Abstract
Objectives
Short-term elevation of ambient particulate air pollution has been associated with autonomic dysfunction and increased systemic inflammation, but the interconnections between these pathways are not well understood. We examined the association between inflammation and autonomic dysfunction and effect modification of inflammation on the association between air pollution and heart rate variability (HRV) in elderly subjects.
Methods
25 elderly subjects in Steubenville, Ohio, were followed up to 24 times with repeated 30-min ECG Holter monitoring (545 observations). C-reactive protein (CRP), fibrinogen, interleukin-6 (IL-6), soluble inter-cellular adhesion molecule 1 (sICAM-1), and white blood cell and platelet counts were measured in peripheral blood samples collected in the first month of the study. Increased systemic inflammation was defined for subjects within the upper 20% of the distribution for each marker. A central ambient monitoring station provided daily fine particle (PM2.5) and sulphate (SO42−) data. Linear mixed models were used to identify associations between inflammatory markers and HRV and to assess effect modification of the association between air pollution and HRV due to inflammatory status.
Results
A 5.8 mg/l elevation in CRP was associated with decreases of between −8% and −33% for time and frequency domain HRV outcomes. A 5.1 μg/m3 increase in SO42− on the day before the health assessment was associated with a decrease of −6.7% in the SD of normal RR intervals (SDNN) (95% CI −11.8% to −1.3%) in subjects with elevated CRP, but not in subjects with lower CRP (p value interaction=0.04), with similar findings for PM2.5.
Conclusions
Increased systemic inflammation is associated with autonomic dysfunction in the elderly. Air pollution effects on reduced SDNN are stronger in subjects with elevated systemic inflammation.
Introduction
Acute exposure to ambient air pollution has been associated with autonomic dysfunction as reflected by decreased heart rate variability (HRV)1–9 and increases in heart rate.2,3,10,11 Pollution-related increases in blood pressure are thought to be mediated through both autonomic and inflammation/oxidative stress-induced endothelial dysfunction.12–17 While pollution effects on autonomic dysfunction have usually been considered separately from their effects on inflammation, it is possible that pollution can lead to autonomic dysfunction through inflammation and oxidative stress. Alternatively people with chronic inflammatory diseases may be more vulnerable to the autonomic effects of pollution. Chronic inflammation, a hallmark of both type 2 diabetes and coronary artery disease, is predictive of increased risk of coronary events.18 It is hypothesised that patients with diabetes are more vulnerable to the clinical and subclinical cardiovascular effects of pollution because of their chronic ongoing systemic inflammation and consequent oxidative stress.19–21 In adult Sprague—Dawley rats, Rhoden and colleagues showed that particulate matter-induced oxidative stress was mediated by autonomic stimulation.22
In a repeated measures study in Steubenville, Ohio, we found that elevated levels of PM2.5 and sulphate were associated with decreased HRV.8 In this analysis we examined whether baseline elevations in systemic inflammatory markers predicted reduced HRV, and whether systemic inflammation or a diabetes diagnosis modified susceptibility to air pollution-associated autonomic dysfunction.
Subjects and Methods
Study population and protocol
Thirty-two non-smoking senior adults from Steubenville, Ohio participated in a study on air pollution and cardiovascular health during the summer and autumn of 2000. Subjects with pacemakers, a recent acute coronary syndrome, atrial flutter or atrial fibrillation and smokers were excluded. The study has been described in detail elsewhere.8 The study design was reviewed and approved by the Human Subjects Committees of the Brigham and Women's Hospital and the Harvard School of Public Health.
Study participants were seen weekly during summer (June 4–August 18) and autumn (September 25–December 15) of 2000. A short questionnaire on recent symptoms, hospital or doctor's visits and medication use was administered, followed by Holter electrocardiogram monitoring (SEER MC; GE Medical Systems, Milwaukee, Wisconsin, USA) with electrodes in a modified V5 and AVF position. The Holter monitoring protocol included: (1) 5 min of rest in a supine position; (2) three supine blood pressure (BP) measurements (NIBP Vital Signs Monitor; Welch Allyn, Skaneateles, New York, USA); (3) 5 min of standing with three standing BP measurements taken after 2 min; (4) 5 min of exercise (walking) outdoors (weather and health permitting); (5) 5 min of rest in a supine position; and (6) 2 min and 20 s of paced breathing.
