Abstract
Objective
Emerging evidence suggests abnormalities in amino acid neurotransmitter function and impaired energy metabolism contribute to the underlying pathophysiology of Major Depressive Disorder (MDD). To test whether impairments in energetics and glutamate neurotransmitter cycling are present in MDD we used in vivo 13C magnetic resonance spectroscopy (13C MRS) to measure these fluxes in individuals diagnosed with MDD relative to non-depressed subjects.
Method
1H MRS and 13C MRS data were collected on 23 medication-free MDD and 17 healthy subjects. 1H MRS provided total glutamate and GABA concentrations, and 13C MRS, coupled with intravenous infusion of [1-13C]-glucose, provided measures of the neuronal tricarboxylic acid cycle (VTCAN) for mitochondrial energy production, GABA synthesis, and glutamate/glutamine cycling, from voxels placed in the occipital cortex.
Results
Our main finding was that mitochondrial energy production of glutamatergic neurons was reduced by 26% in MDD subjects (t = 2.57, p = 0.01). Paradoxically we found no difference in the rate of glutamate/glutamine cycle (Vcycle). We also found a significant correlation between glutamate concentrations and Vcycle considering the total sample.
Conclusions
We interpret the reduction in mitochondrial energy production as being due to either mitochondrial dysfunction or a reduction in proper neuronal input or synaptic strength. Future MRS studies could help distinguish these possibilities.
Introduction
Major depressive disorder (MDD), is a debilitating, and at times, life-threatening psychiatric disorder. MDD affects an estimated 350 million people worldwide, and according to a recent report by the world health organization, is a leading cause of years lost due to disability (1). However, despite the high prevalence and immense economic burden associated with MDD, relatively little is known about the pathophysiology underlying the disorder. Although the monoaminergic neurotransmitter systems had been the primary focus in the majority of investigations exploring the associated pathophysiology for the past 5 decades, there is rapidly accumulating evidence to suggest the amino acid neurotransmitter systems and impaired mitochondrial function contribute to pathophysiological mechanisms of mood and other stress related disorders (2, 3).
Clinical studies using magnetic resonance spectroscopy (MRS) have repeatedly shown abnormal amino acid neurotransmitter (glutamate, glutamine and GABA) content in the brains of patients diagnosed with mood disorders. A meta-analysis of 1H MRS studies found evidence of both diffuse and region specific changes in glutamine and glutamate content (4) and the recent discovery of significant antidepressant effects associated with drugs targeting the glutamatergic system has led to intense speculation on the contributions of the system to mood disorder pathophysiology (5). In addition to evidence linking glutamate to mood disorders, there is a long history of studies suggesting abnormalities in the GABAergic system contribute to the pathophysiology of mood disorders (6). Early reports suggested that GABA levels were reduced in the blood and cerebrospinal fluid of depressed patients (7). Imaging studies have provided fairly consistent reports suggesting GABA content is reduced in the brains of depressed patients, especially in the occipital cortex (8, 9), although it appears to be primarily in subtypes of the disease. Additional studies suggest altered function of the amino acid neurotransmitter systems contribute to changes in cortical excitability and inhibition as well as several other pathophysiological processes related to mood disorder (10, 11).
One potential pathophysiological mechanism that could be related to the findings of altered amino acid neurotransmitter system content and function in mood-disordered individuals is impaired in astrocyte function. Astrocytes provide a primary means of glutamate and GABA clearance through the Excitatory Amino Acid Transports 1 and 2 (EAAT1 and EAAT2) and GABA transporters 1-3 (GAT 1-3) respectively (12, 13). Astrocytes also play a critical role in the metabolism of both glutamate and GABA. Once glutamate is brought into an astrocyte it is rapidly converted into glutamine by glutamine synthetase. The glutamine is then transported back to glutamatergic neurons to replenish glutamate stores by the mitochondrial enzyme glutaminase in a process referred to as the glutamate/glutamine cycle (14). Glutamine is also transported to GABAergic cells where it is first converted into glutamate by glutaminase and then to GABA by glutamic acid decarboxylase in the GABA/glutamine cycle. Evidence suggesting astrocytic pathology is associated with mood disorders and rodent models of mood disorders (see (15, 16) for reviews), suggests the abnormalities observed within the amino acid neurotransmitter systems associated with mood disorders could be secondary to changes in the glutamate/glutamine and GABA/glutamine cycles.
