SUMMARY
Background
Non-tuberculous mycobacteria (NTM) are ubiquitous environmental organisms. Cystic fibrosis (CF) patients are susceptible to NTM, but data about NTM in patients with non-CF bronchiectasis are limited.
Methods
We conducted a retrospective, descriptive study at the University of Illinois Medical Center. All patients diagnosed with bronchiectasis (code 494) using the International Classification of Diseases, ninth revision (ICD-9), between 1999 and 2006, were identified. Clinical data including lung function, radiology studies, and presence of NTM in sputum were abstracted for those who met the study criteria.
Results
One hundred eighty-two patients were enrolled in the study. Patients were divided into two groups: bronchiectasis with NTM isolates (n = 68) and bronchiectasis without isolates (n =114), and compared for clinical characteristics and underlying diseases. Mycobacterium avium complex (MAC) was the most common isolate. Fifty-five patients (30%) met the American Thoracic Society criteria for diagnosis of NTM disease. Gram-negative rods were commonly co-isolated. The probability of NTM isolation was significantly higher in elderly female patients (p = 0.04). Moreover, the probability of NTM isolation was significantly higher in the female group with low body mass index (BMI) (p = 0.002).
Conclusions
NTM infections are common in non-CF bronchiectasis. MAC is the most frequently isolated NTM in these patients. There is also great variability in age and sex characteristics for NTM in non-CF bronchiectasis patients. Female patients with a low BMI are a high risk group for NTM infection in non-CF bronchiectasis. Routine screening for NTM is strongly recommended in this patient population.
Keywords: Non-tuberculous mycobacterial diseases, NTM, Non-CF, Bronchiectasis, BMI
1. Introduction
Bronchiectasis represents a significant disease entity that has been historically under-represented in the medical literature.1 Bronchiectasis is a disease of the bronchi and bronchioles involving a vicious circle of transmural infection and inflammation with mediator release. Bronchiectasis in general can manifest in one of two forms: as a local or focal obstructive process of a segment or lobe of a lung, or as a diffuse process involving most of the lungs.2 When extensive, bronchiectasis has the potential to cause devastating illness by predisposing susceptible individuals to recurrent respiratory infections. While the lower respiratory tract is normally sterile, conditions such as bronchiectasis enable colonization of a variety of microbes. Recent advances in molecular technology have led to the unprecedented ability to analyze complex microbial populations, revealing extensive communities of often unculturable or previously unidentified organisms.3,4
Recently, the incidence of non-tuberculous mycobacterial (NTM) infections has been increasingly reported both in the immunocompromised and immunocompetent population. Pulmonary infections with bronchiectasis due to NTM are also increasing in incidence worldwide.5,6 Much of this increase in incidence is likely due to advancements in diagnostic techniques such as high-resolution computed tomography (HRCT). HRCT has proved to be a reliable and non-invasive method for the diagnosis of bronchiectasis. The pattern and distribution of abnormalities revealed by HRCT scanning are influenced by the underlying cause of bronchiectasis.7 Multiple small nodules, along with the occasional appearance of one or more cavities combined with diffuse bronchiectasis are reported to be the typical HRCT findings of NTM pulmonary infection associated with bronchiectasis.8 In patients with characteristic HRCT findings, 34–50% of patients have an active NTM pulmonary infection. The etiologies of NTM infections are also being identified more readily with improvements in laboratory methodology, liquid culture techniques, and more precise molecular tools.5 The most common organism associated with these infections is Mycobacterium avium complex infection.9
Despite these advances in diagnostics, the diagnosis of NTM pulmonary infection is still often delayed because symptoms are mild and excretion of NTM in sputum is intermittent with few colonies retrievable in culture. Many patients therefore require bronchoscopic examination or a lung biopsy for diagnosis of NTM pulmonary disease.10 Because there is limited information about characteristics of dual diseases of NTM and bronchiectasis, this study evaluated a cohort of patients with adult-onset bronchiectasis to determine the prevalence of NTM in this group. Clinical indices were also compared with patients with bronchiectasis who did not grow NTM to determine whether there was any association with disease severity, antibiotic usage, radiographic changes, or microbiology.
