an antibody to MOR384–398. Images are single optical sections. In unstimulated tissues, the MOR was present at the plasma membrane (arrowheads) and in a few superficial endosomes of the soma, dendrites and axons. After incubation with agonists, the MOR was present in many superficial and perinuclear endosomes, and there was no detectable surface immunoreactivity. Naloxone caused retention of the MOR at the plasma membrane (arrowheads). Scale bar = 10 μm.
Opioid-induced inhibition of stimulated twitch contractions in longitudinal muscle–myenteric plexus preparations
The electrically evoked contractions in longitudinal muscle–myenteric plexus preparations were abolished by both tetrodotoxin (1 μM; n = 4) and atropine (1 μM; n = 4), indicating that they were mediated by activation of cholinergic nerves. Endomorphin-1, endomorphin-2 and DAMGO (each at 30 pM–1 μM) caused a concentration-dependent inhibition of the twitch height, with IC50 values of 4.2 ± 1.1 nM (n = 5), 5.3 ± 0.9 nM (n = 4) and 4.9 ± 1.3 nM (n = 5), respectively. Naloxone (30 nM) produced a parallel rightward shift of the concentration–response curves to both endomorphin-1 and endomorphin-2 (Fig. 4) and DAMGO (data not shown), without depression of their maximum effects. Apparent affinity estimates (pA2) of naloxone versus these compounds were 8.5 ± 0.1, 8.3 ± 0.1 and 8.5 ± 0.1, respectively.
Fig. 4.
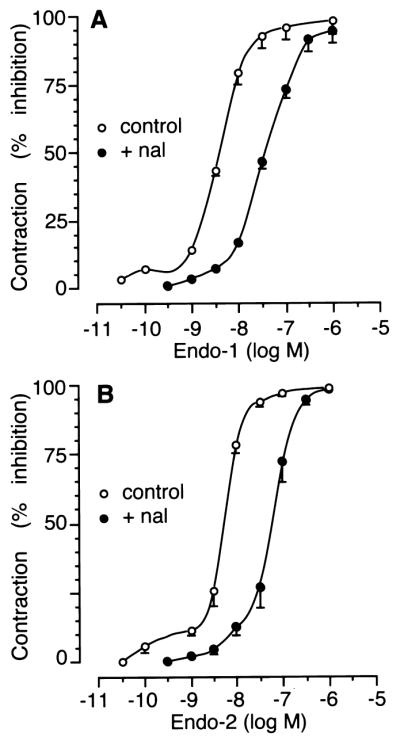
Concentration–response curves for the inhibitory effect of endomorphin-1 (A) and endomorphin-2 (B) on stimulated contractions in the longitudinal muscle–myenteric plexus preparation under control conditions (open symbols) or in the presence of 30 nM naloxone (filled symbols). Values are expressed as the mean ± S.E. of four to five determinations.
DISCUSSION
This study provides direct demonstration that endomorphins specifically activate the MOR and induce MOR endocytosis in transfected cells and enteric neurons that naturally express this receptor. Endomorphin-1 and endomorphin-2 bind the MOR at the cell surface and cause rapid endocytosis with identical potency, similarly to DAMGO, an MOR-specific peptide agonist. Endomorphin-induced activation of the neuronal MOR inhibits electrically evoked muscle contractions due to the stimulation of cholinergic neurons in the myenteric plexus. Endomorphin-induced endocytosis and trafficking of the MOR may mediate receptor desensitization, resensitization and down-regulation, mechanisms that regulate cellular responsiveness to ligand stimulation and that might be important in the development of opiate tolerance and addiction.2 Receptor phosphorylation by G-protein receptor kinases and second messenger kinases and interaction with β-arrestins may uncouple the MOR from G-proteins and mediate desensitization.1 Resensitization may require MOR endocytosis, dissociation of agonist and receptor dephosphorylation in endosomes, and subsequent recycling. In this study, we did not address the issue of whether the MOR recycles following endomorphin-induced internalization. However, this is likely, since the MOR recycles in myenteric neurons several hours following etorphine-or fentanyl-induced endocytosis (Sternini C., Minnis J. and Brecha N. C., unpublished observations).