Mitochondria, in addition to serving a critical role in cellular energy metabolism, are also intimately involved in amino-acid metabolism and brain function. Work over the last two decades has shown that there is a linear relationship between changes in the rates of neurotransmitter glutamate release and recycling and the neuronal TCA cycle, with approximately 80% of neuronal energy metabolism devoted to this function in the resting awake cerebral cortex (17). Therefore, even relatively minor mitochondrial impairment could adversely impact glutamate neurotransmission and brain function. Considering the rapidly mounting evidence suggesting that altered mitochondrial function and amino acid metabolism are associated with depression and other mood disorders (3), we used 13C magnetic resonance spectroscopy (13C MRS) in vivo to determine whether the rate of the neuronal TCA cycle (VTCAN), the glutamate/glutamine cycle (Vcycle), and GABA synthesis (VGAD) are altered in major depressive disorder (MDD). We found a significant reduction in the rate of the neuronal TCA cycle in glutamatergic neurons, implicating the glutamatergic system and mitochondrial energy metabolism as having an important role in the pathology of MDD. Paradoxically we also found that Vcycle was similar in depressed and control subjects implying a possible alteration in neuronal coupling. This also serves as the first study to use 13C MRS in vivo to study the pathophysiology of MDD and provides the first measures of VGAD in humans and to show correlations between 13C MRS and 1H MRS measures of metabolite concentrations in humans.
Methods
Subjects
Men and women between the ages of 18–65 years were recruited into this study. An institutional review board at Yale University approved all study procedures. After complete description of the study to the subjects, written informed consent was obtained. Following comprehensive medical and psychiatric assessments, subjects meeting the study criteria underwent a 1H MRS and then a 13C MRS scan, each on a separate day. Two groups of subjects were enrolled: a group with Major Depressive Disorder (MDD) and a healthy control group. The MDD subjects met the following criteria: (a) medication-free for at least 4 weeks, (b) met DSM-IV criteria for MDD, confirmed by structured interview with the SCID-P, and (c) a Hamilton Depression Rating Scale (HDRS21) scores higher than 21. For the healthy group, study criteria included (a) no personal or first degree family member with a history of axis I DSM-IV disorder, confirmed by SCID-NP, (b) age-, and sex-matched to the MDD group. The exclusion criteria for both groups included (a) history or current major medical or neurological illness, (b) history or current substance abuse or dependence, (c) current use of nicotine, (d) implanted metal, or (e) currently pregnant. Rating scales included HDRS21, Beck Depression Inventory (BDI), and Hamilton Anxiety rating scale (HAM-A) (see Online Supplements for references).
1H MRS Acquisition and Processing
1H MRS acquisitions were performed in the occipital cortex as previously outlined (9). Briefly, metabolite levels were measured in a 13.5 cc voxel (3.0 × 1.5 × 3.0 cm) placed across the midline of the brain, centered 2 cm from the dura. Cortical GABA content was determined using J-editing, where subspectra were acquired with 8K data points over a 410-ms acquisition, a 2.5-second repetition time, and a TE of 68 ms on a 4T Bruker spectrometer at Yale Magnetic Resonance Research Center (MRRC), averaging data in 20-second blocks for 20 minutes. Glutamate and glutamine were measured simultaneously using the unedited subspectra of the J-editing acquisition, with in-house software that uses an LCModel approach (18). The metabolites fitted included GABA, glutamate, glutamine, and creatine. The subspectrum obtained without the editing pulse was fitted simultaneously with the J-edited difference spectrum of GABA. Because of limited resolution in vivo, the results for NAA and NAAG were combined and recorded as NAA, creatine and phosphocreatine were combined and recorded as creatine, and the three choline-containing compounds were combined and recorded as choline. This implementation had no macromolecular contamination of GABA (19), so the basis set for fitting did not include a macromolecular signal. The level of aspartate, though present in the spectra, was poorly determined at the echo time of 68 ms and was not used. Uncertainties of individual measurements were determined using a Monte-Carlo analysis (19), in which the least-squares spectral fits were treated with random Gaussian noise whose standard deviation was equal to that of the raw data and refitted, using 20 repetitions to estimate the standard deviations of the uncertainty for each metabolite measure. For each metabolite, a threshold for rejection was set at twice the average noise-based standard deviation of the respective metabolite. GABA levels whose uncertainties were greater than 11%, glutamine levels whose uncertainties were greater than 20%, and glutamate levels whose uncertainties were greater than 16% were not included in subsequent analysis.
To account for potential differences in tissue composition, a series of 3-mm-thick contiguous images of T1 were used to quantify gray matter, white matter, and cerebrospinal fluid in the voxel of interest (20). The images of T1 were measured using a series of inversion-recovery images that required images of the spatial distribution of the radiofrequency power to overcome the problems associated with radiofrequency inhomogeneity. The means of percent solid tissue in the acquired voxel were not different between groups (p > 0.3). Thus, no co-variance for tissue content was needed. Similarly, the means of Cr/water were not different between groups (p > 0.5). As such, the concentration of brain metabolites was calculated assuming normal Cr concentration of 9 mmol/kg (21).