2. Methods
The study was performed at the University of Illinois Medical Center. A retrospective chart review was conducted after obtaining approval from the Internal Review Board (IRB). All patients diagnosed with bronchiectasis (code 494) using the International Classification of Diseases, ninth revision (ICD-9), between 1999 and 2006, were identified. Consent was waived because of the retrospective nature of the study. A list of 306 patients with ICD code 494 was generated. Patients were included in the study if they were aged 18 years or older, with confirmed radiological changes suggestive of bronchiectasis on chest X-ray or CT scan of the chest reported by a radiologist, which was independently evaluated by two clinicians (Figure 1). The radiological criteria used to define bronchiectasis included: bronchial dilation (bronchial diameter compared to adjacent to pulmonary artery), peri-bronchial wall thickening, and cylindrical changes. Patients with known cystic fibrosis (CF) were excluded (n = 6). The investigation for CF was performed based on clinical suspicion, particularly in patients with bilateral extensive bronchiectasis, by CF genotyping. Only six patients had CF and were excluded.
Figure 1.
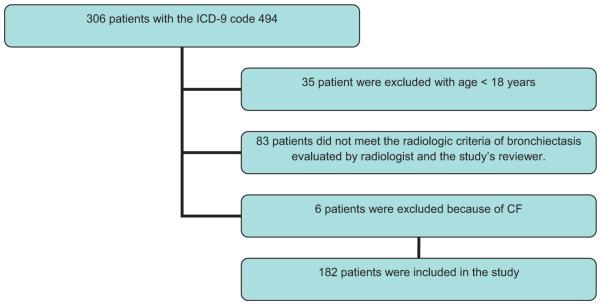
Study flowchart of non-tuberculous mycobacterial disease in patients with non-cystic fibrosis bronchiectasis.
Data regarding microbiology were obtained. All patients had at least two samples of sputum examined. Patients for whom there was a strong clinical suspicion of mycobacterial disease by the clinician, but who had sputum that was negative, underwent bronchoscopy. Only patients with a positive sputum or bronchoalveolar lavage (BAL) that showed growth of mycobacteria were classified as having NTM.
The following data were collected for patients included in this study: (1) demographics including age, sex, race, height, weight, and body mass index (BMI), (2) date of onset of symptoms and the date when the patient was diagnosed with NTM, as well as the method used for the diagnosis, (3) characteristics of the symptoms including cough, sputum production, hemoptysis, dyspnea, and weight loss, and other symptoms if present were recorded, (4) smoking habits including current smoking status and the number of packs smoked, (5) history of recurrent childhood pulmonary infections (≥3 times up to 18 years of age), (6) associated diseases and family history of bronchiectasis, (7) investigations performed to evaluate an etiology including serum immunoglobulins, nasal biopsy for cilia, and other tests, (8) pulmonary function tests, (9) radiology findings, including if the changes were localized or bilateral, (10) sputum microbiology results, (11) bronchoscopy results if a patient had undergone one, (12) therapies that the patient was receiving including antibiotic usage, prednisone courses, clearance devices, and hospitalization record. The interval between the worsening of pulmonary symptoms as recorded from the patient history and the diagnosis of NTM infection was characterized as a delay in diagnosis. This study was reviewed and approved by the ethics committees of the University of Illinois at Chicago with an approval number 2006-0742.
2.1. Statistical analysis
Categorical variables were described as counts and percentages or examined as predictors using the odds ratio (OR) and were tested by the Chi-square test, or, if applicable, Fisher’s exact test. Univariate analysis was used to compare differences in demographic and clinical variables between bronchiectasis patients from whom NTM was isolated and non-NTM patients. Comparisons were unpaired, and all tests of significance were two-tailed. Continuous variables were compared using the Student’s t-test for normally distributed variables and the Wilcoxon rank-sum test for non-normally distributed variables. In order to examine risk factors for NTM isolation, a stepwise logistic regression model was used. The outcome variable was isolation of NTM from respiratory secretions from sputum or BAL and other variables listed in Table 1 that were considered statistically significant upon univariate analysis (p < 0.10) or considered clinically relevant.
Table 1.