The MOR is the target of opiate alkaloids, potent analgesics with high potential for abuse, which exert powerful biological effects, including a profound impairment of gastrointestinal transit, often resulting in severe constipation, which, at least in the guinea-pig, is principally due to MOR-mediated inhibition of excitatory (mainly cholinergic) and inhibitory (non-adrenergic, non-cholinergic) pathways subserving peristalsis.18,36,37 The MOR is also activated by several endogenous opioid peptides, including enkephalins, dynorphins and endorphins, which interact with this receptor with low to moderate specificity.38 Among these opioids, enkephalins are likely candidates as the endogenous ligands for the MOR in the enteric nervous system, since enkephalins are expressed by myenteric neurons and fibers, which are in close vicinity to neurons expressing the MOR.8,33,34 In addition, endomorphins,38 peptides that have recently been shown to be in the CNS and to have high affinity for the MOR, are also candidate endogenous ligands for the MOR. Endomorphins have the highest affinity and selectivity for the MOR than any opioid peptides described to date. Indeed, they bind the MOR with ~10-fold higher affinity than enkephalins, dynorphins and endorphins, and they have a selectivity for the MOR that is several thousand-fold above that for δ and κ receptors. Furthermore, these peptides have potent biological effects that mimic those of other MOR agonists. Indeed, endomorphin-1 induces analgesia in mice with a greater potency than morphine38 and, like morphine and DAMGO, endomorphin-1 and endomorphin-2 inhibit electrically induced contraction of the guinea-pig ileum.
The results of the present study, by showing that endomorphins directly activate and induce endocytosis of the MOR in transfected cells and neurons, provide further evidence for these peptides as potent and selective agonists of the MOR. Firstly, endomorphins induced a marked alteration in the subcellular distribution of the MOR in KNRK-MOR cells and in myenteric neurons that naturally express this receptor,33 in a fashion comparable to the MOR-selective enkephalin analog, DAMGO. Within minutes of exposure to these agonists, the MOR translocated from the cell surface to superficial and then perinuclear endosomes, which were identified as early endosomes by their expression of the transferrin receptor. This confirms previous results with MOR agonist-induced internalization in other cell lines and in enteric neurons in the intact animal.1,11,17,33 In a similar fashion, substance P stimulates endocytosis of its receptor, neurokinin-1, in transfected and highly differentiated cells, and the appearance of endosomes containing this receptor has been used to identify sites of substance P release and action.12,13,21,22 Thus, our observation that endomorphins induce MOR endocytosis in transfected cells and neurons provides direct evidence that they activate the MOR. Secondly, endomorphin-1, endomorphin-2 and DAMGO caused a loss of surface FLAG immunoreactivity from KNRK-MOR cells with comparable potency and time-course, as determined by flow cytometry. Finally, the inhibition of endomorphin-induced endocytosis in KNRK-MOR cells and myenteric neurons by the opioid antagonist, naloxone, indicates that endocytosis was due to receptor activation.
The observation that naturally occurring, high-affinity and selective MOR agonists stimulate endocytosis is significant in view of remarkable differences in the ability of MOR agonists to cause internalization of this receptor. Other peptide and non-peptide agonists of the MOR, including DAMGO, [Lys7]dermorphin and the alkaloid etorphine, also induce receptor internalization in transfected cells and myenteric neurons.1,11,17,33 In marked contrast, morphine does not cause internalization in transfected cells or neurons,1,17,33 even though it has high affinity for the MOR and it activates this receptor through the same signaling pathway as opioids and opiates that are capable of triggering MOR endocytosis.25,28 The explanation for this discrepancy is unknown, but presumably different agonists induce conformational states that affect receptor interaction with G-proteins and the endocytic apparatus. We should point out that endomorphin-1, endomorphin-2 and DAMGO stimulated MOR endocytosis at much higher concentrations than those required to induce functional effects. Indeed, concentrations of >10 nM were required to detect endocytosis in transfected cells by confocal microscopy, whereas DAMGO, endomorphin-1 and endomorphin-2 bind to the MOR with affinities in the subnanomolar range. This could be due to peptide degradation, even though protease inhibitors were included to minimize this possibility, or to the presence of a high proportion of spare receptors.5,27 Alternatively, the possibility that agonists bind to and induce signaling of the MOR with higher affinity than they stimulate endocytosis cannot be discounted, even though such a mechanism is unknown.