13C MRS Acquisition, Processing, and Metabolic Modeling
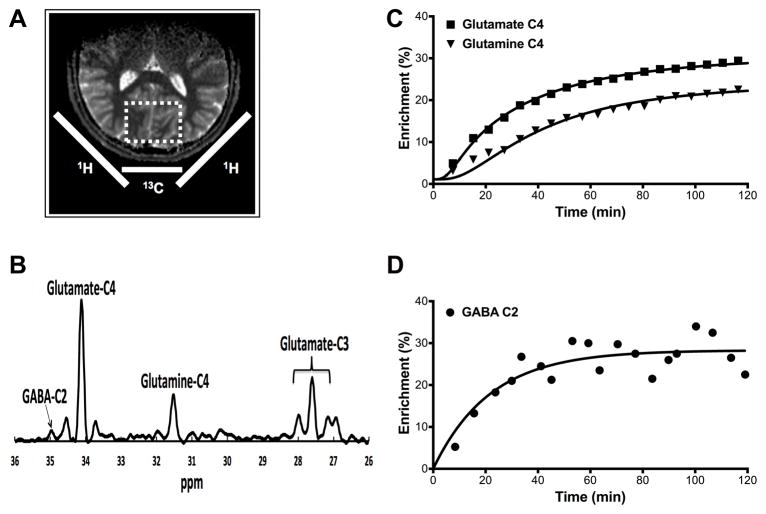
13C MRS acquisition, processing, and kinetic metabolic modeling were performed as described previously (22, 23). In summary, all subjects fasted overnight prior to the 13C MRS acquisition. The 13C MRS studies were performed (mean ±SEM) 6.7 ±1.4 days after completing to the 1H MRS study. In the morning, two intravenous (IV) lines were initiated, one for [1-13C]-glucose infusion and the other for blood sampling. 13C MRS data were acquired on a 4.0 T magnet. Subjects were placed supine with their heads lying on top of a radiofrequency probe consisting of one 8.5-cm diameter circular 13C coil and two circular 12.5-cm diameter, quadrature driven 1H RF coils. The region of interest (ROI) was a voxel (5 × 4 × 4.5 cm) placed across the midline of the occipital-parietal lobe. Following tuning, acquisition of scout images, FASTERMAP shimming, and decoupling power calibration, infusion of [1-13C]-glucose was started and 5-minute blocks of 13C MR spectra were acquired for 120 min using polarization transfer (Fig. 1). The plasma glucose concentration was rapidly titrated to 180–200 mg/dl and maintained near that level for the duration of 13C MRS acquisition. Plasma glucose concentrations and [1-13C] enrichments were determined from blood samples collected at baseline and during the [1-13C]-glucose infusion
Figure 1.
13C MRS Voxel, Spectrum, and Time Courses. (A) Coils and voxels placement. (B) Spectrum acquired over 10 minutes at 4T from the occipital region during [1-13C]-glucose infusion at steady state. (C) Time courses from one participant showing the percent enrichment for each metabolite.
Spectral data were analyzed with −2Hz/6Hz Lorentzian-to-Gaussian conversion and 16-fold zero-filling followed by Fourier transformation. Software developed in-house, using Matlab 7.12.0 (The MathWorks, Natick, MA, USA), was used to determine the peak heights for the C3 and C4 positions of glutamate, C4 position of glutamine, and C2 position of GABA of each spectrum. Peak heights were converted to concentrations of 13C using the fractional enrichment of glutamate C4, determined by isotopomer analysis (24) and the total glutamate concentration measured by 1H MRS. Time courses of glutamate, glutamine, and GABA peaks were analyzed using CWave (25) to implement a three-compartment model of brain metabolism that included astroglial, and glutamatergic and GABAergic neurons (26). The CWave software iterated the values of the rates of GABA synthesis (VGAD), glutamate neurotransmitter cycling (Vcycle), and the neuronal tricaboxilic acid cycle (VTCAN) to obtain a least-squares fit of the model to the time courses of each subject’s data, using the time courses of the individual’s own plasma glucose concentration and fractional enrichment as input functions. The equations used in the kinetic model are shown in Table S1.
Statistical Analyses
Prior to each analysis, outcomes were assessed for normality using normal probability plots and Kolmogorov test statistics. Logarithmic (Log10) transformations were performed as necessary on variables with skewed distribution. Independent t-test and Chi Square test were used to determine differences between groups. Bonferroni correction for multiple comparisons was implemented as described in the Results. Spearman’s rank order was used for correlational analyses. All tests were two-tailed, with significance level set at p ≤ 0.05.
Results
A total of 46 subjects completed all study procedures. Six subjects (3 MDD and 3 healthy) were excluded due to poor 13C spectral quality. Demographic data show that the study subjects were well matched for age, gender, and body mass index (BMI) (Table 1). All MDD subjects were medication-free for at least 4 weeks. Clinical characteristics of the MDD subjects are also presented in Table 1, which are consistent with a moderate level of depression severity with coexisting levels of mild anxiety on average.
