Abstract
Rationale
Previous studies have demonstrated that several N-substituted 4′,4″-diF-benztropine (BZT) analogs with high dopamine transporter affinity selectively decreased cocaine self-administration without affecting food-maintained behavior in rats.
Objectives
The present study examined if the decreases in cocaine self-administration are due to competition from excess behavioral activity (hyperlocomotion or stereotypy) induced by the BZT analogs alone or in combination with cocaine.
Results
Pretreatments with the typical dopamine uptake inhibitor methylphenidate (1.0, 3.2 and 10 mg/kg, i.p.) dose-dependently shifted the cocaine self-administration dose-effect curve (0, 0.032, 0.1, 0.32 1.0 mg/kg/injection) leftward. The shift in the dose-effect curve was obtained at doses of methylphenidate that, when administered alone, also decreased food-maintained behavior and increased locomotor activity and stereotypy. In contrast, the N-substituted BZT analogs, JHW 007 (1.0, 3.2 and 10 mg/kg, i.p.), AHN 1-055 (10 mg/kg), and, AHN 2-005 (10 mg/kg), as previously reported, decreased the maximum for the cocaine self-administration dose-effect curve, and did so at doses that were virtually without effects on food-maintained behavior. Further, the BZT analogs alone had minimal effects on locomotor activity and stereotypies and did not appreciably change the effects of cocaine on these measures when administered in combination with cocaine.
Conclusions
The present results suggest that the decrease in cocaine self-administration produced by the N-substituted BZT analogs is due to an antagonism of the reinforcing effects of cocaine rather than due to interference from competing behavioral overstimulation, and further supports the development of N-substituted BZT analogs as medications to treat cocaine abuse.
Keywords: cocaine, benztropine, self-administration, stereotyped behavior, locomotor activity, dopamine transporter, AHN 1-055, AHN 2-005, JHW 007, methylphenidate
INTRODUCTION
Previous reports from our laboratory have suggested an antagonism of the effects of cocaine by several N-substituted 4′,4″-difluoro-benztropine (BZT) analogs in rodents (Tanda et al. 2009b). For example, oral pretreatments with the N-methyl- (AHN 1-055), N-allyl- (AHN 2-005), and N-butyl-4′,4″-difluoro-BZT (JHW 007) all dose-dependently decreased responding maintained by intravenous injections of a wide range of cocaine doses in rats trained to self-administer cocaine (Hiranita et al. 2009). Each of the BZT analogs produced a dose-related decrease in the maximal self-administration of cocaine and at their highest doses self-administration was not greater than that maintained by vehicle (Hiranita et al. 2009). Further, these effects of the BZT analogs were relatively selective, as food-maintained responding under a similar schedule of reinforcement was not appreciably affected by the doses that decreased cocaine self-administration (Hiranita et al. 2009). Further, responding maintained by intravenous injections of a wide range of doses of those N-substituted BZT analogs was not maintained above vehicle levels when tested in rats trained to self-administer cocaine (Hiranita et al. 2009).
Consistent with results from the drug self-administration procedure, “cocaine antagonist” effects of the BZT analogs were observed in several other procedures that assess in vivo effects often related to drug abuse (Tanda et al. 2009b), such as locomotor activity (Desai et al. 2005; Katz et al. 2004; Velazquez-Sanchez et al. 2009; Velazquez-Sanchez et al. 2010), place conditioning (Li et al. 2005), and in vivo microdialysis (Tanda et al. 2009a). Interestingly, the BZT analogs are highly selective inhibitors of the dopamine transporter (e. g. Katz et al. 2004; Newman et al. 1995), a primary target of the reinforcing effects of cocaine (Ritz et al. 1987). However, most known compounds with affinity for the dopamine transporter, such as WIN 35,428, methylphenidate and nomifensine, have abuse liability like that of cocaine (Ritz et al. 1987), and shift the cocaine self-administration dose-effect curve leftward when administered before sessions of self-administration (Barrett et al. 2004; Hiranita et al. 2011; Hiranita et al. 2009; Schenk 2002). Additionally, typical dopamine uptake inhibitors produce cocaine-like behavioral and neurochemical effects in diverse procedures (Katz et al. 2004; 2003; Li et al. 2006; Tanda et al. 2009a). The cocaine-like effects of the standard dopamine uptake inhibitors are markedly different from “cocaine antagonist” effects of the BZT analogs.
The conclusion that the BZT analogs selectively decreased the self-administration of cocaine followed from the observation that food-maintained responding in one group of subjects was not appreciably affected by doses of the BZT analogs that decreased cocaine self-administration in a second group of subjects (Hiranita et al. 2009). However, given the affinity of the BZT analogs for the dopamine transporter, the primary target involved in many of the behavioral effects of cocaine (Tanda et al. 2009b), it is possible that these compounds induce locomotor activation or stereotypic behaviors either alone or in combination with cocaine. The induction of locomotor activity or stereotypy if excessive could interfere with continued cocaine self-administration. In a previous study, intraperitoneal injections of AHN 1-055 at a selected dose of 10 mg/kg produced a small increase in locomotor activity and stereotypy compared to vehicle injection, with effects less than those produced by cocaine. When combined with a selected intraperitoneal dose of 15 mg/kg of cocaine, however, the selected dose of AHN 1-055 reduced the effects of cocaine (Velazquez-Sanchez et al. 2009). The lack of additive or synergistic effects of the combination was not due to cocaine itself producing increases to the maximum as d-amphetamine further increased the effects of cocaine (Velazquez-Sanchez et al. 2009). Thus AHN 1-055 has some capacity to induce locomotor activity (see also Katz et al. 1997) and stereotypy. However, absent comparisons among doses, it is currently unclear whether the doses that decrease cocaine self-administration are those that induce locomotor activity and stereotypy. Thus the present study examined the effects of several N-substituted BZT analogs for their effectiveness as inducers of locomotor activity and stereotyped behavior both alone and in combination with cocaine and compared the effective doses with those that decrease cocaine self-administration.