Characteristics of the 182 patients enrolled in the studya
| Variable | NTM patient, n (%) |
Non-NTM patient, n (%) |
p-Value |
|---|---|---|---|
| Age >65 years | 45 (66) | 23 (46) | 0.0150 |
| Male | 11 (16) | 30 (34) | 0.0120 |
| Diagnosis delay >12 months | 29 (45) | 34 (42) | 0.6870 |
| Diagnosis delay >24 months | 34 (53) | 46 (56) | 0.6600 |
| BMI >30 kg/m2 | 3 (11.5) | 34 (33) | 0.4000 |
| Sputum production | 48 (72) | 56 (65) | 0.3530 |
| Hemoptysis | 20 (30) | 17 (20) | 0.1510 |
| Cough | 65 (97) | 75 (87) | 0.0470 |
| Dyspnea | 34 (51) | 40 (47) | 0.6030 |
| Chest pain | 2 (3) | 4 (5) | 0.4720a |
| Cancer | 9 (17) | 8 (10) | 0.2160 |
| GERD | 7 (14) | 6 (7) | 0.2580 |
| COPD | 4 (8) | 8 (10) | 0.6690a |
| DM | 1 (2) | 7 (9) | 0.1520a |
| Current smoker | 3 (4) | 12 (14) | 0.3090a |
| Childhood pulmonary infection | 24 (36.4) | 23 (27) | 0.2220 |
| Pneumonia | 22 (40) | 23 (35.9) | 0.6490 |
| Family history of bronchiectasis | 3 (4.4) | 1 (1.1) | 0.3200a |
| PFT obstructive defect | 7 (39) | 34 (51) | 0.3740 |
| PFT restrictive defect | 4 (27) | 23 (39) | 0.5500a |
| Bronchiectasis seen in CXR | 22 (33) | 22 (26) | 0.3490 |
| IV antibiotic therapy | 4 (6) | 16 (18) | 0.0290a |
| Clearance device | 31 (46) | 11 (12) | <0.0001 |
| Flutter valve used | 29 (43) | 2 (2.3) | <0.0001a |
| Mortality | 1 (2) | 5 (6) | 0.1610a |
NTM, non-tuberculous mycobacteria; BMI, body mass index; GERD, gastroesophageal reflux disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PFT, pulmonary function tests; CXR, chest X-ray; IV, intravenous.
Data are number (%) of patients.
bFisher’s exact test.
Results were reported as adjusted ORs with 95% confidence intervals (CI). The goodness-of-fit of the final model was examined by the Hosmer–Lemeshow test. A non-significant p-value implied that the model adequately fits the data. The Kaplan–Meier estimate curve was used to determine the cumulative probability of NTM isolation for age and BMI for each gender; curves obtained for the two groups were compared using the log-rank test.
All data analyses were performed using SPSS for Windows, version 17.0; two-tailed p values were used and p-values <0.05 were considered to be statistically significant.
3. Results
One hundred eighty-two patients diagnosed with non-CF bronchiectasis were included. Table 1 shows the demographic, clinical, and outcome characteristics of the study population.
The mean (standard deviation (SD)) age of patients with a diagnosis of bronchiectasis was 64.5 (15.1) years. Of the 182 patients who were identified with a diagnosis of bronchiectasis, mycobacterial culture was performed for 137 (75%) based on symptoms compatible with mycobacterial diseases or a clinical suspicion. NTM was isolated from sputum or BAL in 68 (37%) patients. Mycobacterium avium complex (MAC) was isolated from 55 (81%), Mycobacterium chelonae from five (7%), Mycobacterium kansasii from two (3%), and other NTM were isolated from six (9%) patients. The diagnosis of bronchiectasis and isolation of NTM was significantly higher in female patients. This was further confirmed by logistic regression analysis (Table 2). Patients in whom NTM was isolated also had medical histories significant for previous childhood infections (Table 2).
Table 2.
Logistic regression analysis results for patients with bronchiectasis with and without NTMa
| Variables | p-Value | OR (95% CI) |
|---|---|---|
| Age >65 years | 0.191 | 0.51 (0.186–1.399) |
| Female gender | 0.051 | 3.828 (0.997–14.704) |
| Recurrent childhood pulmonary infections |
0.087 | 0.426 (0.160–1.132) |
| BMI | 0.008 | 0.876 (0.795–0.966) |
| COPD | 0.487 | 9.584 (0.128–2.662) |
| GERD | 0.932 | 0.935 (0.195–4.478) |
NTM, non-tuberculous mycobacteria; OR, odds ratio; CI, confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease.