Table 1.
Demographics and Clinical Data
| MDD Group (n = 23) | Healthy Group (n = 17) | |||
|---|---|---|---|---|
|
|
||||
| Mean ± SEM | Mean ± SEM | df | Sig. a | |
| Age | 43.0 ± 2.2 | 43.8 ± 3.1 | 38 | 0.83 |
| Female (N; %) | 16 (70%) | 13 (76%) | 38 | 0.63 |
| BMI | 26.3 ± 1.0 | 27.3 ± 1.6 | 38 | 0.58 |
| HDRS25 | 30.1 ± 1.2 | |||
| BDI | 26.3 ± 1.3 | |||
| HAM-A | 15.6 ± 1.0 | |||
| 1st MDE (N; %) | 4 (17%) | |||
| Medication Naïve (N; %) | 8 (35%) | |||
| Subtypes | ||||
| Melancholic (N; %) | 5 (22%) | |||
| Atypical (N; %) | 15 (65%) | |||
| No Subtype (N; %) | 3 (13%) | |||
Independent t-test or Chi square test (2-tailed, significance set at p ≤ .05);
Abbreviations: MDD: Major Depressive Disorder; MDE: Major Depressive Episode; SEM: Standard Error of Mean; HDRS: Hamilton Depression Rating Scale; BDI: Beck Depression Inventory; HAM-A: Hamilton Anxiety Rating Scale.
13C MRS Spectra and Metabolic Modeling
In contrast to the more commonly employed 1H MRS methods, 13C MRS is capable of providing unique information on the dynamic processes of metabolism and neurotransmission. Since 12C, which is invisible to MRS detection methods, comprises nearly 99% of the carbon content in biological systems, it is possible to track and model the labeling of individual molecules over time with exogenously administered 13C, a stable isotope visible by MRS methods. This approach affords the ability to obtain dynamic measures relevant to amino acid neurotransmitter metabolism and neurotransmission. To label the carbon positions of glutamate, glutamine, and GABA, [1-13C]-glucose was infused intravenously over 120 minutes during MR spectroscopy acquisition focused on the region of the occipital cortex (Fig. 1-A). The incorporation of the 13C in glutamate, glutamine and GABA, generates unique signals on the 13C spectrum (Fig. 1-B). 13C MRS spectra were obtained with a 5-minute time resolution. A plot of the time courses of the 13C labeling in C4 glutamate, glutamine and C2 GABA is shown in Figure 1-C & 1-D. In addition the C3 resonances of glutamate and glutamine were measured and used in the modeling. The steady state fractional enrichment of glutamine was lower than that of glutamate in most subjects, consistent with previous findings (27). Using mass and isotope balance equations, the labeling time courses were used to calculate -1- glutamate/glutamine cycling (Vcycle, a measure of neuronal glutamate release and glial reuptake), -2- Neuronal oxidation (VTCAN – mitochondrial energy production specific to glutamatergic neurons), and -3- GABA synthesis (VGAD).
13C MRS Metabolic Fluxes
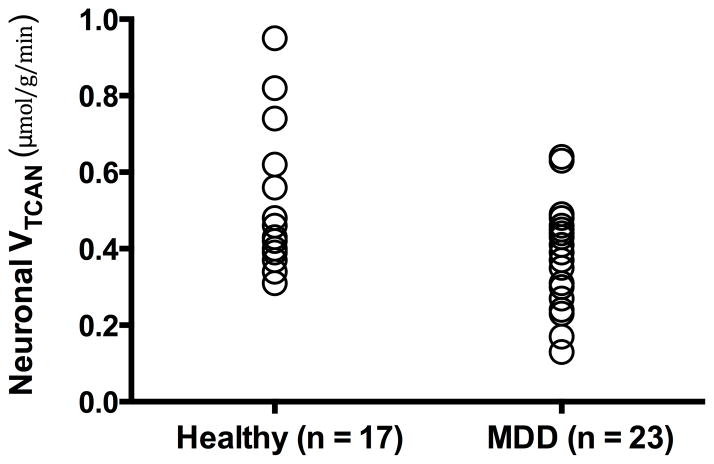
Patients with MDD had a 26% reduction in oxidative mitochondrial energy production of glutamatergic neurons [Mean ±SEM; MDD VTCAN = 0.35 ± 0.03 μmol/g/min, Healthy VTCAN = 0.47 ± 0.05 μmol/g/min, t = 2.57, n = 40, p = 0.01] (Fig. 2). Cohen’s d was 0.84 (95% confidence interval: 0.80–0.89). The VTCAN differences between groups maintain significance following Bonferroni correction for multiple comparisons (p < 0.05/3). Vcycle and VGAD did not differ between the two groups (p > 0.7; Table 2). The values of Vcycle and VTCAN were consistent with previous studies (28). The rate of VGAD is approximately 10–20% of VTCAN, which is consistent with previous animal studies (26, 29).