The Holter tapes were analysed using a Marquette MARS Workstation (GE Medical Systems), with a 125 samples/second sampling rate following HRV guidelines.23 Reviewed time domain HRV measures included the SD of normal RR intervals (SDNN), the mean square of differences between adjacent RR intervals (r-MSSD), and the per cent of RR intervals more than 50 ms different from the prior interval (PNN50). Frequency domain HRV measures included low frequency power and high frequency power. All HRV parameters and average heart rate were obtained for the whole protocol and for each separate interval.
Air pollution concentration and meteorological measurements
We collected 24 h integrated PM2.5, SO42−, elemental carbon and gaseous pollutant (O3, NO2, SO2) samples beginning at 09:00 h each day (except Saturday) using a Harvard multi-pollutant monitor.24 PM2.5 was measured gravimetrically. The filters were analysed by CONSOL Energy Inc. Research and Development (South Park, Pennsylvania, USA), SO42−, O3, NO2 and SO2 through ion chromatography and elemental carbon filters through thermal optical transmission.
Temperature, relative humidity and dew point were obtained from a centrally located ambient monitoring site at the Franciscan University of Steubenville operated by CONSOL. Apparent temperature was calculated as: −2.653+(0.994×Ta)+(0.0153×Td2), where Ta is the air temperature and Td is the dew point.
Blood collection and analysis
A blood sample was drawn from 25 subjects during the fourth week of the summer session. Each sample was analysed promptly for white blood cell count (WBC) using a Coulter Gen S System (Beckman Coulter, Fullerton, California, USA). The remaining plasma was extracted, preserved at −80°C, and shipped to the Clinical and Epidemiologic Research Laboratory at Boston Children's Hospital. Samples stored with sodium citrate were analysed for hs-CRP using immunoturbidimetric assays on the Roche Diagnostics Hitachi 917 system (Indianapolis, Indiana, USA) with reagents and calibrations from Denka Seiken (Niigata, Japan). IL-6 and sICAM-1 were analysed using ELISA assays from R&D Systems (Minneapolis, Minnesota, USA). The concentration of fibrinogen was determined using an immunoturbidimetric assay on the Hitachi 917 analyser (Roche Diagnostics), using reagents and calibrators from Kamiya Biomedical (Seattle, Washington, USA).
Statistical methods
We examined the associations between levels of baseline inflammatory markers and log-transformed HRV parameters and heart rate over the entire 30-min protocol with linear mixed models including random subject effects, inflammatory marker (one at a time), age, gender, race, body mass index (BMI, kg/m2), season, time of day, apparent temperature, and a first-order auto-regressive process for the within-subject residuals.25 The inflammatory markers were considered as continuous and as dichotomous variables. We defined a blood marker as high if it was greater than the 80th percentile within the population.
Effect modification of the association between air pollution and HRV by inflammatory status was examined in regression models including interaction terms between air pollution effects and inflammation marker category, with increased inflammation defined as a subject-specific condition. With a total of 25 subjects, subjects with the five highest CRP measurements were compared to those with the lowest 20 measurements. Based on the results from the main analysis, interaction models are restricted to CRP, fibrinogen and platelets as effect modifiers. In addition, as subjects with diabetes usually have increased chronic inflammation, diabetes was also evaluated as a possible effect modifier.
Results are reported as estimated per cent difference (and 95% CI) in HRV or heart rate associated with an IQR increase in inflammatory marker and, in case of binary markers, as per cent difference between the higher category and the lower category. For the effect modification analysis (interaction model), the estimated per cent differences associated with an IQR increase in air pollution are reported by level of inflammatory marker. All analyses were performed with SAS software version 9.1.
Sensitivity analyses
To check whether the association between a subject's level of inflammatory marker and HRV measurement is decreasing with time after blood draw, we included the time (in weeks) between blood draw and electrocardiogram and an interaction term between the time and blood marker levels in the model.
We also repeated both the main and the interaction analysis while excluding the subject with the highest CRP level of 23.4 mg/l and excluding the two male study participants.
Results
Study population
The distribution of the baseline blood marker levels for the 25 subjects is shown in table 1. We used 9.0 mg/l CRP (80th percentile) as a cut-point to divide the population according to inflammatory status. Although the correlation between CRP and fibrinogen is high in this population (Pearson's correlation coefficient=0.74), only two subjects are in the subgroups with levels above the 80th percentile for both CRP and fibrinogen.