Exposure to endomorphins did not remove all detectable MOR from the surface of transfected cells. Analysis by flow cytometry indicated that <50% of surface MOR internalized in transfected cells, even after incubation with 10 μM endomorphin-1, endomorphin-2 and DAMGO for 120 min. Analysis of transfected cells by immunofluorescence also indicated retention of surface MOR, whereas there was no detectable surface receptor in neurons after agonist stimulation. In support of our results, only 25% of specifically bound ligands internalize in transfected cells expressing the MOR,11 and < 50% of neurokinin-1 and gastrin-releasing peptide receptors13,14 internalize after incubation with supramaximal concentrations of agonists. We do not know why surface receptors are still present on transfected cells following high concentrations of agonists, since these concentrations of agonists are likely to saturate binding sites. One possibility is that not all agonist-occupied receptors internalize. Alternatively, some surface receptors may be in a low-affinity state in cells that overexpress receptors and may be unable to interact with agonists.
Endomorphin activation of the MOR in myenteric neurons is also supported by the concentration-dependent inhibition of electrically stimulated contractions of the ileum, with IC50 values in the nanomolar range, comparable to those observed with DAMGO. This confirms the potent bioactivity of these peptides.38 Furthermore, the apparent affinity estimate of the opioid antagonist naloxone (pA2 ~ 8.4) against the inhibitory action of both peptides (and DAMGO) is consistent with endomorphin agonism at the MOR in myenteric neurons. Finally, the lack of effect of δ- and κ-selective antagonists (namely, naltrindole and nor-binaltorphimine) on the concentration-dependent inhibition of the twitch height in response to endomorphin-1 is an additional indication of the selectivity of this peptide for MOR (Sternini C., Brecha N. C. and Tonini M., unpublished observations).
CONCLUSIONS
Together, the results of the present investigation provide morphological and functional evidence that endomorphin-1 and endomorphin-2 are ligands for the MOR. Endomorphins specifically activate the MOR in transfected and in highly differentiated cells, and induce MOR endocytosis, which might indicate sites of endomorphin release and action.
Acknowledgments
We thank Dr M. von Zastrow (UCSF) for supplying MOR cDNA, Michelle Lovett, Patrick Gamp and Katherine Wen for technical assistance, Dr Paul Dazin (Howard Hughes Medical Institutes) for flow cytometry, and Drs M. Tagliani and E. Fiori for assisting with the longitudinal muscle–myenteric plexus functional assay. This work was supported by NIH grants DK 39957 (N.W.B.), DK43207 (N.W.B.), DK 52388 (E.F.G., N.W.B.), DK 41301 (C.S.), DK 54155 (C.S.), NATO grant CRG971522 (C.S., M.T.), Veterans Affairs Medical Research Funds (N.B.) and MURST 40% 1996 project (M.T.). K.M. was supported by a C. J. Martin Fellowship of the National Health and Medical Research Council of Australia.