Figure 2.
Mitochondrial Energy Production In MDD and Healthy Control
Table 2.
Comparison Of Occipital Metabolic Fluxes
| MDD Group (n = 23) | Healthy Group (n = 17) | ||||
|---|---|---|---|---|---|
|
|
|||||
| Mean ± SEM | Mean ± SEM | t | df | Sig. a | |
| VTCAN | 0.35 ± 0.03 | 0.47 ± 0.05 | 2.57 | 38 | 0.01 b |
| Vcycle | 0.19 ± 0.01 | 0.18 ± 0.01 | − 0.32 | 38 | 0.75 |
| VGAD | 0.04 ± 0.003 | 0.03 ± 0.003 | − 0.35 | 34 c | 0.72 |
Independent t-test (2-tailed, significance set at p ≤ 0.05).
p value maintains significance after adjustment for multiple comparisons.
VGAD was excluded for subjects (n = 5) with noise level higher than 0.05 μmol/g/min;
Abbreviations: MDD: Major Depressive Disorder; SEM: Standard Error of Mean; VTCAN: Neuronal tricaboxilic acid cycle; VGAD: GABA synthesis; Vcycle: glutamate-glutamine cycle. Unit of measure is μmol/g/min.
1H MRS Metabolite Levels
Across all study subjects, we found significant correlations between glutamate level and Vcycle (rs = 0.45, p = 0.004), as well as between glutamate and VTCAN (rs = 0.34, p = 0.04). However, only the association between glutamate and Vcycle maintains significance following Bonferroni correction for multiple comparisons (p < 0.05/9). Associations between metabolic fluxes and amino-acid neurotransmitters level are provided in Table 3. In the current study, the means of amino-acid neurotransmitters level were not different between the healthy and MDD groups (p > 0.4; Table S2). However, there was an interesting pattern suggesting that GABA levels may have been lower in the small sample of melancholic subjects participating in the study (Figure S1).
Table 3.
Correlations Between Metabolic Fluxes and Brain Metabolites
| VTCAN | VCycle | VGAD a | |
|---|---|---|---|
| GABA (rs) | 0.23 | 0.09 | − 0.06 |
| Glutamine (rs) | 0.16 | 0.28 t | 0.12 |
| Glutamate b (rs) | 0.34 * | 0.45 ** c | 0.09 |
VGAD was excluded for subjects (n = 5) with noise level higher than 0.05 μmol/g/min.
Two subjects had poor spectral fitting for glutamate.
Survive Bonferroni correction for multiple comparisons (p < 0.05/9).
Abbreviations: rs: Spearman’s correlation coefficient; GABA: γ-Aminobutyric acid.
p < 0.05;
p < 0.01;
p < 0.1;
Associations Between Clinical and Spectroscopy Measures in the MDD Group
The number of lifetime major depressive episodes negatively correlated with the concentration of glutamate (rs = − 0.59, p = 0.01). HAM-A scores negatively correlated with glutamine concentration (rs = − 0.47, p = 0.03) and with VTCAN at trend level (rs = − 0.37, p = 0.09). However, these correlations did not survive Bonferroni corrections for multiple comparisons (p > 0.05/24). Correlations between clinical and spectroscopy measures are detailed in Table S3.
Discussion
Using state-of-the-art human 13C MRS methods, we studied neuronal oxidative energy production, glutamate-glutamine cycling, and GABA synthesis in vivo in MDD subjects compared to healthy controls. We found a 26% reduction in oxidative energy production specific to glutamatergic neurons. No differences in glutamate-glutamine cycling or GABA synthesis were found. Total glutamate levels correlated with glutamate-glutamine cycling and, to a lower extent, neuronal energy production. However, in contrast to previous studies, amino-acid neurotransmitters did not differ between groups in this cohort (8, 9). Finally, post hoc exploratory analysis – without correction for multiple comparisons – showed a negative association between the glutamate concentration and the number of depressive episodes, as well as between the glutamine concentration and clinician-rated anxiety scores.
The ability of MRS to separate mitochondrial energy production between glutamatergic neurons, GABAergic neurons, and glia is based upon the compartmentation of glutamine synthase and GAD in glia and neurons respectively and the majority of the neuronal glutamate pool being in glutamatergic neurons (see (14) for a review). Because MRS can distinguish 13C labeling in GABA, glutamine, and glutamate, it is then possible to use kinetic modeling to obtain separate measures of the TCA cycle in all three compartments (14, 26, 29).
The findings of reduced neuronal energy production in the depressed subjects are in general consistent with reports of slower energy metabolism, primarily decreased baseline levels of β-nucleoside triphosphate (β-NTP) and total NTP, associated with MDD (30, 31) and reports from PET studies of regional and global reductions in glucose metabolism in MDD (32). In addition, in a rodent model of chronic unpredictable stress reduced 13C labeling from glucose was observed in glutamate, consistent with the human findings (33).