Table 1. Blood marker levels (n=25).
| Mean | Minimum | 20th Percentile |
80th Percentile |
Maximum | IQR | |
|---|---|---|---|---|---|---|
| CRP (mg/l) | 5.7 | 0.4 | 1.0 | 9.0 | 23.4 | 5.8 |
| Fibrinogen (g/l) | 5.1 | 3.8 | 4.4 | 5.9 | 7.3 | 1.2 |
| Platelet count (109/l) | 253 | 95 | 199 | 320 | 388 | 108 |
| IL-6 (pg/ml) | 4.3 | 1.1 | 1.8 | 5.5 | 14.1 | 2.4 |
| sICAM-1 (ng/ml) | 325 | 0.1 | 267 | 389 | 605 | 89 |
| White blood cell count (109/l) | 6.8 | 3.2 | 6.0 | 7.6 | 11.7 | 1.3 |
CRP, C-reactive protein; IL-6, interleukin-6; sICAM-1, soluble inter-cellular adhesion molecule 1.
The 25 subjects with measured blood markers completed a total of 552 health visits, with an average of 22 visits (range 11–24) per subject (table 2). The mean age at screening was 72 years; 92% of the participants were female and 72% were white. Most participants (76%) had one or more cardiovascular conditions, 26% had diabetes and 72% were on one or more medications (table 2). Sixteen subjects (64%) with a BMI greater than 30 kg/m2 were classified as obese and six subjects (24%) with a BMI between 25 and 30 kg/m2 were classified as over-weight. All cardiovascular diagnoses and diseases were more frequent in the group with high CRP levels. The distribution of diagnoses was more even between groups with stratification by fibrinogen and platelet count (results not shown).
Table 2. Participant characteristics (n=25).
| All subjects (n = 25) |
CRP≤9.0 mg/dl (n = 20) |
CRP>9.0 mg/dl (n = 5) |
|
|---|---|---|---|
|
| |||
| Mean (range) | |||
| Number of visits | 22 (11–24) | 22 (11–24) | 22 (21–23) |
| Age, years | 72.4 (55–90) | 71.5 (55–87) | 75.8 (63–90) |
| BMI (kg/m2) | 32.3 (23–47) | 32.3 (23–47) | 32.4 (25–39) |
| N (%) | N (%) | N (%) | |
|
| |||
| Gender | |||
| Male | 2 (8) | 2 (10) | 0 (0) |
| Female | 23 (92) | 18 (90) | 5 (100) |
| Race/ethnicity | |||
| Black | 7 (28) | 6 (30) | 1 (20) |
| White | 18 (72) | 14 (70) | 4 (80) |
| Diagnoses | |||
| Angina | 4 (16) | 3 (15) | 1 (20) |
| Myocardial infarct | 4 (16) | 1 (5) | 3 (60) |
| Coronary artery disease* | 7 (28) | 4 (20) | 3 (60) |
| Congestive heart failure | 4 (16) | 2 (10) | 2 (40) |
| Diabetes | 6 (24) | 3 (15) | 3 (60) |
| Hypertension | 18 (72) | 14 (70) | 4 (80) |
| Chronic obstructive pulmonary disease | 7 (28) | 4 (20) | 3 (60) |
| Medication use† | |||
| Beta blocker | 10 (40) | 7 (35) | 3 (60) |
| Calcium channel blocker | 9 (36) | 8 (40) | 1 (20) |
| Statin | 6 (24) | 3 (15) | 3 (60) |
| ACE inhibitors | 10 (40) | 6 (30) | 4 (80) |
| Digoxin | 2 (8) | 0 (0) | 2 (40) |
| Any of the above listed medications | 18 (72) | 13 (65) | 5 (100) |
Angina or myocardial infarct.
Medication use is defined as reported at least once during the study.
BMI, body mass index; CRP, C-reactive protein.
Ambient air pollutant concentrations
A detailed assessment of the air pollution situation in Steubenville is reported elsewhere.8 Ambient concentrations of the measured criteria gases were moderate in Steubenville (average concentration: 6.0 ppb NO2, 0.4 ppb SO2 and 12.1 ppb O3), while ambient fine particle and sulphate concentrations were relatively high with averages of 19.7 μg/m3 and 6.9 μg/m3 and IQR of 13.4 μg/m3 and 5.1 μg/m3, respectively.
Heart rate variability measurements
All HRV measures were considerably lower and heart rate was slightly higher within the group with higher CRP, fibrinogen and platelet levels (see online table 1).