Adjusted risk of NTM isolation in patients with non-cystic fibrosis bronchiectasis by: age >65 years, male gender, recurrent childhood pulmonary infections, BMI, COPD, gastroesophageal reflux disease (GERD), p > 0.05 by Hosmer– Lemeshow goodness-of-fit test.
The mean (SD) of diagnosis delay per month was 44.95 (56.2). Gram-negative rods (Enterobacteriaceae group and Pseudomonas aeruginosa bacilli) were isolated from 22 patients (14%), of whom 17 (77%) had never been co-isolated NTM (p = 0.031). Forty-one (23%) patients were hospitalized secondary to complications from bronchiectasis, seven (10%) of whom were those with NTM (p = 0.002). Table 2 shows the multivariate model that accounted for the possible confounding effect of several factors, for patients with bronchiectasis with and without NTM.
Our univariate analysis suggests that patients with bronchiectasis and NTM complained of cough as a major symptom, suggesting that the presence of NTM contributed to a worsening of their symptoms. Interestingly, there was no significant difference in the lung function characteristics of patients with bronchiectasis with and without NTM. There was also no significant difference in the presence of comorbidities such as chronic obstructive pulmonary disease (COPD), gastroesophageal reflux disease (GERD), diabetes mellitus (DM), cancer, or HIV in patients with bronchiectasis with or without NTM. The univariate analysis suggested that patients with bronchiectasis and NTM used clearance devices more frequently, although there was no difference in the rate of usage of antibiotics for respiratory infections. Overall, seven patients with bronchiectasis during this period underwent surgery, two of whom had NTM isolated and five patients were from the non-NTM group. The surgical resection was for hemoptysis in three patients and localized disease with continuing symptoms in the remaining four. Five patients underwent lobectomies, one had a pneumonectomy, and one had a cavernostomy.
The American Thoracic Society (ATS) criteria for the diagnosis of NTM disease were met by 55 (30%) patients. Of the patients from whom NTM was isolated, 80% met the ATS criteria for NTM disease. Table 3 depicts the data for patients who were culture-positive for NTM with and without disease.
Table 3.
Characteristics of the patients culture-positive for an NTM with and without disease, based on ATSa
| Variable | NTM disease, n (%) |
Non-NTM disease, n (%) |
p-Value |
|---|---|---|---|
| Age >65 years | 26 (65) | 19 (68) | 0.806 |
| Female | 38 (95) | 19 (68) | 0.008 |
| Diagnosis delay >12 months | 19 (53) | 16 (57) | 0.728 |
| Diagnosis delay >24 months | 18 (50) | 16 (57) | 0.570 |
| BMI >30 kg/m2 | 3 (10) | 0 (0) | 1.000a |
| Sputum production | 26 (67) | 22 (79) | 0.290 |
| Hemoptysis | 12 (31) | 8 (29) | 0.846 |
| Cough | 39 (100) | 26 (93) | 0.900 |
| Dyspnea | 23 (59) | 11 (39) | 0.114 |
| Chest pain | 1 (3) | 1 (4) | 1.000a |
| Cancer | 6 (18) | 3 (16) | 1.000a |
| GERD | 4 (12) | 3 (16) | 0.697a |
| COPD | 3 (9) | 1 (5) | 1.000a |
| DM | 0 (0) | 1 (5) | 0.373a |
| Current smoker | 2 (4) | 1 (4) | 1.000a |
| Childhood pulmonary infection | 15 (39) | 9 (33) | 0.670 |
| Pneumonia | 14 (41) | 8 (38) | 0.821 |
| Family history of bronchiectasis | 3 (8) | 0 (0) | 0.263a |
| PFT obstructive defect | 5 (42) | 2 (33) | 1.000a |
| PFT restrictive defect | 1 (13) | 3 (43) | 0.282a |
| Bronchiectasis seen in CXR | 10 (45) | 12 (44) | 0.100 |
| IV antibiotic therapy | 1 (3) | 3 (11) | 0.298a |
| Clearance device | 20 (50) | 9 (32) | 0.175 |
| Flutter valve used | 29 (43) | 2 (2.3) | 0.146 |
| Mortality | 0 (0) | 1 (4) | 0.406a |
NTM, non-tuberculous mycobacteria; ATS, American Thoracic Society; BMI, body mass index; GERD, gastroesophageal reflux disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; PFT, pulmonary function testing; CXR, chest X-ray; IV, intravenous.