A potential explanation for the reduced neuronal energy production is the presence of mitochondrial impairment in depressed individuals. Mitochondrial dysfunction in mood disorders has been previously suggested [reviewed in (3)]. However, direct in vivo measures of neuronal mitochondrial energy production in MDD were not previously investigated. Impaired oxidative metabolism, in the absence of reduced neuronal activity, would be consistent with prior evidence suggesting an increase in cerebral glycolysis and lactate, as well as reductions in phosphocreatine and pH in patients with mood disorders (3, 34).
An alternate explanation is that the reduced neuronal energy production reflects a downregulation of cortical activity. Extensive animal and human studies have previously demonstrated a strong, close to 1:1, coupling between energy production in the TCA cycle and the glutamate/glutamine cycle (17). Consistent with this relationship, there was a strong trend for a positive relationship between VTCAN and Vcycle (r = 0.436, p = 0.08) observed in the healthy subjects in this study (Table S4). However, in the MDD group the relationship between VTCAN and Vcycle (r = 0.252, p > 0.2) appears to be weakened. This is again reflected in the fact that we found normal rates of glutamate/glutamine cycling despite slower energy production in the MDD subjects.
The seemingly contradictory findings of reduced neuronal energy metabolism with normal levels of glutamate/glutamine cycling could be reconciled by the presence of an overall reduction of glutamatergic synaptic strength. Brain functional energetic needs are largely determined by the ATP needed to maintain ion flows in the post and pre-synaptic terminals of excitatory synapses that are coupled to glutamate neurotransmission (17). Reduced overall synaptic strength in depressed subjects would reduce energetic demands for the same amount of glutamate-glutamine cycling. In line with this hypothesis, chronic stress has been shown to reduce glutamate AMPA and NMDA receptor expression and transmission – the primary determinants of synaptic strength (35).
Alternatively the rate of the glutamate/glutamine cycle in MDD subjects may have been overestimated due to a change in the balance between neuronal and astroglial metabolism in the MDD group. A previous 13C MRS study of healthy aging (22) found that that there is an increase in glial metabolism accompanying the reduction in neuronal metabolism, similar changes in MDD could lead to an overestimate of the rate of the glutamate/glutamine cycle with the kinetic modeling used. Rising evidence suggest astroglial changes in major depression (16, 36) including 13C MRS studies in a rodent chronic unpredictable stress model (33). To overcome this limitation, future studies may employ recently developed 13C methods of combined labeling by 13C-glucose and 13C-acetate (37). Acetate oxidation is limited to astroglial cells, so therefore the simultaneous administration of 13C-glucose and 13C-acetate allows separate measurements of neuronal and astroglial energy production, respectively (14). In addition, double labeling would enhance the precision of glutamate-glutamine cycling estimates, providing additional insight into the relationship between metabolic fluxes in MDD compared to healthy controls.
In addition to the lack of direct measure of astroglial metabolism, other limitations of the current study include the study’s strict criteria to enroll only subjects who have been medication-free for at least 4 weeks in an attempt to minimize any effects of medication withdrawal. This inclusion criterion might have affected the MDD sample characteristic, excluding subjects with more treatment resistant and melancholic type depression. In turn, this could have contributed to the lack of GABAergic differences between groups in contrast to previous reports where reduced GABA was primarily found in subjects with melancholic and treatment resistant depression (8, 9). Although we had a very limited number of subjects meeting criteria for the melancholic subtype of MDD, there was suggestion that they did show lower GABA levels compared to other groups of comparison subjects (Fig S1).
Given the focus on the prefrontal cortex in depression the occipital cortex volume studied could be considered a major limitation, however this is the region where significant changes in amino acid neurotransmitter content has previously been reported (8, 9). Additionally, a growing number of studies have demonstrated abnormal occipital cortex function in individuals diagnosed with depression. The studies most consistently demonstrate altered levels of occipital cortex activation to emotionally laden visual stimuli (38, 39). Interestingly, the abnormal experience stimulus-processing biases seen in depressed patients is reported to normalize with treatment, and a recent study suggests that the magnitude of neural response in the middle occipital cortex may provide a biomarker that predicts response to the rapidly acting antidepressant effects of scopolamine (40).
In conclusion, the data presented here provide evidence of reduced oxidative energy production within glutamatergic neurons from individuals with MDD. The strengths of the current investigation include the use of human 13C MRS methods in the study of psychiatric disorders to interrogate glutamatergic activity in vivo in humans, as well as the first quantitative measurement of the rate of GABA synthesis in human cerebral cortex. The reduction in oxidative mitochondrial energy production in glutamatergic neurons could be related to several possible pathophysiological processes including mitochondrial dysfunction, reduced levels of glutamatergic synaptic activity, and/or altered coupling of neuronal-astroglial metabolism. Studies employing animal models, specifically examining the relationships between these potential factors and oxidative metabolism will help determine the mechanisms underlying the finding. Future studies identifying the pathophysiological changes underlying the reduced levels of oxidative energy production could provide novel targets for the development of new therapeutics.