Adjusted for demographic and meteorological covariates, time and frequency domain HRV parameters including SDNN, r-MSSD, PNN50, high frequency power and low frequency power were negatively associated with levels of CRP, fibrinogen and platelets (table 3). For example, R-MSSD change was −29.4% (95% CI −50% to −0.4%, p=0.047) with each IQR increase in baseline fibrinogen (1.2 g/l). WBC, IL-6 and sICAM-1 levels were not associated with changes in HRV Heart rate was slightly elevated with increased levels of fibrinogen, WBC and platelets. For example, heart rate was elevated by 3.9% (95% CI −3.7% to 12.0%, p=0.32) with each IQR increase in baseline fibrinogen.
Table 3. Estimated per cent changes* (95% CIs) in heart rate variability and heart rate associated with an IQR increase in baseline blood marker.
| IQR | SDNN % Change (95% CI) |
r-MSSD % Change (95% CI) |
PNN50 % Change (95% CI) |
HF % Change (95% CI) |
LF % Change (95% CI) |
Heart rate % Change (95% CI) |
|
|---|---|---|---|---|---|---|---|
| CRP | 5.8 mg/l | −8.0 (−17.9 to 3.0) | −19.1 (−38.0 to 5.5) | −22.4 (−62.1 to 58.7) | −32.2 (−53.2 to −1.8) | −32.6 (−52.2 to −5.0) | 0.8 (−4.8 to 6.8) |
| Fibrinogen | 1.2 g/l | −9.1 (−22.0 to 6.1) | −29.4 (−50.0 to −0.4) | −54.1 (−81.3 to 13.1) | −34.2 (−60.5 to 9.5) | −22.9 (−53.1 to 26.8) | 3.9 (−3.7 to 12.0) |
| IL-6 | 2.4 pg/ml | 0.65 (−7.3 to 9.3) | −7.1 (−22.9 to 12.0) | 4.1 (−36.2 to 70.1) | −2.4 (−25.7 to 28.3) | −5.0 (−26.5 to 22.7) | −1.5 (−5.2 to 2.4) |
| sICAM-1 | 89 ng/ml | 2.8 (−6.0 to 12.3) | −9.3 (−25.6 to 10.5) | 10.4 (−34.5 to 85.8) | −14.4 (−35.9 to 14.2) | −11.5 (−32.7 to 16.3) | −1.0 (−5.0 to 3.1] |
| WBC | 1.3 109/I | −0.07 (−9.0 to 9.7) | 0.5 (−19.1 to 24.7) | 3.8 (−40.7 to 81.7) | −13.4 (−36.0 to 17.2) | −15.7 (−36.4 to 11.7) | 2.4 (−2.0 to 6.9) |
| Platelets | 108 109/l | −15.1 (−27.6 to −0.4) | −41.6 (−58.4 to −18.1) | −74.2 (−89.2 to −38.1) | −61.5 (−74.6 to −41.6) | −54.5 (−70.6 to −29.6) | 6.4 (−1.9 to 15.4) |
Adjusted for age, gender, race, BMI, season, time of day and apparent temperature.
CRP, C-reactive protein; HF, high frequency power; IL-6, interleukin-6; LF, low frequency power; PNN50, the per cent of RR intervals more than 50 msec different from the prior interval; r-MSSD, the mean square of differences between adjacent RR intervals; SDNN, SD of normal RR intervals; sICAM-1, soluble inter-cellular adhesion molecule 1; WBC, white blood cell count.
Considering blood marker levels as dichotomous variables, the pattern was very similar (figure 1). For example, SDNN was 24.7% lower (95% CI −41.1% to −3.7%, p=0.024) for subjects with high CRP compared to subjects with CPR levels up to 9.0mg/l, and r-MSSD was 41.5% lower (95% CI −68.2% to 7.9%, p=0.087) for subjects with high CRP.
Figure 1.
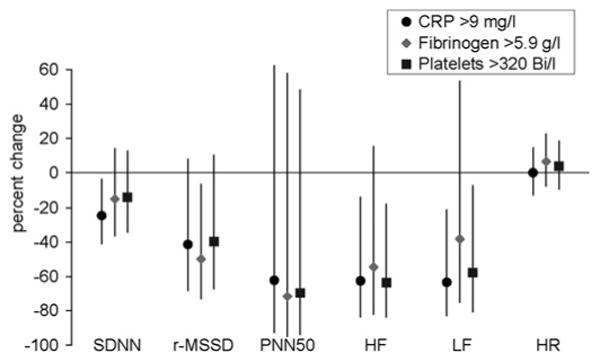
Estimated per cent changes (95% CIs) in heart rate variability parameters and heart rate associated with baseline blood marker levels in the upper 25% of the distribution.