Data are number (%) of patients.
b Fisher’s exact test.
The probability of NTM isolation was significantly higher in elderly female patients with non-CF bronchiectasis (p = 0.04), as shown in Figure 2. Moreover, the probability of NTM isolation was significantly higher in the female group with low BMI (p = 0.002), as shown in Figure 3.
Figure 2.
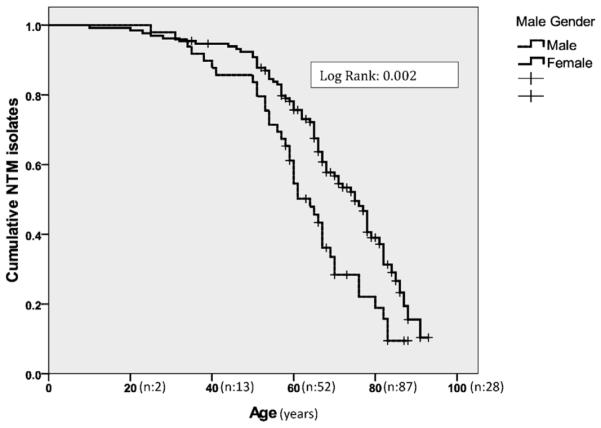
The Kaplan–Meier estimate curve for the cumulative probability of NTM isolation according to age and sex in patients with non-cystic fibrosis bronchiectasis.
Figure 3.
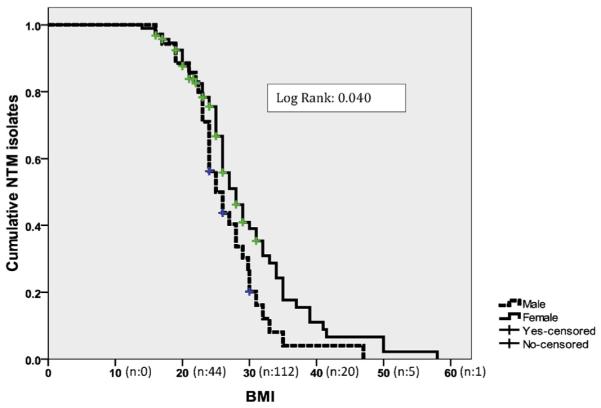
The Kaplan–Meier estimate curve for the cumulative probability of NTM isolation according to body mass index (BMI) and sex in patients with non-cystic fibrosis bronchiectasis.
4. Discussion
This study indicates that NTM is common in non-CF bronchiectasis patients. The frequency of NTM in our bronchiectasis population was 37%, and 30% of these patients met the ATS criteria for NTM disease. NTM is more frequently isolated from elderly female patients (age >65 years). In addition, our data suggest that childhood pulmonary infection is a significant risk factor for acquiring NTM later in life. Furthermore, MAC is the most common isolate (88%) of NTM in patients with bronchiectasis.
NTM have emerged over the past two decades as important pathogens in patients with chronic lung diseases, including patients with CF and patients with non-CF bronchiectasis. The exact prevalence of NTM disease in patients with non-CF bronchiectasis remains to be determined. It is also unclear if the presence of NTM contributes to the pathogenesis of the underlying disease process in patients with non-CF bronchiectasis. Previous studies have shown that NTM are isolated mainly from female patients, although the reasons for this increased susceptibility of elderly women to NTM is unclear and needs further investigation.5,11 Tanaka and colleagues, reported that 34–50% of bronchiectasis patients have active NTM pulmonary infection, especially MAC infection.9 Wickremasinghe and researchers also found MAC to be the predominant NTM species isolated in 72% of sputum samples obtained from their study population of patients with non-CF bronchiectasis.11 These results are consistent with those of our study in which MAC was the leading pathogen isolated from samples obtained from patients with non-CF bronchiectasis. It is likely that structural changes in the airways that cause alterations in mucociliary clearance with an increase in mucus production predispose patients with bronchiectasis to NTM colonization and subsequent infection.13,14 However, it is also plausible that primary alteration in the immune response in patients with non-CF bronchiectasis may also play a pivotal role in the development of NTM infections.15
Although the predisposition of elderly women to disease due to MAC has been described, our data suggest that male gender may partially protect from the acquisition of NTM.12 Gender differences in the predisposition to NTM have previously been noted, however the mechanisms of the causality are speculative. A study by Han and colleagues showed that MAC infections in general are rare in women younger than 50 years, and as the number of postmenopausal years increases, the prevalence rate of MAC increases as well. As a result, with menopausal status playing a role in MAC infection, Han and colleagues hypothesized that estrogen has a protective effect on MAC.16 There are some additional supporting data for this speculation. The serum levels of macrophage colony-stimulating factor fall during the first 10 years of menopause, which is restored with hormone replacement.17 In addition, it has been shown that estrogen protects mice from MAC infection, mainly through augmenting macrophage functions.18 In contrast, Yamamoto et al. showed that testosterone increases susceptibility to NTM in mice.19 Cooper et al. showed that the use of anabolic steroids is associated with an increase in mycobacterial infections.20 Therefore, it appears that there might be other reasons to justify the gender difference in NTM diseases other than sexual hormones. It is plausible that a genetic resistance in males may play a role. Nonetheless, further studies to define the mechanisms that are responsible for these differences are clearly needed.