Supplementary Material
Acknowledgments
Funding was provided by NIH grants R01 MH071676 (G.S.), K02 MH076222 (G.S.), the Stanley Foundation (G.F.M.), NARSAD (G.F.M.), and R01 DA021785 (G.F.M.). Salary support for C.G.A. was provided by NIMH K23 MH101498 and NIDA T32 DA022975 (NeuroImaging Science Training Program).
Footnotes
Previous presentation: Hot Topics, ACNP annual meeting, December 2012, Hollywood Florida USA
Disclosure
L.J., H.M.D.F., M.F., and D.L.R. report no competing financial interests.
CGA has received consulting fees from Genentech.
G.F.M. has received consulting fees from U.C.B. Pharma S.A.
G.S. has received consulting fees from (Note: – The Individual Consultant Agreements listed below are less than $10,000 per year): Abbott, AstraZeneca, Avanier Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly & Co., Hoffman La-Roche, Johnson & Johnson, Novartis, and Takeda Pharmaceuticals over the past 24 months. In addition he received consulting fees greater than $10,000 from Noven Pharmaceuticals. He has also received additional grant support from AstraZeneca, Bristol-Myers Squibb, Hoffman La-Roche, Eli Lilly and Co., Merck & Co., Naurex and Johnson and Johnson over the past 24 months. In addition, he is a co-inventor on a filed patent application by Yale University (PCTWO06108055A1), and holds shares in BioHaven Pharmaceutical Holding Company. Editorial Board: compensation from Neuropsychopharmacology ACNP.
J.H.K. Financial Disclosure includes: Consultant (Note: – The Individual Consultant Agreements listed below are less than $10,000 per year): Aisling Capital, LLC; Astellas Pharma Global Development, Inc.; AstraZeneca Pharmaceuticals; Biocortech; Brintnall & Nicolini, Inc.; Easton Associates; Gilead Sciences, Inc.; GlaxoSmithKline; Janssen Pharmaceuticals; Lundbeck Research USA; Medivation, Inc.; Merz Pharmaceuticals; MK Medical Communications; F. Hoffmann-La Roche Ltd; Sage Therapeutics, Inc.; SK Holdings Co., Ltd; Sunovion Pharmaceuticals, Inc.; Takeda Industries; Teva Pharmaceutical Industries, Ltd. Scientific Advisory Board: Abbott Laboratories; Bristol-Myers Squibb; CHDI Foundation, Inc.; Eisai, Inc.; Eli Lilly and Co.; Forest Laboratories, Inc.; Lohocla Research Corporation; Mnemosyne Pharmaceuticals, Inc.; Naurex, Inc.; Neurobiology Foundation-Research in Schizophrenia and Bipolar Disorder; Pfizer Pharmaceuticals; Shire Pharmaceuticals; StratNeuro Research Program at Karolinska Institute (International Advisory Board). Board of Directors: Coalition for Translational Research in Alcohol and Substance Use Disorders. President: American College of Neuropsychopharmacology. Editorial Board: (Income Greater than $10,000) Editor - Biological Psychiatry. Employment: Yale University School of Medicine; VA CT Healthcare System. Patents and Inventions: 1) Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent #:5,447,948.September 5, 1995; 2) Co-inventor on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1); 3) Intranasal Administration of Ketamine to Treat Depression (pending).