METHODS
Subjects
Male Sprague–Dawley rats (Taconic Farms, Germantown, NY) served as subjects. Animals weighing approximately 300 g were initially acclimated for at least one week to the animal vivarium which was temperature- and humidity-controlled, and had a 12-h light/dark cycle (lights on 07:00 h). Food and water were available at all times except during the experimental tests. Rats in studies of cocaine self-administration were maintained at approximately 320 g throughout the course of the two month study by adjusting their daily food ration. Care of the subjects was in accordance with the guidelines of the National Institutes of Health and the National Institute on Drug Abuse Intramural Research Program Animal Care and Use Program, which is fully accredited by AAALAC International.
Drug Self-administration and Food-Reinforced Responding
Experimental sessions were conducted at the same time daily, seven days per week, during the light phase of the light/dark cycle. Subjects were placed in operant-conditioning chambers (modified ENV-008CT, Med Associates, St. Albans, VT) that measured 25.5 cm x 32.0 cm x 25.0 cm. The chambers were enclosed within sound-attenuating cubicles that were equipped with a fan for ventilation and white noise to mask extraneous sounds. On the front wall of each chamber were two response levers, 5.0 cm from the midline and 4.0 cm above the grid floor. A downward displacement of either lever with a force approximating 20 g defined a response, which activated a relay mounted behind the front wall of the chamber producing an audible “feedback” click. Three light-emitting diodes (LEDs) were located in a row above each lever. A receptacle for the delivery of food pellets was mounted behind a 5.0 × 5.0 cm opening in the front wall 2.0 cm above the floor and midway between the two levers. A pellet dispenser (ENV-203, Med Associates) mounted behind the front wall when activated delivered 20-mg food pellets (BIOSERV, Frenchtown, NJ) to the receptacle. A syringe driver (Model 22, Harvard Apparatus, Holliston, MA) placed above each chamber delivered injections of specified volumes and durations from 10 ml syringes. Syringes were connected by Tygon tubing to a single-channel fluid swivel (375 Series Single Channel Swivels, Plymouth Meeting, PA) which was mounted on a balance arm above the chamber. Tygon tubing from the swivel to the subject’s catheter was protected by a surrounding metal spring and completed the connection to the subject.
Details of the training of subjects have been presented elsewhere (Hiranita et al. 2010). Initially subjects were trained to press the right lever under a fixed-ratio (FR) 5-response schedule of food reinforcement (each fifth response produced a 20-mg food pellet). One group of subjects continued with food reinforcement under contingencies described below. The other group was surgically prepared with jugular catheters and trained to self-administer cocaine. Catheter implantation was performed under anesthesia (ketamine 60.0 mg/kg, i.p. and xylazine 12.0 mg/kg, i.p.). Catheters were surgically implanted in the right or left external jugular vein, exited at the mid-scapular region of the subject’s back, and were infused (0.1 ml of a sterile saline solution containing heparin (30.0 IU/ml), penicillin G potassium (250,000 IU/ml)) daily throughout the study to minimize the likelihood of infection or clot and fibroid formation. All animals were allowed to recover from surgery for approximately one week before studies resumed.
Sessions were approximately 2 hr, and were comprised of five sequential 20-min components each preceded by a 2-min “time out” (TO) during which lights were out and responses had no scheduled consequences. This arrangement allowed the assessment of a different cocaine dose within each component or a different amount of food. Cocaine dose per injection was incremented in the five sequential components by adjusting infusion volumes and durations in the following order: no injection (also referred to as extinction, or EXT, because completion of the FR 5 had no scheduled consequences other than turning off the LEDs for 20 sec), 0.03, 0.10, 0.32, and 1.0 mg/kg/inj. Infusion volumes and durations were respectively 0, 5.6, 18.0, 56.0, 180 μl and 0, 0.32, 1.0, 3.2 10.0 sec, based on a body weight of 0.32 kg. A response-independent injection of cocaine at the corresponding dose was administered at the start of each component.
Training continued until response rates and patterns were stable from one session to the next. Cocaine self-administration sessions continued until: 1) at least 5.0 mg/kg of cocaine was self-administered within a session with less than 20% variation in the total number of cocaine injections compared to the previous session; 2) the dose of cocaine that maintained maximal rates of responding varied by no more than one-half log unit over two consecutive test sessions; and 3) maximum rates of responding were at least five-fold higher than rates of responding maintained during EXT. For the subjects on food reinforcement, daily rations of food (~35 g of 1-gram chocolate-flavored pellet, Bio-Serv) were given 150 min before sessions, so that their response rates approximated those maintained by cocaine.
Once performances were stable from one session to the next the effects of pre-session i.p. injections of saline, methylphenidate, AHN 1-055, AHN 2-005 and JHW 007 on the rates of responding maintained by cocaine injection or food presentation were compared. Pretreatments with either saline or methylphenidate (1.0–10 mg/kg) were administered five-min before, AHN 1-055 (10 mg/kg) or AHN 2-005 (10 mg/kg) 30-min before, and JHW 007 (1-10 mg/kg) 120-min before sessions. These assessments were separated by a minimum of 72 hours, and were conducted only if performances met the training criteria for two consecutive sessions. All of the assessments were conducted with a mixed order of drugs and doses.
Response rates were determined by dividing responses by elapsed times in each component, excluding the TO periods that followed injections or food presentations. Average values across six subjects (with standard error of the mean) are presented below. Data were further analyzed using one- or two-way repeated-measures analysis of variance (ANOVA) as appropriate with Bonferroni post-hoc t-tests.