The Kaplan–Meier curve showed that the probability of NTM isolation was significantly higher in patients with a low BMI. Our study results are in agreement with those of Kim and colleagues who found that patients with NTM infections have a distinct body morphology.21 Ziedalski and colleagues also reported low BMI values in their patients with pulmonary NTM infections.22
The results of our study showed that there is a long NTM diagnostic delay in bronchiectasis patients. We speculate that the absence of a pathognomonic clinical picture, coupled with variable radiologic presentation of findings of NTM disease in the presence of bronchiectasis, can be misleading clinicians, which can delay diagnosis and treatment. Previous studies have reported that patients who have a delay in diagnosis of NTM have a higher rate of recurrence.23 Because of the retrospective design of our study, there is a recall bias in determining the accurate diagnostic delay. Future studies with larger numbers of patients are needed to better evaluate the extent of the association of bronchiectasis with NTM disease, the delay in diagnosis, and to identify associated risk factors.
Our study suggests that a significant number of patients with non-CF bronchiectasis were positive for the isolation of atypical mycobacteria from their sputum. However, further studies are needed to establish whether the presence of NTM infection has an impact on the clinical outcomes of patients with bronchiectasis. The relatively small cohort size of the present study did not allow for this analysis. This study was unable to show any possible association between NTM infection and DM, probably due to the sample size. Therefore, understanding the relationship between NTM infection and DM will require further studies.
This study has several other limitations that should be noted. First, the study population was entered from a single tertiary referral center and, therefore, had multiple risk factors for NTM infection. Secondly, data regarding the use of macrolide therapy were not collected in our study; patients without NTM may have received macrolide therapy which may have led to partial treatment and misclassification. Third, the investigation to diagnose CF was not performed in all patients and there was no documentation of a family history of CF. Therefore patients with heterozygotic status for CF as non-CF may have been misclassified.
In summary, this study shows that NTM is commonly isolated from patients with non-CF bronchiectasis. Additionally MAC appears to be the most common pulmonary NTM in this group. This study also shows that elderly females, particularly those with low BMIs, are at a higher risk for acquiring NTM diseases. A better understanding of the role of NTM in the outcomes of patients with bronchiectasis may generate new therapeutic modalities for patients with severe disease. Furthermore, during the initial assessment of patients with bronchiectasis, physicians might need to consider sputum culture, and possibly bronchoscopy, if the pulmonary imaging study is suggestive of NTM, as a predictive factor for clinical outcomes.
Acknowledgement
The authors thank Mary Beth Allen PhD(c) for editorial assistance.
Footnotes
Ethical approval: The study was approved by the UIC Ethics Committee.
Conflict of interest: None.