References
- 1.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, Anderson W, Dhansay MA, Phillips A, Shurin S, Walport M, Ewart W, Savill SJ, Bordin IA, Costello EJ, Durkin M, Fairburn C, Glass RI, Hall W, Huang Y, Hyman SE, Jamison K, Kaaya S, Kapur S, Kleinman A, Ogunniyi A, Otero-Ojeda A, Poo MM, Ravindranath V, Sahakian BJ, Saxena S, Singer PA, Stein DJ. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 4.Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, Bakker SC. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Krystal JH, Sanacora G, Duman RS. Rapid-Acting Glutamatergic Antidepressants: The Path to Ketamine and Beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petty F. GABA and mood disorders: a brief review and hypothesis. J Affect Disord. 1995;34:275–281. doi: 10.1016/0165-0327(95)00025-i. [DOI] [PubMed] [Google Scholar]
- 8.Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ. Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry. 2009;65:792–800. doi: 10.1016/j.biopsych.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 10.Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ. Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry. 2010;67:458–464. doi: 10.1016/j.biopsych.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Hasler G, Northoff G. Discovering imaging endophenotypes for major depression. Mol Psychiatry. 2011;16:604–619. doi: 10.1038/mp.2011.23. [DOI] [PubMed] [Google Scholar]
- 12.O’Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clinical and experimental pharmacology & physiology. 2002;29:1018–1023. doi: 10.1046/j.1440-1681.2002.03770.x. [DOI] [PubMed] [Google Scholar]
- 13.Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Brain Res Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Current drug targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73:1172–1179. doi: 10.1016/j.biopsych.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyder F, Rothman DL, Bennett MR. Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc Natl Acad Sci U S A. 2013;110:3549–3554. doi: 10.1073/pnas.1214912110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 19.Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G. The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [(1)H]-MRS. Psychiatry Res. 2011;191:122–127. doi: 10.1016/j.pscychresns.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason GF, Rothman DL. Graded image segmentation of brain tissue in the presence of inhomogeneous radio frequency fields. Magn Reson Imaging. 2002;20:431–436. doi: 10.1016/s0730-725x(02)00510-6. [DOI] [PubMed] [Google Scholar]
- 21.Petroff OA, Spencer DD, Alger JR, Prichard JW. High-field proton magnetic resonance spectroscopy of human cerebrum obtained during surgery for epilepsy. Neurology. 1989;39:1197–1202. doi: 10.1212/wnl.39.9.1197. [DOI] [PubMed] [Google Scholar]
- 22.Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason GF, Rothman DL. Basic principles of metabolic modeling of NMR (13)C isotopic turnover to determine rates of brain metabolism in vivo. Metabolic engineering. 2004;6:75–84. doi: 10.1016/j.ymben.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Gruetter R, Novotny EJ, Boulware SD, Mason GF, Rothman DL, Shulman GI, Prichard JW, Shulman RG. Localized 13C NMR spectroscopy in the human brain of amino acid labeling from D-[1-13C]glucose. J Neurochem. 1994;63:1377–1385. doi: 10.1046/j.1471-4159.1994.63041377.x. [DOI] [PubMed] [Google Scholar]
- 25.Mason GF, Falk Petersen K, de Graaf RA, Kanamatsu T, Otsuki T, Rothman DL. A comparison of (13)C NMR measurements of the rates of glutamine synthesis and the tricarboxylic acid cycle during oral and intravenous administration of [1-(13)C]glucose. Brain Research Brain Research Protocols. 2003;10:181–190. doi: 10.1016/s1385-299x(02)00217-9. [DOI] [PubMed] [Google Scholar]
- 26.Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen J, Rothman DL, Behar KL, Xu S. Determination of the glutamate-glutamine cycling flux using two-compartment dynamic metabolic modeling is sensitive to astroglial dilution. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:108–118. doi: 10.1038/jcbfm.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duarte JM, Gruetter R. Glutamatergic and GABAergic energy metabolism measured in the rat brain by (13) C NMR spectroscopy at 14.1 T. J Neurochem. 2013;126:579–590. doi: 10.1111/jnc.12333. [DOI] [PubMed] [Google Scholar]
- 30.Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–118. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- 31.Renshaw PF, Parow AM, Hirashima F, Ke Y, Moore CM, de Frederick BB, Fava M, Hennen J, Cohen BM. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry. 2001;158:2048–2055. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- 32.Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 33.Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G. Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Molecular Psychiatry. 2010;15:501–511. doi: 10.1038/mp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shungu DC, Weiduschat N, Murrough JW, Mao X, Pillemer S, Dyke JP, Medow MS, Natelson BH, Stewart JM, Mathew SJ. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25:1073–1087. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin JL, Magistretti PJ, Allaman I. Regulation of Neurotrophic Factors and Energy Metabolism by Antidepressants in Astrocytes. Current drug targets. 2013 doi: 10.2174/1389450111314110009. [DOI] [PubMed] [Google Scholar]
- 37.Xiang Y, Shen J. Simultaneous detection of cerebral metabolism of different substrates by in vivo (1)(3)C isotopomer MRS. J Neurosci Methods. 2011;198:8–15. doi: 10.1016/j.jneumeth.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keedwell PA, Drapier D, Surguladze S, Giampietro V, Brammer M, Phillips M. Subgenual cingulate and visual cortex responses to sad faces predict clinical outcome during antidepressant treatment for depression. J Affect Disord. 2010;120:120–125. doi: 10.1016/j.jad.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 39.Shaw A, Brealy J, Richardson H, Muthukumaraswamy SD, Edden RA, John Evans C, Puts NA, Singh KD, Keedwell PA. Marked reductions in visual evoked responses but not gamma-aminobutyric acid concentrations or gamma-band measures in remitted depression. Biol Psychiatry. 2013;73:691–698. doi: 10.1016/j.biopsych.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 40.Furey ML, Drevets WC, Hoffman EM, Frankel E, Speer AM, Zarate CA., Jr Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA psychiatry. 2013;70:280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.