In sensitivity analyses, to evaluate whether the effects of inflammatory markers on HRV decreased with the amount of time elapsed between blood test and electrocardiogram, we checked for interactions between the weeks since blood test and the inflammatory markers but found no consistent patterns. Exclusion of the subject with the highest CRP level and exclusion of the two male participants did not lead to any essential changes in results.
Effect modification
The estimates for the effects of pollution on per cent change in almost all HRV outcomes and heart rate were higher for those subjects with elevated CRP, but effect modification by CRP level only reached statistical significance (p<0.05) for the effect of SO42− with the outcome SDNN (figure 2(a); see online table 2). Subjects within the upper 20% of the CRP distribution showed a −6.1% change in SDNN (95% CI −11.6 to −0.2) associated with increased PM2.5 (p value of interaction=0.09) and a −6.7% change in SDNN (95% CI −11.8 to −1.3) associated with increased SO42− (p value of interaction=0.04). Subjects with CRP levels ≤80th percentile showed very little response to increased levels of PM2.5 and sulphate. We did not find any effect modification on the other HRV parameters or heart rate, although most point estimates were stronger in the group with high CRP. Exclusion of the subjects with the highest CRP value (23.4 mg/l) did not change the results. Elevations in fibrinogen increased only the association between air pollution exposure and heart rate but did not modify the associations between air pollution and HRV We did not find any effect modification of high platelet count on the relationship of pollution with any of the cardiovascular outcomes (results not shown). Associations between air pollution and HRV and heart rate were consistently stronger among the subjects with diabetes, and reached statistical significance for high frequency power and borderline significance for r-MSSD (figure 2(b); see online table 2). We did not find any effect modification of blood markers with elemental carbon or any of the gases (results not shown).
Figure 2.
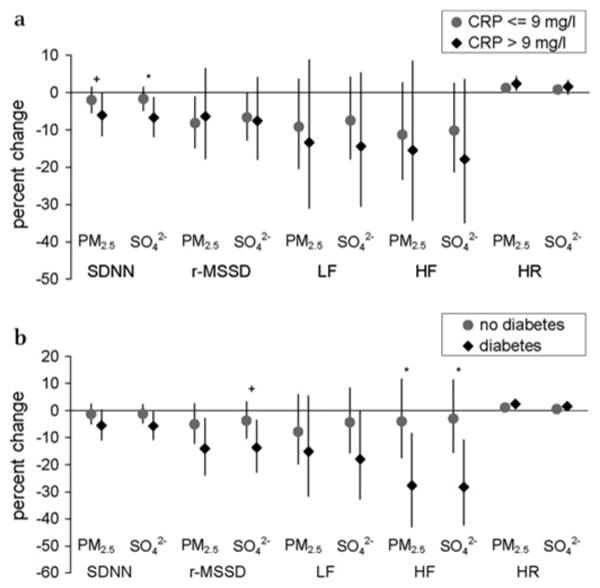
Estimated per cent changes (95% CIs) in heart rate variability parameters and heart rate associated with an IQR increase in exposure (13.4 μg/m3 for PM2.5 and 5.1 μg/m3 for SO42−) by inflammatory status (a) and by diabetes (b) (interaction models). *p<0.10, †p<0.05 for interaction effect.
Discussion
In elderly subjects living in Steubenville, Ohio, we found that elevated levels of baseline CRP and fibrinogen, biomarkers of chronic inflammation, and higher platelet counts were associated with subsequent reduced HRV and increased heart rate in a repeated measures study focused on the physiological effects of air pollution. We also found stronger associations between particulate air pollution and some autonomic HRV endpoints in subjects with elevated CRP and stronger associations between particles and elevated heart rate in subjects with increased fibrinogen levels, suggesting that subjects with increased systemic inflammation are more susceptible to the adverse effects of short-term air pollution.