References
- 1.Smith MP. Non-cystic fibrosis bronchiectasis. J R Coll Physicians Edinb. 2011;41:132–9. doi: 10.4997/JRCPE.2011.217. quiz 9. [DOI] [PubMed] [Google Scholar]
- 2.Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–93. doi: 10.1056/NEJMra012519. [DOI] [PubMed] [Google Scholar]
- 3.Botterel F, Cabaret O, Foulet F, Cordonnier C, Costa JM, Bretagne S. Clinical significance of quantifying Pneumocystis jirovecii DNA by using real-time PCR in bronchoalveolar lavage fluid from immunocompromised patients. J Clin Microbiol. 2012;50:227–31. doi: 10.1128/JCM.06036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat N, O’Brien KL, Karron RA, Driscoll AJ, Murdoch DR. Use and evaluation of molecular diagnostics for pneumonia etiology studies. Clin Infect Dis. 2012;54(Suppl 2):S153–8. doi: 10.1093/cid/cir1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 6.Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997;156:S1–25. doi: 10.1164/ajrccm.156.2.atsstatement. [DOI] [PubMed] [Google Scholar]
- 7.Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004;231:880–6. doi: 10.1148/radiol.2313030833. [DOI] [PubMed] [Google Scholar]
- 8.Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005;235:282–8. doi: 10.1148/radiol.2351040371. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka E, Amitani R, Niimi A, Suzuki K, Murayama T, Kuze F. Yield of computed tomography and bronchoscopy for the diagnosis of Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1997;155:2041–6. doi: 10.1164/ajrccm.155.6.9196113. [DOI] [PubMed] [Google Scholar]
- 10.Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium-intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999;115:1033–40. doi: 10.1378/chest.115.4.1033. [DOI] [PubMed] [Google Scholar]
- 11.Wickremasinghe M, Ozerovitch LJ, Davies G, Wodehouse T, Chadwick MV, Abdallah S, et al. Non-tuberculous mycobacteria in patients with bronchiectasis. Thorax. 2005;60:1045–51. doi: 10.1136/thx.2005.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh WJ, Kwon OJ. Bronchiectasis and non-tuberculous mycobacterial pulmonary infection. Thorax. 2006;61:458. author reply. [PMC free article] [PubMed] [Google Scholar]
- 13.Middleton AM, Chadwick MV, Nicholson AG, Wilson R, Thornton DJ, Kirkham S, et al. Interaction between mycobacteria and mucus on a human respiratory tissue organ culture model with an air interface. Exp Lung Res. 2004;30:17–29. doi: 10.1080/01902140490252876. [DOI] [PubMed] [Google Scholar]
- 14.Middleton AM, Chadwick MV, Nicholson AG, Dewar A, Feldman C, Wilson R. Investigation of mycobacterial colonisation and invasion of the respiratory mucosa. Thorax. 2003;58:246–51. doi: 10.1136/thorax.58.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuschillo S, De Felice A, Balzano G. Mucosal inflammation in idiopathic bronchiectasis: cellular and molecular mechanisms. Eur Respir J. 2008;31:396–406. doi: 10.1183/09031936.00069007. [DOI] [PubMed] [Google Scholar]
- 16.Han XY, Tarrand JJ, Infante R, Jacobson KL, Truong M. Clinical significance and epidemiologic analyses of Mycobacterium avium and Mycobacterium intracellulare among patients without AIDS. J Clin Microbiol. 2005;43:4407–12. doi: 10.1128/JCM.43.9.4407-4412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamada M, Irahara M, Maegawa M, Ohmoto Y, Takeji T, Yasui T, et al. Postmenopausal changes in serum cytokine levels and hormone replacement therapy. Am J Obstet Gynecol. 2001;184:309–14. doi: 10.1067/mob.2001.109940. [DOI] [PubMed] [Google Scholar]
- 18.Tsuyuguchi K, Suzuki K, Matsumoto H, Tanaka E, Amitani R, Kuze F. Effect of estrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol. 2001;123:428–34. doi: 10.1046/j.1365-2249.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Saito H, Setogawa T, Tomioka H. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect Immun. 1991;59:4089–96. doi: 10.1128/iai.59.11.4089-4096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper RG, Kirk JW, Bielenberg P. Mycobacterial infection associated with the use of an anabolic steroid. Med J Aust. 1993;159:216. doi: 10.5694/j.1326-5377.1993.tb137813.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–74. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziedalski TM, Kao PN, Henig NR, Jacobs SS, Ruoss SJ. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest. 2006;130:995–1002. doi: 10.1378/chest.130.4.995. [DOI] [PubMed] [Google Scholar]
- 23.Henry MT, Inamdar L, O’Riordain D, Schweiger M, Watson JP. Nontuberculous mycobacteria in non-HIV patients: epidemiology, treatment and response. Eur Respir J. 2004;23:741–6. doi: 10.1183/09031936.04.00114004. [DOI] [PubMed] [Google Scholar]