Abbreviations
- BSA
bovine serum albumin
- DAMGO
[D-Ala2,MePhe4,Gly-ol5]enkephalin
- FBS
fetal bovine serum
- KNRK
Kristen murine sarcoma virus transformed rat kidney epithelial cell line
- MOR
μ-opioid receptor
- PB
phosphate buffer
- PBS
phosphate-buffered saline
References
- 1.Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK293 cells. J Neurochem. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- 2.Bohm S, Grady EF, Bunnett NW. Mechanisms attenuating signaling by G protein-coupled receptors. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brookes SJ, Steele PA, Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42:863–878. doi: 10.1016/0306-4522(91)90050-x. [DOI] [PubMed] [Google Scholar]
- 4.Bunnett NW, Dazin PF, Payan DG, Grady EF. Characterization of receptors using cyanine 3-labeled neuropeptides. Peptides. 1995;16:733–740. doi: 10.1016/0196-9781(95)00042-i. [DOI] [PubMed] [Google Scholar]
- 5.Chavkin C, Goldstein A. Opioid receptor reserve in normal and morphine-tolerant guinea pig ileum myenteric plexus. Proc natn Acad Sci USA. 1984;81:7253–7257. doi: 10.1073/pnas.81.22.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corbett AD, Paterson SJ, Kosterlitz HW. Selectivity of ligands for opioid receptors. In: Herz A, editor. Handbook of Experimental Pharmacology, Opioids I. Springer; New York: 1993. pp. 645–679. [Google Scholar]
- 7.Di Chiara G, North RA. Neurobiology of opiate abuse. Trends pharmac Sci. 1992;13:185–193. doi: 10.1016/0165-6147(92)90062-b. [DOI] [PubMed] [Google Scholar]
- 8.Furness JB, Costa M, Miller RJ. Distribution and projections of nerves with enkephalin-like immunoreactivity in the guinea-pig small intestine. Neuroscience. 1983;8:644–653. doi: 10.1016/0306-4522(83)90001-5. [DOI] [PubMed] [Google Scholar]
- 9.Furness JB, Johnson PJ, Pompolo S, Bornstein JC. Evidence that enteric motility reflexes can be initiated through entirely intrinsic mechanisms in the guinea-pig small intestine. Neurogastroenterol Motil. 1995;7:89–96. doi: 10.1111/j.1365-2982.1995.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 10.Gaddum JH. Series of drug antagonism. Pharmac Rev. 1957;9:211–218. [PubMed] [Google Scholar]
- 11.Gaudriault G, Nouel D, Dal Farra C, Beaudet A, Vincent JP. Receptor-induced internalization of selective peptidic mu and delta opioid ligands. J biol Chem. 1997;272:2880–2888. doi: 10.1074/jbc.272.5.2880. [DOI] [PubMed] [Google Scholar]
- 12.Grady EF, Gamp PD, Jones E, Baluk P, McDonald DM, Payan DG, Bunnett NW. Endocytosis and recycling of neurokinin 1 receptors in enteric neurons. Neuroscience. 1996;79:1239–1254. doi: 10.1016/0306-4522(96)00357-0. [DOI] [PubMed] [Google Scholar]
- 13.Grady EF, Garland AM, Gamp PD, Lovett M, Payan DG, Bunnett NW. Delineation of the endocytic pathway of substance P and its seven-transmembrane domain NK1 receptor. Molec Biol Cell. 1995;6:509–524. doi: 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grady EF, Slice LW, Brant WO, Walsh JH, Payan DG, Bunnett NW. Direct observation of endocytosis of gastrin releasing peptide and its receptor. J biol Chem. 1995;270:4603–4611. doi: 10.1074/jbc.270.9.4603. [DOI] [PubMed] [Google Scholar]
- 15.Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. Fedn Am Socs exp Biol. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 16.Herz A. Opioids II. Springer; Berlin: 1993. [Google Scholar]
- 17.Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kag L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J biol Chem. 1996;271:19021–19,024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 18.Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmac Rev. 1988;40:121–162. [PubMed] [Google Scholar]
- 19.Law PY, Hom DS, Loh HH. Down-regulation of opiate receptor in neuroblastoma × glioma NG108-15 hybrid cells. Chloroquine promotes accumulation of tritiated enkephalin in the lysosomes. J biol Chem. 1984;259:4096–4104. [PubMed] [Google Scholar]