Locomotor activity and stereotyped behavior
Locomotor activity and stereotyped behavior were assessed in 40- X 40-cm clear acrylic chambers. The acrylic chambers were placed inside monitors (Accuscan Instruments, Inc., Columbus, Ohio, USA), which were equipped with light-sensitive detectors spaced 2.5 cm apart along two perpendicular walls 30 cm above the floor. Mounted on the opposing walls were infrared light sources that were directed at the detectors. One count of horizontal activity was registered each time the subject interrupted a single beam.
Subjects were allowed to habituate for 60 min before injections of either cocaine (1.0–56 mg/kg) or saline, after which subjects were placed in the clear acrylic chambers for assessment of locomotor activity. Pretreatments with either saline or methylphenidate (1.0–30 mg/kg) were administered five-min before, AHN 1-055 (3.0 and 10 mg/kg) or AHN 2-005 (3.0 and 10 mg/kg) 30-min before, and JHW 007 (10 and 17 mg/kg) 120-min before the start of the habituation period, with the subjects spending the interim in their home cages. Each dose or dose combination was studied in six subjects, and subjects were used only once.
Locomotor activity was recorded automatically and continuously over the 60 min following cocaine (or corresponding saline) injections. Locomotor activity was recorded as total beam breaks representing horizontal movement. Stereotyped behavior was recorded by visual observation of each subject for 60 min immediately after the habituation period using a time-sampling procedure. Behavior of each subject was observed by trained observers (L.-B.L. and a research assistant), who were blind to treatments. Observations were made for 1 min, every 10 min over the course of 60 min (i.e., each subject was observed six times at 10-min intervals), with instances of specific stereotyped behaviors recorded as present or absent.
Based on the frequencies of individual behaviors recorded, each subject was rated as belonging within one of the categories of the scale originally described by Kalivas et al. (1988). Each subject was given a single cumulative score on a scale from 1 to 7.5 as follows: 1) asleep or still; 2) grooming (any kind of grooming, scratching, or licking for more than 3 consecutive seconds); 3) locomotion (move more than half of their body length during 10 sec), rearing (both forepaws off from the cage floor) or sniffing (more than 3 consecutive seconds); 4) any combination of two: locomotion, rearing, or sniffing; 7.5) head bobbing. The effects of cocaine were most pronounced within the first 30 min after injection and only those data are presented. Data for activity counts and stereotypy scores were analyzed using one- or two-way measures ANOVA as appropriate with Bonferroni post-hoc t-tests.
Drugs
The drugs used in the present study were as follows: (-)-cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO), methylphenidate (National Institute of Drug Abuse), AHN 1-055, AHN 2-005, and JHW 007 (the latter three being N-substituted benztropine analogs synthesized in the Medicinal Chemistry Section of National Institute on Drug Abuse Intramural Research Program according to procedures previously published: Agoston et al. 1997; Newman et al. 1995). All drug solutions were prepared fresh daily in 0.9% NaCl, with heat and sonication, as necessary. Pretreatment times and doses of drugs used in the present study were chosen based on published reports (Katz et al. 2004) or preliminary data obtained in this laboratory.
RESULTS
Cocaine Self-Administration
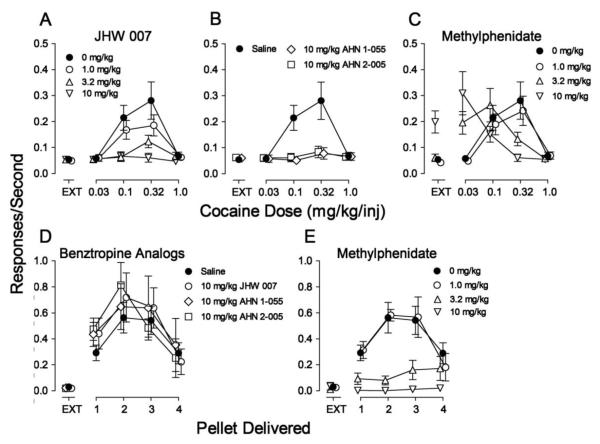
The average response rates maintained by cocaine were a bitonic function of dose (Fig. 1A, filled circles), with a maximum of 0.28 ± 0.07 responses/sec during the fourth component (0.32 mg/kg/inj), which was approximately seven-fold greater than the 0.05 responses/sec occurring during EXT (the first component, Fig. 1A, filled circle above EXT). One-way repeated measures ANOVA indicated a significant effect of component on response rate (F4, 20=11.3, p<0.001).
Figure 1.
Effects of pre-session treatments with the N-substituted BZT analogs (JHW 007, AHN 1-055 and AHN 2-005) and the typical dopamine-uptake inhibitor methylphenidate on responding maintained in rats by a range of doses of cocaine or number of food pellets delivered. Saline (5 min), methylphenidate (5 min), JHW 007 (120 min), AHN 1-055 (30 min), and AHN 2-005 (30 min) were administered intraperitoneally at the designated times before sessions. Each point represents the mean ± SEM (N=6). Panels A-C. Effects on cocaine self-administration. Ordinates: Responses/sec. Abscissae: unit dose of cocaine injection (in mg/kg/inj) or EXT (extinction-no injection). Panel A. JHW 007 treatment: 0 mg/kg (filled circles, saline injections), 1.0 mg/kg (open circles), 3.2 mg/kg (open triangles up), and 10 mg/kg (open triangles down). Panel B. AHN 1-055 and AHN 2-005 treatments: Saline (filled circles), 10 mg/kg of AHN 1-055 (open diamonds), 10 mg/kg of AHN 2-005 (open squares). Panel C. Methylphenidate treatment: 0 mg/kg (filled circles, saline injections), 1.0 mg/kg (open circles), 3.2 mg/kg (open triangles up), and 10 mg/kg (open triangles down). Panels D and E. Effects on food presentation. Ordinates: Responses/sec. Abscissae: number of pellets delivered. Panel D. N-substituted BZT analogs: Saline (filled circles), 10 mg/kg of JHW 007 (open circles), 10 mg/kg of AHN 1-055 (open diamonds), 10 mg/kg of AHN 2-005 (open squares). Panel E. Methylphenidate: 0 mg/kg (filled circles, saline injections), 1.0 mg/kg (open circles), 3.2 mg/kg (open triangles up), and 10 mg/kg (open triangles down).