While many studies have linked chronic inflammation to autonomic dysfunction, and air pollution to acute inflammatory changes, less is known about whether chronic inflammation primes the system for an increased autonomic dysfunction in response to air pollution (figure 3). Prior studies have demonstrated relationships between elevated markers of systemic inflammation and decreased HRV/increased heart rate (figure 3 (1)) both in patients with cardiovascular conditions26,27 and healthy populations. 28–32 In a recent review, Haensel and co-authors concluded that inflammatory markers and HRV are inversely correlated based on the results of 13 studies on inflammation and HRV.33 Air pollution exposure has been associated with both increased levels of inflammatory blood markers34–38 (figure 3 (2)) and decreased cardiac autonomic function2,3,7,8 (figure 3 (3)). In another repeated measures study, chronic illnesses associated with chronic inflammation (eg, diabetes and obesity) increased the risk of acute inflammatory responses to elevated air pollution.39 In this analysis, we also investigate the relationship between systemic inflammation and pollution-related HRV changes (figure 3 (4)).
Figure 3.
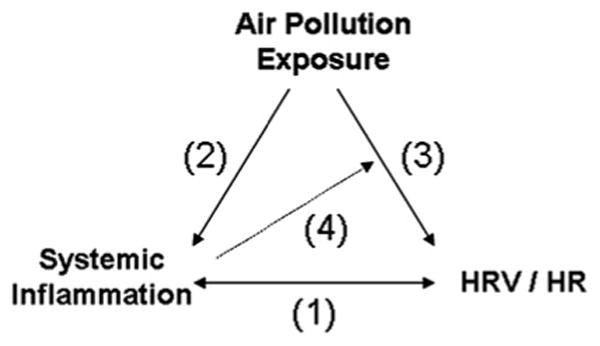
Schematic associations between cardiovascular outcomes and air pollution. Inflammation has direct effects on heart rate variability (HRV) and heart rate (1). Air pollution has direct effects on inflammation (2) and HRV/heart rate (3). Finally, inflammation modifies the association of pollution with HRV (4).
Our greatest limitation is the size of the population, limiting power to detect significant interactions, despite the strong consistent point estimates for the pollution associations with reduced HRV for those with elevated CRP. Small numbers and a predominantly female study population may also limit generalisability of study findings. As three of the six subjects with diabetes also had increased CRP, it is not surprising that effect modification by diabetes led to similar results as effect modification by CRP level, but we did not have the power to distinguish effect modification by CRP level from effect modification by diabetes. Finally, our study was limited in that we did not repeat elevated CRP levels to confirm that they were reproducible and represented the chronic state rather than response to an acute inflammatory condition. However, despite the potential for misclassification, a number of large epidemiological studies40–44 including the Framingham Study45 have effectively used baseline measures of CRP as predictors of long-term adverse cardiac outcomes. Overall, CRP was found to be very consistent and reproducible over up to 12 years in a prospective longitudinal study on cardiovascular disease.46
Our upper 20th percentile for CRP (9 mg/l) was comparable to the 10 mg/l cut-point for CRP levels found in the Framingham Study to predict increased cardiac risk.45 Fibrinogen levels are less standardised and more variable, absolute cut-off points are subsequently less comparable, and studies using fibrinogen to predict cardiac risk have more variable results.18 In a meta-analysis of 31 prospective studies from Europe, North America and Japan, increases of 1.0 g/l in fibrinogen were associated with more than twofold increased risks of coronary heart disease and stroke.47 Fibrinogen levels are linked to thrombosis as well as to inflammation, and haemostatic factors are well known to influence cardiac risk.48 A recent Italian study found that short-term air pollution levels were associated with reduced prothrombin time49 and chronically elevated air pollution levels predicted reduced prothrombin time as well as increased risk of deep venous thrombosis.50 We found that higher platelet counts predicted autonomic dysfunction. Increased mean platelet volume, a known risk factor for adverse coronary outcomes, may reflect increased platelet activation or increased numbers of large, hyperaggregable platelets. Ozdemir and colleagues found that increased mean platelet volume was correlated with autonomic dysfunction and with acute myocardial infarct.
In summary, this study suggests that chronic systemic inflammation is linked to autonomic dysfunction and may also increase vulnerability to the autonomic effects of outdoor air pollution. In the Normative Ageing Study, statins were found to reduce the adverse effects of particle pollution on heart rate variability.52 It is possible that control of chronic inflammation in cardiac disease and in diabetes will protect these vulnerable patients from the adverse autonomic effects of air pollution.
Supplementary Material
What this paper adds.
-
▸
While links between chronic inflammation and autonomic dysfunction and between air pollution and acute inflammatory and autonomic changes are well established, less is known about whether chronic inflammation increases the extent of autonomic responses to air pollution.