- 20.Law PY, Hom DS, Loh HH. Loss of opiate receptor activity in neuroblastoma × glioma NG108-15 hybrid cells after chronic opiate treatment. A multiple-step process. Molec Pharmac. 1982;22:1–4. [PubMed] [Google Scholar]
- 21.Liu H, Mantyh PW, AIB NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 22.Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, Simone DA. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- 23.Mestek A, Hurley JH, Bye LS, Campbell AD, Chen Y, Tian M, Liu J, Schulman H, Yu L. The human mu opioid receptor: modulation of functional desensitization by calcium/calmodulin-dependent protein kinase and protein kinase C. J Neurosci. 1995;15:2396–2406. doi: 10.1523/JNEUROSCI.15-03-02396.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minami M, Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res. 1995;23:121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- 25.Nestler EJ, Hope BT, Widnell KL. Drug addiction: a model for the molecular basis of neural plasticity. Neuron. 1993;11:995–1006. doi: 10.1016/0896-6273(93)90213-b. [DOI] [PubMed] [Google Scholar]
- 26.Paton WD, Zar MA. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol, Lond. 1968;194:13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porreca F, Burks TF. Affinity of normorphine for its pharmacologic receptor in the naive and morphine-tolerant guinea-pig isolated ileum. J Pharmac exp Ther. 1983;225:688–693. [PubMed] [Google Scholar]
- 28.Raynor K, Kong H, Yasuda K, Chen Y, Yu L, Bell GI, Reisine T. Pharmacological characterization of the clones kappa, delta and mu opioid receptors. Molec Pharmac. 1994;45:330–334. [PubMed] [Google Scholar]
- 29.Reisine T, Pasternak G. Opioid analgesics and antagonists. In: Hardman JGL, Limbird LE, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 1996. pp. 521–555. [Google Scholar]
- 30.Sharma SK, Klee WA, Nirenberg M. Opiate-dependent modulation of adenylate cyclase. Proc natn Acad Sci USA. 1977;74:3365–3369. doi: 10.1073/pnas.74.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon EJ. Opioid receptors and endogenous opioid peptides. Med Res Rev. 1991;11:357–374. doi: 10.1002/med.2610110402. [DOI] [PubMed] [Google Scholar]
- 32.Sternini C, Wong H, Wu VS, De Giorgio R, Yang M, Reeve J, Jr, Brecha NC, Walsh JH. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enetrochromaffin-like cells of tha gastrointestinal tract. J comp Neurol. 1997;386:396–408. [PubMed] [Google Scholar]
- 33.Sternini C, Spann M, Anton B, Keith DE, Jr, Bunnett NW, von Zastrow M, Evans C, Brecha NC. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc natn Acad Sci USA. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sternini C, Spann M, De Giorgio R, Anton B, Keith D, Evan C, Brecha NC. Cellular localization of the mu opioid receptor in the rat enteric nervous system. Analgesia. 1995;1:762–765. [Google Scholar]
- 35.Sternini C, Su D, Gamp PD, Bunnett NW. Cellular sites of expression of the neurokinin-1 receptor in the rat gastrointestinal tract. J comp Neurol. 1995;358:531–540. doi: 10.1002/cne.903580406. [DOI] [PubMed] [Google Scholar]
- 36.Tonini M, Onori L, Perucca E, Manzo L, De Ponti F, Crema A. Depression by morphine of the excitability of intrinsic inhibitory neurons in the guinea pig colon. Eur J Pharmac. 1985;115:317–320. doi: 10.1016/0014-2999(85)90708-3. [DOI] [PubMed] [Google Scholar]
- 37.Tonini M, Waterman SA, Candura SM, Coccini T, Costa M. Sites of action of morphine on the ascending excitatory reflex in the guinea-pig small intestine. Neurosci Lett. 1992;144:195–198. doi: 10.1016/0304-3940(92)90748-v. [DOI] [PubMed] [Google Scholar]
- 38.Zadina JE, Hackler L, Ge LJ, Kastin A. A potent and selective endogenous agonist for the μ-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]