Pretreatment with JHW 007 dose-dependently decreased response rates maintained by cocaine (Fig. 1A). The decrease in cocaine self-administration was expressed as a flattening of the cocaine dose-effect curve. At the highest dose of JHW 007, no dose of cocaine maintained responding at levels appreciably greater than those maintained in EXT (Fig. 1A). Two-way repeated measures ANOVA of the effects of JHW 007 on response rates indicated a significant effect of cocaine dose (F4,60=11.5; p<0.001), pre-session JHW 007 treatment (F3,60=14.9; p<0.001), and the interaction of the two (F12,60=12.0; p<0.001). In addition, post-hoc Bonferroni t-tests indicated that 3.2 and 10 mg/kg of JHW 007 significantly decreased rates of responding maintained by a cocaine dose of 0.1 mg/kg/inj (t=7.18 and 7.03, respectively; p values <0.001). Further, all doses of JHW 007 decreased rates of responding maintained by a cocaine dose of 0.32 mg/kg/inj (t=4.52, 7.45 and 10.4, respectively; p values <0.001).
As with JHW 007, 10 mg/kg of each of AHN 1-055 and AHN 2-005 decreased response rates maintained by cocaine (Fig. 1B, open diamonds and squares, respectively). The decreases were expressed as a flattening of the cocaine dose-effect curve. No dose of cocaine maintained responding at levels appreciably greater than those maintained in EXT (Fig. 1B, open symbols). Two-way repeated measures ANOVAs for the effects of each of the BZT analogs on response rates indicated a significant effect of cocaine dose (AHN 1-055: F4,20=9.98; p<0.001; AHN 2-005: F4,20=9.56; p<0.001), pre-session treatment (AHN 1-055: F1,20=13.7; p=0.014; AHN 2-005: F1,20=12.8; p=0.016) and the interactions of the two (AHN 1-055: F4,20=12.6; p<0.001; AHN 2-005: F4,20=13.3; p<0.001). Post-hoc tests indicated decreases in responding maintained by cocaine doses of 0.1 and 0.32 mg/kg/inj by AHN 1-055 (t=5.05 and 6.28, respectively; p values <0.001) and AHN 2-005 (t=4.97 and 6.39, respectively; p values <0.001).
In contrast to the effects of the BZT analogs, pre-session treatment with methylphenidate produced a dose-dependent leftward shift in the cocaine self-administration dose-effect curve, without affecting maximum response rate (Fig. 1C). The lowest dose was inactive; 3.2 mg/kg of methylphenidate shifted the cocaine dose-effect curve approximately threefold leftward; and 10 mg/kg of methylphenidate shifted the cocaine dose-effect curve approximately 10-fold leftward, as well as producing an increase in response rates during EXT. The ANOVA results of methylphenidate pretreatments indicated significant effects of methylphenidate pretreatments (F3,60=10.2; p<0.001), cocaine dose (F4,60=12.3; p<0.001) and the interaction (F12,60=10.9; p<0.001). Post-hoc analyses indicated that 3.2 and 10 mg/kg of methylphenidate increased rates of responding maintained by 0.032 mg/kg/inj of cocaine (t=3.94 and 7.19, respectively; p values ≤0.001) and decreased rates of responding maintained by 0.32 mg/kg/inj of cocaine (t=4.22 and 6.29, respectively; p values <0.001). Further, 10 mg/kg of methylphenidate increased response rates during EXT (t=4.13; p<0.001).
Food-Reinforced Responding
The average response rates maintained by food presentation were a bitonic function of number of pellets delivered (Fig. 1D, filled circles), with a maximum of 0.56 ± 0.12 responses/sec during the third component (2 pellets delivered), which was approximately 19-fold greater than the 0.03 responses/sec occurring during EXT. As with cocaine injection, a one-way repeated measures ANOVA indicated a significant effect of component (number of pellets) on response rate (F4, 20=15.0, p<0.001).
The highest dose of JHW 007 (10 mg/kg) was without effects on response rates maintained by food presentation (Fig. 1D, compare filled to open circles). Two-way repeated measures ANOVA of the effects of JHW 007 on response rates indicated a significant effect of number of pellets (F4,20=11.1; p<0.001), and non-significant effects of pre-session JHW 007 treatment (F1,20=1.57; p=0.266), and the interaction of the treatment and pellet number (F4,20=1.54; p=0.229).
As with JHW 007, neither AHN 1-055 nor AHN 2-005 (each at 10 mg/kg) had effects on response rates maintained by food presentation (Fig. 1D, compare filled circles to open diamonds or squares, respectively). An ANOVA of the effects on response rates of each of these drugs indicated significant effects of number of pellets (F4,20=8.56 and 12.5, respectively; p values < 0.001), and non-significant effects of treatment (F1,20=1.27 and 2.17, respectively; p values ≥ 0.201), and the interaction of the two (F4,60=0.324 and 2.73, respectively; p values ≥0.058). Methylphenidate dose-dependently decreased response rates maintained by food presentation (Fig. 1E). The decreases were expressed as a flattening of the bitonic food reinforcement-effect curve. Two-way repeated measures ANOVA of the effects of methylphenidate on response rates indicated a significant effect of number of pellet delivered (F4,60=13.8; p<0.001), pre-session methylphenidate treatment (F3,60=13.4; p<0.001), and the interaction of the two (F12,60=6.64; p<0.001). Post-hoc tests indicated that 10 mg/kg of methylphenidate decreased rates of responding maintained by presentation of one pellet (t=3.17; p=0.016). Further, 3.2 and 10 mg/kg of methylphenidate decreased rates of responding maintained by presentation of two (t=5.29, and 6.16, respectively; p values <0.001) and three (t=4.18, and 5.81, respectively; p values <0.001) pellets. Finally, 10 mg/kg of methylphenidate decreased rates of responding maintained by presentation of four pellets (t=2.94; p=.030).