-
▸
This paper confirms that increased systemic inflammation, defined by elevated levels of C-reactive protein, fibrinogen and platelets, is associated with autonomic dysfunction in the elderly.
-
▸
Stronger air pollution effects on reduced SD of normal RR intervals were observed in subjects with elevated systemic inflammation and in subjects with diabetes.
Acknowledgments
The authors wish to thank all of the participants of the study as well as Monique Verrier, Meghan Syring, Bruce Nearing, Gail McCallum, Marisa Barr and Marina Jacobson-Canner. The authors are also grateful for CONSOL Energy Inc. Research and Development's laboratory analysis of air pollutant samples and for the provision of continuous ambient monitoring data.
Funding: This work is supported by funding from the National Institute of Environmental Health Sciences (ES-09825 and ES-00002), the U.S. Environmental Protection Agency (R82B780-01-0, R827353-01-0), the Ohio Coal Development Office (CDO/D-98-2) and the U.S. Department of Energy's National Energy Technology Laboratory Award No. DE-FC2B-00NT40771.
Footnotes
Additional tables are published online only. To view these files please visit the journal online (http://oem.bmj.com).
Competing interests: None.
Patient consent: Obtained.
Ethics approval: This study was conducted with the approval of the Human Subjects Committees of the Brigham and Women's Hospital and the Harvard School of Public Health.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Creason J, Neas L, Walsh D, et al. Particulate matter and heart rate variability among elderly retirees: the Baltimore 1998 PM study. J Expo Anal Environ Epidemiol. 2001;11:116–22. doi: 10.1038/sj.jea.7500154. [DOI] [PubMed] [Google Scholar]
- 2.Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–73. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz J, Litonjua A, Suh H, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–61. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope CA, 3rd, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–45. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holguin F, Tellez-Rojo MM, Hernandez M, et al. Air pollution and heart rate variability among the elderly in Mexico City. Epidemiology. 2003;14:521–7. doi: 10.1097/01.ede.0000081999.15060.ae. [DOI] [PubMed] [Google Scholar]
- 6.Liao D, Duan Y, Whitsel EA, et al. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol. 2004;159:768–77. doi: 10.1093/aje/kwh109. [DOI] [PubMed] [Google Scholar]
- 7.Park SK, O'Neill MS, Vokonas PS, et al. Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect. 2005;113:304–9. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luttmann-Gibson H, Suh HH, Coull BA, et al. Short-term effects of air pollution on heart rate variability in senior adults in Steubenville, Ohio. J Occup Environ Med. 2006;48:780–8. doi: 10.1097/01.jom.0000229781.27181.7d. [DOI] [PubMed] [Google Scholar]
- 9.Adar SD, Gold DR, Coull BA, et al. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007;18:95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- 10.Peters A, Perz S, Doring A, et al. Increases in heart rate during an air pollution episode. Am J Epidemiol. 1999;150:1094–8. doi: 10.1093/oxfordjournals.aje.a009934. [DOI] [PubMed] [Google Scholar]
- 11.Pope CA, 3rd, Verrier RL, Lovell EG, et al. Heart rate variability associated with particulate air pollution. An Heart J. 1999;138:890–9. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- 12.Ibald-Mulli A, Stieber J, Wichmann HE, et al. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001;91:571–7. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibald-Mulli A, Timonen KL, Peters A, et al. Effects of particulate air pollution on blood pressure and heart rate in subjects with cardiovascular disease: a multicenter approach. Environ Health Perspect. 2004;112:369–77. doi: 10.1289/ehp.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanobetti A, Canner MJ, Stone PH, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–9. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 15.de Paula Santos U, Braga AL, Giorgi DM, et al. Effects of air pollution on blood pressure and heart rate variability: a panel study of vehicular traffic controllers in the city of Sao Paulo, Brazil. Eur Heart J. 2005;26:193–200. doi: 10.1093/eurheartj/ehi035. [DOI] [PubMed] [Google Scholar]
- 16.Urch B, Silverman F, Corey P, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–5. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurements, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 18.Ridker PM, Brown NJ, Vaughan DE, et al. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation. 2004;109(25 Suppl 1):IV6–19. doi: 10.1161/01.CIR.0000133444.17867.56. [DOI] [PubMed] [Google Scholar]
- 19.Zanobetti A, Schwartz J. Are diabetics more susceptible to the health effects of airborne particles? Am J Respir Crit Care Med. 2001;164:831–3. doi: 10.1164/ajrccm.164.5.2012039. [DOI] [PubMed] [Google Scholar]