Locomotor Activity and Stereotyped Behavior
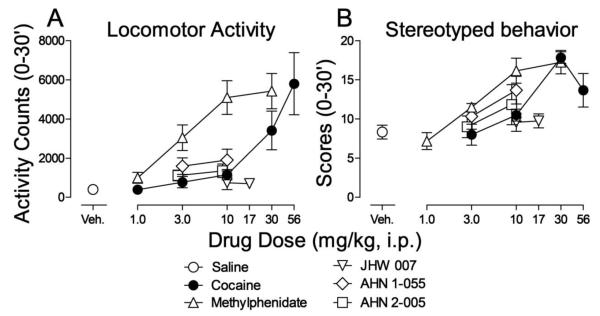
Cocaine increased activity counts in a dose-dependent manner (Fig. 2A, filled symbols). One-way ANOVA indicated a significant effect of cocaine treatment (F5,30=7.81; p<0.001). Post-hoc analysis indicated a significant effect of 56 mg/kg cocaine (t=4.89, p<0.001). The standard dopamine-uptake inhibitor methylphenidate also increased locomotor activity (Fig. 2A, open triangles up) and was approximately ten-fold more potent than cocaine. One-way ANOVA indicated a significant effect of methylphenidate treatment (F4,25=12.8; p<0.001) and post-hoc analysis indicated that doses from 3 to 30 mg/kg were significant (t=2.92, 5.18 and 5.54, p values ≤0.029).
Figure 2.
Effects of injections of dopamine uptake inhibitors alone on spontaneous locomotor activity and stereotyped behavior in naïve rats. Saline (open circles), cocaine (filled circles, 1.0 - 56 mg/kg), methylphenidate (triangles up, 1.0 - 30 mg/kg), JHW 007 (triangles down, 10 and 17 mg/kg), AHN 1-055 (diamonds, 3.0, 10 mg/kg) and AHN 2-005 (squares, 3.0 and 10 mg/kg) were administered i.p. five, five, five, 180, 90, and 90 min, respectively, before recording observations. Each point represents the mean ±SEM (N=6). Panel A. Effects on spontaneous locomotor activity. Ordinates: Activity counts (0-30 min). Abscissae: drug dose in mg/kg/inj. Veh: vehicle. Panel B. Effects on stereotypy scores. Ordinates: Stereotypy scores (0-30 min). Abscissae: drug dose in mg/kg/inj. Veh: vehicle.
None of the BZT analogs appreciably increased activity counts to the same extent as methylphenidate or cocaine (Fig. 2A). Nonetheless, one-way ANOVAs indicated significant effects of treatment on activity counts for AHN 1-055 (F2,15=3.84; p=0.045), but non-significant effects of JHW 007 (F2,15=592; p=0.565) and AHN 2-005 (F2,15=3.68; p=0.050). Despite the overall results with ANOVAs for AHN 1-055, post-hoc analyses indicated no significant effects at any doses of AHN 1-055, AHN 2-005 or JHW 007 (t values ≤2.63, p values ≥0.057).
The average scores for stereotyped behavior produced by cocaine were a bitonic function of dose, with a maximum of 17.8 ± 0.74 at a dose of 30 mg/kg (Fig. 2B, filled symbols). Oneway ANOVA indicated a significant effect of cocaine treatment (F4,25=10.3; p<0.001) and post-hoc analysis indicated significant effects at doses of 30 and 56 mg/kg (t=5.22 and 2.93, respectively, p values ≤0.029). Methylphenidate dose-dependently increased stereotypy (Fig. 2B, open triangles up), with one-way ANOVA indicating a significant effect of methylphenidate treatment (F4,25=15.0; p<0.001). Post-hoc analysis indicated significant effects of doses of 10 and 30 mg/kg (t=4.73 and 5.39, respectively, p values <0.001). In contrast, none of BZT analogs appreciably increased stereotypy scores (Fig. 2B), with one-way ANOVAs indicating non-significant effects (F2,15 values ≤3.29; p values ≥0.065).
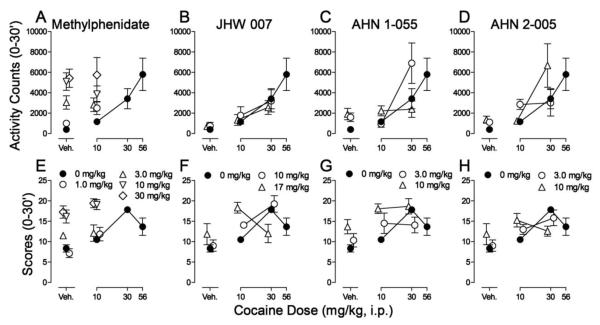
When co-administered with cocaine (10 mg/kg), methylphenidate increased the effects of cocaine on activity counts (Fig 3A). Two-way ANOVA indicated a significant effect of methylphenidate treatment (F4,50=11.8; p<0.001), and non-significant effects of the 10 mg/kg cocaine dose (F1,50=0.198; p=0.658), and the interaction of the two (F4,50=0.811; p=0.524). Methylphenidate also dose-dependently enhanced the effects of cocaine on stereotypy scores (Fig. 3E). Two-way ANOVA indicated significant effects of cocaine dose (F1,50=9.20; p=0.004) and methylphenidate treatment (F4,50=22.3; p<0.001), but a non-significant effect of the interaction of the two (F4,50=0.645; p=0.633).
Figure 3.
Effects of pretreatments with methylphenidate or N-substituted BZT analogs (JHW 007, AHN 1-055 and AHN 2-005) on spontaneous locomotor activity and stereotyped behavior in rats with cocaine injections. Cocaine (10-56 mg/kg), saline, methylphenidate (1.0-30 mg/kg), JHW 007 (10 and 17 mg/kg), AHN 1-055 (3.0 and 10 mg/kg), and AHN 2-005 (3.0 and 10 mg/kg) were administered intraperitoneally respectively five, five, five, 180, 90, and 90 min before sessions. Each point represents the mean ±SEM (N=6). Panel A-D. Effects on spontaneous locomotor activity. Ordinates: Activity counts (0-30 min). Abscissae: cocaine dose in mg/kg/inj. Veh: vehicle. Panel E-H. Effects on stereotypy scores. Ordinates: Stereotypy scores (0-30 min). Abscissae: cocaine dose in mg/kg/inj. Veh: vehicle.
Pretreatment with JHW 007 did not significantly alter the effects of cocaine on locomotor activity (Fig. 3B). Two-way ANOVA indicated a significant effect of cocaine dose (F2,45=10.8; p<0.001), but non-significant effects of JHW 007 treatment (F2,45=0.259; p=0.773) and the interaction of cocaine dose and JHW 007 treatment (F4,45=0.263; p=0.900). A dose-dependent leftward shift in the cocaine dose-effect curve for stereotypy scores was produced by JHW 007 (Fig. 3F). A two-way ANOVA indicated a non-significant effect of JHW 007 treatment (F2,45=1.90; p=0.162), but significant effects of cocaine dose (F2,45=23.5; p<0.001) and the interaction of the two (F4,45=7.80; p<0.001). Post-hoc analysis with Bonferroni t-tests indicated that 10 mg/kg of JHW 007 was without significant effects compared to cocaine alone, whereas 17 mg/kg increased (t=4.22, p<0.001) and decreased (t=3.14, p=0.009) stereotypic scores produced, respectively, by 10 and 30 mg/kg of cocaine.
At most dose combinations, AHN 1-055 did not alter the effects of cocaine on locomotor activity (Fig. 3C). Two-way ANOVA indicated a non-significant effect of AHN 1-055 treatment (F2,45=2.74; p=0.075), but significant effects of cocaine dose (F2,45=12.7; p<0.001), and the interaction of the two (F4,45=2.96; p=0.030). However, post-hoc analysis indicated that 3.0 mg/kg of AHN 1-055 significantly enhanced the effects of 30 mg/kg cocaine (t=2.90, p=0.017). AHN 1-055 produced a leftward shift of the cocaine stereotypy dose-effect curve (Fig. 3G). A two-way ANOVA indicated significant effects of cocaine dose (F2,45=11.2; p<0.001) and AHN 1-055 treatment (F2,45=7.23; p=0.002), but a non-significant effect of the interaction of the two (F4,45=1.89; p=0.129). Post-hoc analysis with Bonferroni t-test indicated that 10 mg/kg of AHN 1-055 significantly increased stereotypy scores produced by 10 mg/kg dose of cocaine (t=3.39, p=0.004).
Pretreatment with AHN 2-005 altered cocaine induced activity counts (Fig. 3D), but did not appreciably affect the cocaine stereotypy dose-effect curve (Fig. 3H). Two-way ANOVA of the locomotor activity data indicated significant effects of cocaine dose (F2,45=9.30; p<0.001) and AHN 2-005 treatment (F2,45=5.46; p=0.008), but a non-significant interaction of the two (F4,45=1.04; p=0.395). However, post hoc tests indicated that 3.0 mg/kg of AHN 2-005 significantly increased the effects of 30 mg/kg cocaine (t=3.69, p=0.002). Two-way ANOVA indicated non-significant effects of AHN 2-005 treatment (F2,45=0.345; p=0.710) on stereotypy scores, but significant effects of cocaine dose (F2,45=11.1; p<0.001) and the interaction of the two (F4,45=3.48; p=0.015). Post-hoc analysis with Bonferroni t-test indicated that 10 mg/kg of AHN 2-005 significantly decreased stereotypy scores produced by 30 mg/kg dose of cocaine (t=2.50, p=0.049).
DISCUSSION
A previous study demonstrated that pretreatment with oral doses of N-substituted BZT analogs (JHW 007, AHN 1-055, and AHN 2-005) dose-dependently blocked the self-administration of cocaine, and that those effects were obtained at doses that had no effect on comparable responding maintained by food presentation under a similar schedule of reinforcement (Hiranita et al. 2009). The present study replicated that effect with IP injections of the BZT analogs, and as in previous studies found that the typical dopamine uptake inhibitor methylphenidate dose-dependently shifted the dose-effect curve for cocaine self-administration to the left. That shift in the cocaine self-administration dose-effect curve was obtained at doses of methylphenidate that also decreased rates of food-maintained behavior. Thus the present study extended the previous study (Hiranita et al. 2009) and further confirmed that the interactions of self administered cocaine with methylphenidate differed substantially from those obtained with the BZT analogs.
The selective decrease in cocaine self-administration may suggest that the N-substituted BZT analogs antagonized the reinforcing effects of cocaine. However, the conclusion might be premature, as there is a pharmacological difference in procedures for cocaine or food reinforcement. Repeated injections of cocaine over the course of a session could promote an interaction between the BZT analogs and cocaine with the effects of the drug combination altering ongoing responding, an effect that would not occur with food reinforcement. In addition, it remains possible that the decreases in the self-administration of cocaine might result from other effects of the BZT analogs rather than a blockade of cocaine reinforcement. Given the affinity of N-substituted BZT analogs for the dopamine transporter along with their relative selectivity (Agoston et al. 1997; Katz et al. 2004; Newman et al. 1995), it is possible that the N-substituted BZT analogs induce stereotypic behaviors either by themselves or in combination with cocaine, and that these stereotyped behaviors interfere with cocaine self-administration. Indeed, a number of studies have reported the induction of such stereotyped behaviors by cocaine and other dopamine uptake inhibitors (Kalivas et al. 1988; Sahakian et al. 1975; Velazquez-Sanchez et al. 2009). Thus the present study examined whether the induction of stereotyped behaviors could account for the decreases in cocaine self-administration induced by the N-substituted BZT analogs.