- 20.Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology. 2002;13:588–92. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–20. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 22.Rhoden CR, Wellenius GA, Ghelfi E, et al. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim Biophys Acta. 2005;1725:305–13. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Committee of the Environmental and Occupational Health Assembly of the American Thoracic Society. Health effects of outdoor air pollution. Part 2. Am J Respir Crit Care Med. 1996;153:477–98. doi: 10.1164/ajrccm.153.2.8564086. [DOI] [PubMed] [Google Scholar]
- 24.Demokritou P, Kavouras IG, Ferguson ST, et al. Development and laboratory performance evaluation of a personal multipollutnant sampler for simultaneous measurements of particulate and gaseous pollutants. Aerosol Sci Technol. 2001;35:741–52. [Google Scholar]
- 25.Diggle P, Heagerty P, Liang KY, et al. Analysis of longitudinal data. 2nd. Oxford; Oxford University Press; 2002. [Google Scholar]
- 26.Hamaad A, Sosin M, Blann AD, et al. Markers of inflammation in acute coronary syndromes: association with increased heart rate and reductions in heart rate variability. Clin Cardiol. 2005;28:570–6. doi: 10.1002/clc.4960281207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madsen T, Christensen JH, Toft E, et al. C-reactive protein is associated with heart rate variability. Ann Noninvasive Electrocardiol. 2007;12:216–22. doi: 10.1111/j.1542-474X.2007.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sajadieh A, Nielsen OW, Rasmussen V, et al. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–70. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Chuang KJ, Chan CC, Su TC, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–6. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 30.Sloan RP, McCreath H, Tracey KJ, et al. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13:178–84. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein PK, Barzilay JI, Chaves PH, et al. Higher levels of inflammation factors and greater insulin resistance are independently associated with higher heart rate and lower heart rate variability in normoglycemic older individuals: the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:315–21. doi: 10.1111/j.1532-5415.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 32.Lampert R, Bremner JD, Su S, et al. Decreased heart rate variability is associated with higher levels of inflammation in middle-aged men. Am Heart J. 2008;156:759, e1–7. doi: 10.1016/j.ahj.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haensel A, Mills PJ, Nelesen RA, et al. The relationship between heart rate variability and inflammatory markers in cardiovascular diseases. Psychoneuroendocrinology. 2008;33:1305–12. doi: 10.1016/j.psyneuen.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz J. Air pollution and blood markers of cardiovascular risk. Environ Health Perspect. 2001;109(Suppl 3):405–9. doi: 10.1289/ehp.01109s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seaton A, Soutar A, Crawford V, et al. Particulate air pollution and the blood. Thorax. 1999;54:1027–32. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters A, Fröhlich M, Doling A, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- 37.Riediker M, Cascio WE, Griggs TR, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169:934–40. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- 38.Ruckerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173:432–41. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 39.Dubowsky SD, Suh H, Schwartz J, et al. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–8. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–8. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 41.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the health ABC study. Circulation. 2003;108:2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 44.Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- 45.Dhingra R, Gona P, Nam BH, et al. C-reactive protein, inflammatory conditions, and cardiovascular disease risk. Am J Med. 2007;120:1054–62. doi: 10.1016/j.amjmed.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 47.Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 48.Juhan-Vahue I. Haemostatic parameters and vascular risk. Atherosclerosis. 1996;124:S49–55. doi: 10.1016/0021-9150(96)05857-1. [DOI] [PubMed] [Google Scholar]
- 49.Baccarelli A, Zanobetti A, Martinelli I, et al. Effects of exposure to air pollution on blood coagulation. J Thromb Haemost. 2007;5:252–60. doi: 10.1111/j.1538-7836.2007.02300.x. [DOI] [PubMed] [Google Scholar]
- 50.Baccarelli A, Martinelli I, Zanobetti A, et al. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Intern Med. 2008;168:920–7. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozdemir O, Soylu M, Alyan O, et al. Association between mean platelet volume and autonomic nervous system functions: increased mean platelet volume reflects sympathetic overactivity. Exp Clin Cardiol. 2004;9:243–7. [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz J, Park SK, O'Neill MS, et al. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med. 2005;172:1529–33. doi: 10.1164/rccm.200412-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