Cocaine and methylphenidate when administered alone dose-dependently increased both locomotor activity and stereotypy. In contrast, none of the N-substituted BZT analogs when administered alone substantially increased either. Thus the effects of the BZT analaogs on cocaine self administration cannot be attributed primarily to increases in either of these outcomes. Further, any direct effect of the BZT analogs that might have interfered with cocaine self-administration did not interfere with comparable responding maintained by food reinforcement. These considerations suggest that the effects of the BZT analogs on cocaine self-administration were specific to behavior reinforced with cocaine injections.
It remains possible that the combinations of the BZT analogs with cocaine produced an enhanced locomotor activation or induced stereotypy that interfered with continued cocaine self-administration and given the design of this study could not have interfered with food-reinforced behavior. Although methylphenidate produced dose-related enhancements of the locomotor stimulant and stereotypy-inducing effects of cocaine, the BZT analogs did not. Nonetheless, there were occasional combinations of doses of the BZT analogs that increased the effects of cocaine on one or the other measure. However, those effects were not systematic and were obtained at doses that were uniformly greater than the doses that decreased cocaine self-administration. Thus there is little or no evidence that an induction of locomotor activity or stereotypy greater than that induced by cocaine interferes with its self-administration to produce the observed antagonist effects. Indeed, methylphenidate which induced locomotor activity and stereotypy and added to the effects of cocaine shifted the cocaine self-administration dose-effect curve leftward, whereas the BZT analogs which were ineffective in producing locomotor enhancements and stereotypy shifted the cocaine self-administration dose-effect curve downward.
In a previous study, AHN 1-055 at a dose of 10 mg/kg (i.p.) did not enhance the effects of cocaine (15 mg/kg, i.p.) on locomotor activity or stereotypy (Velazquez-Sanchez et al. 2009). Further, the absence of a change in these effects of cocaine by AHN 1-055 pretreatment was not due to the fact that a maximum effect had been obtained with cocaine alone, as a dose of d-amphetamine (4.0 mg/kg, i.p.) further increased the locomotor-stimulating and stereotypy-inducing effects of cocaine (Velazquez-Sanchez et al. 2009). This previous study along with the present results suggest that the enhancement of locomotor activity or the induction of stereotypy is not a necessary condition for the blocking of cocaine self-administration by the N-substituted BZT analogs.
Previous studies have indicated that cocaine and cocaine-like dopamine uptake inhibitors act at the dopamine transporter preferentially when it is in an outward-facing conformation (Ferrer and Javitch 1998; Loland et al. 2012; Loland et al. 2002). In contrast, such a preference is substantially reduced with a number of BZT analogs, including JHW 007 and AHN 1-005, and these compounds appear to shift the conformational equilibrium towards inward facing (Loland et al. 2008). In the study of Loland and colleagues, the decreases in potency for the inhibition of dopamine uptake in cells transfected with wild-type dopamine transporter compared with a Y335A point-mutated dopamine transporter, which assumes an inward-facing conformation, predicted whether the effectiveness of compounds in producing locomotor stimulation or cocaine-like discriminative stimulus effects. Those that exhibited relatively large decreases in potency, such as the standard dopamine uptake inhibitor WIN 35,428, were as effective as cocaine, whereas the BZT analogs that exhibited smaller changes in potency had substantially reduced cocaine-like effects (Loland et al. 2008). These results lead to the hypothesis that the unique effects of BZT analogs may involve their effects on DAT conformational status.
In the present study i.p. injections of N-substituted BZT analogs blocked cocaine self-administration, and did so at doses that had no effect on comparable responding maintained by food reinforcement. These results extend previous ones with the same compounds showing selective effects on cocaine self-administration when the compounds were administered orally. Comparing across the two studies the compounds, when administered i.p., were about ten-fold more potent in blocking the self-administration of cocaine than when administered orally. In addition, neither AHN 2-005 nor JHW 007 was self-administered in rats trained to self-administer cocaine, whereas AHN 1-055 was, but substantially less than cocaine (Hiranita et al. 2009). Several other studies have found similar outcomes in a variety of self-administration procedures (Ferragud et al. 2009; Woolverton et al. 2001; 2000), as well as place-conditioning procedures (Li et al. 2011; 2005; Veláquez-Sánchez et al. 2009; 2010). At least under the selection of experimental conditions examined to this point, these BZT analogs have little liability for abuse and block the self-administration of cocaine; and that blockade is not due to a potentiation of psychomotor effects of cocaine that interfere with its continued self-administration. Taken together with previous findings the present results support the further development of N-substituted BZT analogs as medications for cocaine abuse.
ACKNOWLEDGMENTS
We thank Dawn French-Evans for technical assistance, Patty Ballerstadt for administrative assistance, and J. J. Cao for the synthesis of compounds. Portions of this manuscript were presented at the Annual Meeting of the American Society for Pharmacology and Experimental Therapeutics, Apr 24-28, 2010; Anaheim, CA. The work reported herein was supported by the Intramural Research Program of the National Institute on Drug Abuse.
Non-standard abbreviations
- AHN 1-055
N-methyl-benztropine
- AHN 2-005
N-allylbenztropine
- BZT
benztropine
- JHW 007
N-butyl-benztropine
REFERENCES
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, Newman AH. Novel N-substituted 3 alpha-[bis(4′-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. Journal of medicinal chemistry. 1997;40:4329–39. doi: 10.1021/jm970525a. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–73. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL. Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci. 2005;25:1889–93. doi: 10.1523/JNEUROSCI.4778-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferragud A, Velazquez-Sanchez C, Hernandez-Rabaza V, Nacher A, Merino V, Carda M, Murga J, Canales JJ. A dopamine transport inhibitor with markedly low abuse liability suppresses cocaine self-administration in the rat. Psychopharmacology. 2009;207:281–9. doi: 10.1007/s00213-009-1653-x. [DOI] [PubMed] [Google Scholar]
- Ferrer JV, Javitch JA. Cocaine alters the accessibility of endogenous cysteines in putative extracellular and intracellular loops of the human dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9238–43. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH, Tanda G, Katz JL. Decreases in Cocaine Self-Administration with Dual Inhibition of the Dopamine Transporter and {sigma} Receptors. The Journal of pharmacology and experimental therapeutics. 2011;339:662–77. doi: 10.1124/jpet.111.185025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, Katz JL. Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. The Journal of pharmacology and experimental therapeutics. 2009;329:677–86. doi: 10.1124/jpet.108.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Katz JL. Reinforcing effects of sigma-receptor agonists in rats trained to self-administer cocaine. The Journal of pharmacology and experimental therapeutics. 2010;332:515–24. doi: 10.1124/jpet.109.159236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, DuMars LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. The Journal of pharmacology and experimental therapeutics. 1988;245:485–92. [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, Newman AH. Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. The Journal of pharmacology and experimental therapeutics. 2004;309:650–60. doi: 10.1124/jpet.103.060525. [DOI] [PubMed] [Google Scholar]
- Katz JL, Libby TA, Kopajtic T, Husbands SM, Newman AH. Behavioral effects of rimcazole analogues alone and in combination with cocaine. European journal of pharmacology. 2003;468:109–19. doi: 10.1016/s0014-2999(03)01638-8. [DOI] [PubMed] [Google Scholar]
- Katz JL, Newman AH, Izenwasser S. Relations between heterogeneity of dopamine transporter binding and function and the behavioral pharmacology of cocaine. Pharmacology, biochemistry, and behavior. 1997;57:505–12. doi: 10.1016/s0091-3057(96)00441-8. [DOI] [PubMed] [Google Scholar]
- Li SM, Campbell BL, Katz JL. Interactions of cocaine with dopamine uptake inhibitors or dopamine releasers in rats discriminating cocaine. The Journal of pharmacology and experimental therapeutics. 2006;317:1088–96. doi: 10.1124/jpet.105.100594. [DOI] [PubMed] [Google Scholar]
- Li SM, Kopajtic TA, O’Callaghan MJ, Agoston GE, Cao J, Newman AH, Katz JL. N- substituted benztropine analogs: selective dopamine transporter ligands with a fast onset of action and minimal cocaine-like behavioral effects. The Journal of pharmacology and experimental therapeutics. 2011;336:575–85. doi: 10.1124/jpet.110.173260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SM, Newman AH, Katz JL. Place conditioning and locomotor effects of N-substituted, 4′,4″-difluorobenztropine analogs in rats. The Journal of pharmacology and experimental therapeutics. 2005;313:1223–30. doi: 10.1124/jpet.105.084541. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, Gether U. Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Molecular pharmacology. 2008;73:813–23. doi: 10.1124/mol.107.039800. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S, Kopajtic T, Shi L, Katz JL, Tanda G, Newman AH. R-Modafinil (Armodafinil): A Unique Dopamine Uptake Inhibitor and Potential Medication for Psychostimulant Abuse. Biological psychiatry. 2012 doi: 10.1016/j.biopsych.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, Gether U. Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1683–8. doi: 10.1073/pnas.032386299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Kline RH, Allen AC, Izenwasser S, George C, Katz JL. Novel 4′-substituted and 4′,4″-disubstituted 3 alpha-(diphenylmethoxy)tropane analogs as potent and selective dopamine uptake inhibitors. Journal of medicinal chemistry. 1995;38:3933–40. doi: 10.1021/jm00020a006. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science (New York, NY. 1987;237:1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Robbins TW, Morgan MJ, Iversen SD. The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats. Brain research. 1975;84:195–205. doi: 10.1016/0006-8993(75)90975-0. [DOI] [PubMed] [Google Scholar]
- Schenk S. Effects of GBR 12909, WIN 35,428 and indatraline on cocaine self- administration and cocaine seeking in rats. Psychopharmacology. 2002;160:263–70. doi: 10.1007/s00213-001-0972-3. [DOI] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, Katz JL. Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. The Journal of pharmacology and experimental therapeutics. 2009a;330:802–9. doi: 10.1124/jpet.109.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman AH, Katz JL. Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Advances in pharmacology (San Diego, Calif. 2009b;57:253–89. doi: 10.1016/S1054-3589(08)57007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez-Sanchez C, Ferragud A, Hernandez-Rabaza V, Nacher A, Merino V, Carda M, Murga J, Canales JJ. The dopamine uptake inhibitor 3 alpha-[bis(4′-fluorophenyl)metoxy]-tropane reduces cocaine-induced early-gene expression, locomotor activity, and conditioned reward. Neuropsychopharmacology. 2009;34:2497–507. doi: 10.1038/npp.2009.78. [DOI] [PubMed] [Google Scholar]
- Velazquez-Sanchez C, Ferragud A, Murga J, Carda M, Canales JJ. The high affinity dopamine uptake inhibitor, JHW 007, blocks cocaine-induced reward, locomotor stimulation and sensitization. Eur Neuropsychopharmacol. 2010;20:501–8. doi: 10.1016/j.euroneuro.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Hecht GS, Agoston GE, Katz JL, Newman AH. Further studies of the reinforcing effects of benztropine analogs in rhesus monkeys. Psychopharmacology. 2001;154:375–82. doi: 10.1007/s002130000616. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK, Wilcox KM, Paul IA, Kline RH, Newman AH, Katz JL. 3′- and 4′-chloro-substituted analogs of benztropine: intravenous self-administration and in vitro radioligand binding studies in rhesus monkeys. Psychopharmacology. 2000;147:426–35. doi: 10.1007/s002130050012. [DOI] [PubMed] [Google Scholar]